Introduction
Knee osteoarthritis (OA) is one of the predominant
causes of knee disability, which is characterized by the gradual
deterioration of the articular cartilage, hypertrophic inflammatory
synovium and subchondral osteophyte formation (1). OA causes joint pain, limitation of
joint function and impaired mobility. This disease is widely
regarded as being multifactorial rather than a simple degradative
disease. Accumulating evidence has shown that low-grade chronic
synovial inflammation is involved in the entire pathological
process of OA (2). Proinflammatory
cytokines, including interleukin-1β (IL-1β), tumor necrosis factor
α (TNF-α) and interleukin 6 (IL-6), have been consistently found to
be elevated in the synovial fluid, synovial tissues and
extracellular matrix around the cartilage (3). Hyperactive and hyperplastic synovial
tissues can lead to the synthesis and release of abundant
cytokines, chemokines, matrix metalloproteinases and collagenases,
which can in turn trigger macrophage infiltration, cartilage
degradation and chondrocyte apoptosis (2,4).
However, the molecular mechanism underlying the hyperproliferation
of osteoarthritis synovial fibroblasts (OASFs) remains unclear.
A large number of genes have been found to be
aberrantly expressed in OASFs compared with non-OASFs (5). According to a previous gene expression
profiling analysis performed in our laboratory, it was found from
microarray assays that growth arrest-specific gene 1 (GAS1)
was downregulated in OASFs (Jin et al, unpublished). Growth
arrest-specific gene 1 (GAS1) is a 37 kDa
glycosyl-phosphatidylinositol-anchored protein (6). It has been shown to serve as a
suppressive regulator of cell growth and as a mediator of apoptosis
in a variety of normal or neoplastic of cell types, including mouse
fibroblasts, human glioma, thyroid and breast cancer cells
(7–9). The expression of GAS1 has been found to
be reduced in, and negatively associated with the progression and
metastasis of, various malignancies, including gastric, breast and
colorectal cancer, and glioblastoma (9–13).
Previous studies have revealed that GAS1 can directly inactivate
RET, thereby inhibiting the RET-mediated PI3K-Akt/Bad tumorigenesis
pathway (14,15). However, the biological role of GAS1
in SFs during OA pathogenesis is poorly understood. Therefore, it
may be hypothesized that the downregulation of GAS1 can contribute
to the proliferative phenotype of OASFs to aggravate inflammation
in OA joints.
MicroRNAs (miRNAs or miRs) belong to a family of
single-stranded non-coding RNA molecules (~20–26 nucleotides in
length) that regulate mRNA translation and stability in multiple
physiological and pathological settings (16). Accumulating evidence demonstrates
that miRNAs can alter the epigenetic profiles of multiple cell
types, in turn affecting their downstream biological and
pathological processes, including cell proliferation,
differentiation, invasion and angiogenesis (17). Indeed, numerous studies have reported
that miR-146a, miR-155, miR-124a and miR-126 are widely implicated
in inflammation or hyperplasia in synovial tissues and OA
pathogenesis (18–20). Considering miRNA sequencing data
(21–23) and Targetscan 7.2 prediction results,
it was hypothesized that potential miRNA candidates that can
directly regulate GAS1 expression may exist in OASFs.
The present study sought to elucidate the role of
GAS1 in hyperplastic OASFs in addition to the molecular mechanisms
involved. Subsequent experiments were applied to verify and
investigate potential miRNA candidates that can regulate
GAS1 expression in OASFs.
Materials and methods
Synovial tissue specimens
All human synovial specimens were acquired in the
Department of Orthopedics, Tangdu Hospital of the Fourth Military
Medical University (Xi'an, China) between May 2016 and September
2017. A total of 10 OA synovial tissue specimens were collected
from end-stage OA patients (sex, 4 males and 6 females; age range,
55–78 years) with total knee arthroplasty and from seven non-OA
counterparts (sex, 5 males and 2 females; age range, 34–67 years)
following traumatic amputation, respectively. Patients with
rheumatoid arthritis, potential bacterial infection of the knee
joint and other inflammatory arthritis, including Lupus arthritis,
ankylosing arthritis and seronegative arthritis were excluded from
the study. Informed consent was obtained from all the participants
concerned in the present study, and the study was approved by the
Institutional Review Board of Tangdu Hospital, Fourth Military
Medical University (Xi'an, China).
Primary culture of synovial
fibroblasts and IL-1β stimulation
Synovial tissues freshly obtained from OA and non-OA
patients (average tissue size, 1×1×0.5 cm) were washed with
phosphate-buffered saline (PBS), minced thoroughly using a scalpel,
and subjected to 2 h enzymatic digestion in DMEM/F12 (HyClone; GE
Healthcare Life Sciences) supplemented with 1 mg/ml type I
collagenase (Worthington Biochemical Corporation) on a shaking
incubator (speed, 100 rpm) at 37°C. Cell suspensions were filtered
using a 100-µm cell strainer (BD Biosciences) and placed in 35
cm2 tissue culture flasks containing DMEM/F12
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin. The primary medium
was then switched after 48 h incubation (37°C, 5% CO2)
to remove dead tissue and non-adherent cells. A total of 1–2
passages were performed after 5 days of culture when the adherent
cells approached 70% confluence. Following three generations of
passage, highly purified SFs were used for further assays.
Luciferase assays were performed in 293T cell lines, which were
maintained in DMEM with 10% FBS in a constant-temperature incubator
(37°C, 5% CO2). For IL-1β stimulation, the sub-cultured
SFs at passage 3 were seeded into 6-well dishes at a density of
2×105 cells/well. After 48 h incubation, when the cell
confluence was ~80%, cells were exposed to conditioned medium
(DMEM/F12 with 1% FBS) supplemented with various concentrations (5,
10 or 20 ng/ml) of IL-1β (R&D Systems, Inc.). A negative
control group was established by using an equivalent volume of PBS
instead of IL-1β.
RNA extraction, cDNA synthesis and
reverse transcription-quantitative PCR (RT-qPCR)
Total cellular RNA from non-OASFs and OASFs was
isolated and purified using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. Subsequent cDNA synthesis for miRNA detection was
performed using miDETECT A Track™ miRNA qPCR Starter Kit (Guangzhou
RiboBio Co., Ltd.) from 1 µg total RNA of each sample, according to
manufacturer's protocol. The temperature protocol for cDNA was set
as follows: Poly (A) tailing at 37°C for 1 h, reverse transcription
reaction at 42°C for 1 h, followed by inactivation at 72°C for 10
min. The qPCR reaction was performed in a Rotor-Gene Q PCR cycler
system (Qiagen GmbH). The temperature protocol for miRNA qPCR was
set as follows: Initial enzyme activation at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 2 sec, annealing
at 60°C for 20 sec, extension and fluorescence detection at 70°C
for 10 sec. Subsequent cDNA synthesis for mRNA detection was
performed using 5X All-In-One RT MasterMix kit (Applied Biological
Materials, Inc.) from 1 µg total RNA, according to the
manufacturer's protocol. The temperature program was set as
follows: Incubation at 25°C for 10 min, followed by 42°C for 15 min
and, finally, inactivation at 85°C for 5 min. Subsequent qPCR was
performed using the EvaGreen® 2X qPCR MasterMix kit
(Applied Biological Materials, Inc.) also in the Rotor-Gene Q PCR
cycler system, according to the manufacturer's protocol. The
thermocycling conditions for qPCR were set as follows: Initial
enzyme activation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing and extension at 60°C
for 1 min.
For miRNA expression detection, U6 was also measured
and served as the internal control, whilst GAPDH was used as the
internal control for mRNA expression detection. The oligonucleotide
primers for qPCR were purchased from Sangon Biotech Co., Ltd.
miScript Primers for miR-34a-5p, miR-203a-3p, miR-181a-5p and U6
were purchased from Qiagen, Inc. The sequences of qPCR primers used
for mRNA and miRNA detection are listed in Table I. Results were normalized to the
respective internal controls using the 2−ΔΔCq method
(24).
 | Table I.Sequences for primers, siRNAs, miRNA
mimics and inhibitors. |
Table I.
Sequences for primers, siRNAs, miRNA
mimics and inhibitors.
