Introduction
Mexico is considered one of the most biodiverse
countries in the world. In this country, a wide variety of flowers
grow and an estimated 23,000–30,000 species of vascular plants have
been reported (1), 3,000–5,000 of
which have medicinal properties (2).
Since pre-Hispanic times, it has been noted that plants are an
excellent source of therapeutic compounds and have been used in
Mexican Traditional Medicine (3–5). The
Eupatorium genus of the Asteraceae family comprises ~1,200 species
that mainly grow in tropical regions (4). Eupatorium aschenbornianum is an
endemic herb from Morelos, Mexico; it grows in altitudes of
>2,000 meters above sea level, specifically around the Tepozteco
National Park, and is known locally as Axihuitl. In traditional
medicine, the leaves of Axihuitl are commonly prepared as a tea
taken orally to treat tumors, skin ailments, wounds, aphthae and
gastric ulcers (6,7). Previous studies have indicated that
hexane extracts of E. aschenbornianum have gastroprotective
effects (7), as well as
anti-microbial and anti-fungal activities on diverse strains
(8,9). Those effects are attributed to the
major active compounds of E. aschenbornianum, including
terpenes, flavonoids and alkaloids (9–12).
Peptic ulcer disease represents an important public
health problem worldwide. This disease arises from acid peptic
injury of the digestive tract, which results in mucosal breaks that
reach the submucosal epithelium (13). Helicobacter pylori infection
and non-steroidal anti-inflammatory drugs (NSAIDs) are the major
risk factors for the development of gastric ulcers; however, it has
been reported that 70% of gastric ulcers are linked to H.
pylori and only a small proportion of individuals taking NSAIDs
develop ulcers (13,14). This suggests that other risk factors,
including the consumption of pepsin, certain foods, drugs,
excessive alcohol intake, bacterial or viral infections, and
physiological and psycho-sociological stresses may contribute to
the development of ulcers (15–19).
Treatments for gastric ulcers include proton pump inhibitors, which
reduce the production of stomach acid. Although these inhibitors
help to decrease mortality and morbidity rates, these
pharmaceutical products are costly and may cause adverse effects.
Therefore, products obtained from natural sources may represent
therapeutic alternatives for the treatment of this condition
(20). To date, certain studies have
determined the potential toxicity of plants used in traditional
medicine. Recent surveys have indicated that numerous medicinal
plants applied in traditional medicine have adverse effects
(21); thus, it is important to
determine the toxicology of these medicinal plants. Therefore, the
possible anti-ulcerative effects, as well as the acute toxicity of
the powdered dried stem of E. aschenbornianum, were assessed
in a rat model of ASA-induced gastric ulcers.
Materials and methods
Plant material
The stem of E. aschenbornianum plants were
harvested from Morelos, Mexico, which was made in 2015 during the
spring season and were identified by Professor Carlos Francisco
Cortés García, a Specialist in the Department of Taxonomy at the
University of Guadalajara, Mexico. Leaves, branches and flowers of
the plant were excluded, the remaining stem was dried at room
temperature until the moisture content was ~7%, the stems were then
immediately chopped and pulverized with a blender, the resultant
powder obtained was filtered through a 30-mesh (0.595 mm). Finally,
the E. aschenbornianum powder was vacuum-packed at room
temperature for one week to avoid alteration of the sample
(22).
Animals
A total of 23 male Wistar rats (age, 8 weeks;
weight, 180–220 g) were purchased from the animal facility of the
University of Guadalajara. All animal procedures were in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institute of Health from 1985. Animals were housed,
handled and cared for in accordance with the official Mexican
standards for the care and use of laboratory animals (no.
NOM-062-ZOO-1999). An internal bioethical committee at the Centro
de Investigación y Asistencia en Tecnología y Diseño del Estado de
Jalisco (Guadalajara, Mexico) reviewed and approved the animal
study. Animals were housed with controlled conditions, light cycle
(12-h light/dark cycle), relative humidity (46–50%) and temperature
(22°C). The animals had free access to rat chow and water. All were
maintained individually in polyethylene cages. The rats were fasted
for 24 h prior to the start of the experiment but had free access
to water (23).
