Introduction
Endothelial progenitor cells (EPCs), derived from
bone marrow or peripheral blood cells, have been shown to be
incorporated into the foci of physiological and pathological
neovascularization (1). EPCs can
home to sites of ischemia, differentiate into endothelial cells and
can contribute to postnatal neovascular formation (2). The beneficial angiogenic properties of
EPC for cell therapy have attracted the attention of numerous
researchers (3,4); however, the initial clinical use of
EPCs has not yielded the predicted positive outcomes (5). One of the reasons is that patients with
coronary artery disease (CAD) already possess risk factors, such as
diabetes, hypertension and smoking, which could reduce EPC number
and impair EPC migration (6). EPCs
isolated from patients with type I or type II diabetes exhibited
impaired proliferation, adhesion and incorporation into vascular
structures (7). High glucose (HG)
also impairs the number and function of EPCs (8,9). The
mechanism of HG-induced EPC impairment is related to the activation
of the p38 mitogen-activated protein kinase (MAPK) pathway
(10). Additionally, a number of
studies have shown that HG could reduce EPC proliferation and
migration by exerting a deleterious effect on the
PI3K/Akt/endothelial nitric oxide (NO) synthase (eNOS) signaling
cascade (11,12).
Icariin (C33H40O15;
molecular weight, 676.66), a flavonoid extracted from several
plants in the genus Epimedium, exhibits various pharmacological
activities, including enhancing immune function, stimulating
osteoblast proliferation, antioxidative stress, antiapoptosis,
stimulation of angiogenesis and improving cardiovascular function
(13–16). Icariin has been shown to protect
against endothelial cell dysfunction by activating eNOS and
increasing NO production (17).
However, the role of icariin in HG-induced EPC dysfunction is yet
to be elucidated. In the present study, it was hypothesized that
the administration of icariin could reduce glucose-induced EPC
dysfunction.
Materials and methods
Cell culture and icariin
treatment
Male Sprague Dawley (SD) wild-type rats (SPF grade;
180–200 g; 2–3 weeks; n=3) were obtained from Wuhan University
Experiment Animal Center. These rats were allowed free access to
standard rat chow and water, and were kept in an environment with
controlled temperature and lighting (24°C; 12/12 h-light/dark
cycle; humidity, 50–60%). Mononuclear cells were isolated from bone
marrow from the femurs and tibias of SD rats, and cultured in
endothelial basal medium (EBM-2 SingleQuots; Lonza Group, Ltd.)
containing 5% FBS, human vascular endothelial growth factor A,
human fibroblast growth factor-2, human epidermal growth factor,
insulin-like growth factor-1 and ascorbic acid (EBM-2 SingleQuots;
Lonza Group, Ltd.) to induce mononuclear cells differentiation into
EPCs at 37°C in an atmosphere containing 95% air and 5%
CO2. After 3 days in culture, the non-adherent cells
were removed, and the adherent cells were maintained in new media.
EPCs were characterized by FITC-Ulex europaeus agglutinin I (cat.
no. L9006; Sigma-Aldrich; Merck KGaA) and DiI-acetylated
low-density lipoprotein (cat. no. H7970; Beijing Solarbio Science
& Technology Co., Ltd.) as previously described (18). Icariin (≥94% purity as determined by
high-performance liquid chromatography analysis by the supplier)
was purchased from Sigma-Aldrich (Merck KGaA) and dissolved in
dimethyl sulfoxide at a concentration of 10 mmol/l for storage. The
cytotoxicity of icariin toward EPC was evaluated using a cell
viability assay. Following incubation of EPCs with icariin (0.01,
0.1 or 1 µM), cells were exposed to 0.4% trypan blue solution (cat.
no. T6146; Sigma-Aldrich; Merck KGaA) for 5 min and viewed under a
light microscope (magnification, ×100). Cell viability was defined
as the ratio of unstained cells to the total number of cells. The
EPCs were cultured in 5.5 mM glucose (Control group) or 25 mM
glucose (HG group) for 3 days at 37°C and used for subsequent
experiments (19). In the
proliferation assay, the cells were cultured in serum-free EBM-2
for 12 h for synchronization and then treated with or without
icariin (0.01, 0.1 or 1 µM) under a high glucose condition for 24
h. The EPCs were treated with or without icariin (1 µM) under high
glucose condition for 4 h in the migration assay, for 8 h in
Matrigel tube formation assay, and for 30 min in the measurement of
NO production at room temperature. In the western blot analysis,
the cells were stimulated with or without icariin (1 µM) under a
high glucose condition for 30 min. The animal protocol in the
present study was approved by the Institutional Animal Care and Use
Committee, the Animal Care and Use Committee of Wuhan University
(permit no. WDRM20161204).
Cell proliferation assay
Cell proliferation was assessed using a CCK-8 kit
(Dojindo Molecular Technologies, Inc.). In each well of a 96-well
plate, 5,000 EPCs were seeded and cultured for 12 h at 37°C in an
atmosphere containing 95% air and 5% CO2. After
synchronization in EBM-2 with 0.1% FBS for 12 h, the EPCs were
treated with icariin at three different concentrations (0.01, 0.1
or 1 µM) for 24 h at 37°C (20).