| Name | Sequence (5′ to
3′) |
|---|
| qPCR primers for
mRNA detection |
|
|
GAPDH |
|
|
Forward |
GGAGCGAGATCCCTCCAAAAT |
|
Reverse |
GGCTGTTGTCATACTTCTCATGG |
|
GAS1 |
|
|
Forward |
ATGCCGCACCGTCATTGAG |
|
Reverse |
TCATCGTAGTAGTCGTCCAGG |
| qPCR primers for
miRNA detection |
|
|
Hsa-miR-34a_1 Forward |
UGGCAGUGUCUUGGUUGU |
|
Hsa-miR-181a_2 Forward |
AACAUUCAACGCUGUCGGUGAGU |
|
Hsa-miR-203_1 Forward |
GUGAAAUGUUUAGGACCACUAG |
| U6
Forward |
CGCAAGGATGACACGCAAATTC |
|
Universal Reverse |
GACGAGGACTCGAGCTCAAGCT |
| Primers for GAS1
coding sequence subcloning |
|
|
Forward |
ATCGAATTCCTTCCTGGTAATTCTTCACCTCTT |
|
Reverse |
ATCGCTCGAGAGTGGCCGATTGAAAGGTATATT |
| Primers for
Luciferase assay |
|
|
GAS1-wt-3′UTR |
|
|
Forward |
ATCGCTCGAGGTCCCACTTACCGATTCATTCT |
|
Reverse |
ATATGCGGCCGCTCACAATGGACTGTGGGTTT |
| GAS1-mt-3′UTR for
miRNA-34a-5p |
|
| Primer
1 |
|
|
Forward |
TAAAAAAGCTCTGCTCTGCCATGTATGAAAGTCTC |
|
Reverse |
GAGACTTTCATACATGGCAGAGCAGAGCTTTTTTA |
| Primer
2 |
|
|
Forward |
AAAAAAGCTCTGCTGTGCCATGTATGAAAGTCTCT |
|
Reverse |
AGAGACTTTCATACATGGCACAGCAGAGCTTTTTT |
| Primer
3 |
|
|
Forward |
AAAAGCTCTGCTGAGCCATGTATGAAAGTCTC |
|
Reverse |
GAGACTTTCATACATGGCTCAGCAGAGCTTTT |
| Primer
4 |
|
|
Forward |
AAAAGCTCTGCTGACCCATGTATGAAAGTCTCTTT |
|
Reverse |
AAAGAGACTTTCATACATGGGTCAGCAGAGCTTTT |
| Primer
5 |
|
|
Forward |
TAAAAAAGCTCTGCTGACGCATGTATGAAAGTCTC |
|
Reverse |
GAGACTTTCATACATGCGTCAGCAGAGCTTTTTTA |
| GAS1-mt-3′UTR for
miRNA-181a-5p |
|
| Primer
1 |
|
|
Forward |
GTTTAAATATGCGGAGTTTGTATATTGCCTCTGCTCC |
|
Reverse |
GGAGCAGAGGCAATATACAAACTCCGCATATTTAAAC |
| Primer
2 |
|
|
Forward |
GTTTAAATATGCGGAGTTACTATATTGCCTCTGCTCC |
|
Reverse |
GGAGCAGAGGCAATATAGTAACTCCGCATATTTAAAC |
| siRNAs |
|
|
SiRNA-GAS1 |
|
|
Sense |
CUACUACGACGAAGAAUAUTT |
|
Anti-sense |
AUAUUCUUCGUCGUAGUAGTT |
|
SiRNA-NC |
|
|
Sense |
UUCUCCGACGUGUCACGUTT |
|
Anti-sense |
ACGUGACACGUUCGGAGAATT |
| miRNA mimics and
inhibitors |
|
|
miR-34a-5p mimics |
UGGCAGUGUCUUAGCUGGUUGUAACCAGCUAAGACACUGCCAUU |
|
miR-181a-5p mimics |
AACAUUCAACGCUGUCGGUGAGUUCACCGACAGCGUUGAAUGUUUU |
|
miR-NC |
CAGUACUUUUGUGUAGUACAA |
|
miR-34a-5p inhibitor |
ACAACCAGCUAAGACACUGCCA |
|
miR-181a-5p inhibitor |
ACUCACCGACAGCGUUGAAUGUU |
GAS1 plasmid construction
pcDNA3.1(+) plasmid (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for GAS1 overexpression vector
construction. To obtain a GAS1 coding sequence (CDS) for
subcloning, a PCR procedure was performed to generate a 1600-bp DNA
product using a pair of primers containing EcoRI and
XhoI restricting sites, using Taq DNA polymerase
(Invitrogen; Thermo Fisher Scientific, Inc.) and template cDNA
produced by from reverse transcription from non-OASF mRNA. The
thermocycling protocol for this PCR reaction was set as follows:
Initial denaturation at 95°C for 3 min, followed by 35 cycles of
denaturation at 95°C for 30 sec, annealing at 56°C for 45 sec, and
extension at 72°C for 1 min; The final extension is at 72°C for 10
min. primers for the PCR sub-cloning of GAS1 CDS are listed in
Table I. The PCR products and
pcDNA3.1(+) vector were first digested with EcoRI and
XhoI and subsequently ligated. The assembled
pcDNA3.1(+)-GAS1 (pcDNA-GAS1) was then ready for transfection, and
pcDNA3.1(+) (pcDNA-vector) was also used as the negative
control.
Nucleotide transfection into
cells
Cells used for nucleotide transfection were
distributed into the following groups: siRNA-GAS1, siRNA-NC,
pcDNA-GAS1, pcDNA-vector and Blank control group. SFs in the
siRNA-GAS1 and siRNA-NC groups were transfected with GAS1-specific
siRNA and siRNA negative control, respectively. SFs in pcDNA-GAS1
and pcDNA-vector groups were transfected with reconstructed
pcDNA3.1(+)-GAS1 plasmid and pcDNA3.1(+) plasmid, respectively. For
miRNA transfection, miR-34a-5p and miR-181a-5p mimics and
corresponding inhibitors were transfected into non-OASFs. miRNA
mimics NC were used as negative control for miRNA mimics. siRNAs,
miRNA mimics and inhibitors were purchased from Sangon Biotech Co.,
Ltd., and the sequences of these nucleotide products are listed in
Table I. For the transfection
procedure, SFs at passage 3 were trypsinized, distributed equally
(2×105 per well) into 6-well plates and incubated under
the condition of 37°C, 5% CO2. When 60% cell confluence
was achieved, 2.5 µg plasmids, 75 pmol siRNA, miRNA mimics or
inhibitors were added by mixing with 5 µl Lipofectamine®
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and 250 µl Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.)
per well, according to manufacturer's protocol. A blank group was
set up by using the same volume of PBS in place of the nucleotides
and Lipofectamine 2000. After 6 h incubation under 37°C and 5%
CO2, the medium was changed to normal DMEM/F12. After
another 48 h incubation under the same condition, cells were ready
for subsequent experiments. The GAS1 mRNA expression and miRNAs
expression were assessed using RT-qPCR 48 h following transfection
according to standard protocols. To examine further if PI3K-Akt
pathway activity is involved after GAS1 knockdown in non-OASFs,
LY294002, an inhibitor of PI3K (50 µM; MedChemExpress) was added to
the wells 2 h prior to siRNA-GAS1 transfection of non-OASFs, which
is set as the siRNA-GAS1-LY group,
Immunofluorescence
Immunofluorescence detection was performed to verify
siRNA transfection efficiency. At 48 h after transfection, cells
from each group were inoculated into 8-well chamber slides (Thermo
Fisher Scientific, Inc) at a density of 1×104
cells/well. Cells were subsequently cultured (37°C, 5%
CO2) until 70% confluence was reached, following which
they were washed with PBS and fixed with 4% paraformaldehyde at
room temperature for 10 min, followed by permeabilization with 0.5%
Triton X-100 (Sigma-Aldrich; Merck KGaA) for 30 min at room
temperature. For subsequent blocking, 5% bovine serum albumin (BSA)
solution (Sigma-Aldrich; Merck KGaA) was added to each chamber at
room temperature for 1 h. The cells were then incubated with rabbit
polyclonal anti-GAS1 antibody (1:200; cat. no. 17903-1-AP;
ProteinTech Group, Inc.) at 4°C overnight, followed by washing with
PBS and incubation with goat Cy3-conjugated anti-rabbit IgG
secondary antibody (1:100; cat.no. SA00009-2; ProteinTech Group,
Inc.) in the dark at room temperature for 1 h. Subsequently, the
cells were incubated with DAPI Staining Solution (1:1,000; cat. no.
28718-90-3; Beyotime Institute of Biotechnology) in the dark at
room temperature for 10 min and washed with PBS. Images of the
cells were captured using Olympus IX71 fluorescent microscope
(magnification, ×400; Olympus Corporation).
Protein extraction and western
blotting assay
Cells at 80% confluence were lysed on ice using RIPA
lysis buffer (Sigma-Aldrich; Merck KGaA) supplemented with
proteinase inhibitor cocktail (100X; Beijing ComWin Biotech Co.,
Ltd.) and phosphatase inhibitor cocktail (Beijing Solarbio Science
& Technology Co., Ltd.). After 30 min incubation on ice, cell
protein lysates were collected and centrifuged under 15,000 × g for
10 min at 4°C. Protein concentrations were quantified using a
bicinchoninic acid assay (Beijing ComWin Biotech Co., Ltd.). A
total of 40 µg protein samples were separated by 10% SDS-PAGE
(Bio-Rad Laboratories, Inc.) and transferred onto PVDF membranes
(Thermo Fisher Scientific, Inc.). Membranes were first blocked in
5% (w/v) non-fat milk solution for 1 h at room temperature before
being incubated in specific primary antibody (Ab) solutions at 4°C
overnight. The primary Abs used were as follows: GAS1 polyclonal
rabbit Ab (1:1,000; cat. no. 17903-1-AP; ProteinTech Group, Inc.);
PI3K (p85) polyclonal rabbit Ab (1:1,000; cat. no. 4292; Cell
Signaling Technology, Inc.); phosphorylated (p)-Akt (Ser473)
polyclonal rabbit Ab (1:1,000; cat. no. 9271; Cell Signaling
Technology, Inc.); total Akt polyclonal rabbit Ab (1:1,000; cat.
no. 9272; Cell Signaling Technology, Inc.); cyclin-dependent kinase
2 (Cdk2) polyclonal rabbit Ab (1:1,000; cat. no. 2546; Cell
Signaling Technology, Inc.); Bax polyclonal rabbit Ab (1:750; cat.
no. ab199677; Abcam); and GAPDH polyclonal rabbit Ab (1:2,000; cat.
no. 2118; Cell Signaling Technology, Inc.). Subsequently, membranes
were incubated in room temperature with goat horseradish
peroxidase-conjugated anti-rabbit IgG secondary Ab (1:5,000; cat.
no. 7074; Cell Signaling Technology, Inc.) for 2 h, followed by
extensive washing for 15 min three times. Protein bands were
visualized using Immobilon Western Chemiluminescent HRP Substrate
(EMD Millipore), and band intensities were measured using a
ChemiDoc XRS chemiluminescence imaging system (Bio-Rad
Laboratories, Inc.), according to the manufacturer's protocols.
Densitometric analysis and quantification were performed using
ImageJ (version 1.52a; National Institutes of Health) and were
normalized to GAPDH. The Akt phosphorylation ratios were calculated
using the following formula: (p-Akt/GAPDH)/(total Akt/GAPDH).
Cell viability assay
A Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was applied to assess the cell viability
of SFs. Transfected SFs were trypsinized and seeded in 96 well
plates (1×104 cells/well) in triplicate followed by
incubation at 37°C and 5% CO2. A total of 10 µl CCK-8
solution was then added to each well 0, 24, 48, 72 and 96 h after
culture for 1 h, following which optical density (OD) was measured
at 450 nm using an Infinite M200 Pro multifunctional microplate
reader (Tecan Group, Ltd.).
