Introduction
Legionella pneumophila (Lp), a gram-negative
intracellular bacterium, is a common pathogen that causes
community-acquired and hospital-acquired pneumonia. It is one of
the three most common causative agents of severe pneumonia with an
incidence of 2–9% and a mortality rate of 10% in Europe and North
America (1). In particular,
Immunosuppression as a result of a number of factors, including
chronic disease, malignant tumors or the administration of
immunosuppressive drugs is an important risk factor for Lp
infection (2,3). Immunosuppressed hosts infected with Lp
have an elevated risk of developing severe pneumonia, with the
mortality rate of the disease potentially reaching 20–70% in these
cases (2,4).
The most common pathophysiological characteristics
associated with severe pneumonia are increased pulmonary capillary
permeability (PCP) and pulmonary edema (5). It is well documented that infectious
pulmonary edema is primarily caused by increased PCP, which is also
an important indicator of the severity of lung injury (5). Lp infection induces severe pneumonia
more readily in immunosuppressed hosts compared with
immunocompetent hosts (6). However,
whether the increase of PCP or pulmonary edema are more severe in
Lp-infected immunosuppressed hosts compared with hosts with normal
immune function remains unclear.
Damage to the vascular endothelial barrier leads to
the exudation of protein-rich pulmonary edema fluid (7). Vascular endothelial cadherin
(VE-cadherin) is an important component for maintaining PCP
balance. VE-cadherin internalization increases vascular
permeability and endothelial cell migration, leading to pulmonary
edema (8).
Currently, most studies of Lp infection in
immunocompromised hosts have been case reports (9,10), and
there are insufficient data on the differences in Lp infection
between immunocompromised hosts and those with normal immune
function. It is conceivable to speculate that injury caused by Lp
infection is more severe in immunosuppressed hosts compared with
hosts with normal immune function, and that the major pathological
characteristic is the increase in PCP. To test this hypothesis, in
the present study an IL-2 isolated perfused system was applied to
investigate the changes in the ex vivo weights of lung
tissues from Lp-infected immunosuppressed guinea pigs and those
with normal immunity. Variations in PCP in Lp-infected animals with
different immune status were also evaluated.
Materials and methods
Laboratory animals, immunosuppression
induction and grouping
A total of 144 specific-pathogen-free male Hartley
guinea pigs (age, 4–5 weeks; weight range, 300–350 g) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. Each guinea pig was raised in an individually ventilated
cag and maintained at 23°C environment under a 12 h light/dark
cycle. The animals had free access to sterile feed and water. This
experiment was approved by the Experimental Animal Welfare and
Ethics Committee of the Chinese Medical University (Shenyang,
China).
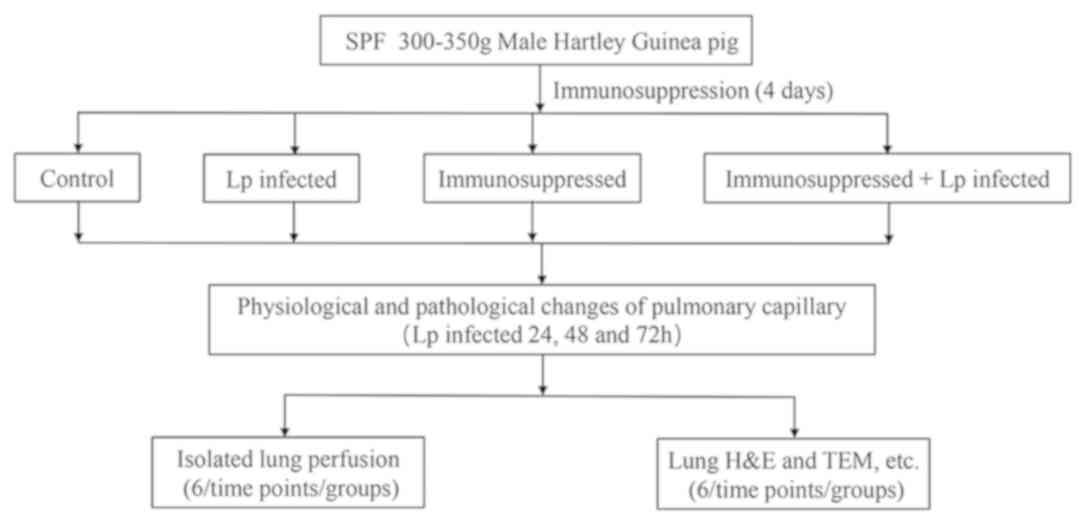
The experimental animals were divided into four
groups: Control, Lp-infected, immunosuppressed and immunosuppressed
+ Lp-infected (Fig. 1).
Immunosuppressed guinea pigs (11)
were obtained by treating the guinea pigs with Triamcinolone
acetonide (Beijing Solarbio Science & Technology Co., Ltd) and
cyclophosphamide (Cayman Chemical Company). A subcutaneous
injection of triamcinolone acetonide (20 mg/kg) was administered to
each animal daily for four days. On the fourth day, an
intraperitoneal injection of cyclophosphamide (300 mg/kg) was also
given to the same animal. This transient immunosuppressive
treatment resulted in a reduction of total white blood cell counts
to <1×109/l, with this immunosuppression lasting
seven days, consistent with previous literature reports (11). On the fifth day, the animals in the
Lp-infected and immunosuppressed Lp-infected groups were
anesthetized with an intraperitoneal injection of sodium
pentobarbital (40 mg/kg). Their neck skin was cut and 0.3 ml Lp
serogroup 1 (1×106 bacteria/guinea pig) suspended in
saline of was injected into the main airway using a 1 ml syringe
(12). For the animals in the
control and the immunosuppressed groups, 0.3 ml sterile saline was
injected into the main airway under the same conditions. All
animals regained consciousness ~1 h following Lp challenge.
Each experimental group contained 36 guinea pigs.
The experiments were conducted at three time points (24, 48 and 72
h after Lp infection). At each time point, 12 animals in each group
were randomly selected. Of the 12 guinea pigs, six were designated
for isolated lung perfusion whilst the other six were designated
for histology and biochemical analyses.
In isolated lung perfusion, following anesthesia by
an intraperitoneal injection of sodium pentobarbital (40 mg/kg),
hearts and lungs were removed from each animal for maintenance
under extracorporeal pulmonary circulation.
For histological and biochemical analyses, following
anesthesia with an intraperitoneal injection of sodium
pentobarbital (40 mg/kg), an incision was made in the chest of the
animals along the midline. A total of 2 ml blood was subsequently
collected from the right ventricle before the lungs were extracted.
Of the blood sample, 1 ml was divided into an anticoagulant tube
for the measurement of white blood cell count using Auto Hematology
Analyzer (BC-2800Vet; Shenzhen Mindray Bio-Medical Electronics Co.,
Ltd). A section of the left lower lung tissue (1 mm3)
was cut and fixed using 2.5% glutaraldehyde at 4°C for 3 days for
electron microscopic analyses, whereas the remaining left lung
tissues were fixed with 4% formalin (room temperature) for 2 days
at room temperature for hematoxylin and eosin (H&E) and
immunofluorescence staining. Remaining blood and lung tissue
samples were stored in −80°C.
