Introduction
To date, clinical studies have shown that large
defects of the jaw due to traumatic injury or tumor resection do
not heal themselves (1). Clinically,
autologous bone, allogeneic bone and xenografts are often used to
repair bone defects. However, these treatments have their
drawbacks, including limited availability, infection in the donor
area and immune rejection (2).
Modern tissue engineering proposes a new direction for the
treatment of bone defects, by designing a porous biomimetic natural
extracellular matrix (ECM) scaffold, to provide cells with a
microenvironment for growth, proliferation and differentiation.
Compared to the ECM scaffold, the high-porosity nanofiber scaffold
provides more space for cells, facilitating the exchange of
nutrients and oxygen and the excretion of metabolic waste (3). Degradable polymer scaffolds also play
an important role in modern tissue engineering. If the implanted
materials degrade too fast, before the new bone has formed, the
scaffold will not be able to provide cells with a microenvironment
for growth. However, if the materials are non-degradable after the
implantation, and continue to remain in the form of foreign matter,
the risk of infection is increased. It is therefore required that
the degradation rate of bone scaffolds should match the formation
rate of new bone and that the degradation products of the scaffolds
are not toxic.
In recent years, polymer composite scaffolds as drug
or gene delivery carriers have been widely used in tissue
engineering (4).
Poly(lactic-co-glycolic acid) (PLGA) is a degradable, functional
compound with good biocompatibility and mechanical properties. It
is widely used in pharmaceutical and medical engineering materials,
and is certified by FDA (5,6). Because PLGA lacks the ability to
promote osteoblast differentiation, strategies are needed to
combine it with other materials. Bioactive glass (BG) is a material
that can repair, replace and regenerate body tissues, and can form
a bond between tissues and materials (7). The degradation products of BG can
promote growth factor production, cell proliferation, gene
expression of osteoblasts and growth of bone tissue (8,9). Animal
experiments have shown that a combination of BG and PLGA can
accelerate bioceramic degradation and promote bone defect
regeneration (10–13).
Although PLGA combined with BG has been reported to
have a positive catalytic significance in bone healing in several
studies (7,10–13), the
long-term degradation effects of BG and PLGA-composite scaffolds
in vivo have not been extensively studied. In the present
study, a PLGA/BG composite scaffold was prepared by thermal phase
separation. The purpose of the present study was to evaluate the
characteristics and physicochemical properties of the composite
scaffold. MG-63 cells were used to evaluate the in vitro
biocompatibility of the scaffold. Scaffold degradation was assessed
after subcutaneous transplant into New Zealand white rabbits.
Materials and methods
Preparation of PLGA/BG composite
scaffold
Micro-nano powders of BG (medical grade; donated by
South China University of Technology) were added to 10 ml
tetrahydrofuran (analytical pure; Guangzhou Jinhua Chemical Reagent
Co., Ltd.) and dispersed using ultrasonic oscillation
(SCIENTZ-1500F; Ningbo Scientz Biotechnology Co., Ltd.). PLGA
(medical grade; Shenzhen Guanghua Weiye Industrial Co., Ltd;
PLGA:BG ratio, 9:1) was then weighed and added to the
tetrahydrofuran solution containing BG. The PLGA/BG solution was
incubated in a 60°C water bath and stirred for 2 h until all
powders had dissolved. The resulting PLGA/BG solution was
transferred into a mold and immediately placed in a −20°C freezer
for at least 2 h to solidify into a gel. The gel was then
transferred to 4°C for 1 day of water displacement, followed by
incubation in a Genesis freeze dryer (VirTis; SP Scientific; −55°C,
pressure <50 Pa) to lyophilize for 48 h to obtain the PLGA/BG
nanofiber composite scaffold.
Analysis of surface morphology
The surface morphology of the composite scaffold was
evaluated by scanning electron microscopy (SEM). A 4×4 mm composite
scaffold was adhered to a copper substrate, and sprayed with gold
under a vacuum. A JSM-6330F cold cathode field emission SEM (JEOL,
Ltd.; provided by Sun Yat-sen University Test Center) was used to
analyze the surface morphology of the gold-coated PLGA/BG composite
scaffold.
Determination of porosity
A liquid (absolute ethanol) replacement method was
used to measure porosity. The proportion pycnometer was filled with
ethanol (analytically pure; Sinopharm Chemical Reagent Co., Ltd.)
to a value of W1 (g). The sample [with the quality set
as WS (g)] was immersed in the pycnometer filled with
ethanol to de-aerate and allow the ethanol to fill the membrane's
pores, followed by replacement of the ethanol. The value of the
pycnometer containing the sample and the ethanol was defined as
W2 (g). After removing the sample from the ethanol, the
value of the pycnometer containing the remaining ethanol was
defined as W3 (g).
The porosity of the sample scaffold was calculated
based on the following equation. Here, P represents the
porosity (%) of the material; V0 is the volume of
material under normal conditions, named as the apparent volume
(cm3); and V represents the volume of compacted
material (cm3).
P=V0-VV0×100%=W2-W3-WsW1-W3×100%
Biocompatibility and osteogenic
properties of a PLGA/BG composite scaffold
MG-63 osteosarcoma cell culture. Human MG-63
osteosarcoma cells (Nanjing Kehao Biotechnology Co., Ltd.) were
transferred to a culture flask containing 10% fetal bovine serum
(Beijing Solarbio Science & Technology Co., Ltd.), 100 U/ml
penicillin, and 100 µg/ml streptomycin in low-glucose DMEM (Beijing
Solarbio Science & Technology Co., Ltd.), which was then
incubated at 37°C in a 5% CO2 incubator. Once the cells
reached 80% confluence, the culture medium was removed and the
cells harvested using a 0.02% EDTA solution containing 0.25%
trypsin. The number of cells was counted and the cell density
adjusted to a 1:3 ratio for further subculture.