Apoptosis and cell cycle detection by
flow-cytometry
For apoptosis assays, SFs in suspension were
collected, rinsed with ice-cold PBS and kept on ice at a
concentration of 1×106 cells/ml in a volume of 500 µl 48
h after transfection. In total, 3 µl Annexin V-FITC (Nanjing Keygen
Biotech. Co. Ltd.) was added to each sample and incubated in the
dark room for 15 min at 4°C. Subsequently, 5 µl propidium iodide
(PI; Nanjing Keygen Biotech. Co. Ltd.) was added and incubated for
5 min at 4°C. Following staining, samples were then subjected to
flow cytometry analysis (NovoCyte®; ACEA Bioscience,
Inc.).
For cell cycle analysis, transfected SFs were washed
three times and fixed with 70% ethanol at 4°C overnight to a final
concentration of 1×106 cells/ml. The cells were then
rinsed with PBS and centrifuged (500 × g and 5 min at room
temperature) to eliminate the fixation buffer, before they were
mixed with 500 µl PI/RNase solution and incubated for 60 min in the
dark at room temperature Finally, cell samples were measured using
flow cytometry (NovoCyte) at a wavelength of 450 nm using the
NovoExpress™ software (version 1.2.1; ACEA Biosciences, Inc.).
miRNA prediction
Candidate miRNAs and corresponding binding sites
were predicted with the help of Targetscan website (version 7.2;
http://www.targetscan.org/vert_72/).
By entering GAS1 in the ‘Gene symbol’, a list of miRNA families
whose seed regions match the 3′untranslated region (3′UTR) of GAS1
were obtained. In total, 18 miRNA families and corresponding
binding sites were listed as ‘broadly conserved among vertebrates.’
Following a literature search, two previous studies were found
showing differentially expressed miRNAs between OA and non-OA
synovial tissues, synovial fluids or synovial fibroblasts (21,23). By
pooling the data obtained from the predicted miRNA list and these
two studies, three miRNA candidates potentially regulating GAS1
expression in OASFs were shortlisted.
Luciferase assay
For the luciferase assay, the psi-CHECK2 vector
(Promega Corporation) was used to construct luciferase reporter
plasmids. The wild-type fragment of GAS1 3′untranslated
region (GAS1-wt-3′UTR) containing the binding sites for candidate
miRNAs, was sub-cloned by PCR using specific primers containing
restriction enzyme cutting sites for XhoI and NotI.
The template cDNA used for cloning was acquired following reverse
transcription from non-OASF mRNA. Mutant 3′UTR fragments of
GAS1 (GAS1-mut-3′UTR), mutated at the predicted miR-34a-5p
or miR-181a-5p binding sites, were created by site-directed
mutagenesis in which consecutive bases were replaced to disrupt
miR-34a-5p or miR-181a-5p binding. The sequences of GAS1-wt-3′UTR
and GAS1-mt-3′UTR primers used were listed in Table I. PCR products and the psi-CHECK2
reporter vector were digested with XhoI and NotI for
16 h at 37°C and ligated overnight at 16°C. The assembled
luciferase reporter vectors were amplified and purified, then 2.5
µg reconstructed reporter vector was co-transfected alongside 75
pmol miRNA mimics into 6-well plates of 293T cells using
Lipofectamine 2000 reagent and Opti-MEM, according to
manufacturers' protocols. The cells were then seeded into 96 well
plates at a concentration of 1×104 cells/well. After 48
h incubation, luciferase activity was tested in each well using the
Luc-Pair™ Duo-Luciferase HS Assay Kit (GeneCopoeia, Inc.) on an
Infinite M200 Pro Multifunctional microplate reader (Tecan Group,
Ltd.). All firefly luciferase activity was normalized to
Renilla luciferase activity.
Statistical analysis
For RT-qPCR, CCK-8 cell viability and luciferase
assays, data were normalized to corresponding controls and are
presented as the mean ± SEM from ≥3 separate replicates. One-way
ANOVA was performed for multiple comparisons, followed by Tukey's
honest significant difference test for pairwise comparisons.
Statistical analysis was performed using GraphPad prism 7.00
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
GAS1 expression is downregulated in
OASFs
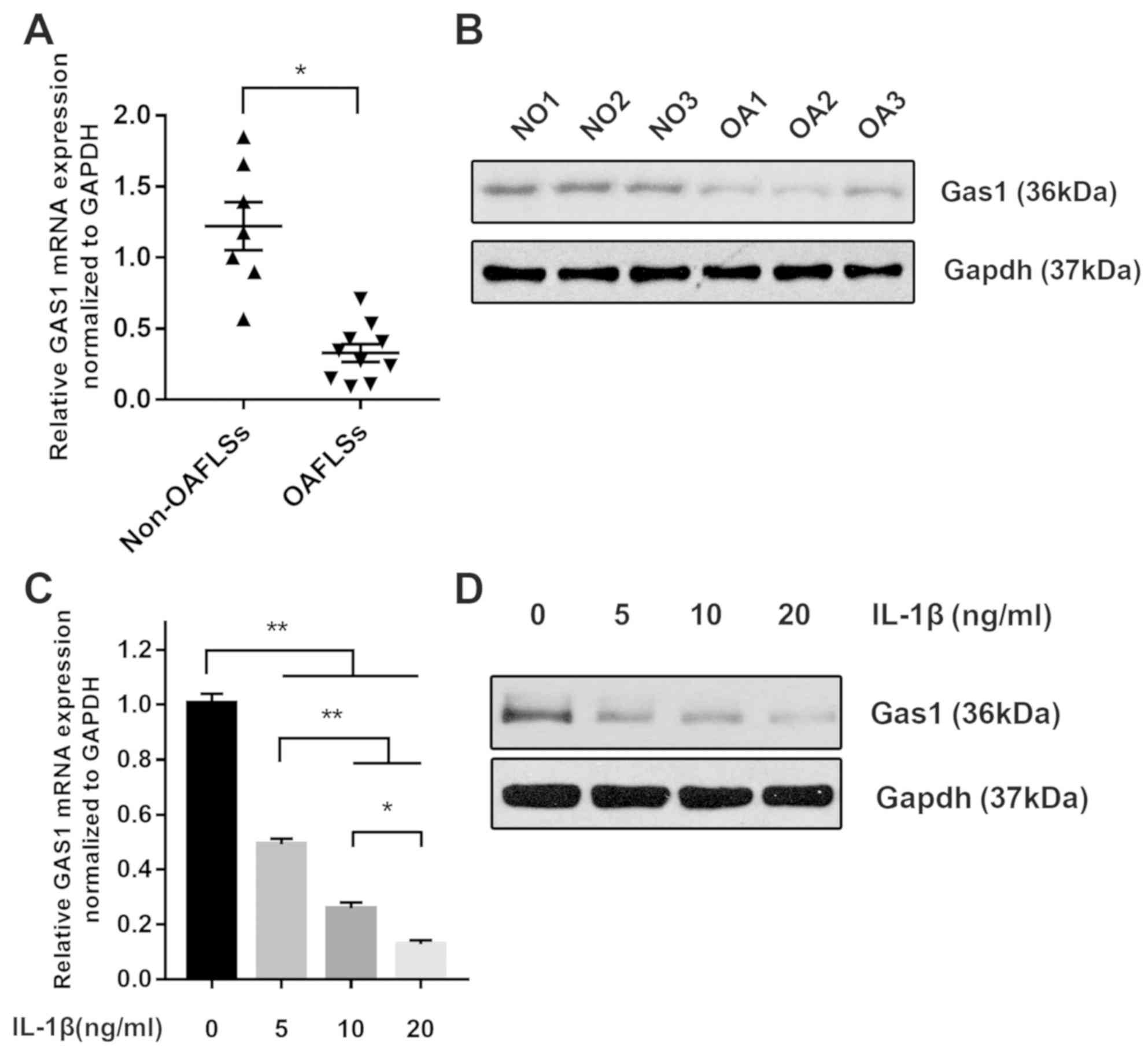
The expression of GAS1 was first assessed
using RT-qPCR and western blotting in non-OASFs and OASFs. In
total, seven non-OASF and 10 OASFs samples were examined for
RT-qPCR. For western blotting, three individual protein samples
were randomly selected from each group for protein measurements.
The expression of GAS1 was found to be downregulated in
OASFs compared with non-OASFs on mRNA and protein levels (Fig. 1A and B), consistent with the findings
of our previous gene profiling (Jin et al, unpublished),
which led to further study into the function of GAS1 in OASFs.
IL-1β treatment downregulates GAS1
expression in non-OASFs
IL-1β is a well-known pro-inflammatory cytokine
which has been reported to exhibit growth-promoting properties in
synovial tissue of OA patients (25). Therefore, multiple concentrations of
IL-1β (5, 10 and 20 ng/ml) were used to stimulate non-OASFs. The
expression of GAS1 in the IL-1β-stimulated groups was
reduced at the mRNA and protein levels compared with corresponding
the PBS control group, in a dose-dependent manner (Fig. 1C and D). This suggested that IL-1β
stimulation may suppress GAS1 expression in a dose-dependent
manner.
GAS1 overexpression inhibits SF
proliferation and promotes apoptosis
To evaluate the function of GAS1 in SFs, GAS1
expression was either overexpressed or knocked down in non-OASFs.
Transfection efficiency was subsequently verified using qPCR and
immunofluorescence (Fig. S1). The
cell viability assay was assessed at different indicated time
points using CCK-8 assay in each group (Fig. 2A). At 96 h timepoint, the OD values
of the siRNA-GAS1 group were significantly higher compared with
those in the siRNA-NC group (P=0.0013). In contrast, the OD value
for the pcDNA-GAS1 group at 96 h timepoint was significantly lower
compared with that in the pcDNA-vector group (P=0.0439). By
observing the cell viability curves for each group, it was noticed
that the siRNA-GAS1 group grew faster compared with other groups,
whereas those in the pcDNA-GAS1 group grew at a less rapid rate.
