Introduction
Some antidepressants and some antipsychotics act as
antagonists on the 5-HT3 receptor. This receptor has binding sites
for antidepressant and antipsychotic drugs, so that the 5-HT3
receptor is a therapeutic target in the treatment of depression and
psychosis as an additional mechanism to the already known classical
ones (1).
5-HT3 receptors belong to the superfamily of
receptors coupled to ion channels, structurally similar to GABA A
receptors, nicotinic receptors and glycine receptors. Localized in
central and peripheral neurons, they generate rapid depolarization
resulting from the opening of cationic channels (Na+,
K+ and Ca2+ ionic currents). Structurally, it
is a five-unit pentamer that surrounds a central channel. There are
numerous 5HT3 receptor subfamilies (2).
There are studies that have shown that tricyclic
antidepressants such as imipramine, desipramine, doxepin, monoamine
oxidase inhibitors (MAOIs) such as phenelzine (3), along with SSRIs such as fluoxetine
uncompetitively inhibit 5-HT3 transreceptor currents (4). Also, endogenous substances such as
sphingolipids and cholesterol regulate channel opening of 5-HT3
receptors (5,6).
Selective serotonin reuptake inhibitors (SSRIs) may
produce digestive side effects such as nausea and vomiting,
diarrhoea and decreased appetite. These side effects are determined
by the increase in serotonin availability at 5-HT3 receptors
(7).
Granisetron, a serotonin 5-HT3 receptor antagonist,
is expected to antagonize the digestive adverse effects of
serotonin reuptake inhibitors. However, the question is to what
extent granisetron influences the antidepressant effect of these
substances.
The aim of the present study was determine the dose
of fluoxetine that has an antidepressant effect in the Porsolt test
(classical variant of the forced swimming test) and the interaction
between fluoxetine and granisetron with respect to the
antidepressant effect in the Porsolt test.
Materials and methods
NMRI Swiss albino strain mice, 25–30 g, from the
‘Carol Davila’ University hatchery were used. Animals were brought
to the accommodation at least 3 days before the experiments and
were kept under standard laboratory conditions, housed in
plexiglass cages with sawdust, 12 mice per cage, and were supplied
with granulated food and water ad libitum, at an ambient
temperature of 21–24°C and a relative humidity of 45–60%, in a
normal dark-light cycle (07:00-19:00 h). The number of animals per
group was set at 15 (experiment 1) and 10 (experiment 2). The
experiments were approved by the Institutional Ethics Committee of
Faculty of Medicine, UMF Carol Davila (Bucharest, Romania).
Substances used were: granisetron solution 1 mg/ml
(Granisetron Kabi 1 mg/ml), fluoxetine powder (Medochemie, Cyprus,
an internal standard) administered intraperitoneally in
concentrations calculated to administer 5 ml/kg body weight.
Experiment 1 used 3 groups: The control group,
saline; group 1, fluoxetine 10 mg/kg; group 2, fluoxetine 20 mg/kg,
all administered intraperitoneally 30 min before testing.
In experiment 2, 6 groups were used: Control group,
saline; group 2, granisetron 0.1 mg/kg; group 3, granisetron 1
mg/kg; group 4, fluoxetine 20 mg/kg; group 5, granisetron 0.1 mg/kg
+ fluoxetine 20 mg/kg; group 6, granisetron 1 mg/kg + fluoxetine 20
mg/kg, administered intraperitoneally 30 min before testing.
Berzelius glasses 18/10 cm height/diameter filled
with water at a height of 12 cm and temperature of 28°C and video
recording system were used.
The tests were conducted in natural light between
8.30 and 16.30. The parameter used for processing was the swimming
time in the last 4 min of a 6-min swimming session. The reading of
the results was blind (mice were divided into three Berzelius
glasses simultaneously, varying the position of introduction into
glass 1, 2 or 3 of each group of animals), according to a test
protocol. Swimming was defined as active horizontal or vertical
movements at the surface of the water.
Antidepressant drugs increase animal mobility during
the last 4 min of the test. It is considered that the duration of
the mobility time in these last 4 min is directly proportional with
the intensity of the antidepressant effect of a substance.
Statistical analysis
Microsoft Excel and SPSS 25 were used for
statistical analysis. For each group means and standard errors were
calculated. ANOVA and post-hoc test Tukey were used because the
groups had homogeneous variance (Levene statistic test >0.05).
P<0.05 was considered as statistically significant.