Since the lungs were removed from the thoracic
cavities of all experimental animals during the operation
procedure, all animals were sacrificed.
Bacterial strain and culture
The Lp strain used in this study was clinically
isolated in The Respiratory Department of Shengjing Hospital
affiliated to the Chinese Medical University (Shengyang, China).
The strain was verified to be of Lp serogroup 1 using slide
agglutination serological test, with the protocol described as
follows (Fig. S1): A total of 25 µl
Lp 1 serum was dropped onto a microscopic slide, following which a
colony of Lp 1 was added into it. The slides were subsequently
shaken for 1 min and then assessed for agglutination. Sterile water
was used as control. The bacteria were cultured in Legionella
CYE-Agar (Base) medium (CM0665B; Oxoid, Ltd.; Thermo Fisher
Scientific, Inc.) containing Legionella BCYE growth supplement
(SR0110C; Oxoid, Ltd.; Thermo Fisher Scientific, Inc.) at 37°C
under 5% CO2. After four days in culture, the bacteria
were resuspended in sterile normal saline at a concentration of
3.33×106 cfu/ml.
In vivo pulmonary edema
At 24, 48 and 72 h following infection with Lp, six
animals were selected in each group with the weights of their lungs
were measured to evaluate the severity of pulmonary edema in
vivo.
Ex vivo lung perfusion
In the present study, the IPL-2 Isolated Perfused
Lung System (Harvard Apparatus) (Fig.
S2) (13,14) was used to perform ex vivo lung
perfusion experiment. Changes in the weights of the lungs in
vivo were measured in constant-pressure perfusion mode, which
was used to directly measure changes in PCP. A total of six animals
in each group were selected and anesthetized with an
intraperitoneal injection of 40 mg/kg pentobarbital sodium 24, 48
and 72 h after Lp infection (15).
The trachea of each guinea pig was cut, followed by intubation and
maintenance of positive pressure ventilation (2–10
cmH2O) at a respiratory rate of 60 beats/min. An
incision was made in the abdomen from the bottom to top along the
midline and the bilateral femoral arteries were cut off for
exsanguination. An additional incision was made in the thoracic
cavity along the midline to expose the heart and lung, and 1 ml
0.9% sodium chloride solution containing 250 IU heparin sodium was
injected into the right ventricle to prevent thrombosis within the
pulmonary vessels. An arterial cannula was inserted into the
pulmonary artery via an incision in the right ventricle whereas a
venous cannula was inserted into the left atrium via an incision in
the left ventricle. The lungs were subsequently separated from the
surrounding tissues and incorporated into the artificial chest of
IPL-2 system. The ventilation mode was changed from
positive-pressure ventilation to negative-pressure (between −2 and
−10 cmH2O). Perfusion of airflow was then commenced at a
rate of 5 ml/min before being gradually increased and the mode was
changed to constant-pressure perfusion at 10 cmH2O,
which is considered to be close to that of the normal average
pulmonary artery pressure (PAP) (16). The ex vivo weight of the lung
was then adjusted to 0 mg and the subsequent values for the ex
vivo weight of the lung was recorded every 5 min for 30 min.
The perfusate composition (Krebs-Henseleit solution) (15) was: NaCl, 118 mM; KCL, 4.7 mM;
KH2PO4, 1.2 mM; MgSO4, 1.2 mM;
CaCl2, 2.5 mM; NaHCO3, 24.9 mM; bovine serum
albumin (BSA) (Amresco, LLC), 2%; glucose, 5.56 mM; and HEPES, 12.6
mM. The temperature of the perfusate and the artificial chest was
maintained at 37°C.
Histology and immunofluorescence
Following fixation, paraffin-embedded lung tissue
samples were cut into 4 µm sections and stained with H&E for a
total of 6 min at room temperature. Inflammation area in H&E
lung sections from six guinea pigs were analyzed and quantified by
three senior pathologists, using the Image J software (version
1.48; National Institutes of Health) (17).
For immunofluorescence staining. All sections were
blocked for 30 min using 5% BSA at 20°C. A primary anti-VE-cadherin
antibody (cat. no. ab33168; 1:400; Abcam) was diluted in
QuickBlock™ Primary Antibody Dilution Buffer for staining (Beyotime
Institute of Biotechnology) which were incubated overnight at 4°C.
The sections were then incubated with an Alexa Fluor®
488-conjugated anti-rabbit IgG (H + L), F(ab)2 fragment antibody
(cat. no. 4412S, 1:1,000, Cell Signaling Technology, Inc.) for 1 h,
stained with 4′,6-diamidino-2-phenylindole (DAPI) for 6 min, and
imaged using a fluorescence microscope. A total of three sections
were selected for each time point (24, 48 and 72 h) in each group
and three visual fields were randomly selected for each section.
ImageJ software was used to measure IOD values for each
condition.
Ultrastructure assessment of lung
injury
Following fixation, the isolated lung tissues were
rinsed in 0.1 mol/l phosphate buffer (pH 7.4) and dehydrated in a
graded ethanol series. The tissues were subsequently infiltrated
and embedded in epoxy-resin at 60°C for 48 h. The ultrathin tissue
sections (70 nm) were stained using uranyl acetate and lead citrate
at 20°C and then examined using transmission electron microscopy
(Hitachi-HT7700; Hitachi, Ltd.).
Statistical analysis
Statistical analysis was performed using SAS 9.4
software (SAS Institute, Inc.). The statistical description of
quantitative variables was expressed as mean ± standard deviation.
Analysis between different treatment groups and time points was
performed using factorial design ANOVA followed by the LSD-t test
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of the immunosuppressed
guinea pig model
Consistent with the results of Kirkpatrick et
al (11), in the present study,
treatment with a combination of triamcinolone acetonide and
cyclophosphamide was used to establish a transient immunosuppressed
guinea pig model. The white blood cell, neutrophil and lymphocyte
counts of immunosuppressed guinea pigs were significantly reduced
compared with Control (Table I).
 | Table I.The white blood cell, neutrophil and
lymphocyte counts in guinea pigs from the four experimental
groups. |
Table I.