For the cell cycle analysis of each group were subsequently
evaluated using flow cytometry at 48 h after transfection (Fig. 2B-D). siRNA-GAS1 transfection
significantly increased the frequency of non-OASFs in the G2-M
phase compared with cells transfected with siRNA-NC (P=0.038;
Fig. 2B and C); while the frequency
of cells in the G0-G1 phase was increased in the pcDNA-GAS1 group
compared with those in the pcDNA-vector group (P=0.039; Fig. 2B and D). In terms of apoptosis
(Fig. 3A and B), cells from the
pcDNA-GAS1 group exhibited a significantly higher frequency of
end-stage apoptosis compared with those in the pcDNA-vector group
(P<0.001; Fig. 3A and B); cells
in the siRNA-GAS1 group exhibited a slight but significant
reduction in the frequency of early-stage apoptosis (P=0.004;
Fig. 3A and B) Taken together, these
findings suggested that GAS1 overexpression inhibit SF cell
viability whilst promoting apoptosis, whereas GAS1 knockdown
increases SF proliferation and inhibit apoptosis.
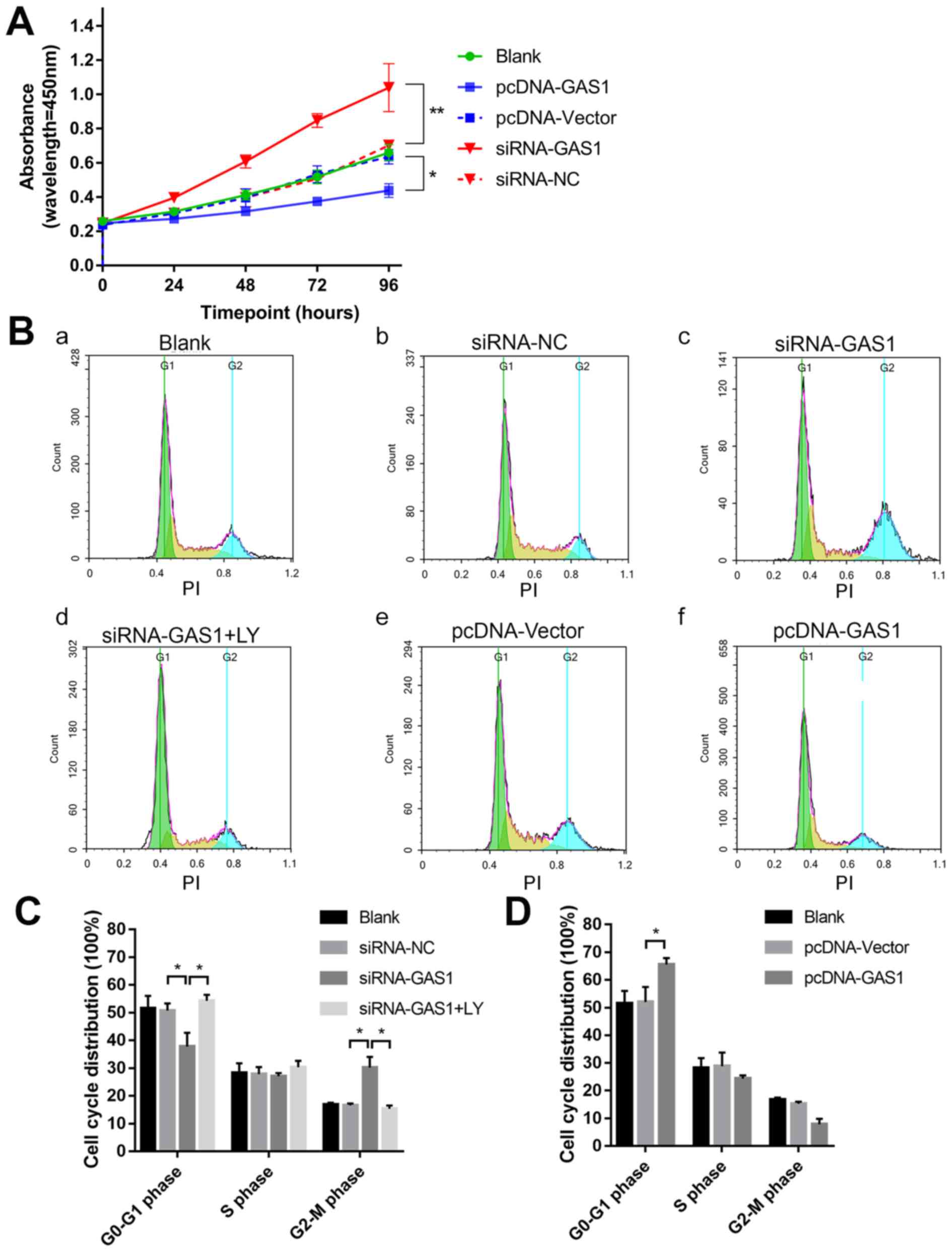 | Figure 2.GAS1 inhibits SF cell viability. (A)
Cell viability curves of non-OASFs transfected with pcDNA-GAS1,
pcDNA-vector, siRNA-GAS1 or siRNA-NC. *P<0.05 and **P<0.01 at
96 h. (B) Representative cell cycle plots of (Ba) the blank control
group, and non-OASFs transfected with (Bb) siRNA-NC, (Bc)
siRNA-GAS1, (Bd) siRNA-GAS1 (plus treatment with LY), (Be)
pcDNA-vector and (Bf) pcDNA-GAS1, as measured using flow cytometry.
(C) Quantified data of non-OASFs transfected with siRNA-GAS1,
siRNA-NC and cells transfected with siRNA-GAS1 and treated with LY.
(D) Quantified data of non-OASFs transfected with pcDNA-GAS1 or
pcDNA-vector. *P<0.05. PI, propidium iodide; SF, synovial
fibroblasts; OASFs, osteoarthritis synovial fibroblasts; GAS1,
growth arrest specific-1; NC, negative control; siRNA, small
interfering RNA; LY, LY294002. |
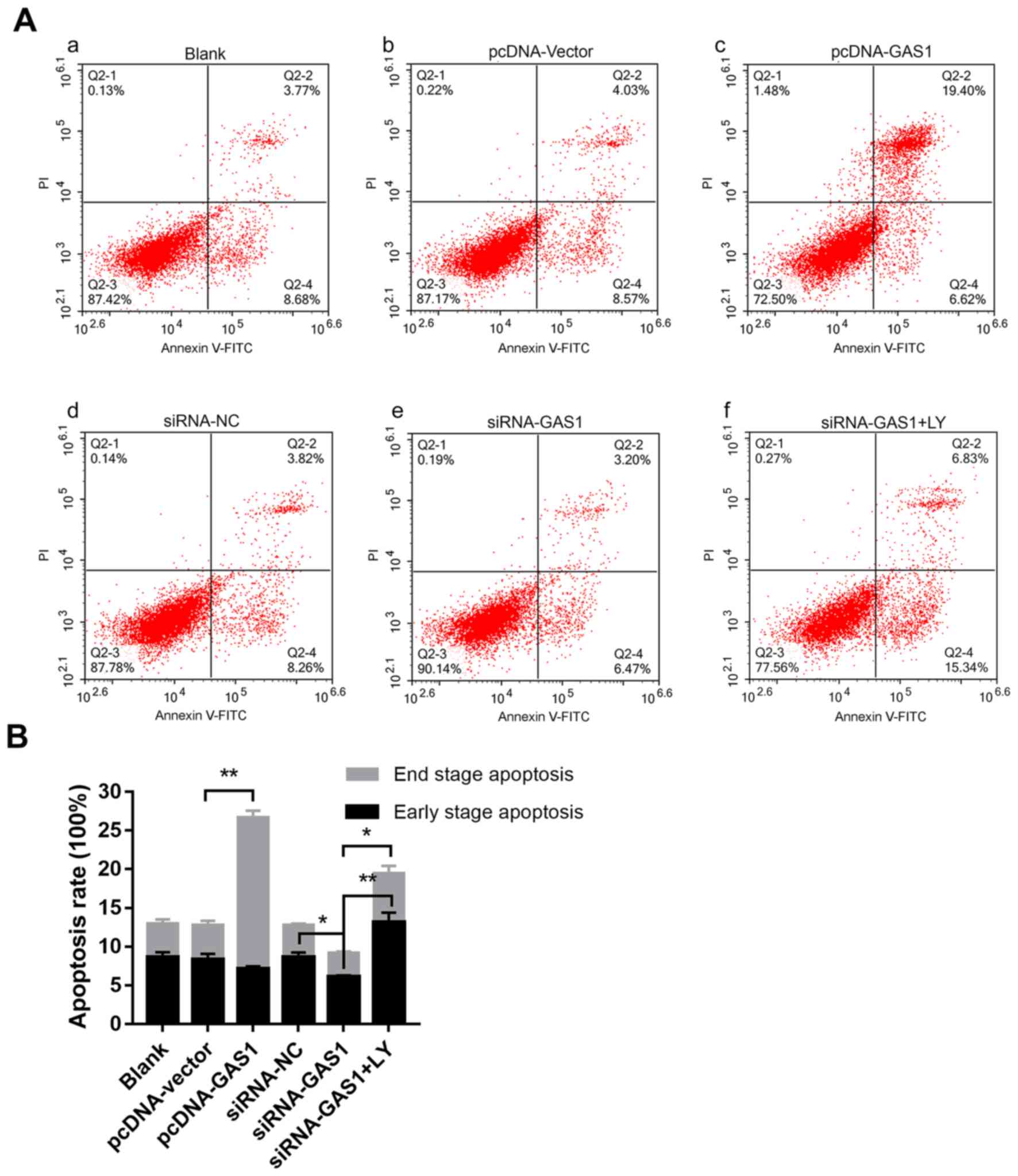 | Figure 3.GAS1 promotes SF apoptosis. (A)
Representative flow cytometry dot plots of (Aa) the blank control
group, and non-OASFs transfected with (Ab) pcDNA-vector, (Ac)
pcDNA-GAS1, (Ad) siRNA-NC, (Ae) siRNA-GAS1 and (Af) siRNA-GAS1 + LY
treatment. (B) Quantified data and corresponding statistical
analysis of early- and end-stage apoptotic cells from each of the
aforementioned groups. *P<0.01 and **P<0.001. PE,
phycoerythrin; APC, allophycocyanin; SF, synovial fibroblasts;
OASFs, osteoarthritis synovial fibroblasts; GAS1, growth arrest
specific-1; NC, negative control; siRNA, small interfering RNA; LY,
LY294002. |
GAS1 regulates SF proliferation and
apoptosis through PI3K-Akt signaling
To further investigate the anti-proliferative
effects of GAS1 in non-OASFs, the activity of the PI3K-Akt pathway,
previously demonstrated to be crucial for cell proliferation as in
prostate, lung and colorectal cancer cells (26–28) and
previously reported to lie downstream of GAS1 (15), was next examined by applying
LY294002, a pharmacological PI3K inhibitor. Transfection with
siRNA-GAS1 increased the levels of PI3K (p85), p-Akt (Ser473) and
Cdk2 expression whilst reducing the levels of Bax, a protein
associated with apoptosis, compared with siRNA-NC group (Fig. 4A and C). The level of Akt
phosphorylation was significantly reduced by treatment with
LY294002 prior to siRNA-GAS1 transfection (referred to as the
siRNA-GAS1 + LY group). Additionally, LY294002 treatment reduced
Cdk2 expression whilst increasing Bax expression compared with
untreated cells (Fig. 4A). In
contrast, transfection with pcDNA-GAS1 reduced the level of PI3K
(p85), p-Akt (Ser473) and Cdk2 whilst increasing the expression of
Bax compared with those transfected with cells transfected with the
pcDNA-vector (Fig. 4B and C). The
role of PI3K/Akt signaling in the anti-proliferative effects of
GAS1 in SFs was functionally assessed by analyzing the cell cycle
and apoptosis in the presence of LY294002. Compared the siRNA-GAS1
group, SFs in the siRNA-GAS1 + LY group exhibited lower frequencies
in G2-M phase (P=0.007) and higher frequencies of early-
(P<0.001) and end-stage (P=0.009) apoptosis (Figs. 2B and C, and 3). The results of the present study
suggested that GAS1 may function as a SF growth suppressor by
inhibiting the PI3K-Akt pathway.