Thereafter, a total of 20 µl Cell Counting Kit-8 (CCK-8) reagent
was added to each well and the cells were incubated at 37°C for a
further 4 h, The absorbance at 450 nm was subsequently measured.
The results are expressed as the fold change of the optical density
value divided by that of the control group.
Cell migration assay
To evaluate the migratory ability of EPCs, a
Transwell chamber assay (Corning, Inc.) was performed. Briefly,
EPCs were seeded at a density of 5×104 cells/well in the
upper chamber with serum-free EBM-2 and different stimulation
conditions, and the lower chamber was filled with serum-free EBM-2
containing stromal cell-derived factor 1a (SDF-1a; 100 ng/ml).
After incubation for 4 h at 37°C, the cells on the top of the
filter were removed, and the migrated cells on the bottom of the
filter were fixed in 95% alcohol for 30 min and stained with 0.1%
crystal violet for 10 min at room temperature. Then, the cells on
the filter were counted manually in at least three random selected
high-power fields (magnification, ×100) in each well under a light
microscope (Olympus Corporation).
Matrigel tube formation assay
A 24-well culture plate was coated with Matrigel (BD
Biosciences), which was allowed to solidify for 30 min at 37°C.
EPCs (5×104/well) were seeded and incubated at 37°C for
8 h. Tube formation was defined as a structure exhibiting a length
four times its width. The total length of the tube formation was
measured in three random fields (magnification, ×100; Olympus
Corporation) per group using Adobe Photoshop CS5 software (Adobe
Systems, Inc.) (21).
Measurement of NO production
NO production was measured in culture medium with a
total NO assay kit (cat. no. S0023; Beyotime Institute of
Biotechnology). Briefly, EPCs (density, 1×104/ml) were
plated on dishes and exposed to various treatments. Then, the
supernatants were collected following centrifugation (140 × g; 10
min) at room temperature and analyzed according to the
manufacturer's protocol. The total NO production of EPCs was
determined by measuring the concentrations of nitrate and nitrite
using the Griess method, and was normalized to standards in the
total NO assay kit.
Western blot assay
Cells were lysed in a RIPA lysis buffer according to
the manufacturer's protocol (BioVision, Inc.). Protein
concentrations were determined by a bicinchoninic acid protein
assay (Beyotime Institute of Biotechnology). Proteins samples (4.5
µg/µl; 20 µl per lane) were separated using 10% SDS-PAGE and
transferred onto PVDF membranes. For western blot analysis, the
PVDF membranes were blocked at room temperature. with 5% non-fat
dried milk that was dissolved in Tris-buffered saline containing
0.1% tween-20 for 90 min and probed with antibodies (1:1,000)
against phosphorylated (p)-p38 (cat. no. sc-7973; Santa Cruz
Biotechnology, Inc.), p38 (cat. no. ab7952; Abcam), protein-CREB
(cat. no. 11273; Signalway Antibody LLC), CREB (cat. no. 9197; Cell
Signaling Technology, Inc.), p-Akt (cat. no. 4060; Cell Signaling
Technology, Inc.), Akt (cat. no. 21054; Signalway Antibody LLC),
p-eNOS (cat. no. 9574; Cell Signaling Technology, Inc.), eNOS (cat.
no. 21170; Signalway Antibody LLC) and GAPDH (cat. no. sc-365062;
Santa Cruz Biotechnology, Inc.) overnight at 4°C. After washing
three times, the membranes were incubated with
peroxidase-conjugated secondary antibodies (Goat Anti Rabbit
IgG/HRP; 1:50,000; cat. no. 31460; Pierce; Thermo Fisher
Scientific, Inc.; Goat Anti Mouse IgG/HRP; 1:50,000; cat. no.
31430; Pierce; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, and enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.) was performed. The autoradiographs were scanned
using Adobe Photoshop CS5 software, and the protein ratios were
calculated (22).