Measurement of alkaline phosphatase
(ALP) activity of MG-63 cells on the composite scaffolds
A 100-µl cell suspension containing 1×105
MG-63 cells was seeded into a 24-well plate containing two groups
of scaffold (PLGA only and PLGA/BG). The cell suspension was
incubated with each scaffold for 3, 7 or 14 days. Culture medium
was then removed and plates rinsed 3 times with PBS, before cells
were lysed with 200 µl 1% Triton X-100 solution for 10–15 min at
4°C. A 30-µl solution was removed from each well and added to a
fresh 96-well plate for the ALP assay, using the ALP Assay kit
(Beijing Solarbio Science & Technology Co., Ltd.), according to
the manufacturer's instructions. The absorbance of each well was
measured at 520 nm and the optical density (OD) was calculated.
Observation of MG-63 cell morphology
on a composite scaffold
The PLGA/BG composite scaffolds were pre-wetted in
the cell culture solution as described above. Pre-wetted composite
scaffolds were placed in a 24-well plate and 100 µl of a cell
suspension containing 1×104 MG-63 cells added to each
well. The scaffolds were incubated in the cell suspension for 3 or
7 days, before removal of the culture medium and fixation of the
cells with 2.5% pentanediol at 4°C overnight. The scaffolds were
then dehydrated in graded ethanol, and coated with colloidal gold
before cell adhesion, cell proliferation and calcium nodule
formation were observed using SEM.
Degradation experiments in vivo
Animal model creation
All animal experiments in this study were approved
by the Animal Ethical and Welfare Committee of Sun Yat-sen
University (IACUC-DB-15-0502) and all experiments were conducted in
accordance with their guidelines. Operations were performed with
the animals under general anesthesia using an intravenous injection
of 30 mg/kg 3% sodium pentobarbital (Wuhan Dongkang Source
Technology Co., Ltd). Nine female New Zealand white rabbits aged
7–8 weeks were obtained from Guangdong Medical Laboratory Animal
Center and one transplant was performed on each rabbit ear. A
transverse incision was made on the appropriate area of the rabbit
ear, and a subcutaneous pocket was created between the rabbit ear
epidermis and the cartilage. The PLGA/BG scaffolds were implanted
in the pockets, and the incisions were closed with sutures. Rabbits
were sacrificed after 2 weeks, or at 3 or 6 months post-surgery.
Tissues from the surgical areas, limited to ~3 mm outside the
transplanted areas, of full ear thickness were obtained, with 6
tissue samples obtained per time period.
Histological analysis
Tissues were fixed in 10% formalin at room
temperature for 48 h and decalcified with EDTA decalcification
solution at room temperature for 2 weeks. Tissues were then
dehydrated in a gradient ethanol solution and embedded in paraffin
using an automatic biological tissue-embedding machine TB-718
(Hubei Taiva Medical Technologies Co., Ltd.). Paraffin-embedded
sections with a thickness of 5–7 µm were obtained after sectioning
using a rotary microtome (Leica Microsystems Shanghai Ltd.). For
hematoxylin and eosin (H&E) staining at room temperature,
paraffin-embedded sections were routinely de-waxed, soaked for 15
min in hematoxylin, and rinsed of excess stain under running water.
Sections were then soaked in 1% hydrochloric acid ethanol for 5
sec, rinsed under running water for 15 min and soaked in eosin dye
solution for 5 min. The sections then underwent gradient alcohol
dehydration and were sealed with neutral gum, before they were
observed under an ordinary light microscope (optical microscope
BX41TF; Olympus Corporation). Four H&E-stained paraffin slices
from the same surgical region of each sample were randomly selected
at 2 weeks, 3 months and 6 months. At ×400 magnification, 5 fields
of view were randomly selected and images gathered of each slice of
the transplanted area.
For Sirius red staining, paraffin-embedded sections
were routinely de-waxed and stained with Sirius red droplets
(Beijing Leagene Biotech. Co., Ltd.) at room temperature for 1 h.