Results and Discussion
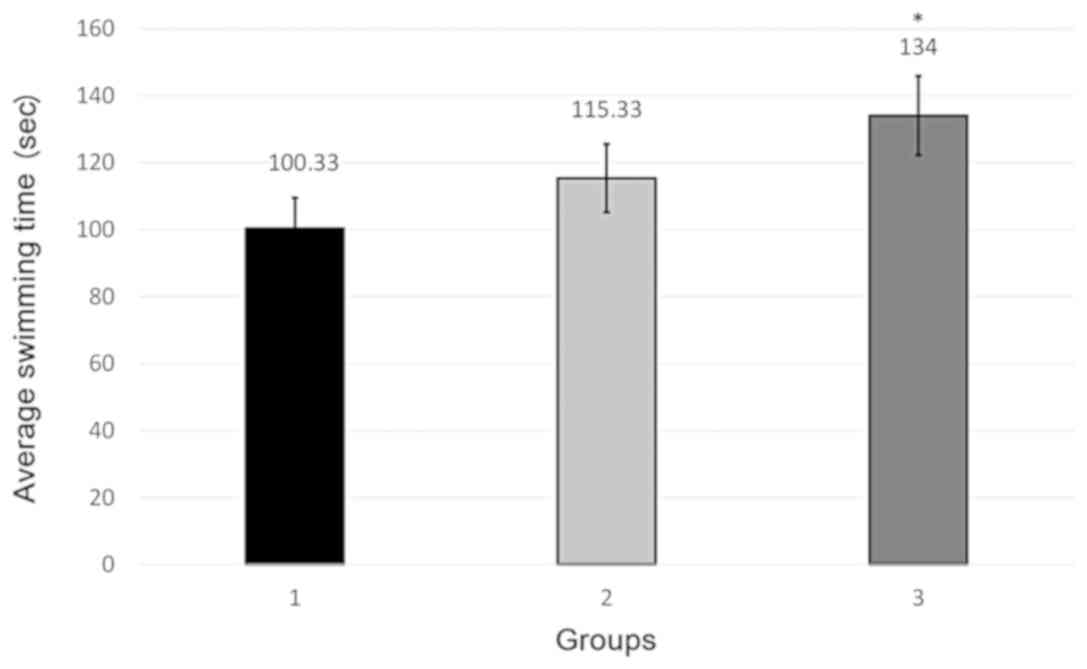
Only the 20 mg/kg dose of fluoxetine significantly
increased the mobility time (Fig. 1;
Table I). Increase in the mobility
time in Porsolt test is considered throughout the literature as an
expression of an antidepressant effect. Since only this dose of
fluoxetine had statistically significant antidepressant effect vs.
control, this dose was used in experiment 2.
 | Table I.P-values of the tests of significance
in experiment 1. |
Table I.
P-values of the tests of significance
in experiment 1.
| Experiment 1 | Group 1 | Group 2 | Group 3 |
|---|
| Group 1 | – | 0.549 | 0.049 |
| Group 2 | 0.549 | – | 0.398 |
| Group 3 | 0.049 | 0.398 | – |
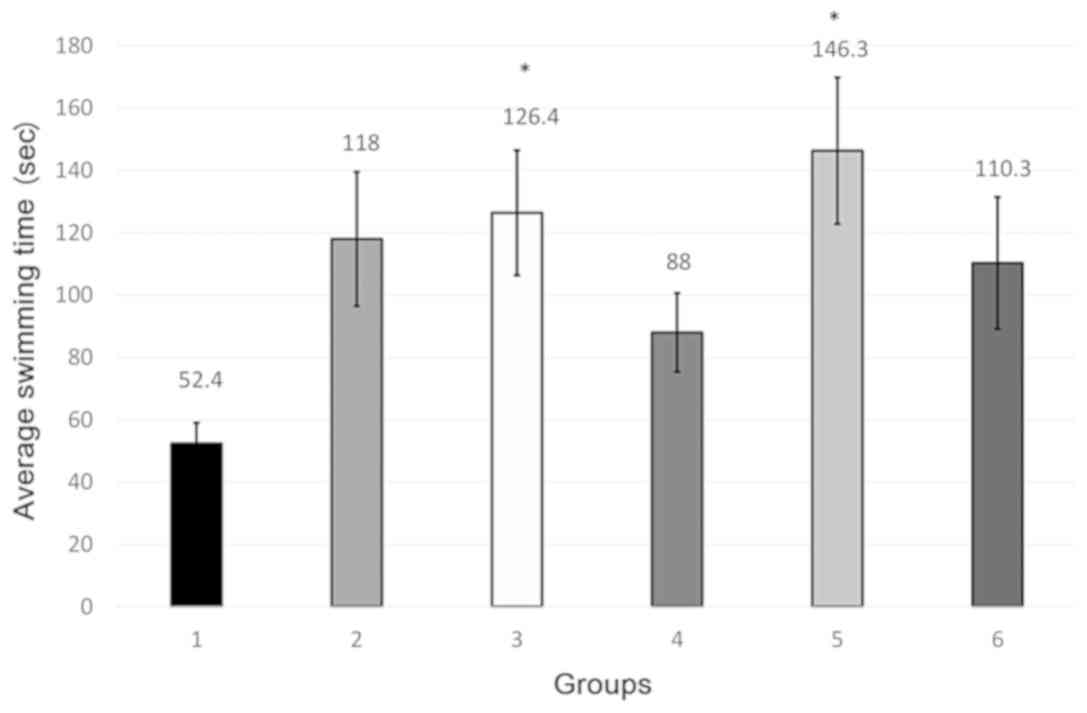
In experiment 2, only group 3, granisetron 1 mg/kg
bw and group 5 fluoxetine 20 mg/kg bw + granisetron 0.1 mg/kg bw
significantly increased the mobility time of the animals in the
Porsolt test, which is consistent with a statistically significant
antidepressant effect (Fig. 2;
Table II).
 | Table II.P-values of the tests of significance
in experiment 2. |
Table II.