Animal experiment to assess anti-ulcer
activity
The induction of gastric ulcers was performed
according to the method described by Konturek et al
(24), Lewis and Shaw (25) and Narayan et al (26). Rats were randomly divided into three
groups containing six animals each. The first and second groups
were induced by acetylsalicylic acid (ASA) by intragastric gavage
at a dose of 150 mg/kg body weight to generate gastric ulcers,
while the third group was administered saline solution. Immediately
after induction, the second group was fed E. aschenbornianum
powder at a dose of 400 mg/kg body weight (previous studies with
few animals suggested anti-ulcerative effects at this dose)
(22) mixed in standard rat chow,
while the other groups were fed in the same manner without E.
aschenbornianum powder. Animals were maintained individually in
cages and were fed for a period of five days. The next day, all
animals were euthanized by an intraperitoneal injection of sodium
pentobarbital (120 mg/kg). Animals were dissected and the stomachs
were carefully extracted. An incision was made on the stomach along
the greater curvature, and the stomachs were then washed with PBS.
Mucosal lesions were evaluated by macroscopic analysis.
Ulcerative index (UI) and protective
effect
Taking into account the severity of damage in the
gastric mucosa, the UI was determined as follows: UI=[1× (number
lesions of ≤1 mm) + 2× (number lesions of 1–2 mm) + 3× (number
lesions >2 mm)]/10.
The overall score was divided among the Ulcer Index
designated as 10 (27,28), while the percentage of ulcer
protection was calculated as follows: Ulcer protection (%)=[(UI
ulcer-induced group)-(UI treated group)]/(UI ulcer-induced group)
×100.
Lipid peroxidation
Gastric mucosal tissues (100 mg/ml) were placed into
20 mM Tris-HCl buffer (pH 7.4) at 4°C and homogenized using a
Tissue-Tearor (BioSpec Products Inc.). The homogenates were
centrifuged at 2,000 × g, at 4°C for 10 min; the supernatant was
collected and stored at 4°C until use. The degree of lipid
peroxidation was determined as the amount of malondialdehyde (MDA)
and 4-hydroxynonenal (HNE) using the Lipid Hydroperoxide Assay kit
(Bioquochem SL). In brief, 200 µl of the supernatant and 650 µl
10.3 nM N-methyl-2-phenylindole were added to a 1:3 mixture of
acetonitrile and methanol. Subsequently, 150 µl methanesulfonic
acid was added and the reaction mixture was incubated at 40°C for
40 min. Subsequently, the tubes were centrifuged at 5,000 × g at
room temperature for 5 min. Finally, 200 µl of the supernatant was
collected and the absorbance was measured at 586 nm. A standard
calibration curve was generated simultaneously to establish the
sample concentration.
Acute toxicity
According to the OECD 425 guidelines, it is possible
to perform a limit test (a maximum fixed dose) with five animals
using a dose of 2,000 mg/kg E. aschenbornianum as a
suggestion (29); it was then
decided to use a single administration of 400 mg/kg E.
aschenbornianum in order to demonstrate that intake at this
dose is safe. E. aschenbornianum powder was administered to
five rats by intragastric gavage at a dose of 400 mg/kg body
weight. Animals were observed for one week to detect any toxicity
signs and an additional week for any delayed toxicity. Mortality,
body weight and clinical signs were recorded. Blood samples were
collected from the tail vein 14 days after E.
aschenbornianum administration and separated to obtain serum,
which was stored at −80°C until use for the analysis of biochemical
parameters. Bilirubin was measured using the method of Jendsrassik
and Grof (30), while the activities
of alanine transaminase (ALT) and aspartate transaminase (AST) were
measured according to the Reitman and Frankel method (31). Albumin was estimated by the method of
Doumas et al (32), while
alkaline phosphatase (ALP) activity was estimated by the method of
Bessey et al (33).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistically significant differences between groups were
determined by one-way analysis of variance, followed by a Tukey's
post-hoc test. P<0.05 was considered to indicate statistical
significance. Statistical analyses were performed using SigmaStat
8.0 software (Systat Software, Inc.).