Sections were then rinsed under running water and Mayer's
hematoxylin staining solution was used to stain nuclei at room
temperature for 10 min. The stained sections underwent graded
alcohol dehydration and were sealed with neutral gum before they
were viewed under a polarized light microscope (STEMI 2000; Zeiss
GmbH). At ×200 magnification, three fields of view were randomly
selected and images gathered of each slice of the transplanted
area.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for
statistical analyses. All measurement data are presented as the
mean ± SD. A one-way analysis of variance was used for data
analysis. LSD was used as the post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Micromorphology of the composite
scaffolds
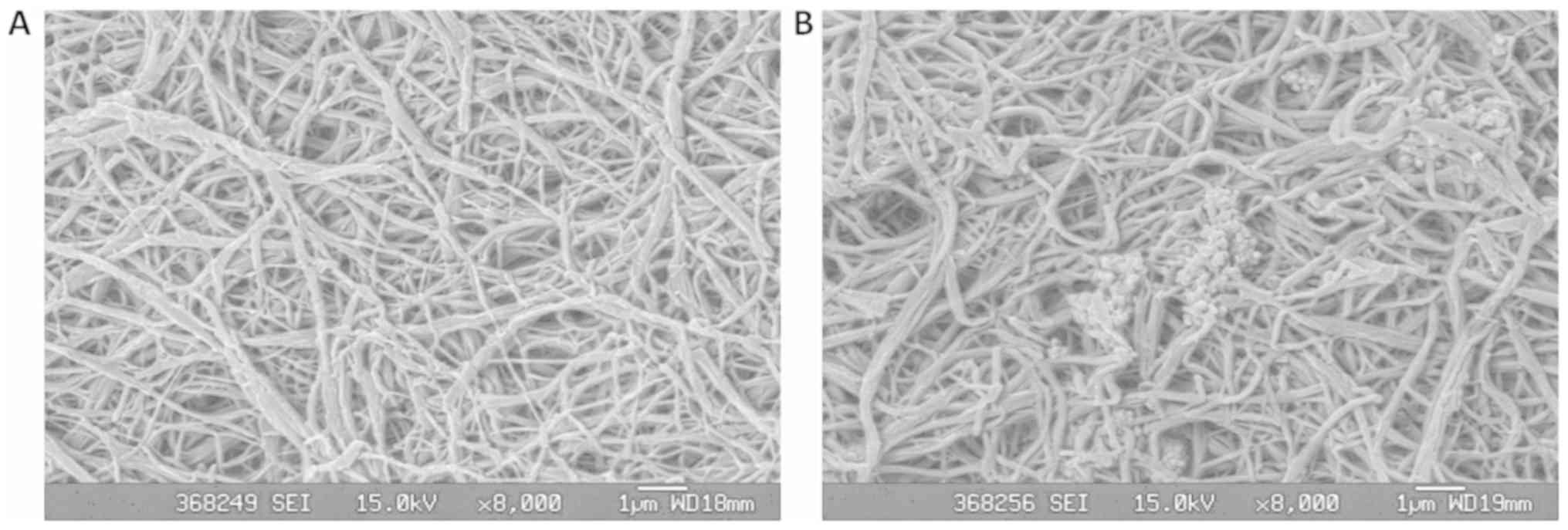
As shown in Fig. 1A,
the PLGA scaffold generated from experimentally-induced phase
separation formed a three-dimensional network structure. The fiber
material diameter was 160–320 nm with a uniform pore distribution
and pore sizes of 1–7 µm. Fig. 1B
shows that BG particles adhered to the PLGA matrix and dispersed in
the porous material. The fiber material diameter was 160–320 nm
with a uniform pore distribution and pore sizes of 1–7 µm. The
fiber diameter and porosity of the composite material showed no
obvious change in comparison to the scaffold generated with PLGA
alone. The two scaffolds formed nano-diameter fibers with
micron-sized pores.
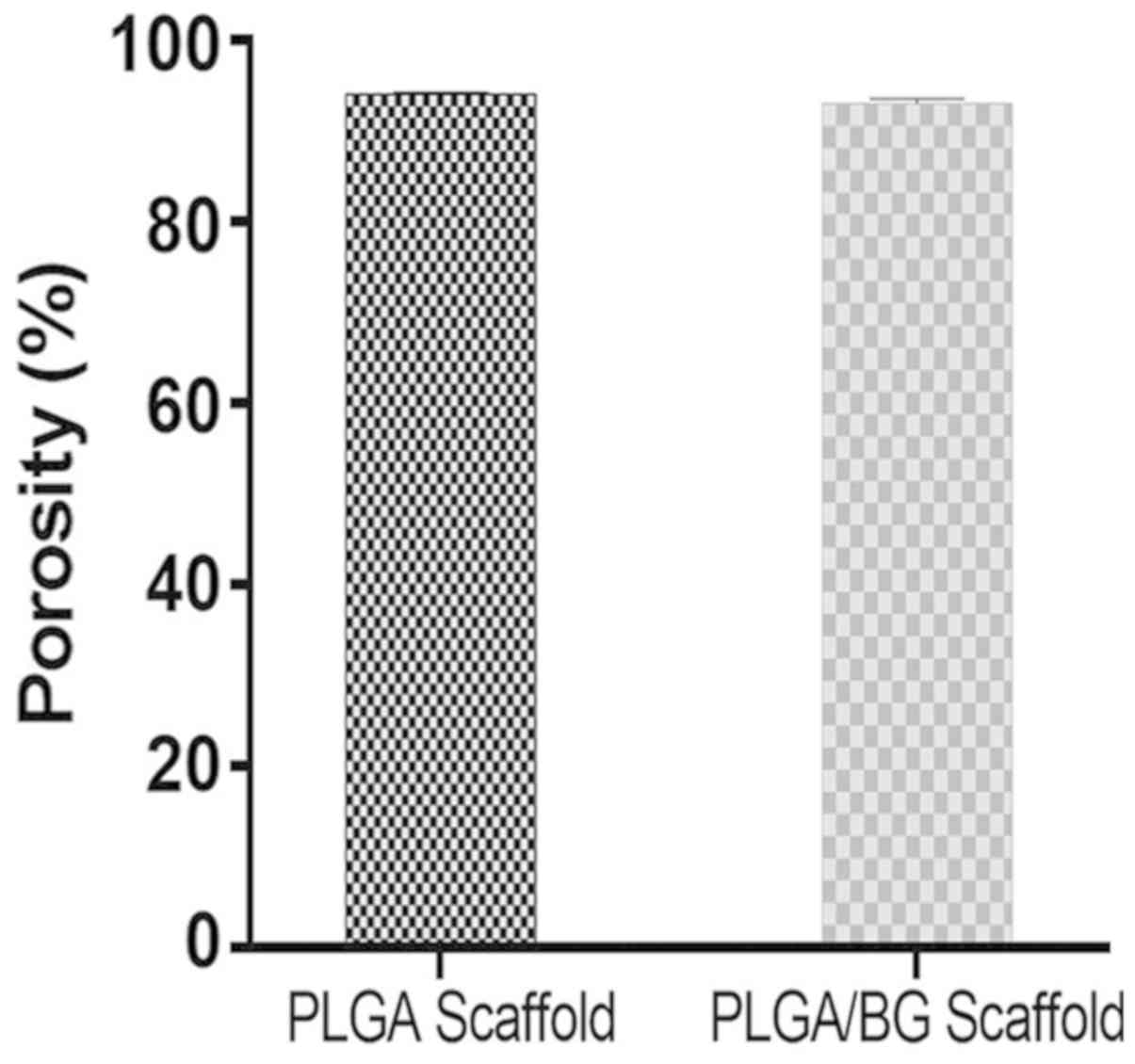
Porosity of the composite
scaffolds
The PLGA scaffold had a high porosity
(93.926±0.094%). The porosity of the PLGA/BG composite scaffold was
93.048±0.121%, which was slightly lower, but in general the
structure of the scaffold did not appear altered. There was no
significant difference in porosity between the two groups and both
groups showed a high porosity (Fig.