Statistical analysis
The experimental data are presented as the mean ±
SD. One-way analysis of variance followed by Bonferroni post hoc
test was used for comparisons between continuous variables. All
statistical analyses were performed using SPSS 19.0 for Windows
(IBM Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
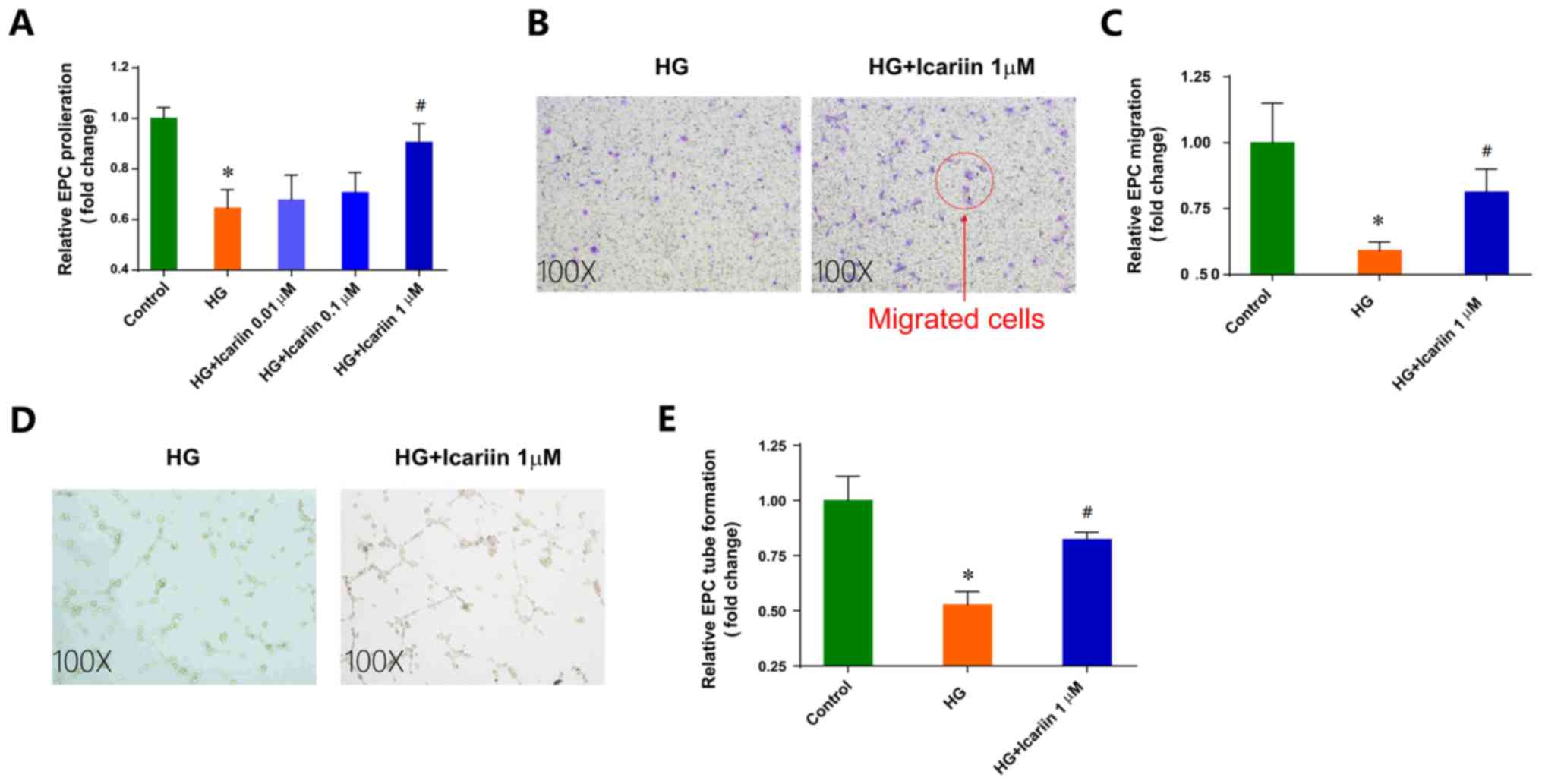
Icariin attenuates the EPC dysfunction
induced by HG
High glucose, which is a risk factor for CAD
(23), significantly impaired EPC
proliferation, migration and tube formation (Fig. 1). Icariin, a flavonoid extracted from
genus Epimedium, exhibits various pharmacological activities
(24). The differences between the
HG group and icariin group were analyzed to evaluate the hypothesis
that icariin could attenuate the impairment of EPC function induced
by HG. Icariin had no notable effects on EPC viability (data not
shown). Treatment with icariin ameliorated the inhibition of EPC
proliferation in HG conditions in a dose-dependent manner in
vitro, with maximal improvement observed following treatment
with 1 µM icariin (Fold change: 0.64±0.07; P=0.0019 HG group vs.
control group; Fold change: 0.90±0.07, P=0.0124 1 µM icariin group
vs. HG group; Fig. 1A).
Additionally, 1 µM icariin treatment significantly increased
HG-impaired EPC migration toward SDF-1a (Fold change: 0.59±0.03;
P=0.0100 HG group vs. control group; Fold change: 0.81±0.08,
P=0.0148 1 µM icariin group vs. HG group; Fig. 1B and C). Furthermore, icariin
improved the in vitro tube-structure formation ability of
EPCs in HG-stimulated conditions (Fold change: 0.52±0.06; P=0.0070
HG group vs. control group; Fold change: 0.82±0.03, P=0.0214; 1 µM
icariin group vs. HG group; Fig. 1D and
E).