Results
Analysis of gastric lesions
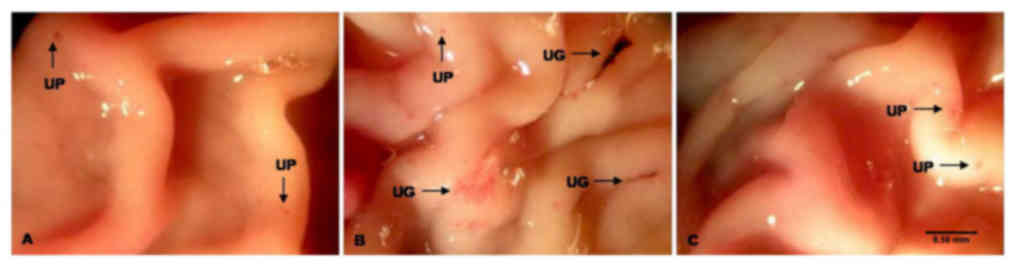
In the ulcer-induced group, gastric mucosal lesions
with a diameter of >2 mm were observed in the glandular regions
of the stomach. These mucosal lesions appeared to be black and dark
red with elongated bands. Of note, a gastroprotective effect was
observed in rats administered E. aschenbornianum powder
after the development of ASA-induced gastric ulcers. The severity
of these mucosal gastric lesions was similar to that of the
non-induced group, and the lesions were <1 mm in diameter
(Fig. 1).
Anti-ulcerative effects of E.
aschenbornianum
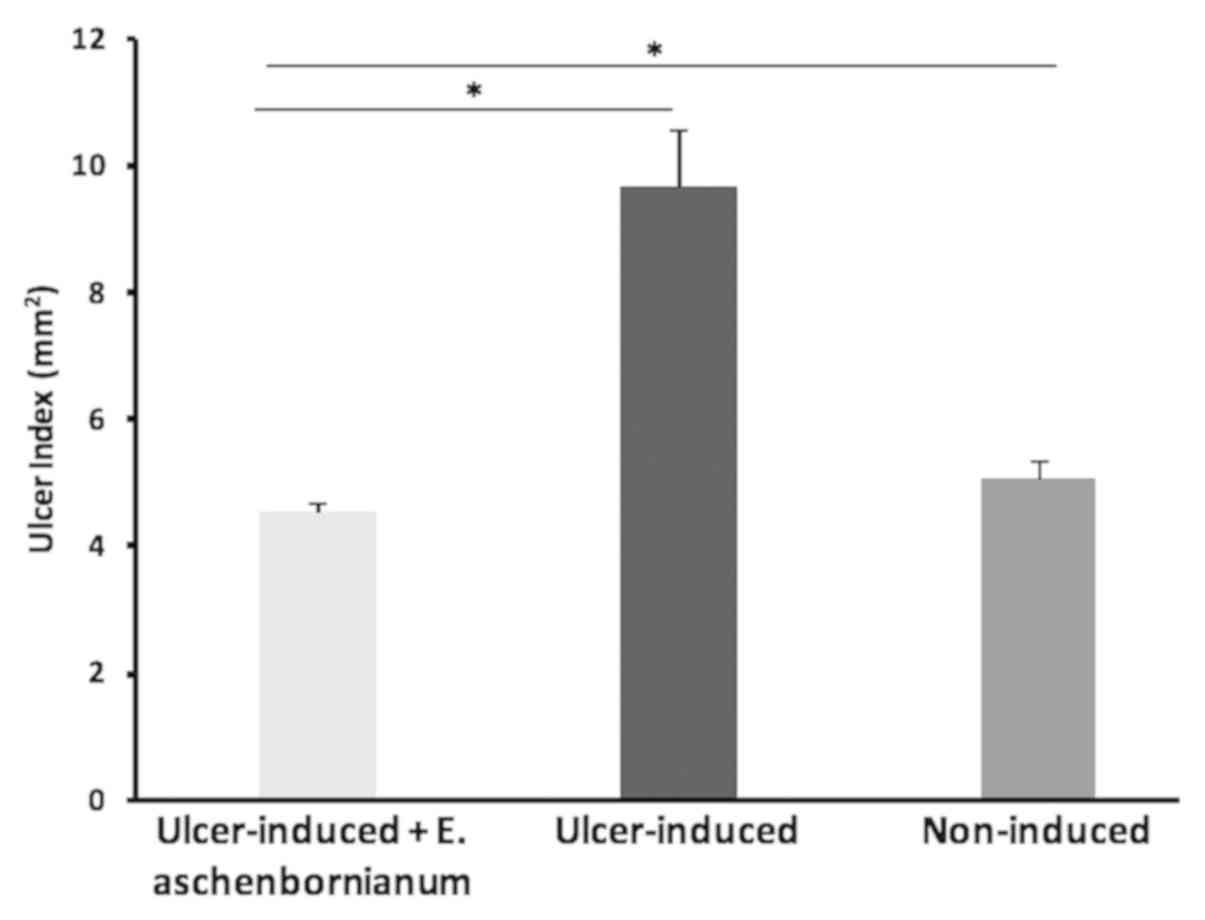
Administration of ASA caused severe gastric lesions
to develop with a UI value of 9.65±0.89 in the ulcer-induced group.