2).
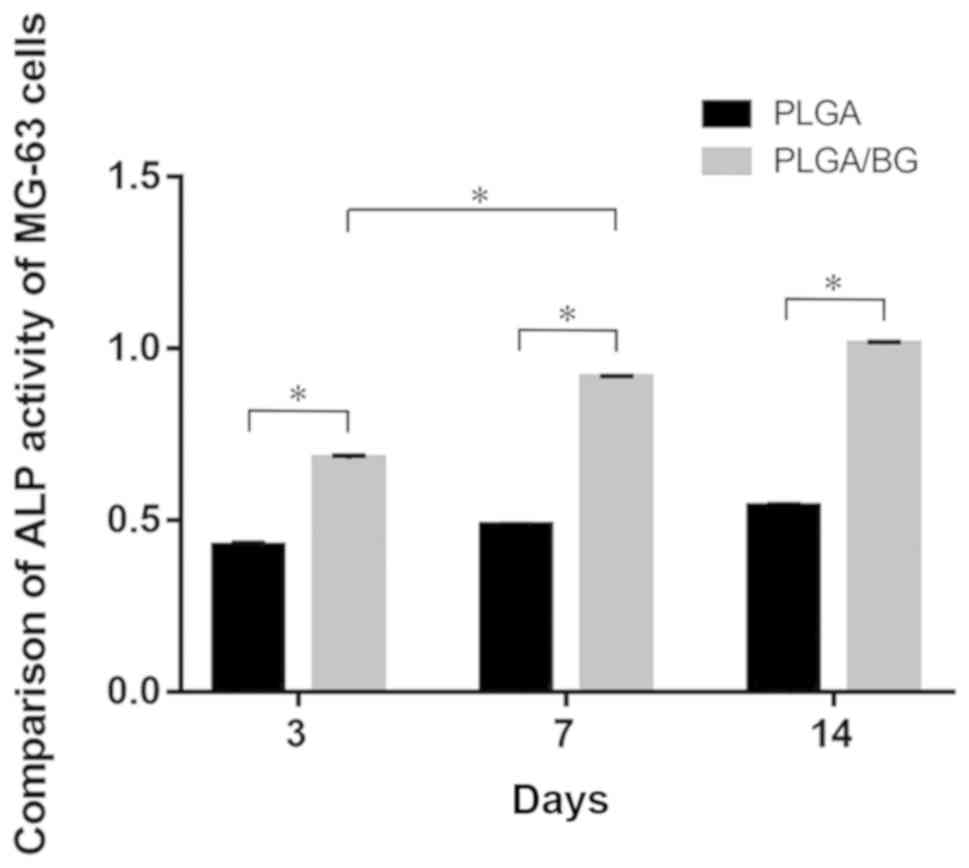
Measurement of ALP activity of MG-63
cells
The overall ALP activity of MG-63 cells in each
group appeared to increase over time. The OD value of cells on the
PLGA/BG scaffold was higher than that of cells grown on the PLGA
scaffold at each of three time points studied (P<0.01). The OD
value of the cells grown on the PLGA/BG for 7 days was
significantly higher than the OD value of the cells grown on the
scaffold for 3 days (P<0.01). There was no significant
difference in OD value between cells cultured on the PLGA/BG for 7
days and the cells cultured on the PLGA/BG scaffold for 14 days
(Fig. 3).