The white blood cell, neutrophil and
lymphocyte counts in guinea pigs from the four experimental
groups.
|
| Control | Lp |
Immunosuppressed | Immunosuppressed +
Lp |
|---|
|
|
|
|
|
|
|---|
|
x109/l | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| WBC | 4.95±0.96 | 4.69±2.41 | 4.37±1.04 |
3.61±0.96a |
2.06±1.12b,d |
1.73±0.85c,d |
2.14±0.71a,d |
1.43±0.22b |
0.5±0.21c,f,g |
3.41±0.87a,g |
0.17±0.15be,h,i |
0.17±0.06c,f,i |
| N | 1.59±0.59 | 1.39±0.75 | 1.57±0.53 |
2.4±0.71a |
1.04±0.72d |
0.56±0.37c,d | 1.89±0.68 | 1.24±0.2 |
0.25±0.16c,g,h |
3.15±0.92a,d,g |
0.07±0.06be,h,i |
0.08±0.03c,i |
| L | 2.97±0.65 | 2.92±1.6 | 2.6±0.65 |
1.05±0.24a |
0.92±0.5b |
0.96±0.56c |
0.22±0.1a,d |
0.17±0.11b,e |
0.22±0.07c,f |
0.23±0.13a,d |
0.14±0.11b,e |
0.09±0.03c,f |
Lung tissue injury is more severe in
the immunosuppressed Lp-infected group compared with the
Lp-infected group with increasing infection time
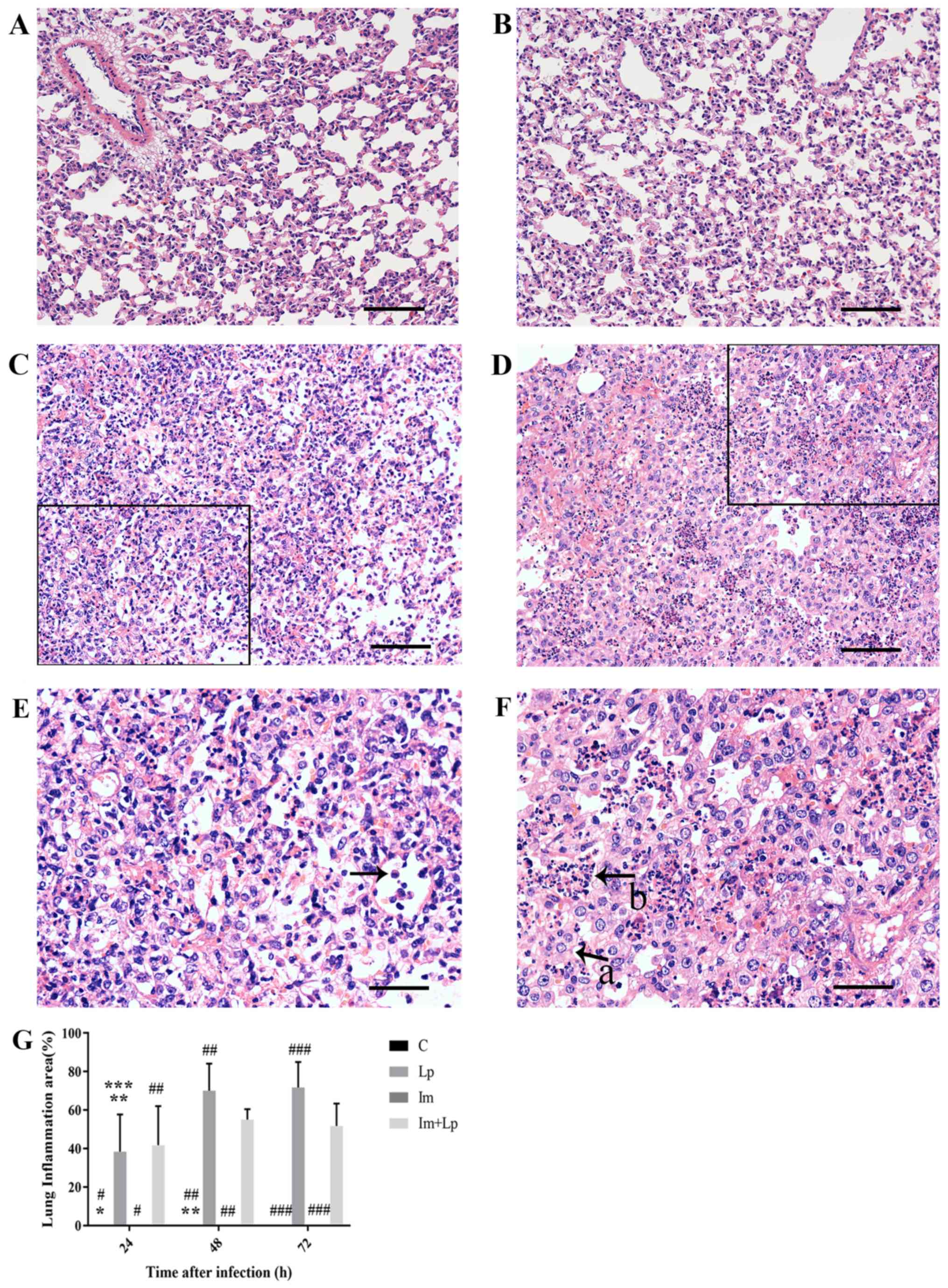
At each time point, the lung tissue structure of the
Control group was normal (Fig. 2A).
As the infection time increased from 24 to 72 h, the
H&E-stained lung tissue samples from the Lp-infected group
exhibited progressive damage, with the most prominent observed at
72 h. After 72 h infection, the tissue structure was disordered
with the structures of the alveoli and alveolar walls becoming
particularly unclear and a large number of inflammatory cell
infiltration (mainly neutrophils; Fig.
2C and E). In tissues from the immunosuppressed Lp-infected
group at the same timepoint, the pathological changes in the lung
tissues were more aggravated with extensive structural disorder. A
large number of alveoli were filled with inflammatory exudate, the
alveolar walls were extensively thickened and reductions in the
number of alveoli was observed (Fig. 2D
and F). At 72 h, the infection area of Lp infected group was
larger compared with that of the immunosuppressed Lp-infected
group, but the damage was not as extensive (Fig. 2G). A small level neutrophil
infiltration was observed in the alveolar walls of the
immunosuppressed group at 24 h after infection (data not shown),
and the lung tissues then largely returned to normal at 72 h
(Fig. 2B).
As the infection time increased, the ultrastructure
of the lung tissue from the Control group was normal (Fig. 3A), whereas that from the Lp-infected
group was aggravated, with the structure of the lung tissue
becoming disordered observed at 72 h (Fig. 3B). The surfaces of the alveolar
capillary endothelial cells and alveolar epithelial cells were
uneven and the basement membranes between them becoming blurred.
This damage was more severe in the lung tissues from the
immunosuppressed Lp-infected group, which exhibited extensive
damage to the lung tissue structure at 72 h with a certain amount
of Lp bacterial infiltration (Fig.
3D). In the immunosuppressed group, the capillary endothelial
cells were swollen at 24 h and the mitochondria had degenerated. As
the infection time increased, the ultrastructure of the lung tissue
gradually recovered to normal (Fig.
3C).