Rats of the ulcer-induced group administered E.
aschenbornianum powder exhibited a statistically significant
reduction in the severity and number of gastric lesions with a UI
of 4.52±0.14. Unexpectedly, this group also exhibited a significant
reduction in the UI value compared with the non-induced group
(UI=5.04±0.30; Fig. 2).
Effect of E. aschenbornianum on lipid
peroxidation
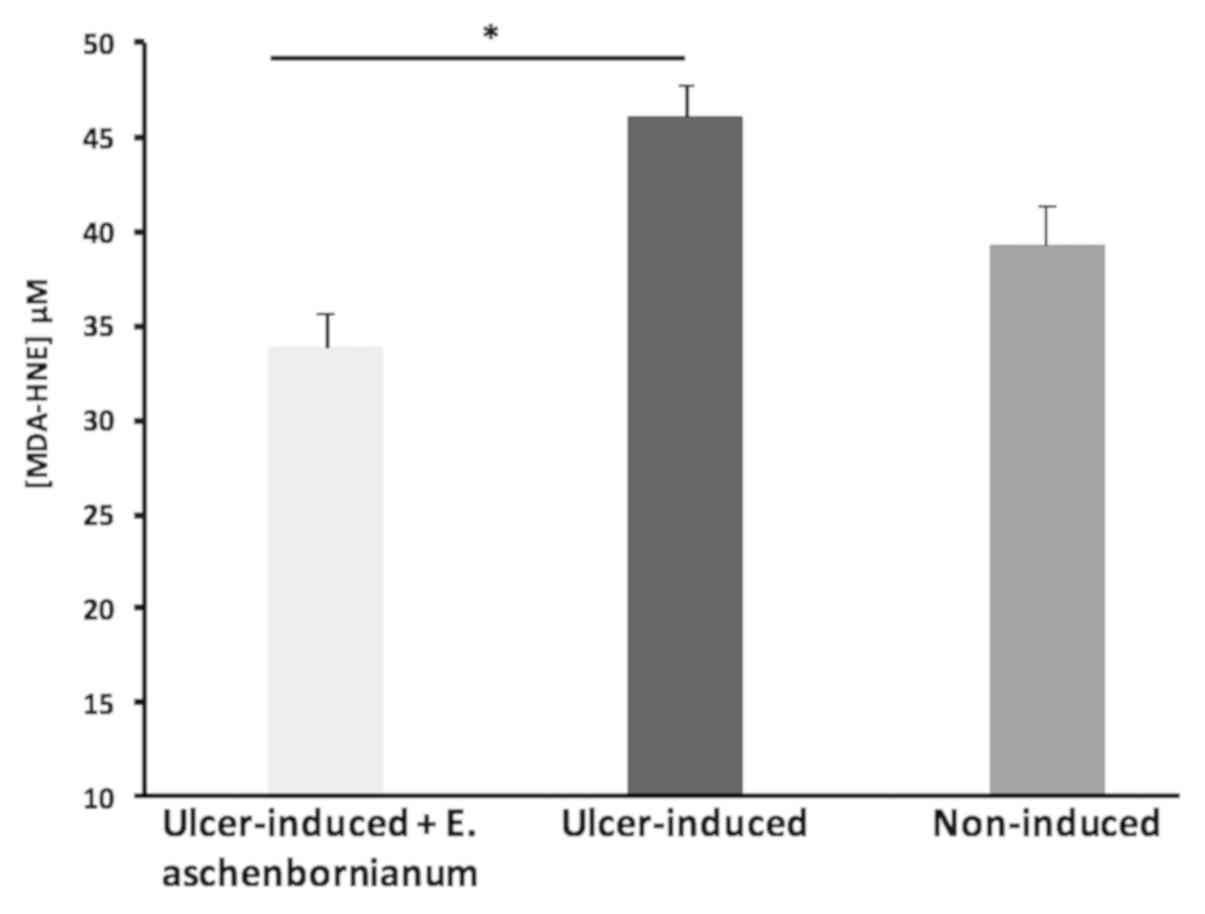
Treatment with E. aschenbornianum powder in
animals with gastric ulcers induced by ASA resulted in
significantly reduced levels of MDA-HNE aldehydes (33.79±1.84 µM)
in the gastric mucosa compared with those in the ASA-induced ulcer
group (46.00±1.65 µM); of note, the MDA-HNE aldehydes in the
treatment group were not significantly different compared with
those in the non-induced group (39.16±2.15 µM; Fig. 3).