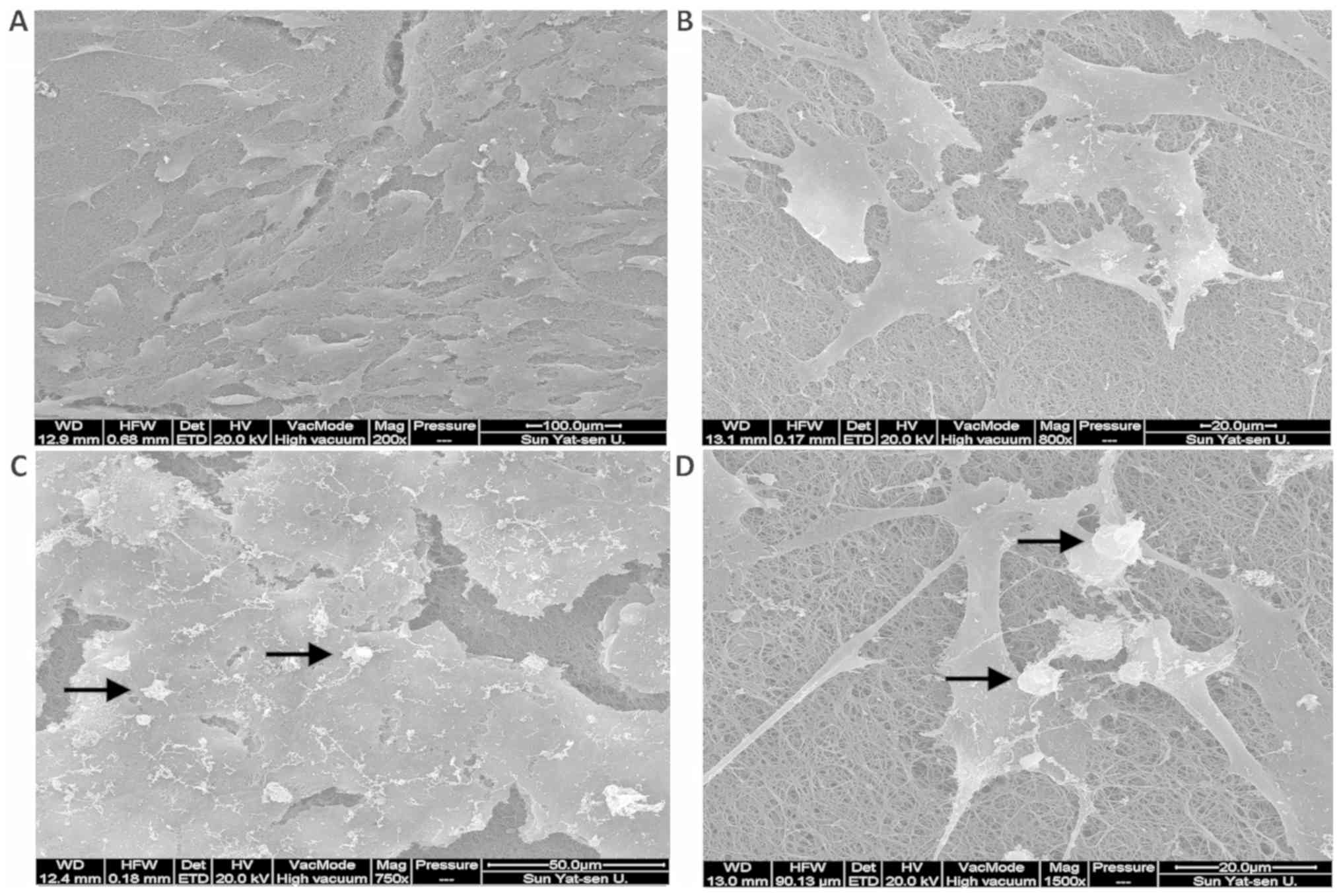
Morphological observation of MG-63
cells
SEM data indicated that after 3 days of culture,
MG-63 cells were spindle-shaped and adhered to the surface of the
PLGA/BG composite scaffold. These cells began to extend pseudopodia
in all directions to cross-link with the composite scaffold
(Fig. 4A and B). After 7 days of
culture, the MG-63 cells formed colonies after aggregating on
multiple layers of the scaffold. At the center of the colony,
overlapping cells blurred the cell boundaries and projections of
the peripheral cells were intertwined. The cells also created
calcified nodules (Fig. 4C and
D).
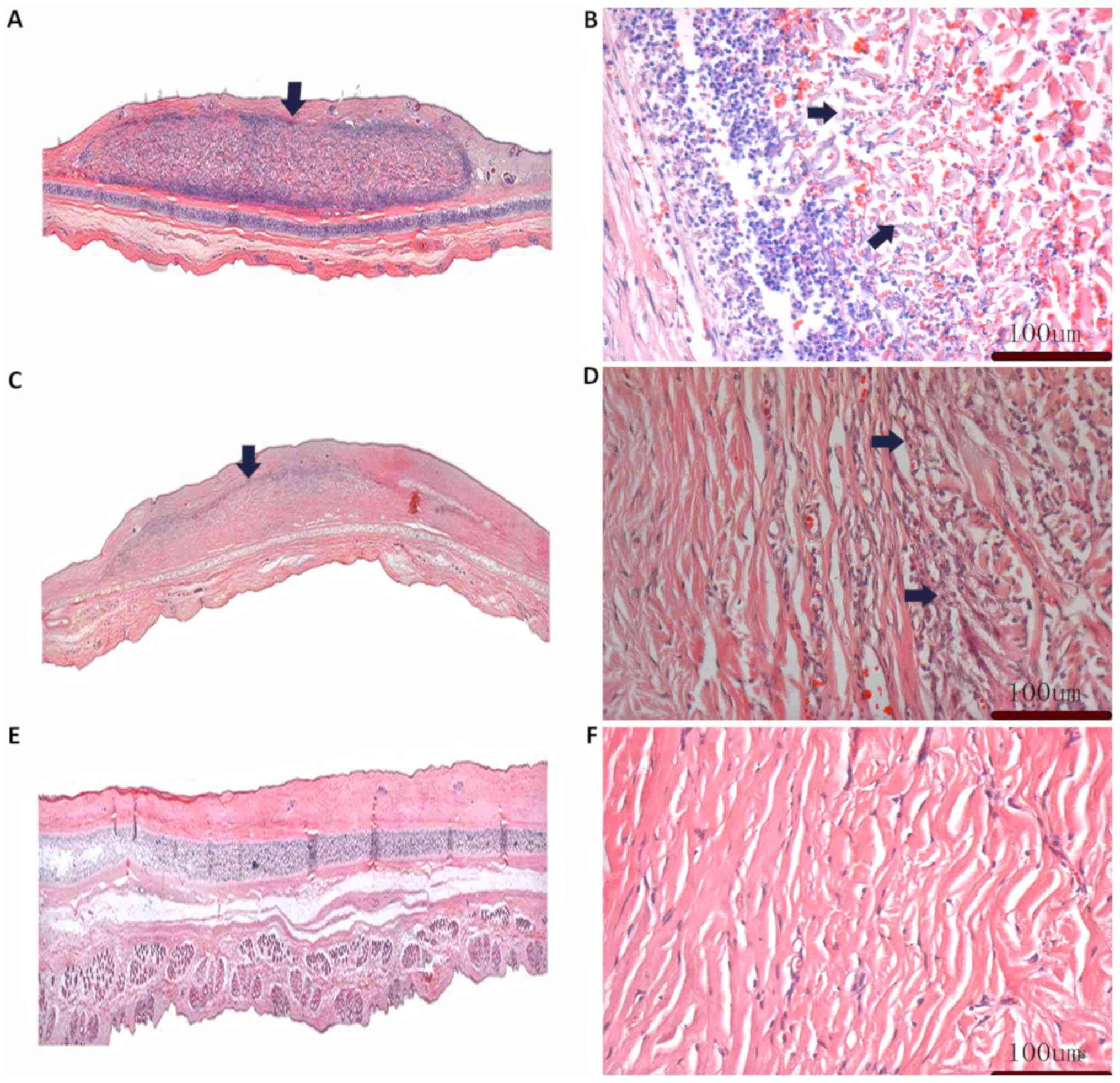
Histological analysis
A total of 2 weeks after transplantation into the
rabbit ear, a large number of lymphocytes and erythrocytes could be
seen near the border of the PLGA/BG scaffold, and the tissue
arrangement in the scaffold was disordered (Fig. 5A and B). In PLGA/BG scaffolds 3
months post-transplantation, the numbers of lymphocytes and
erythrocytes were markedly reduced than at 2 weeks and the
composite scaffold was partly degraded. Histological analysis
showed neovascularization in the transplanted area. The arrangement
of fibers inside the infiltrating tissue began to align in parallel
and the structure became denser. Additionally, the boundary between
the graft material and the surrounding tissue became blurred