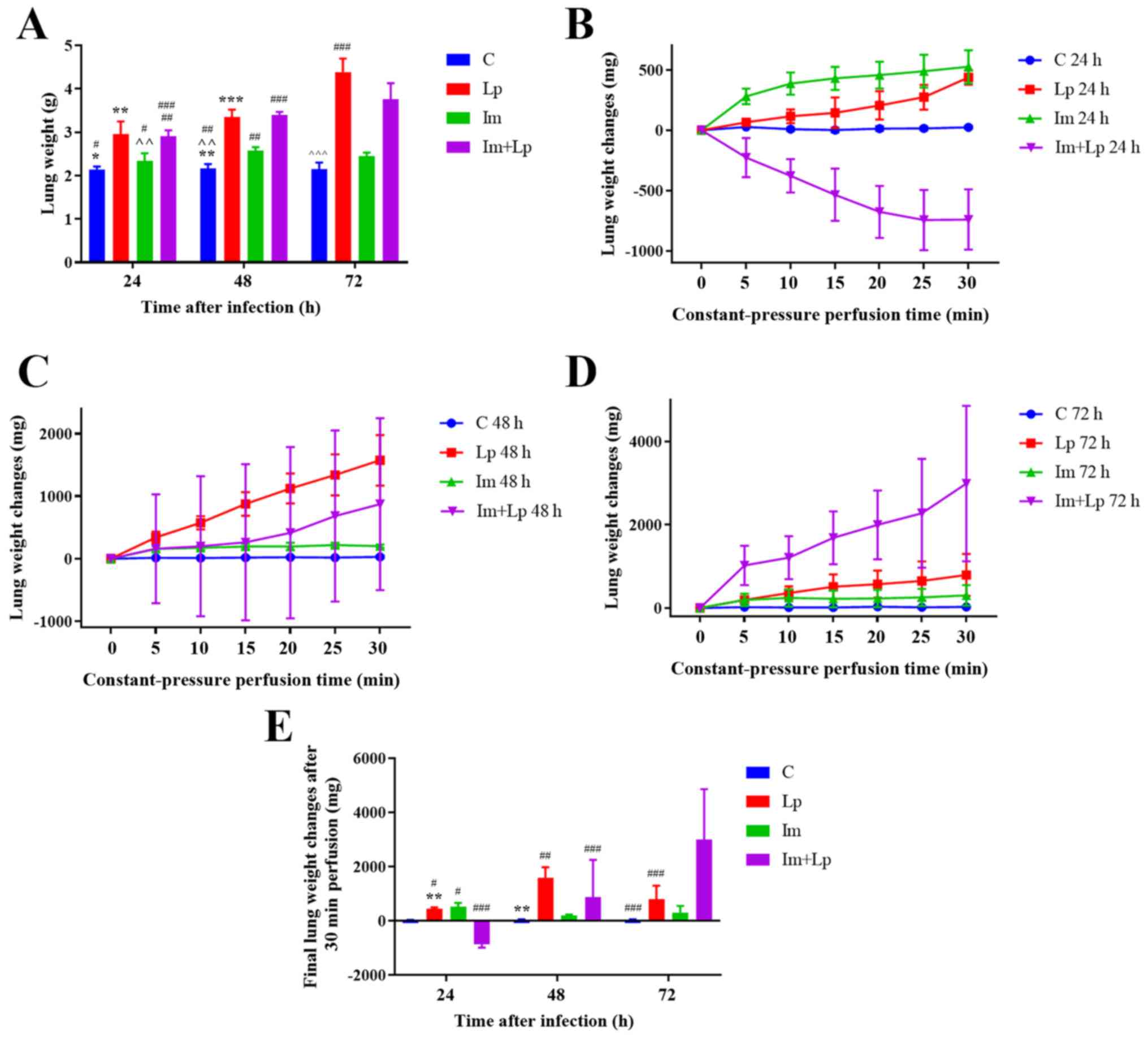
The weight of lungs in animals from
the immunosuppressed Lp-infected group increases gradually with
infection time, but is less compared with that in the Lp-infected
group at 72 h
The severity of pulmonary edema was assessed by
measuring the weights of lungs from the animals at different time
points. At 72 h, the mean weight of the lungs was greater in the
Lp-infected group compared with that in the immunosuppressed
Lp-infected group (P<0.05), whereas that in the immunosuppressed
group was only slightly higher compared with that in the control
group, but there was no significant difference (Fig. 4A).
The weights of the lungs from the
immunosuppressed Lp-infected group first decreases and then
increases as the infection time increased
As the infection time increased, under perfusion at
constant pressure, the weights of the ex vivo lungs from the
Lp-infected group increased, with this gain being most significant
at 48 h (Fig. 4E). In the
immunosuppressed Lp-infected group, the weights of the ex
vivo lungs first decreased following 24 h but then gradually
increased from 48 h onwards and finally the increase at 72 h was
greater compared with the immunocompetent Lp-infected group
(P<0.05; Fig. 4B-E). The weights
of the ex vivo lungs in the immunosuppressed group increased
at all time points, but the rate of increase gradually decreased
(Fig. 4B-E). The changes in the
weights of the ex vivo lungs were significantly greater in
Lp-infected guinea pigs compared with uninfected guinea pigs
(P=0.0008), and the change exhibited by immunosuppressed guinea
pigs was significantly greater compared with those without
immunosuppression (P<0.05). Therefore, this observation
indicates a synergistic effect between infectiousness and
immunosuppression (P<0.05).
Ultrastructural damage to endothelial
junctions of pulmonary capillaries is significantly greater in the
immunosuppressed Lp-infected group
The lung capillary endothelial cells were swollen in
the lung tissues from the Lp-infected group 24 h following
infection with the cell junctions partially open (Fig. 5); at 48 h, the capillary endothelial
cell junctions were completely open before finally at 72 h, the
junctions were blurred and partially open (Fig. 5). In the lung tissues from the
immunosuppressed Lp-infected group, the capillary endothelial cells
were swollen with a reduction in the density and number of cell
junctions 24 h after infection, which were already partially open
(Fig. 5); at 48 h, the number of
capillary endothelial cell junctions had decreased further with
their densities was intermittently reduced, which were partially
open with scattered Lp bacterium visible (Fig. 5). Finally, at 72 h the lung tissue
was notably damaged with the cell membranes of the capillary
endothelial cells no longer resolvable, the nuclei were swollen and
the cell junctions disappeared (Fig.
5). In the lung tissues from the immunosuppressed group, the
capillary endothelial cell junctions were partially open at 24 h
(Fig. 5); at 48 h the density of
cell junctions had decreased and were intermittently opened
(Fig. 5). However, the endothelial
cell junctions had recovered approach to normal by 72 h (Fig. 5).