Acute toxicity
No mortality, clinical signs of toxicity or effects
on behavior or appearance, including lethargy and immobility, were
observed throughout the study. All animals survived during the
whole study period. Similar food intake and consistent changes in
body weight were observed in the non-induced and ulcer-induced
groups (data not shown). The ulcer-induced group administered 400
mg/kg of E. aschenbornianum powder did not exhibit any
alterations in the biochemical parameters analyzed compared with
the non-induced group and the reference range (Table I) (34).
 | Table I.Effect of E. aschenbornianum
(400 mg/kg) on liver and kidney function 14 days after
administration. |
Table I.
Effect of E. aschenbornianum
(400 mg/kg) on liver and kidney function 14 days after
administration.
| Parameter | E.
aschenbornianum treatment | Control | P-value | Reference
range |
|---|
| Total bilirubin
(mg/dl) | 0.19±0.02 | 0.23±0.03 | 0.127 | 0.04–0.23> |
| Direct bilirubin
(mg/dl) | 0.05±0.01 | 0.04±0.01 | 0.288 | 0.03–0.06 |
| AST (U/l) | 120.46±2.28 | 116.00±3.06 | 0.113 | 64–222 |
| ALT (U/l) | 71.10±0.40 | 70.20±0.50 | 0.072 | 14–64 |
| ALP (U/l) | 409.20±15.82 | 356±16.43 | 0.323 | 62–230 |
| GGT (U/l) | 1.89±0.18 | 2.20±0.21 | 0.124 | <1 |
| Creatinine
(mg/dl) | 0.83±0.03 | 0.79±0.04 | 0.238 | 0.3–0.60 |
| Urea (mg/dl) | 60.28±5.45 | 51.04±4.30 | 0.082 | 13.2–27.1 |
| Albumin (g/dl) | 3.69±0.35 | 3.32±0.24 | 0.206 | 3.6–4.7 |
Discussion
Medicinal plant studies are important for the
identification of potential therapeutic agents in the treatment of
gastrointestinal disorders. In the present study, the
anti-ulcerative effects of E. aschenbornianum powder against
ASA-induced gastric ulcers were investigated in rats. The rat model
of ASA-induced gastric ulcers has been used to evaluate the
gastroprotective effects of certain compounds, gastrointestinal
irritation, stomach bleeding, lipid peroxidation and carbonylated
protein content, as well as increases in gastric myeloperoxidase
activity (35–37). Ingestion of E. aschenbornianum
powder led to a significant reduction in the UI compared with that
in the ASA-induced ulcer group; treatment with E.
aschenbornianum powder resulted in a 53.16±2.60%
gastroprotective effect in animals with ASA-induced gastric ulcers.
These results obtained with E. aschenbornianum treatment are
consistent with those of a study by Sánchez-Mendoza et al
(7), in which a hexane extract of
the leaves of E. aschenbornianum provided a gastroprotective
effect in rats with ethanol-induced gastric ulcers. Secondary
metabolites of plants, including terpenes, flavonoids and
alkaloids, have been documented to possess anti-ulcer activity
(38), and are the major active
components of plants of the Eupatorium genus (10–12).
ASA induces the production of reactive oxygen
species, which leads to increased lipid peroxidation, damaging
lipids of the cell membrane (36).
MDA and HNE aldehydes are important toxic byproducts of lipid
peroxidation and are used as indicators of tissue damage (38). The levels of MDA and HNE in gastric
mucosal tissues of rats administered E. aschenbornianum
powder were significantly lower compared with those in the
ASA-induced ulcer group, these results about the possible
antioxidant activity was similar to those reported by Tuluce et
al 2011 (37) and Krishnan et
al (39) using 50%
aqueous-ethanolic small centaury in acute gastric ulcer model and
methanolic fractions of Eupatorium triplinerve on acetic
acid induced ulcerative colitis mice model, respectively. Although
these observations suggest that E. aschenbornianum powder
exerted gastroprotective effects at the dose applied, the ability
of ASA to induce-ulcers could have been reduced in the present
study by the diet intake due to quenching by the food provided to
the rats immediately after ASA administration. Although numerous
traditional medicines are widely used to treat and prevent
diseases, their safety remains in question, as these medicinal
plants also contain several bioactive ingredients that have the
potential to cause harmful or detrimental effects. For this reason
and due to their increasing use worldwide, the safety and
pharmacological efficacy of traditional and alternative medicines
require to be evaluated (10,40,41).