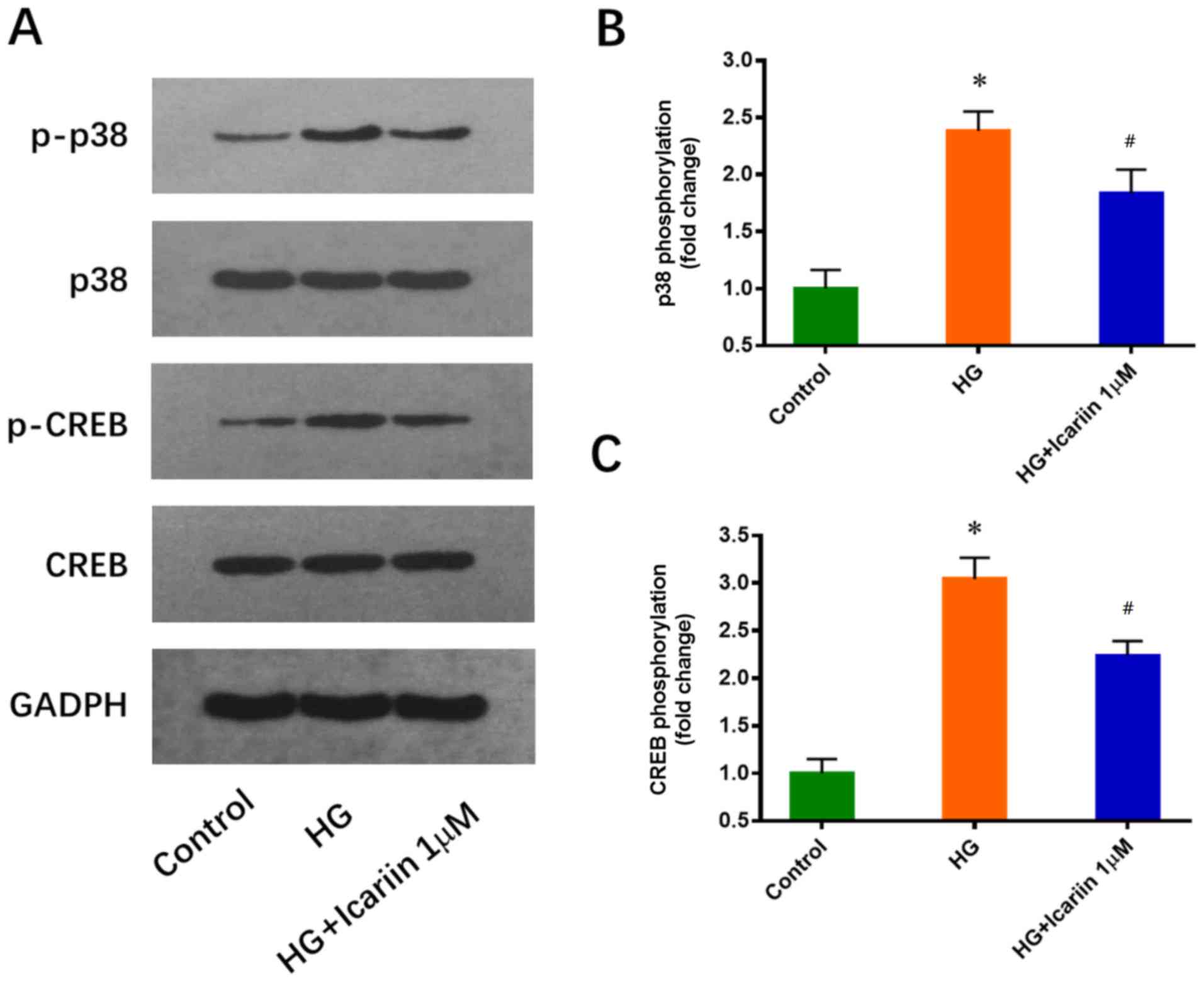
Icariin inhibits the HG-induced
activation of p38 and CREB
p38 and its downstream target CREB have been
reported to play a critical role in EPC downregulation induced by
HG (25). Consistent with this
previous study, increased p38 and CREB phosphorylation levels were
observed in EPCs cultured in 25 mM glucose for 3 days. These
effects were significantly inhibited by icariin (1 µM, 30 min), and
the treatments did not notably alter total p38 and CREB expression
levels (p38 phosphorylation: Fold change: 2.38±0.17; P=0.0005 HG
group vs. control group; Fold change: 1.84±0.21; P=0.0238 icariin
group vs. HG group; CREB phosphorylation: Fold change: 3.04±0.22;
P=0.0002 HG group vs. control group; Fold change: 2.24±0.15,
P=0.0068 icariin group vs. HG group; Fig. 2).