(Fig. 5C and D). At 6 months
post-transplantation, the PLGA/BG scaffold was fully degraded and
had been replaced by newly grown fibers from the host, which were
similar to the tissues surrounding the non-transplanted area
(Fig. 5E and F).
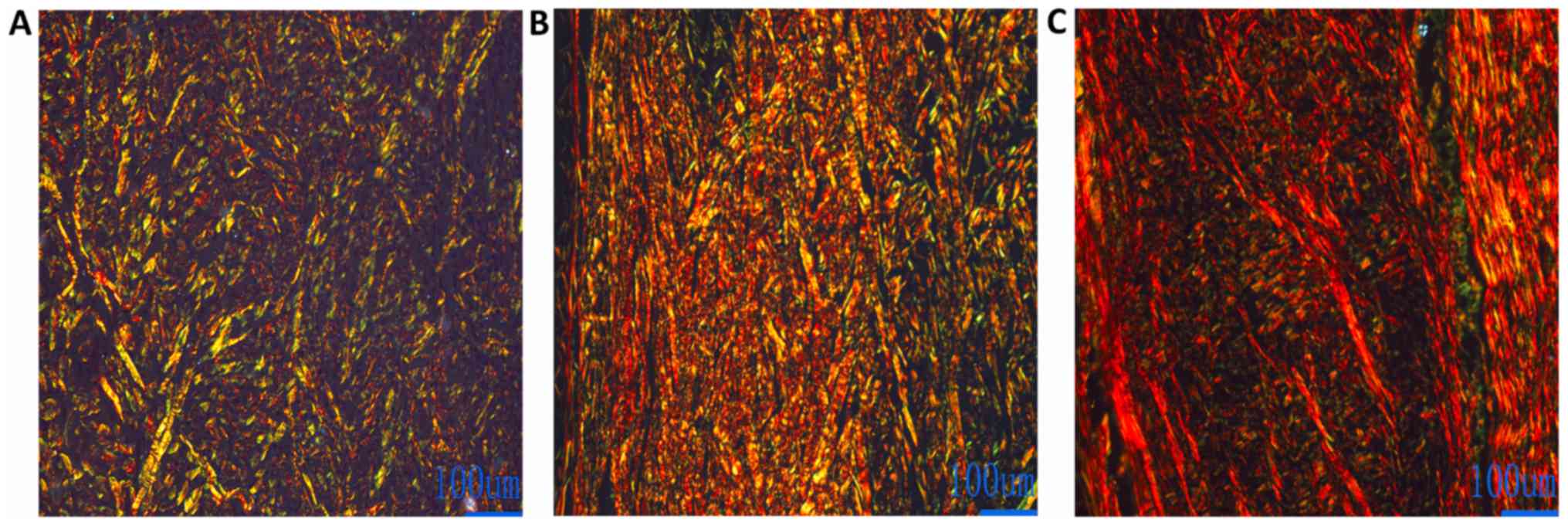
Observation of Sirius red-stained paraffin sections
under a polarized light microscope showed type I collagen fibers as
red and yellow and type III collagen fibers as green. At 2 weeks
post-transplantation, the area of green type III collagen fibers in
the PLGA/BG scaffold was higher than the area of red type I
collagen fibers (Fig. 6A). At 3
months post-transplantation, the area red type I collagen fibers in
the transplanted area increased, and the area of the type III
collagen fibers decreased in comparison with the result 2 weeks
post-transplantation (Fig. 6B). The
type I collagen fibers were the main collagen fibers in the graft
areas. Type III collagen fibers were gradually replaced by red type
I collagen fibers (Fig. 6C).
Discussion
The development of scaffolds with osteoinductivity,
high porosity and stable degradation profiles is a key challenge in
the field of tissue engineering. Although the relationship between
pore size and bone formation is inconclusive, it is clear that
porous structures provide more space and surface area for bone cell
adhesion and bone growth (14).