The acute toxicity of E. aschenbornianum powder at 400 mg/kg
was tested; all animals exhibited notable tolerance without any
mortality or toxicity. Changes in body weight have been used as an
indicator of adverse effects of drugs and chemicals (42). No differences in body weight and food
intake were noted in the present study. The levels of biochemical
indicators used to evaluate liver function, including serum
bilirubin, AST, ALT, ALP and gamma-glutamyl transferase (43) were not significantly altered compared
with those in the non-induced group. Renal biomarkers, including
creatinine, urea and albumin indicated that kidney function was
comparable for the two groups (44,45). As
no toxic effects were observed, the consumption of E.
aschenbornianum powder applied at a unique dose may be
considered safe. Further experiments comparing the effect of E.
aschenbornianum with that of other anti-ulcer drugs are
required.
In conclusion, the present study suggested that
E. aschenbornianum powder had anti-ulcerative and
gastroprotective effects in a rat model of ASA-induced gastric
ulcers, suggesting the potential use of E. aschenbornianum
in the prevention of gastric ulcers. In addition, the consumption
of E. aschenbornianum powder was determined to be
pharmacologically safe after acute oral administration, as no
indications of liver or kidney damage were observed.
Acknowledgements
Not applicable.
Funding
Postdoctoral fellowship (grant no. 33208) by The
National Council for Science and Technology to PBRR.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JMF-F, CPB-A and PBR-R drafted the manuscript.
PBR-R, EP-C and NED-M designed the study. CPB-A, OF-F, JMF-F and
NED-M analyzed and interpreted the data. CPB-A, OF-F and JMF-F
performed the experiments, treated the animals and were responsible
for statistical analysis. EP-C, PBR-R and JMF-F revised the
manuscript. EP-C, JMF-F and PBR-R supervised and coordinated the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Animal Care and Use
Committee of the Centro de Investigación y Asistencia en Tecnología
y Diseño del Estado de Jalisco (Guadalajara, Mexico) and was
performed in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals and the official
Mexican standard for the care and use of laboratory animals
(approval no. NOM-062-ZOO-1999).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Badillo LMD, Espinosa-Madrigal RM,
Martínez-Muñoz RE, Ron-Echeverría OA, Salgado-Garciglia R,
Flores-García A, Gonzalez DR and Pacheco MMM: The Mexican medicinal
plants with antifungal properties are an economic and health
opportunity area. Pharmacologyonline. 3:61–77. 2008.
|
|
2
|
Palma-Tenango M, Miguel-Chávez RS and
Soto-Hernández RM: Aromatic and medicinal plants in Mexico,
aromatic and medicinal plants Hany El-ShemyIntechOpen; 2017
|
|
3
|
Alonso-Castro AJ, Domínguez F,
Maldonado-Miranda JJ, Castillo-Pérez LJ, Carranza-Álvarez C, Solano
E, Isiordia-Espinoza MA, Del Carmen Juárez-Vázquez M,
Zapata-Morales JR, Argueta-Fuertes MA, et al: Use of medicinal
plants by health professionals in Mexico. J Ethnopharmacol.
198:81–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobrinho ACN, de Souza EB, Rocha MFG,
Albuquerque MRJR, Bandera PN, dos Santos HS, Morais SM, Raquel ODS
and Carolina SPC: Cytotoxicity, antifungal and antioxidant
activities of the essential oil from Eupatorium
ballotifolium Kunth (Asteraceae). Afr J Pharm Pharmacol.
10:346–355. 2016. View Article : Google Scholar
|
|
5
|
Heinrich M, Frei Haller B and Leonti M: A
perspective on natural products research and ethnopharmacology in
Mexico: The eagle and the serpent on the prickly pear cactus. J Nat
Prod. 77:678–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Madaleno IM: A comparative study of
medicinal plant cultivation and uses in six Latin American cities.
Adv Environ Biol. 5:307–314. 2011.
|
|
7
|
Sánchez-Mendoza ME, Reyes-Trejo B,
Sánchez-Gómez P, Rodríguez-Silverio J, Castillo-Henkel C,
Cervantes-Cuevas H and Arrieta J: Bioassay-guided isolation of an
anti-ulcer chromene from Eupatorium aschenbornianum: Role of
nitric oxide, prostaglandins and sulfydryls. Fitoterapia. 81:66–71.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Navarro García VM, Gonzalez A, Fuentes M,
Aviles M, Rios MY, Zepeda G and Rojas MG: Antifungal activities of
nine traditional Mexican medicinal plants. J Ethnopharmacol.