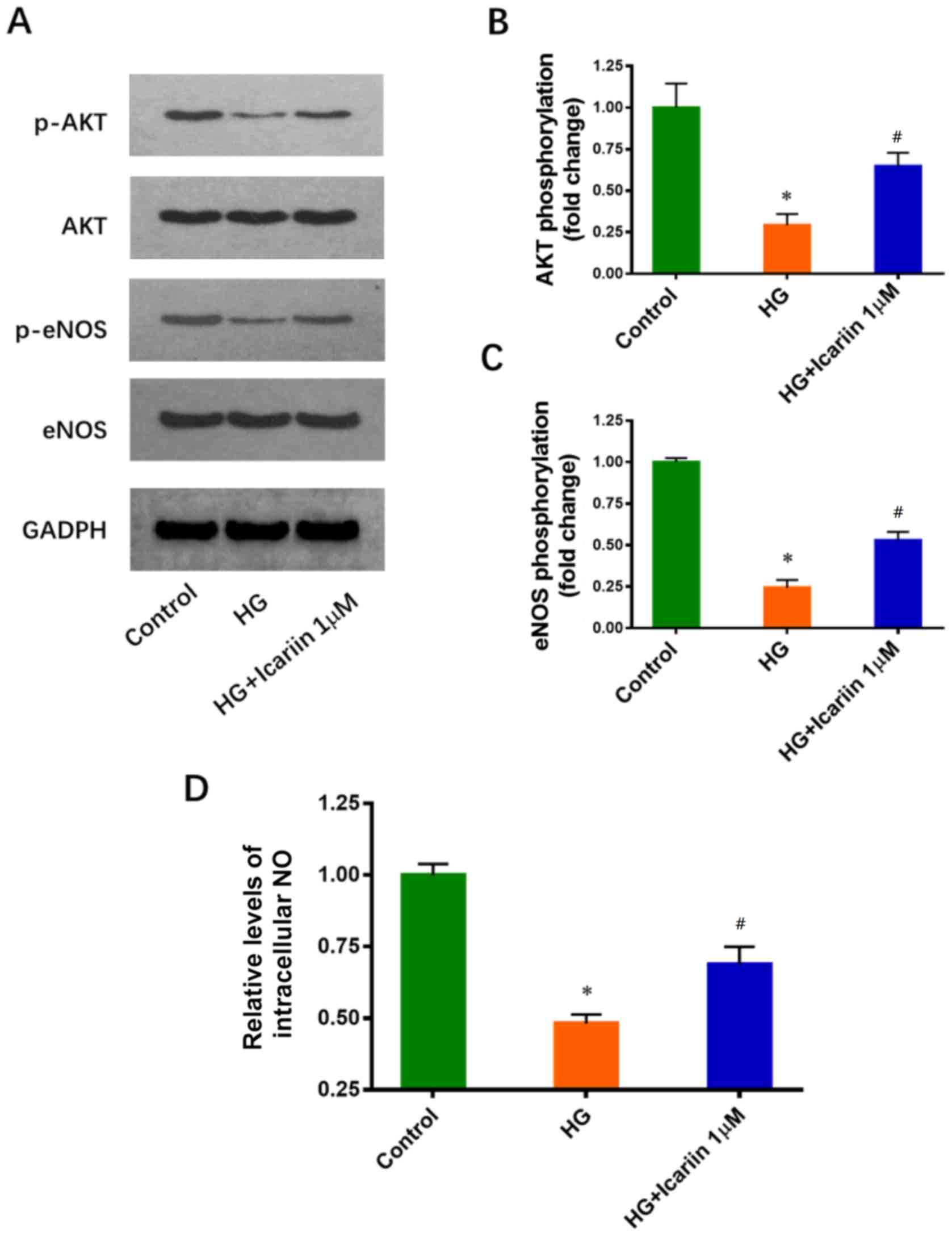
Icariin activates Akt and eNOS, and
promotes NO production in HG-treated EPCs
It has been reported that the inhibitory effects of
HG on Akt and eNOS phosphorylation are involved in HG-induced EPC
dysfunction (11). The effects of
icariin on Akt and eNOS phosphorylation were determined via western
blot analysis. Icariin treatment increased significantly Akt and
eNOS phosphorylation compared with HG treatment only (Akt
phosphorylation: Fold change: 0.29±0.07; P=0.0016 HG group vs.
control group; Fold change: 0.64±0.08; P=0.0047 icariin group vs.
HG group; eNOS phosphorylation: Fold change: 0.24±0.05; P<0.0001
HG group vs. control group; Fold change: 0.53±0.05, P=0.0019
icariin group vs. HG group; Fig.
3A-C). Furthermore, icariin significantly attenuated the
HG-induced inhibition of NO production (Fold change: 0.48±0.03;
P<0.0001 HG group vs. control group; Fold change: 0.69±0.06;
P=0.0064 icariin group vs. HG group; Fig. 3D).
Discussion
The present study demonstrated that icariin could
ameliorate the inhibition of EPC proliferation, migration and tube
formation induced by HG. Additionally, icariin significantly
reduced the activation of the p38/CREB pathway and stimulated the
Akt/eNOS/NO pathway in EPCs treated with HG. These results
indicated potential mechanisms underlying the protective effects of
icariin on EPCs, and suggested that icariin may be a useful agent
for improving EPC function in a HG microenvironment.
Previous studies reported that icariin exerts
endothelial protection effects (17,26).
Icariin stimulated human umbilical vein endothelial cell
proliferation, migration (27) and
NO release (28). Icariin also
delayed homocysteine-induced senescence (27) and inhibited oxidation-induced
apoptosis (29). However, the
effects of icariin on EPC function remain unclear. Numerous
clinical trials are attempting to elucidate the therapeutic effects
of EPCs in cardiovascular diseases, but the CAD risk factors that
may reduce the number and biological activity of EPC limit the
success of EPC transplantation in patients (6,30).