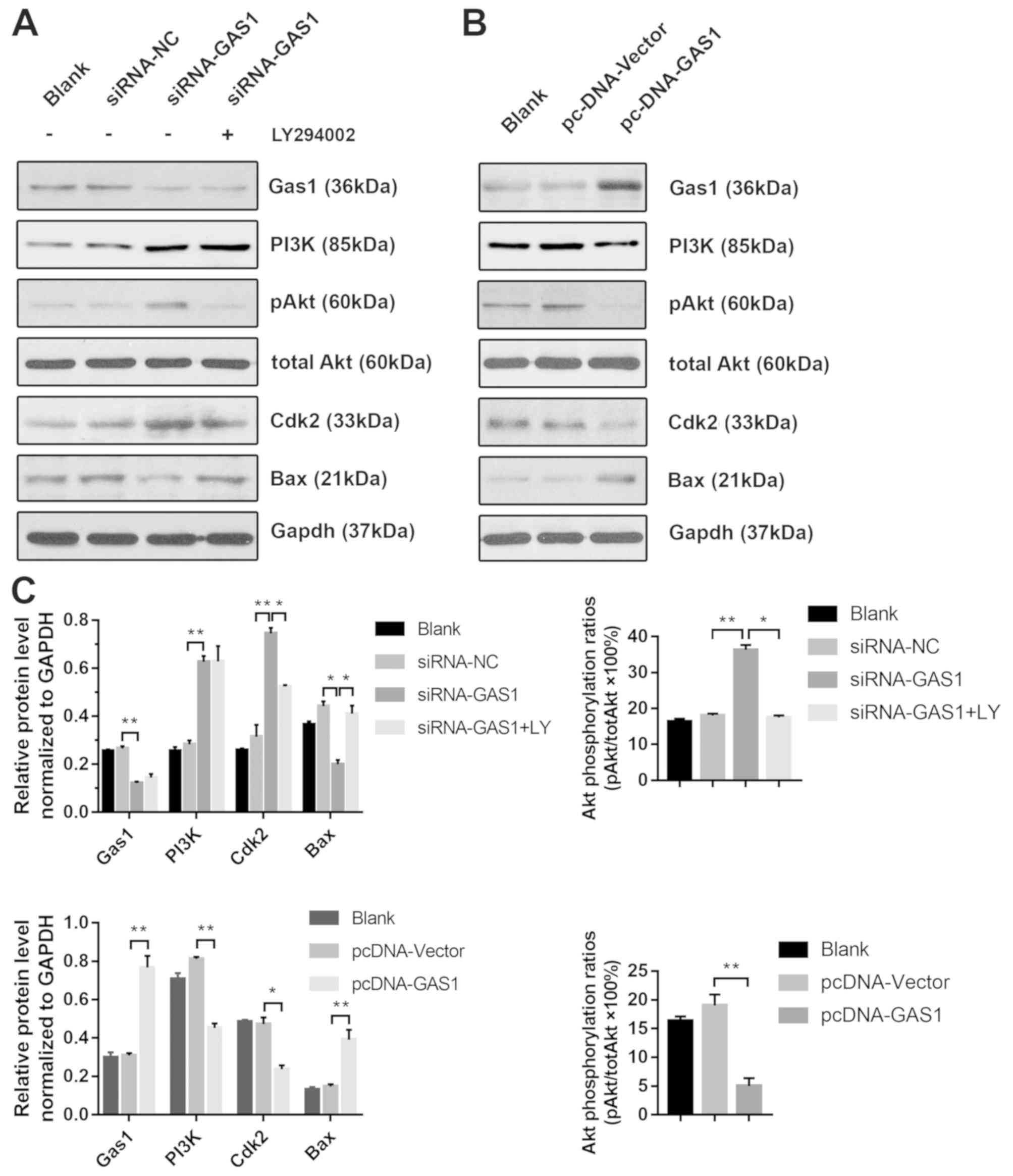 | Figure 4.GAS1 regulates SF proliferation and
apoptosis through the PI3K-Akt pathway. (A) Representative blots
demonstrating the expression of GAS1 and proteins associated with
the PI3K/Akt pathway and apoptosis (PI3K, p-Akt, total Akt, Cdk2
and Bax) from non-OASFs transfected with siRNA-GAS1, siRNA-NC or
cells transfected with siRNA-GAS1 + LY (50 µM) treatment. (B)
Representative blots demonstrating the expression of GAS1 and
proteins associated with the PI3K/Akt pathway and apoptosis (PI3K,
p-Akt, total Akt, Cdk2 and Bax) from non-OASFs transfected with
pcDNA-vector or pcDNA-GAS. (C) Quantification of densitometric data
and Akt phosphorylation. *P<0.01 and **P<0.001. SF, synovial
fibroblasts; OASFs, osteoarthritis synovial fibroblasts; p-Akt,
phosphorylated Akt; Cdk2, cyclin-dependent kinase 2; GAS1, growth
arrest specific-1; NC, negative control; siRNA, small interfering
RNA; LY, LY294002. |
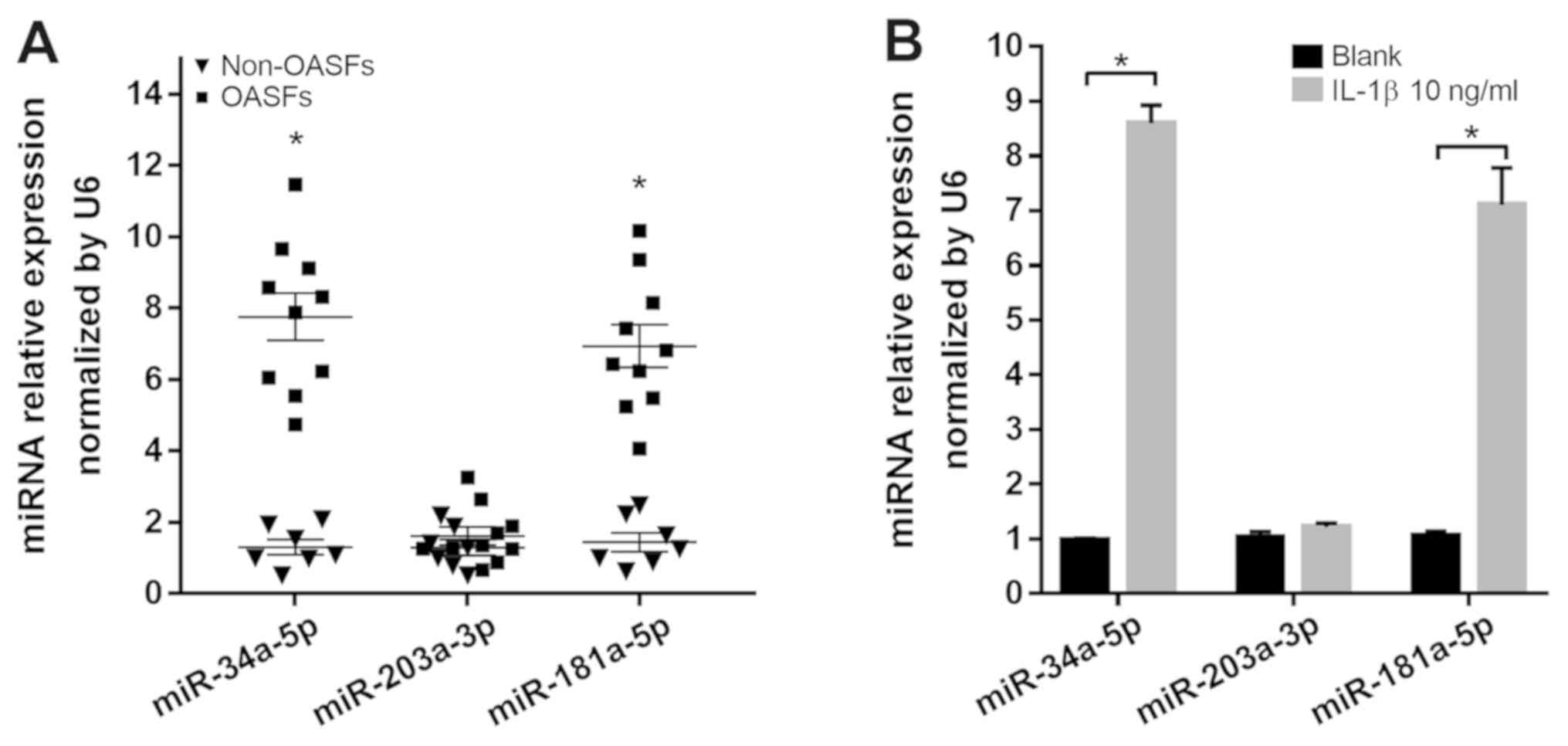
miR-34a-5p and miR-181a-5p directly
downregulate the expression of GAS1 in SFs
The mechanism by which IL-1β induces the
downregulation of GAS1 remains unclear; therefore, it was
hypothesized that endogenous small miRs, induced by
pro-inflammatory factors, may modulate GAS1 expression by
directly targeting the 3′UTR of GAS1 mRNA. In particular,
three miRs, miR-34a-5p, miR-203a-3p and miR-181a-5p, were found to
be overexpressed in OASFs according to a number of previously
published reports (21,23) and were capable of binding to the
3′UTR of GAS1 mRNA, according to the Targetscan 7.2
predictions. Therefore, the expression of these three miRs in OASFs
and non-OASFs were measured using RT-qPCR. OASFs exhibited higher
levels of miR-34a-5p and miR-181a-5p expression, but not
miR-203a-3p, compared with non-OASFs (Fig. 5A). In addition, miR-34a-5p and
miR-181a-5p expression were significantly higher in non-OASFs
stimulated with IL-1β (10 ng/ml) compared with the corresponding
blank controls (Fig. 5B). To examine
the relationship between the miRNA candidates and GAS1 expression
in SFs, non-OASFs were transfected with miRNA mimics or inhibitors.