87:85–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rios MY, Aguilar-Guadarrama AB and Navarro
V: Two new benzofuranes from Eupatorium aschenbornianum and
their antimicrobial activity. Planta Med. 69:967–970. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia S, Lopez R, Torres R and Pacheco M:
A revision of Eupatorium (Compositae omEupatorieae) from Michoacan.
Phyton (B Aires). 80:139–146. 2011.
|
|
11
|
Liu PY, Liu D, Li WH, Zhao T, Sauriol F,
Gu YC, Shi QW and Zhang ML: Chemical Constituents of Plants from
the Genus Eupatorium (1904–2014). Chem Biodivers. 12:1481–1515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ML, Wu M, Zhang JJ, Irwin D, Gu YC
and Shi QW: Chemical constituents of plants from the genus
Eupatorium. Chem Biodivers. 5:40–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lanas A and Chan FKL: Peptic ulcer
disease. Lancet. 390:613–624. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ford AC, Gurusamy KS, Delaney B, Forman D
and Moayyedi P: Eradication therapy for peptic ulcer disease in
Helicobacter pylori-positive people. Cochrane Database Syst Rev.
4:CD0038402016.PubMed/NCBI
|
|
15
|
Boeing T, da Silva LM, Somensi LB, Cury
BJ, Michels Costa AP, Petreanu M, Niero R and de Andrade SF:
Antiulcer mechanisms of Vernonia condensata Baker: A medicinal
plant used in the treatment of gastritis and gastric ulcer. J
Ethnopharmacol. 184:196–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cárdenas-Mondragón MG, Torres J,
Flores-Luna L, Carreón-Talavera R, Camorlinga-Ponce M and
Fuentes-Pananá EM: Epstein-Barr virus association with peptic ulcer
disease. Anal Cell Pathol (Amst). 2015:1648402015.PubMed/NCBI
|
|
17
|
Paguigan ND, Castillo DH and
Chichioco-Hernandez CL: Anti-ulcer activity of leguminosae plants.
Arq Gastroenterol. 51:64–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toma W, Gracioso Jde S, de Andrade FD,
Hiruma-Lima CA, Vilegas W and Souza Brito AR: Antiulcerogenic
activity of four extracts obtained from the bark wood of Quassia
amara L. (Simaroubaceae). Biol Pharm Bull. 25:1151–1155. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsamakidis K, Panotopoulou E,
Dimitroulopoulos D, Xinopoulos D, Christodoulou M,
Papadokostopoulou A, Karagiannis I, Kouroumalis E and Paraskevas E:
Herpes simplex virus type 1 in peptic ulcer disease: An inverse
association with Helicobacter pylori. World J Gastroenterol.
11:6644–6649. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panda VS and Khambat PD: Antiulcer
activity of Garcinia indica fruit rind (kokum berry) in rats.
Biomed Aging Pathol. 4:309–316. 2014. View Article : Google Scholar
|
|
21
|
Yuet Ping K, Darah I, Chen Y, Sreeramanan
S and Sasidharan S: Acute and subchronic toxicity study of
Euphorbia hirta L. methanol extract in rats. BioMed Res Int.
2013:1820642013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Padilla-Camberos E, Fernández-Flores O,
Canales-Aguirre A and Flores-Fernández JM: Subchronic toxicological
evaluation of Eupatorium Aschenbornianum in wistar rats.
IOSR J Environ Sci. 10:1–3. 2016.
|
|
23
|
Wang Y, Huang S, Wang Z, Chen F, Chen P,
Zhao X, Lin H, Ge R, Zirkin B and Chen H: Long-term maintenance of
luteinizing hormone-responsive testosterone formation by primary
rat Leydig cells in vitro. Mol Cell Endocrinol. 476:48–56. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konturek SJ, Brzozowski T, Drozdowicz D
and Beck G: Role of leukotrienes in acute gastric lesions induced
by ethanol, taurocholate, aspirin, platelet-activating factor and
stress in rats. Dig Dis Sci. 33:806–813. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lewis DA and Shaw GP: A natural flavonoid
and synthetic analogues protect the gastric mucosa from
aspirin-induced erosions. J Nutr Biochem. 12:95–100. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Narayan S, Devi RS, Jainu M, Sabitha KE
and Shyamala Devi CS: Protective effect of a polyherbal drug,
ambrex in ethanolinduced gastric mucosal lesions in experimental
rats. Indian J Pharmacol. 36:34–37. 2004.