Therefore, this study focused mainly on the effects of icariin on
EPCs under HG conditions, which is one of the major CAD risk
factors (23). HG has been shown to
adversely affect the number and function of EPCs, leading to
reductions in the angiogenic abilities of EPCs (31). Consistent with the current study, the
present data demonstrated that incubation with HG induces adverse
effects on EPC function. Of note, the present findings showed that
icariin treatment attenuates HG-induced EPC dysfunction.
Several mechanisms may be involved in the HG-induced
reduction in EPC number, and impairment in EPC proliferative and
migratory abilities. p38 MAPK and its downstream target CREB have
been shown to decrease the number and proliferation of EPCs
(25). HG induced the p38-dependent
phosphorylation of CREB, thereby inhibiting proliferation (25). As icariin has been reported to
modulate p38 phosphorylation in other cell types (32,33),
this may be a potential effector signaling mechanism via which
icariin attenuated the impaired proliferation of HG-treated EPCs.
In the present study, p38 and CREB were demonstrated to be
phosphorylated under high glucose conditions, and icariin could
reduce these effects.
Another important mechanism involved with HG-induced
impairments in EPC migration is the inhibition of PI3K/Akt/eNOS
activation and NO production (11),
suggesting that the effects of icariin on Akt/eNOS may ameliorate
this dysfunction. Icariin is also known to stimulate angiogenesis
by activating PI3K/Akt/eNOS-dependent signaling pathways in human
endothelial cells (28). Akt/eNOS
activation is also known to increase NO activation (34), and NO is known to regulate the
migration of EPCs (35). The
restored migration induced by icariin may be due to upregulated
Akt/eNOS phosphorylation and NO production. In the present study,
it was demonstrated that high glucose could inhibit the
phosphorylation of Akt/eNOS and the production of NO, and these
effects were significantly attenuated by icariin.
There are certain limitations to the present study.
Only in vitro experiments were performed to show that
icariin could reduce HG-induced EPC dysfunction. EPC function in HG
microenvironments was only evaluated in vitro, while the
damage produced by HG in humans is observed after several years
in vivo. Although the present study may provide a certain
degree of insight into the mechanisms involved in vivo,
further study in vivo is required to clarify the exact
mechanisms. Additionally, gene silencing technology could be
employed to further demonstrate the exact role of the p38/CREB and
Akt/eNOS signaling pathways in the effects induced by icariin.
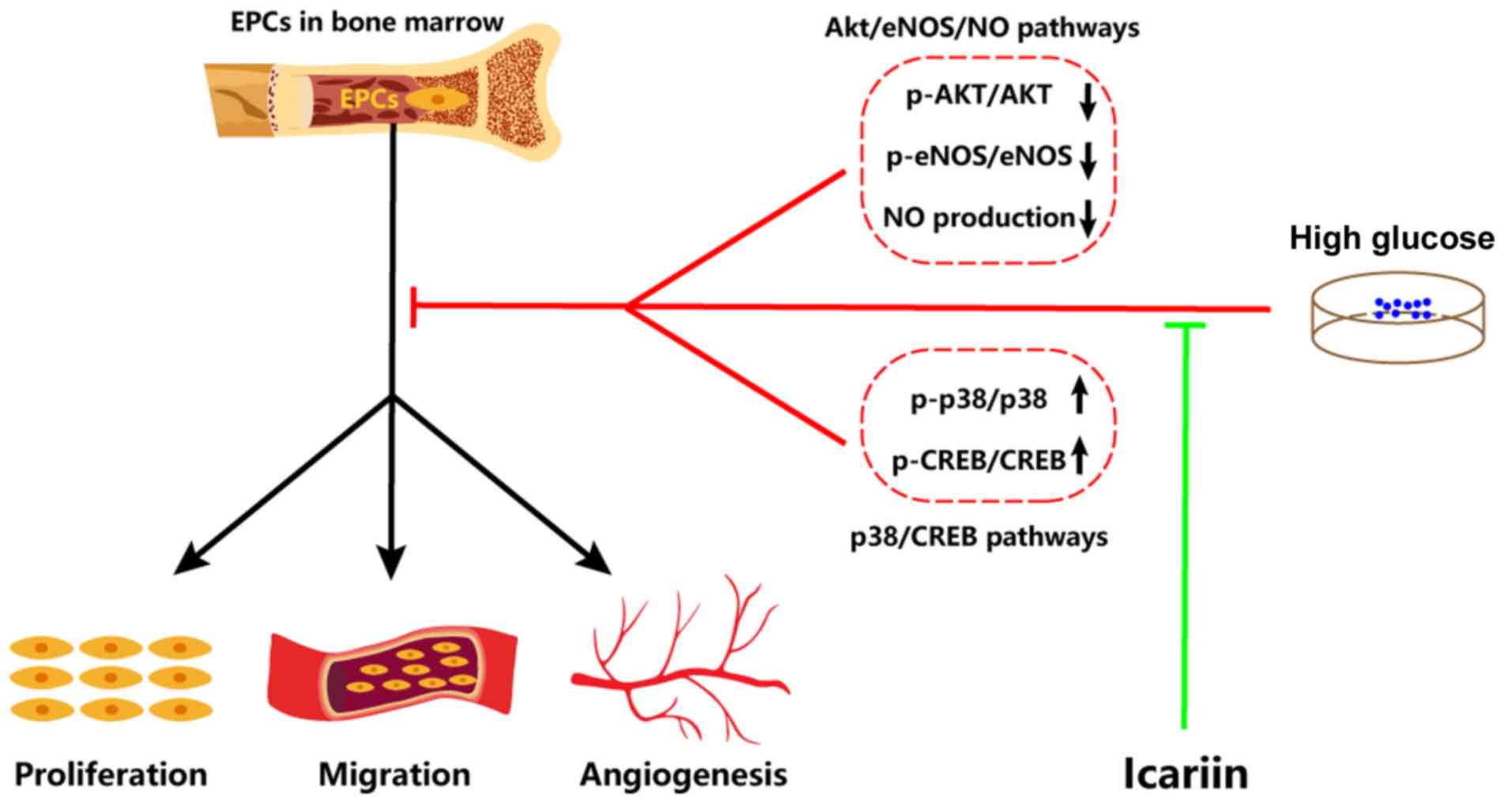
Collectively, the results of the present study
demonstrated that icariin can attenuate HG-induced EPC dysfunction
in vitro, including improving proliferation, migration and
tube formation. Furthermore, the possible molecular mechanisms
involved were identified as the inhibited activation of the
p38/CREB signaling pathway and the promotion of the Akt/eNOS/NO
signaling pathway (Fig. 4).
Therefore, icariin may be a potentially promising tool for
protecting EPC function against HG.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81600226).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJ designed and directed the experiments. SC, ZW and