Transfection efficiency was assessed by RT-qPCR (Fig. S2). GAS1 protein expression was
downregulated when non-OASFs were transfected with miR-34a-5p or
miR-181a-5p mimics compared with the negative control and blank
groups (Fig. 6A). Consistently,
pre-transfection with miR-34a-5p or miR-181a-5p inhibitors rescued
the reduction in GAS1 expression induced by IL-1β, suggesting that
IL-1β-induced GAS1 downregulation is potentially mediated by
miR-34a-5p and miR-181a-5p (Fig.
6B). To further examine whether miR-34a-5p and miR-181a-5p
could directly regulate GAS1 expression, psi-CHECK-2 reporter
vectors containing the GAS1-wt-3′UTR and GAS1-mt-3′UTR were
reconstructed (Fig. 6C and D), which
were then transfected in 293T cells alongside miR-34a-5p or
miR-181a-5p mimics. Luciferase assays confirmed that the reporter
activity was significantly reduced following co-transfection with
psiCHECK2-GAS1-wt-3′UTR and miR-34a-5p (P<0.001) or miR-181a-5p
(P=0.002) mimics, compared with cells co-transfected with
psiCHECK2-GAS1-mut-3′UTR and miR-34a-5p or miR-181a-5p mimics
(Fig. 6E), suggesting that GAS1 is a
direct target gene of miR-34a-5p and miR-181a-5p. Taken together,
these observations suggested that IL-1β-induced GAS1 downregulation
may be mediated by miR-34a-5p and miR-181a-5p.
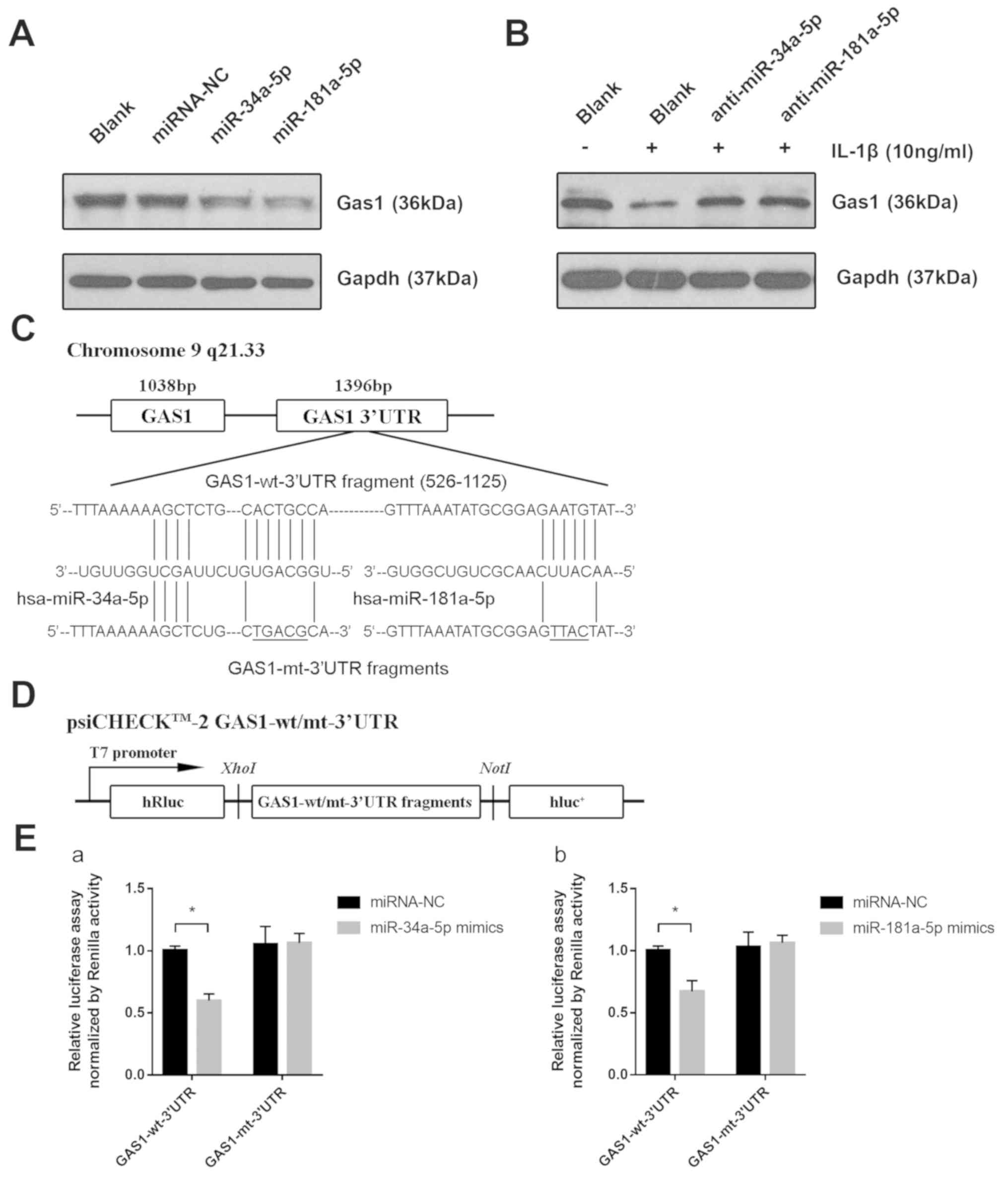 | Figure 6.miR-34a-5p and miR-181a-5p directly
downregulate the expression of GAS1 by targeting the GAS1
3′UTR. (A) Expression of GAS1 proteins in non-OASFs transfected
with miRNA-NC, miR-34a-5p or miR-181a-5p mimics, determined by
western blotting. (B) Expression of GAS1 protein in IL-1β (10
ng/ml)-stimulated non-OASFs transfected with miR-34a-5p or
miR-181a-5p inhibitors. (C) Schematic diagram showing the sequences
of potential binding sites for miR-34a-5p and miR-181a-5p in the
3′UTR of GAS1, as predicted using Targetscan 7.2 software.
(D) Schematic diagram of the luciferase reporter vector constructs
containing wild-type or mutated GAS1 3′UTR sequences. (E)
Luciferase activities in 293T cells co-transfected with
psiCHECK2-wt/mut-3′UTR and (Ea) miR-34a-5p or (Eb) miR-181a-5p
mimics. *P<0.01. OASFs, osteoarthritis synovial fibroblasts;
miR, microRNA; IL-1β, interleukin 1β; GAS1, growth arrest
specific-1; 3′UTR, 3′untranslated region; NC, negative control; wt,
wild-type; mut, mutant; luc, luciferase. |
Discussion
OA is the most prevalent joint disease worldwide and
is characterized by progressive degradation of the articular
cartilage, sequentially leading to a loss of joint function and
disability (1). However, the
molecular mechanism of OA pathogenesis remains unclear. Oehler
et al (29) reported that
hyperplastic and inflammatory SFs are widely found in early- and
late-stage OA by arthroscopic biopsy histology. According to a
retrospective trial by Ayral et al (30), adjacent synovitis of the OA knee was
found to be associated with the severity of cartilage lesions or
chondropathy. Based on this evidence, it is reasonable to conclude
that aberrant hyperplasia of SFs is involved in the progression of
OA. In the local microenvironment of the OA joint, inflamed SFs
proliferate and cumulatively release proinflammatory cytokines and
matrix metalloproteinases, further aggravating inflammation and
cartilage damage (31,32). Notably, in the present study it was
observed that GAS1 was downregulated in OASFs and in
IL-1β-stimulated non-OASFs. The level of GAS1 expression was
negatively associated with the concentration of IL-1β exposure,
which has not been previously reported.
GAS1 was first identified as a growth
suppressive gene in 1988 (7).
Schneider et al reported that in the mouse-derived
fibroblast cell line NIH3T3, cell cycle arrest occurred in the G0-S
phase when GAS1 was overexpressed (7). Del Sal et al (33) found that overexpression of GAS1
induced cell growth arrest in a p53-dependent manner. The
inhibitory effect of GAS1 on tumorigenesis was subsequently
investigated in a number of malignant neoplasms. According to gene
expression profiling analyzes, GAS1 was found to be notably
downregulated in various cancer cell lines compared with their
non-cancerous counterparts (34),
whilst several studies have also reported that the chromosomal site
of the GAS1 gene is deleted in bladder and colorectal
cancers (35,36). Lee et al (37) found that GAS1 expression can be
repressed by proliferative factors, including c-Myc and v-Src, and
Zhao et al (38) found that
overexpression of GAS1 can retard cell growth during the
pathogenesis of gastric cancer. Experiments from the present study
also demonstrated the growth-inhibitory effects on SFs, in that
knockdown of GAS1 expression significantly increased
non-OASF proliferation as measured using CCK-8 assay, whilst
overexpression of GAS1 resulted in the opposite effect, with
cells arrested at the G0/G1 cell cycle phase.