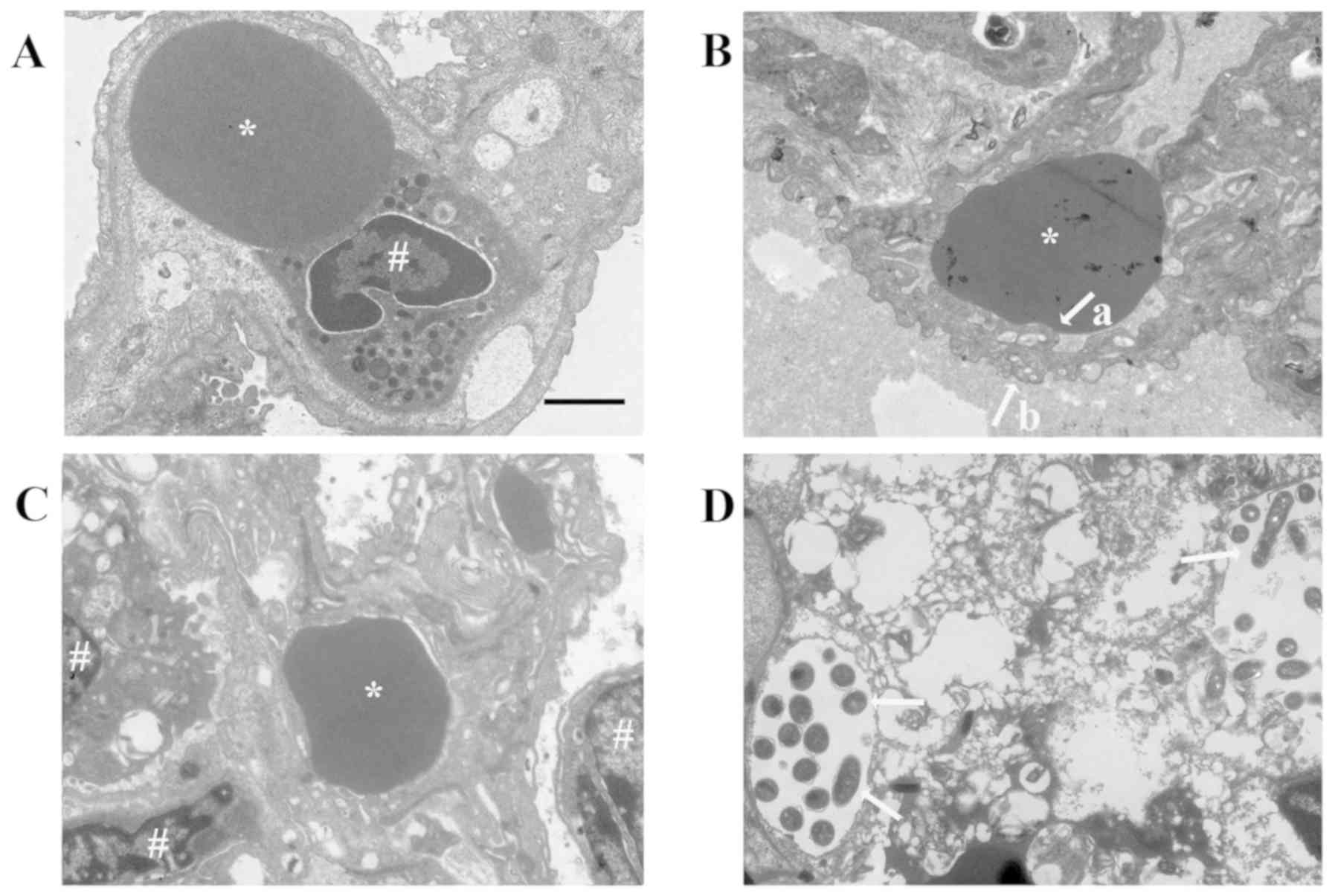
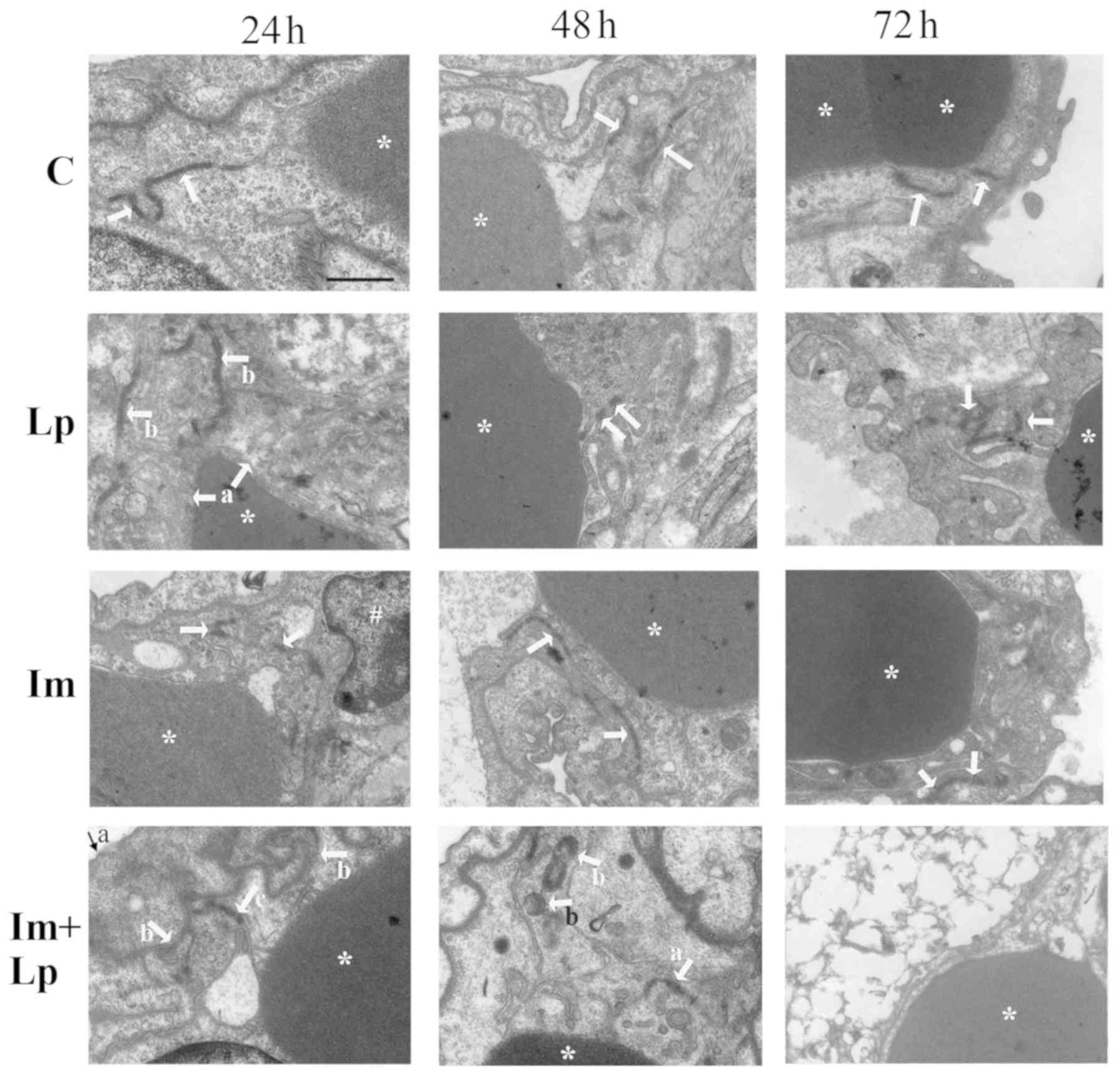 | Figure 5.Transmission electron microscopy
images of pulmonary capillary endothelial cell junctions in lung
tissues isolated from guinea pigs from the experimental groups.