HZ performed the experiments. SC, ZW, HB and DH collected and
analyzed the experimental data. SC and HJ wrote the manuscript. HZ
and HB investigated the relevant literature and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Institutional
Animal Care and Use Committee, the Animal Care and Use Committee of
Wuhan University (permit no. WDRM20161204).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin P, Li T, Li X, Shen X and Zhao Y:
Suppression of oxidative stress in endothelial progenitor cells
promotes angiogenesis and improves cardiac function following
myocardial infarction in diabetic mice. Exp Ther Med. 11:2163–2170.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaipersad AS, Lip GY, Silverman S and
Shantsila E: The role of monocytes in angiogenesis and
atherosclerosis. J Am Coll Cardiol. 63:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Odent Grigorescu G, Preda MB, Radu E,
Rosca AM, Tutuianu R, Mitroi DN, Simionescu M and Burlacu A:
Combinatorial approach for improving the outcome of angiogenic
therapy in ischemic tissues. Biomaterials. 60:72–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shantsila E, Watson T and Lip GY:
Endothelial progenitor cells in cardiovascular disorders. J Am Coll
Cardiol. 49:741–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fadini GP, Sartore S, Albiero M, Baesso I,
Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A,
Agostini C, et al: Number and function of endothelial progenitor
cells as a marker of severity for diabetic vasculopathy.
Arterioscler Thromb Vasc Biol. 26:2140–2146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fadini GP, Miorin M, Facco M, Bonamico S,
Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A,
Agostini C, et al: Circulating endothelial progenitor cells are
reduced in peripheral vascular complications of type 2 diabetes
mellitus. J Am Coll Cardiol. 45:1449–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukada S, Masuda H, Jung SY, Yun J, Kang
S, Kim DY, Park JH, Ji ST, Kwon SM and Asahara T: Impaired
development and dysfunction of endothelial progenitor cells in type
2 diabetic mice. Diabetes Metab. 43:154–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang J, Li Y, Huang Y, Lam KS, Hoo RL,
Wong WT, Cheng KK, Wang Y, Vanhoutte PM and Xu A: Adiponectin
prevents diabetic premature senescence of endothelial progenitor
cells and promotes endothelial repair by suppressing the p38 MAP
kinase/p16INK4A signaling pathway. Diabetes. 59:2949–2959. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun N, Wang H and Wang L: Vaspin
alleviates dysfunction of endothelial progenitor cells induced by
high glucose via PI3K/Akt/eNOS pathway. Int J Clin Exp Pathol.
8:482–489. 2015.PubMed/NCBI
|
|
12
|
Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR,
Huang PH, Liu PL, Chen YL and Chen JW: High glucose impairs early
and late endothelial progenitor cells by modifying nitric
oxide-related but not oxidative stress-mediated mechanisms.
Diabetes. 56:1559–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen R and Wang JH: The effect of icariin
on immunity and its potential application. Am J Clin Exp Immunol.