In the present study, the overexpression of GAS1
induced late-stage apoptosis, whereas the silencing of GAS1 could
slightly, but significantly, reduce early-stages of apoptosis.
Indeed, another previous study had also reported the pro-apoptotic
effects of GAS1 by activating caspase 3 in glioma cells (13), and Wang et al (11) found that GAS1 can promote
chemotherapy-induced cell apoptosis in gastric cancer cell lines.
GAS1 has also been tested using xenografts in vivo. Nude
mice injected with lung cancer cells overexpressing GAS1 exhibited
reductions in tumor growth (10).
Interestingly, an opposite effect has also been reported by another
study, which showed that in vascular endothelial cells,
upregulation of GAS1 which is mediated by VE-cadherin and vascular
endothelial growth factor signaling, can prevent cell apoptosis
(39). The effect of GAS1 on
apoptosis being dependent on the cellular context could be a
reasonable explanation for the apparent discrepancy between these
aforementioned studies. The results of the present study,
in-keeping with the general pattern of other studies, confirmed the
pro-apoptotic effects of GAS1 in SFs. However, there was only a
slightly reduced apoptosis rate in response to GAS1 downregulation.
In conclusion, the suppressive effects of GAS1 may maintain the
balance of non-OASF growth, whereas its downregulation could induce
hyperplasia in SFs during OA pathogenesis, suggesting GAS1 to be a
potential target for the treatment of synovial hyperplasia in OA
management.
Research from Cabrera et al (14) detailed the downstream signaling
pathway of GAS1 in neural tumors. According to sequence alignments
and secondary structure predictions, similar to glial cell-derived
neurotrophic factor family receptor α (GFRα), GAS1 has a
RET-binding domain (40). In the N2a
neuroblastoma cell line, GAS1 can inactivate the PI3K-Akt pathway
by directly inhibiting RET phosphorylation (14). The overactivation of the PI3K-Akt
pathway and downstream signaling have been linked to SF survival
and proliferation in abnormal synovial states, including rheumatoid
arthritis and synovitis (41). Based
on these findings, it was speculated in the present study that the
apoptotic and cell cycle arrest effects of GAS1 may be dependent on
the PI3K-Akt pathway (14,42). The expression levels of PI3K, p-Akt
(Ser473) and Cdk2, a cell cycle regulator, were upregulated after
GAS1 silencing, but were all conversely downregulated after GAS1
overexpression. In contrast, Bax, a gene associated with
apoptosis involved in the PI3K-Akt downstream pathway, was
downregulated after GAS1 overexpression. Treatment of SFs with
LY294002, a PI3K-Akt inhibitor, following GAS1 knockdown,
recovered the expression of Cdk2 and Bax to normal levels
comparable with the negative control group, suggesting that the
silencing of GAS1 promoted the hyperproliferation of SFs in a
PI3K-Akt-dependent manner. Further cell cycle and apoptosis
detection experiments on the siRNA-GAS1-LY group also provided
support to this conclusion. As to why RET was not detected in the
present study, it was suspected that inactivation of the PI3K-Akt
pathway by GAS1 was not mediated by RET. Therefore, the molecular
mechanisms underlying the functional role of GAS1 in cell growth
suppression and in promoting apoptosis in SFs require further
investigation.
The mechanism of IL-1β-induced GAS1 downregulation
remains poorly understood. It was speculated that miRs could be
important mediators of GAS1 expression due to their reported
regulatory functions by post-transcriptionally binding to specific
sequences of target gene 3′UTRs. Following investigations into
miRNA-gene matching using a combination of miRNA expression profile
data of OA and non-OASFs from other studies (21,23),
three miRNAs, miR-34a-5p, miR-203a-3p and miR-181a-5p, which are
found to be upregulated in OASFs and have potential binding sites
in the GAS1 3′UTR as predicted by Targetscan 7.2, were
selected for further analysis in the present study. miR-34a-5p and
miR-181a-5p, but not miR-203a-3p, were found to be significantly
overexpressed with a >7-fold change, in OASFs and IL-1β-treated
non-OASFs, by RT-qPCR. Their potential contributions to regulating
GAS1 expression in non-OASFs during the OA process were
subsequently functionally validated. GAS1 expression was
demonstrated to be downregulated in SFs transfected with miR-34a-5p
or miR-181a-5p mimics, whilst IL-1β-induced GAS1 downregulation was
reversed by transfection with miR-34a-5p and miR-181a-5p
inhibitors. Luciferase assays verified further that miR-34a-5p and
miR-181a-5p directly target the GAS1 3′UTR. miR-34a-5p has
been reported to induce divergent effects on cell growth and
apoptosis among different cell types (15,43–46). In
some studies, miR-34a-5p was regarded as a tumor growth suppressor
and apoptotic factor in multiple cancer cell types (43–46). By
contrast, in papillary thyroid carcinoma, miR-34a-5p was
demonstrated to be a tumorigenic and anti-apoptotic factor by
directly downregulating GAS1, sequentially hindering cancer
cell apoptosis through the PI3K-Akt/Bad pathway (15). Li et al (21) found that miR-34a-5p can be detected
at relatively higher levels in the synovial fluid of late stage OA
patients compared with early stage OA patients. Several researchers
have confirmed that miR-34a can induce chondrocyte apoptosis during
OA progression by targeting genes, including sirtuin 1, cellular
communication network factor 1 and δ-like canonical Notch ligand 1
(47–49). The present study strongly suggested
that OASFs, in addition to IL-1β-stimulated SFs, overexpressed
miR-34a-5p, which is consistent with previous data regarding OA
chondrocytes and the present observation that miR-34a-5p directly
targets the GAS1 3′UTR. However, additional experiments are
required to prove that miR-34a-5p can promote the
hyperproliferation of SFs by directly targeting GAS1.
miR-181a-5p, a member of the miR-181 family, has not been well
studied in OA and other arthritic disease models. Elevated
expression of miR-181a-5p was observed by miRNA expression
sequencing profiling acquired in a post-traumatic OA mouse model
(23). In cancer research, another
study have previously proved that miR-181a-5p is a proliferative
factor which can facilitate cell cycle progression in melanoma
(50). In the present study,
miR-181a-5p was found to be upregulated in OASFs or
IL-1β-stimulated SFs, which negatively regulated GAS1
expression by directly targeting the GAS1 3′UTR, in a
similar mechanism to that of miR34a-5p. To the best of our
knowledge, the present study was the first to report the potential
proliferative effects of miR-181a-5p in OASFs. As such, miR-34a-5p
and miR-181a-5p could serve as novel therapeutic targets in OA
treatment, though further comprehensive experimental analyzes would
need to be performed.
In conclusion, the present study confirmed the
downregulation of GAS1 in SFs of OA joints or in
cytokine-induced SFs. By overexpressing or silencing GAS1
expression in non-OASFs, the suppressive role of GAS1 on SF
hyperproliferation was observed, which was mechanistically found to
involve inhibiting PI3K-Akt signaling. Finally, two miRNA
candidates, miR-34a-5p and miR-181a-5p, were found to downregulate
GAS1 expression in OASFs or in IL-1β-stimulated SFs, by
directly targeting the GAS1 3′UTR. According to the present
study, GAS1 could be regarded as a key mediator in regulating SF
proliferation. In addition, a unique
miR-34a-5p/miR-181a-5p-GAS1-PI3K-Akt axis has been potentially
uncovered in the regulation of SF hyperplasia in OA joints, which
could serve as a potential therapeutic target for preventing
synovial hyperplasia in OA progression.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Hua Long and Dr
Jun Chen, The Second Affiliated Hospital of Air Force Medical
University (Tangdu Hospital of Fourth Military Medical University),
Xi'an, China for collecting the synovial tissue specimens. The
authors would also like acknowledge the technical assistance of Ms
Shun Guo, The Second Affiliated Hospital of Air Force Medical
University (Tangdu Hospital of Fourth Military Medical University)
and Dr Weigang Zhang (Xijing Hospital, Xi'an, China) for technical
assistance and manuscript revision. The authors are sincerely
grateful to Professor Shizhen Emily Wang (University of California,
San Diego, CA, USA) for laboratory technique training.
Funding
The present study was supported by grant from the
Natural Science Foundation of China (grant no. 81072194).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CD and BAM designed the study. CD, XLW, NL and XYW
performed the primary cultures of SFs. CD, XLW and NL performed the
mRNA quantification and protein detection. CD, KLZ and XYW
performed cell viability, apoptosis, and cell cycle experiments. CD
and XLW performed luciferase assays. CD and KLZ prepared the
figures. CD, HMZ, HPW, BW and MA collected and analyzed the data.
CD, KLZ and HMZ wrote the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures in this study were in accordance with
ethical standards, and were approved by the Institutional Review
Board of Tangdu Hospital, Fourth Military Medical University
(Xi'an, China) with informed consent obtained from all the
participants concerned.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Robinson WH, Lepus CM, Wang Q, Raghu H,
Mao R, Lindstrom TM and Sokolove J: Low-grade inflammation as a key
mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:580–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Del Rey MJ, Usategui A, Izquierdo E,
Cañete JD, Blanco FJ, Criado G and Pablos JL: Transcriptome
analysis reveals specific changes in osteoarthritis synovial
fibroblasts. Ann Rheum Dis. 71:275–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CS, Buttitta L and Fan CM: Evidence
that the WNT-inducible growth arrest-specific gene 1 encodes an
antagonist of sonic hedgehog signaling in the somite. Proc Natl
Acad Sci USA. 98:11347–11352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schneider C, King RM and Philipson L:
Genes specifically expressed at growth arrest of mammalian cells.