|
|
27
|
Ajaikumar KB, Asheef M, Babu BH and
Padikkala J: The inhibition of gastric mucosal injury by
Punicagranatum L. (pomegranate) methanolic extract. J
Ethnopharmacol. 96:171–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Main IH and Whittle BJ: Investigation of
the vasodilator and antisecretory role of prostaglandins in the rat
gastric mucosa by use of non-steroidal anti-inflammatory drugs. Br
J Pharmacol. 53:217–224. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
OECD, . 425: Acute oral toxicity:
Up-and-down procedure. OECD Guidelines for the testing of
chemicalsParis: OECD Publishing; 2008
|
|
30
|
Jendrassik L and Grof P: Vereinfachte
photo-metrische Methoden zur Bestimmung des Blutbilirubins.
Biochemische Z Band. 297:81–89. 1938.
|
|
31
|
Reitman S and Frankel S: A colorimetric
method for the determination of serum glutamic oxalacetic and
glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doumas BT, Watson WA and Biggs HG: Albumin
standards and the measurement of serum albumin with bromcresol
green. 1971. Clin Chim Acta. 31:87–96. 1977. View Article : Google Scholar
|
|
33
|
Bessey OA, Lowry OH and Brock MJ: A method
for the rapid determination of alkaline phosphates with five cubic
millimeters of serum. J Biol Chem. 164:321–329. 1946.PubMed/NCBI
|
|
34
|
Giknis M and Clifford C: Clinical
laboratory parameters for Crl:WI(Han) rats. Accel Drug Dev. 1–14.
2008.
|
|
35
|
El-Shinnawy NA, Abd-Elmageid SA and
Alshailabi EM: Evaluation of antiulcer activity of
indole-3-carbinol and/or omeprazole on aspirin-induced gastric
ulcer in rats. Toxicol Ind Health. 30:357–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jainu M and Devi CS: Effect of ambrex (an
amber based formulation) on gastric mucosal damage: Role of
antioxidant enzymes and lipid profile. Indian J Physiol Pharmacol.
48:343–347. 2004.PubMed/NCBI
|
|
37
|
Tuluce Y, Ozkol H, Koyuncu I and Ine H:
Gastroprotective effect of small centaury (Centaurium
erythraea L) on aspirin-induced gastric damage in rats. Toxicol
Ind Health. 27:760–768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jain P: Secondary metabolites for
antiulcer activity. Nat Prod Res. 30:640–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krishnan M, Jayaraj RL, Megala J and
Elangovan N: Antioxidant mediated antiulcer effect of Eupatorium
triplinerve Vahl against acetic acid induced ulcerative colitis
in mice. Biomed Aging Pathol. 4:153–160. 2014. View Article : Google Scholar
|
|
40
|
Das SK and Roy C: The protective role of
Aegle marmelos on aspirin-induced gastro-duodenal ulceration in
albino rat model: A possible involvement of antioxidants. Saudi J
Gastroenterol. 18:188–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Firenzuoli F and Gori L: Herbal medicine
today: Clinical and research issues. Evid Based Complement Alternat
Med. 4 (Suppl 1):S37–S40. 2007. View Article : Google Scholar
|
|
42
|
Santos SR, Rangel ET, Lima JC, Silva RM,
Lopes L, Noldin VF, Cechinel Filho V, Delle Monache F and Martins
DT: Toxicological and pheytochemical studies of Aspidosperma
subincanum Mart. stem bark (Guatambu). Pharmazie. 64:836–839.
2009.PubMed/NCBI
|
|
43
|
Gowda S, Desai PB, Hull VV, Math AA,
Vernekar SN and Kulkarni SS: A review on laboratory liver function
tests. Pan Afr Med J. 3:172009.PubMed/NCBI
|
|
44
|
Wang L, Li Z, Li L, Li Y, Yu M, Zhou Y, Lv
X, Arai H and Xu Y: Acute and sub-chronic oral toxicity profiles of
the aqueous extract of Cortex Dictamni in mice and rats. J
Ethnopharmacol. 158:207–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Adeyemo-Salami OA and Makinde JM: Acute
and sub-acute toxicity studies of the methanol extract of the
leaves of Paullinia pinnata (Linn.) in Wistar albino mice
and rats. Afr J Med Med Sci. 42:81–90. 2013.PubMed/NCBI
|