Simultaneously, the interconnection between pores provides a
pathway for cell migration and vascularization in vivo
(15–17). The scaffold used in the present study
had a porous three-dimensional nanofibrous structure with 160–320
nm diameter fibers, a uniform pore distribution, and pore sizes of
1–7 µm. In the in vitro experiment, the PLGA/BG nanofiber
scaffold had a large surface area and provided a large number of
cell contact points, which is beneficial to cell adhesion, invasion
and proliferation (7). Studies have
confirmed that nanofiber scaffolds are structurally closer to
natural ECM, and are more conducive to cell adhesion and growth
than traditional micron scaffolds (18,19).
Expression of ALP is an early marker of a maturing
extracellular matrix. It is an important marker for the in
vitro differentiation of osteoblasts into mature osteocytes
that reach the matrix mineralization stage (9). In in vitro experiments, ALP is
used as an early marker for osteoblast differentiation and
maturation. The level of ALP secretion is positively correlated
with the capacity for in vitro mineralization (20–22). It
has previously been shown that bioactive glass that degrades in the
early stage, releases calcium, phosphorus and silicon from the
material into the plasma, and this stimulates the expression of ALP
(23,24). Studies such as that by Bellows et
al (25) have shown that each
calcium nodule is formed by a nodular osteoblast that undergoes 2–3
weeks of proliferation and differentiation. The formation of
calcified nodules is due to the multi-layer growth of osteoblasts,
which must undergo three stages: Rapid proliferation, maturity of
the extracellular matrix and mineralization of the matrix. Only
after entering maturity, does mineralization occur. The results of
SEM showed that, after 7 days of culture in the scaffold, cell
proliferation and the number of calcified nodules increased
gradually.
Stable degradation in vivo is a prerequisite
for ideal bio-scaffolds. Premature degradation, or late or absent
degradation, will affect bone remodeling, leading to the failure of
bone defect repair. Novel scaffolds with good osteoconductivity and
osteoinductivity have been prepared from PLGA and BG, and their
biocompatibility was previously reported in the literature
(10–13). However, long-term studies on the
stable degradation of PLGA/BG scaffolds in animals have, to the
best of our knowledge, not yet been published.
In the animal experiments conducted in the present
study, inflammatory reactions appeared in the PLGA/BG
scaffold-transplanted area in the early post-operative period. A
previous study has shown that the early inflammatory response is
mediated by neutrophils releasing reactive oxygen species and
inflammatory cytokines to clean the dead cells from the graft area
(26). Additionally,
neovascularization could be seen in the implanted areas in this
present study. A previous study revealed that vascularization of
the graft is not only key to graft survival, but that it is also
important for osteogenesis. Mazio et al (27) aimed to obtain graft vascularization
by promoting host vessel invasion of the scaffold, as well as by
developing pre-vascularized constructs. An active blood vessel
network helps grafts to survive and integrate with existing host
tissue. Bone is a highly vascularized tissue that relies on tight
connections between blood vessels and bone cells to maintain
integrity (28). An inflammatory
cell response could be observed in the transplant area and nascent
immature type III collagen fibers were gradually replaced by type I
collagen fibers, which were structurally and functionally more
mature. Collagen is the most widespread fibrillar secretory protein
in the body (29). It is a part of
all tissues that require a framework or mechanical scaffold
functionalized with growth factors, biological signals or specific
structures allowing cells to adhere, proliferate and differentiate
(29).
In the present study, the PLGA/BG scaffold had good
biocompatibility and capacity for successful vascularization, and
induced tissue maturation in the transplanted area. The scaffold
could also be fully degraded after serving its purpose. The
limitations of this study need to be further explored in future
experiment. Results of the histological analysis are only
qualitative in this study and detailed statistical analysis and
comparison will be further studied. In future research, the
physical and mechanical properties of the PLGA/BG scaffolds will
also be further investigated as will cell adhesion based on primary
human mesenchymal stem cells with the goal of examining the
efficacy of the PLGA/BG scaffolds for the treatment of bone
defects.
In summary, the PLGA/BG scaffold used in the current
study had a three-dimensional network structure and a porosity of
93.048±0.121%. The scaffold promoted cell adhesion, had good
biocompatibility and provided a calcified matrix that promoted
osteoblast formation. It may be a candidate bio-scaffold for the
repair of bone defects.
Acknowledgements
Not applicable.
Funding
This work was supported by the Medical Science and
Technology Research Fund Project of Guangdong Province (grant no.
A2017547).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY conceived and designed the study. SL performed
the experiments and acquired the data. YC analyzed the data,
prepared the figures and drafted the manuscript. JC and WF
interpreted the results. WF edited and revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments in this study were approved
by the Animal Ethical and Welfare Committee of Sun Yat-sen
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Furia JP, Rompe JD, Cacchio A and Maffulli
N: Shock wave therapy as a treatment of nonunions, avascular
necrosis, and delayed healing of stress fractures. Foot Ankle Clin.
15:651–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lichte P, Pape HC, Pufe T, Kobbe P and
Fischer H: Scaffolds for bone healing: Concepts, materials and
evidence. Injury. 42:569–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palecek SP, Loftus JC, Ginsberg MH,
Lauffenburger DA and Horwitz AF: Integrin-ligand binding properties
govern cell migration speed through cell-substratum adhesiveness.
Nature. 385:537–540. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Félix Lanao RP, Leeuwenburgh SC, Wolke JG
and Jansen JA: Bone response to fast-degrading, injectable calcium
phosphate cements containing PLGA microparticles. Biomaterials.