Pulmonary capillary endothelial cell junctions in the control group
were normal (indicated by arrows). In the Lp-infected group, at 24
h, abnormal changes that could be observed included swollen
vascular endothelial cells (indicated by arrows a) and partially
opened cell junctions (indicated by arrows b); at 48 h, the
endothelial cell junctions were completely opened (indicated by
arrows); at 72 h, the cell junctions were obscure and partially
opened (indicated by arrows). In the immunosuppressed group, at 24
h, the endothelial cell junctions were partially opened (indicated
by arrows); at 48 h, the density of the cell junctions was reduced
and the cell junctions were intermittently opened (indicated by
arrows); at 72 h, the endothelial cell junctions had recovered to
normal (indicated by arrows). In the immunosuppressed Lp-infected
group, at 24 h, the alveolar epithelial cells were swollen
(indicated by arrow a), the basement membrane was shrunken
(indicated by arrows b), the density and number of cell junctions
were reduced and the junctions were partially opened (indicated by
arrow c); at 48 h, the number and densities of cell junctions were
reduced and partially opened (indicated by arrow a), Lp bacteria
were visible (indicated by arrows b); at 72 h, destruction of
tissue structure was evident, the cell membrane was unclear, the
nuclei were swollen, and the cell junctions had disappeared.
*Denotes red blood cells and #denotes the nucleus.
Scale-bars, 1 µm. C, control; Lp, Legionella pneumophila;
Im, immunosuppressed. |
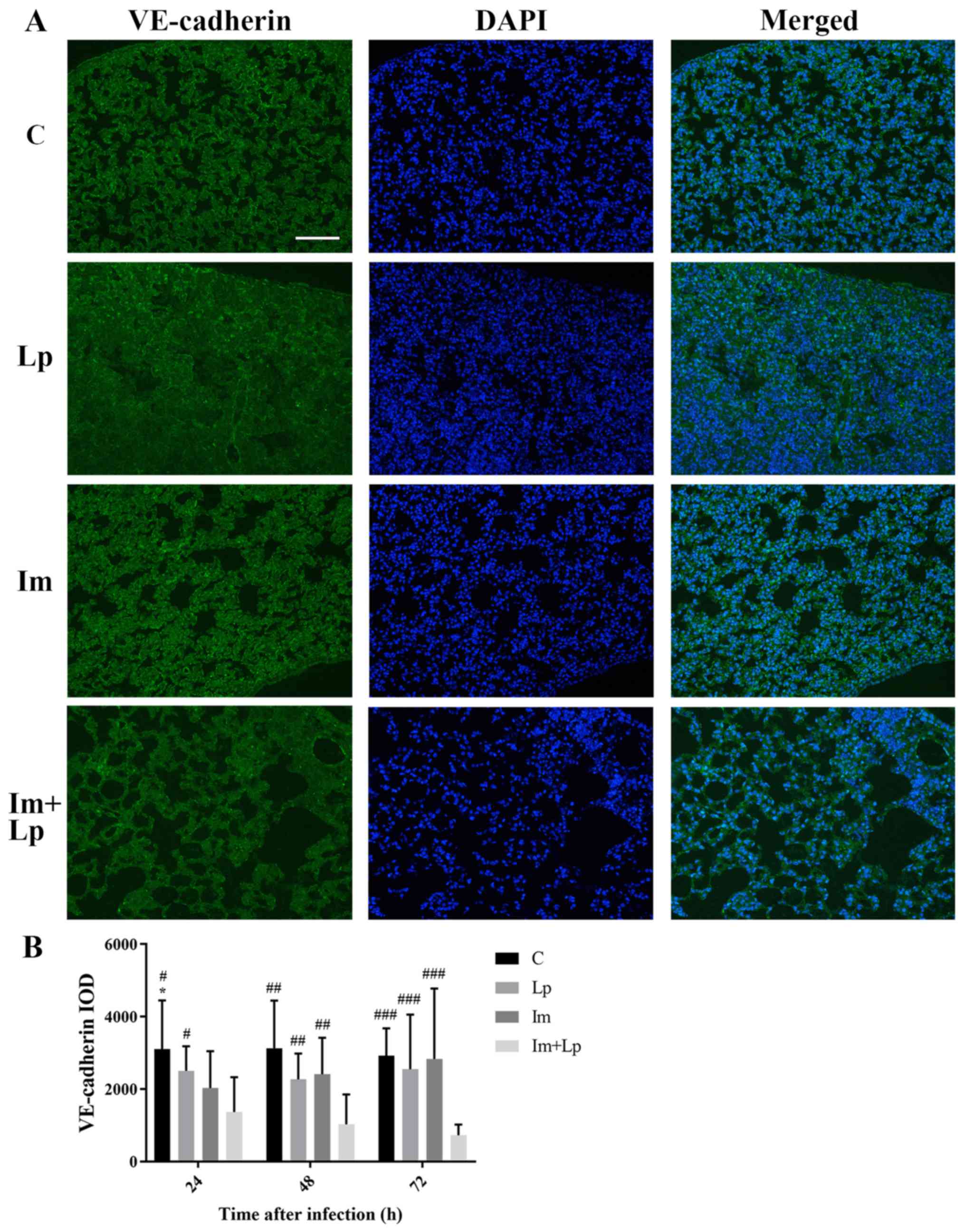
VE-cadherin expression is
significantly lower in the lung tissues from the immunosuppressed
Lp-infected group compared with the Lp-infected group
An immunofluorescence assay of VE-cadherin in the
lung tissues showed that the integral optical density (IOD) value
for VE-cadherin fluorescence in the lung tissues from the
immunosuppressed Lp-infected group was gradually decreased from 24
to 72 h and was lower compared with the Lp-infected group at each
time point (P<0.05; Fig. 6A and
B). The expression of VE-cadherin in lung tissues was also
measured using western blot analysis (Fig. S3). The expression of VE-cadherin
appeared to be reduced in Lp-infected group and immunosuppressed
Lp-infected group, but no significant differences between each
group were observed.
Discussion
Lp infection is more likely to result in severe
pneumonia in immunosuppressed hosts compared with immunocompetent
hosts (2,4). The typical pathological changes in
severe pneumonia are increased PCP and the consequent formation of
pulmonary edema. In the present study, using H&E staining and
transmission electron microscopy, it was found that the opening of
capillary endothelial cell junctions and the injury to the lung
tissue were more serious in immunosuppressed guinea pigs infected
with Lp in compared with in the animals infected with Lp alone,
despite the area of lung inflammation being larger in the latter.
This is consistent with a previous clinical report that Lp
infection is positively associated with the severity of the
condition in immunosuppressed hosts compared within normal hosts
(9).
Existing methods used to study PCP, including the
measurement of Evans blue content, fluorescein-isothiocyanate
(FITC)-labeled BSA and the ratio of albumin in the plasma to that
in the alveolar lavage fluid, are indirect detection methods that
are unsuitable for severe cases due to the fact they are
susceptible to interference by other factors such as
hypoalbuminemia (18). Wang et
al (18) used an Evans blue
assay to compare the changes in PCP in Streptococcus
pneumonia-infected mice with normal immunity and mice with
cyclophosphamide-induced leukopenia at different time points (4,
24, 48, 72 and 96 h). They found that the leukopenic group
exhibited higher PCP compared with the normal immunity group only
at 48 h. The present study focused on the application of IPL-2
Isolated Perfused Lung System to remove the influence of
extrapulmonary factors to directly measure PCP in guinea pigs
infected with Lp.