Cell. 54:787–793. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benitez JA, Arregui L, Vergara P and
Segovia J: Targeted-simultaneous expression of Gas1 and p53 using a
bicistronic adenoviral vector in gliomas. Cancer Gene Ther.
14:836–846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiménez A, López-Ornelas A, Estudillo E,
González-Mariscal L, González RO and Segovia J: A soluble form of
GAS1 inhibits tumor growth and angiogenesis in a triple negative
breast cancer model. Exp Cell Res. 327:307–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evdokiou A and Cowled PA:
Tumor-suppressive activity of the growth arrest-specific gene GAS1
in human tumor cell lines. Int J Cancer. 75:568–577. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Zhou X, Zhang Y, Zhu H, Zhao L,
Fan L, Wang Y, Gang Y, Wu K, Liu Z and Fan D: Growth
arrest-specific gene 1 is downregulated and inhibits tumor growth
in gastric cancer. FEBS J. 279:3652–3664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Z, Xu Y and Cai S: Down-regulated
GAS1 expression correlates with recurrence in stage II and III
colorectal cancer. Hum Pathol. 42:361–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zamorano A, Lamas M, Vergara P, Naranjo JR
and Segovia J: Transcriptionally mediated gene targeting of gas1 to
glioma cells elicits growth arrest and apoptosis. J Neurosci Res.
71:256–263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cabrera JR, Sanchez-Pulido L, Rojas AM,
Valencia A, Mañes S, Naranjo JR and Mellström B: Gas1 is related to
the glial cell-derived neurotrophic factor family receptors alpha
and regulates Ret signaling. J Biol Chem. 281:14330–14339. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Qin H and Cui Y: MiR-34a targets
GAS1 to promote cell proliferation and inhibit apoptosis in
papillary thyroid carcinoma via PI3K/Akt/Bad pathway. Biochem
Biophys Res Commun. 441:958–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stanczyk J, Pedrioli DM, Brentano F,
Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S and Kyburz
D: Altered expression of MicroRNA in synovial fibroblasts and
synovial tissue in rheumatoid arthritis. Arthritis Rheum.
58:1001–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamachi Y, Kawano S, Takenokuchi M,
Nishimura K, Sakai Y, Chin T, Saura R, Kurosaka M and Kumagai S:
MicroRNA-124a is a key regulator of proliferation and monocyte
chemoattractant protein 1 secretion in fibroblast-like synoviocytes
from patients with rheumatoid arthritis. Arthritis Rheum.
60:1294–1304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao J, Zhou XL, Kong RN, Ji LM, He LL and
Zhao DB: microRNA-126 targeting PIK3R2 promotes rheumatoid
arthritis synovial fibro-blasts proliferation and resistance to
apoptosis by regulating PI3K/AKT pathway. Exp Mol Pathol.
100:192–198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YH, Tavallaee G, Tokar T, Nakamura A,
Sundararajan K, Weston A, Sharma A, Mahomed NN, Gandhi R, Jurisica
I and Kapoor M: Identification of synovial fluid microRNA signature
in knee osteoarthritis: Differentiating early- and late-stage knee
osteoarthritis. Osteoarthritis Cartilage. 24:1577–1586. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murata K, Yoshitomi H, Tanida S, Ishikawa
M, Nishitani K, Ito H and Nakamura T: Plasma and synovial fluid
microRNAs as potential biomarkers of rheumatoid arthritis and
osteoarthritis. Arthritis Res Ther. 12:R862010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kung LHW, Ravi V, Rowley L, Bell KM,
Little CB and Bateman JF: Comprehensive expression analysis of
microRNAs and mRNAs in synovial tissue from a mouse model of early
post-traumatic osteoarthritis. Sci Rep. 7:177012017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue H, Takamori M, Nagata N, Nishikawa
T, Oda H, Yamamoto S and Koshihara Y: An investigation of cell
proliferation and soluble mediators induced by interleukin 1beta in
human synovial fibroblasts: Comparative response in osteoarthritis
and rheumatoid arthritis. Inflamm Res. 50:65–72. 2001.PubMed/NCBI
|
|
26
|
Li L, Ittmann MM, Ayala G, Tsai MJ, Amato
RJ, Wheeler TM, Miles BJ, Kadmon D and Thompson TC: The emerging
role of the PI3-K-Akt pathway in prostate cancer progression.
Prostate Cancer Prostatic Dis. 8:108–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarris EG, Saif MW and Syrigos KN: The
biological role of PI3K pathway in lung cancer. Pharmaceuticals.
5:1236–1264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng Y, Qian W, Zhang Y, Peng W, Li J, Gu
Q, Ji D, Zhang Z, Wang Q, Zhang D and Sun Y: CDCA2 promotes the
proliferation of colorectal cancer cells by activating the
AKT/CCND1 pathway in vitro and in vivo. BMC Cancer. 19:5762019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oehler S, Neureiter D, Meyer-Scholten C
and Aigner T: Subtyping of osteoarthritic synoviopathy. Clin Exp
Rheumatol. 20:633–640. 2002.PubMed/NCBI
|
|
30
|
Ayral X, Pickering EH, Woodworth TG,
Mackillop N and Dougados M: Synovitis: A potential predictive
factor of structural progression of medial tibiofemoral knee
osteoarthritis-results of a 1 year longitudinal arthroscopic study
in 422 patients. Osteoarthritis Cartilage. 13:361–367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sieghart D, Liszt M, Wanivenhaus A, Bröll
H, Kiener H, Klösch B and Steiner G: Hydrogen sulphide decreases
IL-1β-induced activation of fibroblast-like synoviocytes from
patients with osteoarthritis. J Cell Mol Med. 19:187–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eymard F, Pigenet A, Citadelle D,
Flouzat-Lachaniette CH, Poignard A, Benelli C, Berenbaum F,
Chevalier X and Houard X: Induction of an inflammatory and
prodegradative phenotype in autologous fibroblast-like synoviocytes
by the infrapatellar fat pad from patients with knee
osteoarthritis. Arthritis Rheumatol. 66:2165–2174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Del Sal G, Ruaro EM, Utrera R, Cole CN,
Levine AJ and Schneider C: Gas1-induced growth suppression requires
a transactivation-independent p53 function. Mol Cell Biol.
15:7152–7160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang Y, Prasad M, Lemon WJ, Hampel H,
Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, et
al: Gene expression in papillary thyroid carcinoma reveals highly
consistent profiles. Proc Natl Acad Sci USA. 98:15044–15049. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moriarty HT and Webster LR: Fragile sites
and bladder cancer. Cancer Genet Cytogenet. 140:89–98. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Konstantinova LN, Fleischman EW, Knisch
VI, Perevozchikov AG and Kopnin BP: Karyotype peculiarities of
human colorectal adenocarcinomas. Hum Genet. 86:491–496. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee TC, Li L, Philipson L and Ziff EB: Myc
represses transcription of the growth arrest gene gas1. Proc Natl
Acad Sci USA. 94:12886–12891. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao L, Pan Y, Gang Y, Wang H, Jin H, Tie
J, Xia L, Zhang Y, He L, Yao L, et al: Identification of GAS1 as an
epirubicin resistance-related gene in human gastric cancer cells
with a partially randomized small interfering RNA library. J Biol
Chem. 284:26273–26285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spagnuolo R, Corada M, Orsenigo F, Zanetta
L, Deuschle U, Sandy P, Schneider C, Drake CJ, Breviario F and
Dejana E: Gas1 is induced by VE-cadherin and vascular endothelial
growth factor and inhibits endothelial cell apoptosis. Blood.
103:3005–3012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Airaksinen MS, Holm L and Hätinen T:
Evolution of the GDNF family ligands and receptors. Brain Behav
Evol. 68:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hayer S, Pundt N, Peters MA, Wunrau C,
Kühnel I, Neugebauer K, Strietholt S, Zwerina J, Korb A, Penninger
J, et al: PI3Kgamma regulates cartilage damage in chronic
inflammatory arthritis. FASEB J. 23:4288–4298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mograbi B, Bocciardi R, Bourget I, Busca
R, Rochet N, Farahi-Far D, Juhel T and Rossi B: Glial cell
line-derived neurotrophic factor-stimulated phosphatidylinositol
3-kinase and Akt activities exert opposing effects on the ERK
pathway: Importance for the rescue of neuroectodermic cells. J Biol
Chem. 276:45307–45319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou Y, Ding BZ, Lin YP and Wang HB:
MiR-34a, as a suppressor, enhance the susceptibility of gastric
cancer cell to luteolin by directly targeting HK1. Gene. 644:56–65.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li T, Li L, Li D, Wang S and Sun J:
MiR-34a inhibits oral cancer progression partially by repression of
interleukin-6-receptor. Int J Clin Exp Pathol. 8:1364–1373.
2015.PubMed/NCBI
|
|
45
|
Zhao J, Guerrero A, Kelnar K, Peltier HJ
and Bader AG: Synergy between next generation EGFR tyrosine kinase
inhibitors and miR-34a in the inhibition of non-small cell lung
cancer. Lung Cancer. 108:96–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi H, Zhou S, Liu J, Zhu J, Xue J, Gu L
and Chen Y: miR-34a inhibits the in vitro cell proliferation and
migration in human esophageal cancer. Pathol Res Pract.
212:444–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang B, Ni J, Long H, Huang J, Yang C and
Huang X: IL-1β-induced miR-34a up-regulation inhibits Cyr61 to
modulate osteoarthritis chondrocyte proliferation through ADAMTS-4.
J Cell Biochem. 119:7959–7970. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang W, Hsu P, Zhong B, Guo S and Zhang
C, Wang Y, Luo C, Zhan Y and Zhang C: MiR-34a enhances chondrocyte
apoptosis, senescence and facilitates development of osteoarthritis
by targeting DLL1 and regulating PI3K/AKT pathway. Cell Physiol
Biochem. 48:1304–1316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang L, He X, Li F, Pan H, Huang X, Wen
X, Zhang H, Li B, Ge S, Xu X, et al: The miR-181 family promotes
cell cycle by targeting CTDSPL, a phosphatase-like tumor suppressor
in uveal melanoma. J Exp Clin Cancer Res. 37:152018. View Article : Google Scholar : PubMed/NCBI
|