7:50–56. 2018.PubMed/NCBI
|
|
14
|
Jin J, Wang H, Hua X, Chen D, Huang C and
Chen Z: An outline for the pharmacological effect of icariin in the
nervous system. Eur J Pharmacol. 842:20–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Wang D, Yang D, Zhen W, Zhang J
and Peng S: The effect of icariin on bone metabolism and its
potential clinical application. Osteoporos Int. 29:535–544. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qian ZQ, Wang YW, Li YL, Li YQ, Ling-Zhu
and Yang DL: Icariin prevents hypertension-induced cardiomyocyte
apoptosis through the mitochondrial apoptotic pathway. Biomed
Pharmacother. 88:823–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu HB and Huang ZQ: Icariin enhances
endothelial nitric-oxide synthase expression on human endothelial
cells in vitro. Vascul Pharmacol. 47:18–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song E, Lu CW, Fang LJ and Yang W: Culture
and identification of endothelial progenitor cells from human
umbilical cord blood. Int J Ophthalmol. 3:49–53. 2010.PubMed/NCBI
|
|
19
|
Hamed S, Brenner B, Abassi Z, Aharon A,
Daoud D and Roguin A: Hyperglycemia and oxidized-LDL exert a
deleterious effect on endothelial progenitor cell migration in type
2 diabetes mellitus. Thromb Res. 126:166–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koizumi H, Yu J, Hashimoto R, Ouchi Y and
Okabe T: Involvement of androgen receptor in nitric oxide
production induced by icariin in human umbilical vein endothelial
cells. FEBS Lett. 584:2440–2444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma FX, Chen F, Ren Q and Han ZC:
Lovastatin restores the function of endothelial progenitor cells
damaged by oxLDL. Acta Pharmacol Sin. 30:545–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng L, Xu J, Qian YY, Pan HY, Yang H,
Shao MY, Cheng R and Hu T: Interaction between mDia1 and ROCK in
Rho-induced migration and adhesion of human dental pulp cells. Int
Endod J. 50:15–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ross S, Gerstein HC, Eikelboom J, Anand
SS, Yusuf S and Paré G: Mendelian randomization analysis supports
the causal role of dysglycaemia and diabetes in the risk of
coronary artery disease. Eur Heart J. 36:1454–1462. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Li Q, Mei Q and Lu T:
Pharmacological effects and pharmacokinetic properties of icariin,
the major bioactive component in Herba Epimedii. Life Sci.
126:57–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seeger FH, Haendeler J, Walter DH,
Rochwalsky U, Reinhold J, Urbich C, Rössig L, Corbaz A, Chvatchko
Y, Zeiher AM, et al: p38 mitogen-activated protein kinase
downregulates endothelial progenitor cells. Circulation.
111:1184–1191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao HB, Liu ZK, Lu XY, Deng CN and Luo
ZF: Icariin regulates PRMT/ADMA/DDAH pathway to improve endothelial
function. Pharmacological reports: PR. 67:1147–1154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao-Hong D, Chang-Qin X, Jian-Hua H,
Wen-Jiang Z and Bing S: Icariin delays homocysteine-induced
endothelial cellular senescence involving activation of the
PI3K/AKT-eNOS signaling pathway. Pharm Biol. 51:433–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chung BH, Kim JD, Kim CK, Kim JH, Won MH,
Lee HS, Dong MS, Ha KS, Kwon YG and Kim YM: Icariin stimulates
angiogenesis by activating the MEK/ERK- and PI3K/Akt/eNOS-dependent
signal pathways in human endothelial cells. Biochem Biophys Res
Commun. 376:404–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song YH, Cai H, Zhao ZM, Chang WJ, Gu N,
Cao SP and Wu ML: Icariin attenuated oxidative stress
induced-cardiac apoptosis by mitochondria protection and ERK
activation. Biomed Pharmacother. 83:1089–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HY, Gao PJ, Ji KD, Shen WF, Fan CL,
Lu L and Zhu DL: Circulating endothelial progenitor cells,
C-reactive protein and severity of coronary stenosis in Chinese
patients with coronary artery disease. Hypertension Research:
Official Journal of the Japanese Society of Hypertension.
30:133–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang PH, Chen JW, Lin CP, Chen YH, Wang
CH, Leu HB and Lin SJ: Far infra-red therapy promotes
ischemia-induced angiogenesis in diabetic mice and restores high
glucose-suppressed endothelial progenitor cell functions.
Cardiovasc Diabetol. 11:992012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding L, Liang XG, Hu Y, Zhu DY and Lou YJ:
Involvement of p38MAPK and reactive oxygen species in
icariin-induced cardiomyocyte differentiation of murine embryonic
stem cells in vitro. Stem Cells Dev. 17:751–760. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin S, Zhou W, Liu S, Chen P and Wu H:
Icariin stimulates the proliferation of rat bone mesenchymal stem
cells via ERK and p38 MAPK signaling. Int J Clin Exp Med.
8:7125–7133. 2015.PubMed/NCBI
|
|
34
|
Taguchi K, Hida M, Hasegawa M, Matsumoto T
and Kobayashi T: Dietary polyphenol morin rescues endothelial
dysfunction in a diabetic mouse model by activating the Akt/eNOS
pathway. Mol Nutr Food Res. 60:580–588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamed S, Brenner B and Roguin A: Nitric
oxide: A key factor behind the dysfunctionality of endothelial
progenitor cells in diabetes mellitus type-2. Cardiovasc Res.
91:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|