In the present study, the IPL-2 Isolated Perfused
Lung System was used to study the effects of immunosuppression and
infection on PCP over time. This system has two modes of perfusion,
constant-pressure and constant-current perfusion. The former not
only simulates the in vivo lung circulation, but also
excludes the influence of extrapulmonary factors (13,14). The
changes in the weights of lungs ex vivo directly reflects
changes in PCP, which can be used to evaluate PCP in conditions in
which the degree of lung injury varies (13,14). In
the control group, PCP was normal and the weights of the lungs
ex vivo exhibited no notable changes under constant-pressure
perfusion mode.
However, as the infection time increased, the
weights of the lungs ex vivo in the immunosuppressed
Lp-infected group did not continue to increase; instead, the weight
of the lungs gradually changed from displaying a significant
reduction to a significant increase. These changes could be caused
by a gradual increase in PCP in this group. Another important cause
could be that the average PAP in the Lp-infected immunosuppressed
guinea pigs gradually changed from significantly higher to lower
compared with the normal average pressure. At 24 h after infection,
PCP increased in the Lp-infected immunosuppressed guinea pigs, by
which time the average PAP was already significantly higher
compared with the normal pressure in vivo. When the lung was
perfused with normal average PAP, the pulmonary capillary
hydrostatic pressure decreased relative to the in vivo
state, causing the edema fluid flowed out of the lung into the
perfusate, reducing the weights of the lungs ex vivo. PCP
increased further 48 h after infection, whereas in vivo, the
average PAP was lower compared with 24 h. It was considered that at
48 h, the average PAP in the Im+Lp group in vivo remained
higher compared with the controls in some animals, but in other
animals, the average PAP had decreased to below the normal level.
Therefore, under perfusion with normal average PAP, the ex
vivo weight of the lungs both increased and decreased in the
Lp-infected immunosuppressed animals at 48 h. At 72 h, PCP was
significantly increased, whilst in vivo, the average PAP was
below the normal level and the pulmonary capillary hydrostatic
pressure had decreased. This led to insufficient lung perfusion and
a lower weight of the lungs in the immunosuppressed Lp-infected
group compared with in the Lp-infected group. Perfusion with the
normal average PAP elevated the pulmonary capillary hydrostatic
pressure and significantly increased the ex vivo weight of
the lungs. It has been clinically observed that immunosuppressive
therapy can reduce the average PAP in patients with pulmonary
hypertension caused by systemic lupus erythematosus or mixed
connective tissue disease (19,20),
suggesting that immunosuppressive therapy may also reduce the
average PAP in hosts with normal immunity. However, the reason for
the mean PAP being elevated in the early stages of infection in the
Lp-infected immunosuppressed group requires further study.
The vascular endothelial cell junction protein
VE-cadherin serves an important role in the regulation of the
vascular endothelial cell barrier function, where its
internalization increases PCP (21–24). In
the presence of inflammation, activated neutrophils accumulate in
the microvasculature and release proteolytic enzymes (25). This enzyme cleaves the extracellular
region of VE-cadherin (26),
increasing vascular permeability in the area of leukocyte
accumulation. In the present study, immunofluorescence assay showed
that the expression of VE-cadherin was lower in the
immunosuppressed Lp-infected group compared with that in the
Lp-infected alone group. Neutrophil infiltration was observed in
both groups from H&E staining. Electron microscopy showed that
as the infection time increased, the damage to the pulmonary
capillary endothelial cells was more extensive in the
immunosuppressed Lp-infected group compared with in the Lp-infected
alone group. Therefore, it could be concluded from these findings
that the increase in PAP in the Lp-infected immunosuppressed guinea
pigs resulted from the synergistic effects of multiple factors,
including the internalization and cleavage of VE-cadherin and the
tissue damage caused by Lp. Pulmonary inflammation was
heterogeneous throughout the tissue. Therefore, the
immunofluorescence staining of VE-cadherin in the lung tissues from
the Lp-infected and immunosuppressed Lp-infected groups was
non-uniform, which could provide an explanation for the large
variation observed in the corresponding VE-cadherin western blots
of the lung tissues (Fig. S3). It
was hypothesized in the present study that the distribution of
VE-cadherin could be observed more accurately in the tissue using
immunofluorescence compared with Western blot, where errors could
be reduced. Therefore, immunofluorescence was used to detect the
expression of VE-cadherin. The mechanism underlying the reduction
of VE-cadherin expression remains unclear and require further
study, along with changes in pulmonary vascular resistance and the
pulmonary artery pressure in response to Lp infection.
Pentobarbital is not suitable for use as an
anesthetic in guinea pigs as it can cause cardiovascular and
respiratory depression, however, these side effects can be avoided
by using the IPL-2 Isolated Perfused Lung System (27,28).
This is because the ventilation mode of this system is by tracheal
intubation and the application of negative pressure ventilation
(−2–10 cmH2O), with the respiration of this isolated
lung carried out only under this negative pressure. The heart only
acts as a vehicle for intubation and therefore does not participate
in pulmonary circulation under this system.
The application of IPL-2 Isolated Perfused Lung
System requires substantial preparation (procedures including
preparation of perfusion fluid, cardiopulmonary extraction and
machine operation), which can potentially lead to a loss of time
required for the detection of bacterial load. There have been
numerous reports of Legionella pneumophila infection in
laboratory animals, where the correlation between the bacterial
concentration in the lungs and the severity of pneumonia is low
(12,29,30).
Therefore, the bacterial load in the lungs in the present study was
not measured.
In the immunosuppressed Lp-infected group, under
constant-pressure perfusion, the weight of the lungs ex vivo
decreased significantly 24 h after infection, but increased
significantly after 72 h, indicating that PCP increased in the
immunosuppressed Lp-infected hosts, and that the average PAP also
had a profound effect on the degree of in vivo pulmonary
edema. Indeed, Lp infection in immunosuppressed hosts is more
likely to develop into severe pneumonia. Most patients with severe
pneumonia receive intensive therapies, including mechanical
ventilation and vasoactive drugs, leading to an elevation in PAP
and the aggravation of pulmonary edema (31,32).
Therefore, data from the present study suggest that during the
intensive treatment of Lp infection immunosuppressed patients, PCP
will increase and therefore the PAP should be monitored for each
patient to avoid aggravating pulmonary edema.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2017YFC1309702) and the
National Natural Science Foundation of China (grant no.
81170009).