32:8839–8847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chereddy KK, Vandermeulen G and Préat V:
PLGA based drug delivery systems: Promising carriers for wound
healing activity. Wound Repair Regen. 24:223–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao S, Tang G, Hua D, Xiong R, Han J,
Jiang S, Zhang Q and Huang C: Stimuli-responsive bio-based
polymeric systems and their applications. J Mater Chem B Mater Biol
Med. 7:709–729. 2019. View Article : Google Scholar
|
|
7
|
Kido HW, Brassolatti P, Tim CR,
Gabbai-Armelin PR, Magri AM, Fernandes KR, Bossini PS, Parizotto
NA, Crovace MC, Malavazi I, et al: Porous poly
(D,L-lactide-co-glycolide) acid/biosilicate® composite
scaffolds for bone tissue engineering. J Biomed Mater Res B Appl
Biomater. 105:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peitl O, Zanotto ED, Serbena FC and Hench
LL: Compositional and microstructural design of highly bioactive
P2O5-Na2O-CaO-SiO2
glass-ceramics. Acta Biomater. 8:321–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Granito RN, Rennó AC, Ravagnani C, Bossini
PS, Mochiuti D, Jorgetti V, Driusso P, Peitl O, Zanotto ED,
Parizotto NA, et al: In vivo biological performance of a novel
highly bioactive glass-ceramic (Biosilicate®): A
biomechanical and histomorphometric study in rat tibial defects. J
Biomed Mater Res B Appl Biomater. 97:139–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Habraken WJ, Wolke JG, Mikos AG and Jansen
JA: PLGA microsphere/calcium phosphate cement composites for tissue
engineering: In vitro release and degradation
characteristics. J Biomater Sci Polym Ed. 19:1171–1188. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruhé PQ, Boerman OC, Russel FG, Mikos AG,
Spauwen PH and Jansen JA: In vivo release of rhBMP-2 loaded porous
calcium phosphate cement pretreated with albumin. J Mater Sci Mater
Med. 17:919–927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding J, Zhang J, Li J, Li D, Xiao C, Xiao
H, Yang H, Zhuang X and Chen X: Electrospun polymer biomaterials.
J.Prog Polym Sci. 90:1–34. 2019. View Article : Google Scholar
|
|
13
|
Plachokova A, Link D, van den Dolder J,
van den Beucken J and Jansen J: Bone regenerative properties of
injectable PGLA-CaP composite with TGF-beta1 in a rat augmentation
model. J Tissue Eng Regen Med. 1:457–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu BK, Choi DJ, Park SJ, Kim YJ and Kim
CH: 3D bioprinting technologies for tissue engineering
applications. Adv Exp Med Biol. 1078:15–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mastrogiacomo M, Scaglione S, Martinetti
R, Dolcini L, Beltrame F, Cancedda R and Quarto R: Role of scaffold
internal structure on in vivo bone formation in macroporous calcium
phosphate bioceramics. Biomaterials. 27:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deing A, Luthringer B, Laipple D, Ebel T
and Willumeit R: A porous TiAl6V4 implant material for medical
application. Int J Biomater. 2014:9042302014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen G, Liu B, Liu H, Zhang H, Yang K,
Wang Q, Ding J and Chang F: Calcium phosphate cement loaded with
10% vancomycin delivering high early and late local antibiotic
concentration in vitro. Orthop Traumatol Surg Res. 104:1271–1275.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jun HW, Paramonov SE, Dong H, Forraz N,
McGuckin C and Hartgerink JD: Tuning the mechanical and
bioresponsive properties of peptide-amphiphile nanofiber networks.
J Biomater Sci Polym Ed. 19:665–676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murphy WL and Mooney DJ: Molecular-scale
biomimicry. Nat Biotechnol. 20:30–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Proff P and Römer P: The molecular
mechanism behind bone remodelling: A review. Clin Oral Investig.
13:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu B, Xu W, Liu J, Ding J and Chen X:
Osteoinductive agents-incorporated three-dimensional biphasic
polymer scaffold for synergistic bone pegeneration. J. ACS Biomater
Sci Eng. 5:986–995. 2019. View Article : Google Scholar
|
|
22
|
Fávaro-Pípi E, Bossini P, de Oliveira P,
Ribeiro JU, Tim C, Parizotto NA, Alves JM, Ribeiro DA, Selistre de
Araújo HS and Renno AC: Low-intensity pulsed ultrasound produced an
increase of osteogenic genes expression during the process of bone
healing in rats. Ultrasound Med Biol. 36:2057–2064. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renno AC, van de Watering FC, Nejadnik MR,
Crovace MC, Zanotto ED, Wolke JG, Jansen JA and van den Beucken JJ:
Incorporation of bioactive glass in calcium phosphate cement: An
evaluation. Acta Biomater. 9:5728–5739. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xynos ID, Edgar AJ, Buttery LD, Hench LL
and Polak JM: Gene-expression profiling of human osteoblasts
following treatment with the ionic products of Bioglass 45S5
dissolution. J Biomed Mater Res. 55:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bellows CG, Aubin JE, Heersche JN and
Antosz ME: Mineralized bone nodules formed in vitro from
enzymatically released rat calvaria cell populations. Calcif Tissue
Int. 38:143–154. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu K, Cai J, Li H, Feng J, Feng C and Lu
F: The disturbed function of neutrophils at the early stage of fat
grafting impairs long-term fat graft retention. Plast Reconstr
Surg. 142:1229–1238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mazio C, Casale C, Imparato G, Urciuolo F,
Attanasio C, De Gregorio M, Rescigno F and Netti PA:
Pre-vascularized dermis model for fast and functional anastomosis
with host vasculature. Biomaterials. 192:159–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barabaschi GD, Manoharan V, Li Q and
Bertassoni LE: Engineering pre-vascularized scaffolds for bone
regeneration. Adv Exp Med Biol. 881:79–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turkevych M, Turkevych A, Kadjaya A, Gold
MH, Lotti T and Sulamanidze G: Pathomorphological criteria of use
efficiency of resorbable and permanent implants in aesthetic
medicine and cosmetic dermatology. J Cosmet Dermatol. 17:731–735.
2018. View Article : Google Scholar : PubMed/NCBI
|