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
JK, YC, WW and XC designed the experiments, and XC,
NY, JM, WYL and MX performed them. EL and MZ collected and analyzed
the data. XC wrote the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The Experimental Animal Welfare and Ethics Committee
of the Chinese Medical University (Shenyang, China) approved the
experimental protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Lp
|
Legionella pneumophila
|
|
PCP
|
pulmonary capillary permeability
|
|
PAP
|
pulmonary artery pressure
|
References
|
1
|
Stout JE and Yu VL: Legionellosis. New Eng
J Med. 337:682–687. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helms CM, Viner JP, Weisenburger DD, Chiu
LC, Renner ED and Johnson W: Sporadic legionnaires' disease:
Clinical observations on 87 nosocomial and community-acquired
cases. Am J Med Sci. 288:2–12. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pedro-Botet ML, Sabria-Leal M, Sopena N,
Manterola JM, Morera J, Blavia R, Padilla E, Matas L and Gimeno JM:
Role of immunosuppression in the evolution of Legionnaires'
disease. Clin Infect Dis. 26:14–19. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beaute J, Zucs P and de Jong B; European
Legionnaires' Disease Surveillance Network, : Legionnaires disease
in Europe, 2009–2010. Euro Surveill. 18:204172013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark SB and Soos MP: Noncardiogenic
Pulmonary Edema. StatPearls. StatPearls Publishing LLC. (Treasure
Island (FL)). 2019.https://www.ncbi.nlm.nih.gov/books/NBK542230/
|
|
6
|
Htwe TH and Khardori NM: Legionnaire's
disease and immunosuppressive drugs. Infect Dis Clin North Am.
31:29–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lo SK, Everitt J, Gu J and Malik AB: Tumor
necrosis factor mediates experimental pulmonary edema by ICAM-1 and
CD18-dependent mechanisms. J Clin Invest. 89:981–988. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donners MM, Wolfs IM, Olieslagers S,
Mohammadi-Motahhari Z, Tchaikovski V, Heeneman S, van Buul JD,
Caolo V, Molin DG, Post MJ and Waltenberger J: A disintegrin and
metalloprotease 10 is a novel mediator of vascular endothelial
growth factor-induced endothelial cell function in angiogenesis and
is associated with atherosclerosis. Arterioscler Thromb Vasc Biol.
30:2188–2195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edelstein PH: Legionella jamestowniensis
fatal pneumonia in an immunosuppressed man. J Infect Chemother.
23:59–61. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nanovic Z: Legionnaires' disease and use
of tumor necrosis factor-αlpha inhibitors: A forthcoming problem?
Macedonian. J Med Sci. 6:465–472. 2013.
|
|
11
|
Kirkpatrick WR, McAtee RK, Fothergill AW,
Rinaldi MG and Patterson TF: Efficacy of voriconazole in a guinea
pig model of disseminated invasive aspergillosis. Antimicrob Agents
Chemother. 44:2865–2868. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gamradt P, Xu Y, Gratz N, Duncan K, Kobzik
L, Högler S, Kovarik P, Decker T and Jamieson AM: The influence of
programmed cell death in myeloid cells on host resilience to
infection with legionella pneumophila or streptococcus
pyogenes. PLoS Pathog. 12:e10060322016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ismael-Badarneh R, Guetta J, Klorin G,
Berger G, Abu-Saleh N, Abassi Z and Azzam ZS: The role of
angiotensin II and cyclic AMP in alveolar active sodium transport.
PLoS One. 10:e01341752015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ong HX, Benaouda F, Traini D, Cipolla D,
Gonda I, Bebawy M, Forbes B and Young PM: In vitro and ex vivo
methods predict the enhanced lung residence time of liposomal
ciprofloxacin formulations for nebulisation. Eur J Phar Biopharm.
86:83–89. 2014. View Article : Google Scholar
|
|
15
|
Kadlecek S, Shaghaghi H, Siddiqui S,
Profka H, Pourfathi M and Rizi R: The effect of exogenous substrate
concentrations on true and apparent metabolism of hyperpolarized
pyruvate in the isolated perfused lung. NMR Biomed. 27:1557–1570.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dominguez-Fandos D, Valdes C, Ferrer E,
Puig-Pey R, Blanco I, Tura-Ceide O, Paul T, Peinado VI and Barberà
JA: Sildenafil in a cigarette smoke-induced model of COPD in the
guinea-pig. Eur Respir J. 46:346–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He X, Liang Y, LaValley MP, Lai J and
Ingalls RR: Comparative analysis of the growth and biological
activity of a respiratory and atheroma isolate of chlamydia
pneumoniae reveals strain-dependent differences in inflammatory
activity and innate immune evasion. BMC Microbiol. 15:2282015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang E, Simard M, Ouellet N, Bergeron Y,
Beauchamp D and Bergeron MG: Pathogenesis of pneumococcal pneumonia
in cyclophosphamide-induced leukopenia in mice. Infect Immun.
70:4226–4238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Machireddy K, Myint Z, Dein E, Mathai SC,
Seo P, Haque U, Manno R and Timlin H: Mycophenolate mofetil in a
lupus patient with pulmonary hypertension. Cureus.
10:e21212018.PubMed/NCBI
|
|
20
|
Jais X, Launay D, Yaici A, Le Pavec J,
Tchérakian C, Sitbon O, Simonneau G and Humbert M:
Immunosuppressive therapy in lupus- and mixed connective tissue
disease-associated pulmonary arterial hypertension: A retrospective
analysis of twenty-three cases. Arthritis Rheum. 58:521–531. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bates DO: Vascular endothelial growth
factors and vascular permeability. Cardiovas Res. 87:262–271. 2010.
View Article : Google Scholar
|
|
22
|
Dejana E and Giampietro C: Vascular
endothelial-cadherin and vascular stability. Curr Opin Hematol.
19:218–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komarova Y and Malik AB: Regulation of
endothelial permeability via paracellular and transcellular
transport pathways. Ann Rev Physiol. 72:463–493. 2010. View Article : Google Scholar
|
|
24
|
Komarova YA, Mehta D and Malik AB: Dual
regulation of endothelial junctional permeability. Sci STKE.
2007:re82007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weiss SJ: Tissue destruction by
neutrophils. N Engl J Med. 320:365–376. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lampugnani MG, Resnati M, Raiteri M,
Pigott R, Pisacane A, Houen G, Ruco LP and Dejana E: A novel
endothelial-specific membrane protein is a marker of cell-cell
contacts. J Cell Biol. 118:1511–1522. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lan CC, Peng CK, Tang SE, Wu SY, Huang KL
and Wu CP: Anti-vascular endothelial growth factor antibody
suppresses ERK and NF-κB activation in ischemia-reperfusion lung
injury. PLoS One. 11:e01599222016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rieg AD, Suleiman S, Perez-Bouza A,
Braunschweig T, Spillner JW, Schröder T, Verjans E, Schälte G,
Rossaint R, Uhlig S and Martin C: Milrinone relaxes pulmonary veins
in guinea pigs and humans. PLoS One. 9:e876852014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Viswanathan VK, Edelstein PH, Pope CD and
Cianciotto NP: The legionella pneumophila iraAB locus is required
for iron assimilation, intracellular infection, and virulence.
Infect Immun. 68:1069–1079. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edelstein PH: The guinea pig model of
legionnaires' disease. Methods Mol Biol. 954:521–540. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Ma S, Liu Y, Xu W and Li Z:
Effects and mechanism analysis of combined infusion by levosimendan
and vasopressin on acute lung injury in rats septic shock. Cell
Biochem Biophys. 70:1639–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Daudel F, Tuller D, Krahenbuhl S, Jakob SM
and Takala J: Pulse pressure variation and volume responsiveness
during acutely increased pulmonary artery pressure: An experimental
study. Crit Care. 14:R1222010. View
Article : Google Scholar : PubMed/NCBI
|