P-values of the tests of significance
in experiment 2.
| Experiment 2 | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
|---|
| Group 1 | – | 0.107 | 0.048 | 0.710 | 0.005 | 0.203 |
| Group 2 | 0.107 | – | 0.999 | 0.833 | 0.864 | 1.000 |
| Group 3 | 0.048 | 0.999 | – | 0.640 | 0.967 | 0.987 |
| Group 4 | 0.710 | 0.833 | 0.640 | – | 0.197 | 0.946 |
| Group 5 | 0.005 | 0.864 | 0.967 | 0.197 | – | 0.700 |
| Group 6 | 0.203 | 1.000 | 0.987 | 0.946 | 0.700 | – |
Granisetron at the high dose (1 mg/kg bw) had a
statistically significant antidepressant effect vs. control.
Fluoxetine 20 mg/kg bw had no statistically significant effect in
this test, result that may be considered unexpected taking into
account the significance obtained at the same dose in the first
experiment.
Fluoxetine 20 mg/kg bw associated with a small dose
of granisetron (0.1 mg/kg bw) produced a significant antidepressant
effect vs. control. This shows that low doses of granisetron
associated with fluoxetine might produce a significant
antidepressant effect, suggesting a potentiation between these two
drugs used in sub-effective antidepressant doses.
Fluoxetine 20 mg/kg bw associated with a high dose
of 1 mg/kg bw of granisetron had no antidepressant effect vs.
control.
Based on the above aspects and the data obtained, we
state that in our experimental conditions, it cannot be asserted
that the association of granisetron to fluoxetine has a potential
risk of diminishing the antidepressant effect of fluoxetine.
Moreover, in our experimental conditions, granisetron seems to have
an antidepressant effect per se.
At low doses, granisetron seems to potentiate the
effect of fluoxetine, but this increase disappears at high doses of
granisetron. Based on the results obtained in this study, we cannot
reliably consider the mechanism of interactions between fluoxetine
and granisetron relating to the antidepressant effect.
In literature, one can find that stimulation of
5-HT3 receptors activates the nitric oxide-cyclic guanosine
monophosphate pathway, which is involved in regulating behaviour
and emotional functions (8).
A research study on an antidepressant effect of a
5HT3 receptor antagonist
N-(benzo[d]thiazol-2-yl)-3-methoxyquinoxalin-2-carboxamide (6z)
showed improvements of swimming in forced swimming test at
psychomotor non-stimulating doses (9). Besides the above results, ondansetron,
tropisetron, code-named substance QCF-3 ((4-benzylpiperazin-1-yl)
(quinoxalin-2-yl) methanone), code-named substance MDL 72222 (1
alpha H, 3 alpha, 5 alpha H-tropan-3-yl-3,5-dichlorobenzoate) and
2-(4-methyl piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile proved
antidepressant effects in some paradigms of antidepressant activity
(10–14).
Corroborating all the experimental results obtained
in this investigation, we can say that since SSRI antidepressants
increase serotonin availability in serotonin synapses, granisetron
may antagonize the digestive side effects of these antidepressants.
Moreover, granisetron seems to have an antidepressant effect per
se. At low doses, granisetron appears to increase the
antidepressant effect of selective serotonin reuptake inhibitors,
an effect that disappears with high doses.
In conclusion, based on the data obtained in our
experimental conditions, we can assume that granisetron in low
doses could be used to combat intestinal transit disorders produced
by SSRI antidepressants. These low doses are preferred, because
based on our experimental data they increase the antidepressant
effect of these SSRIs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data could be consulted upon request.
Authors contributions
MC made substantial contributions to the
acquisition, analysis, and interpretation of data for the study and
participated in the drafting of the study. HP made substantial
contributions to the design of the study and participated in the
drafting of the study and in revising it critically for important
intellectual content. OAC made substantial contributions to the
analysis and interpretation of data for the study and participated
in the drafting of the study and in revising it critically for
important intellectual content. LC made substantial contributions
to the analysis and interpretation of data for the study and
participated in the drafting of the study. IF made substantial
contributions to the conception and design of the study and
participated in revising it critically for important intellectual
content. All authors gave their final approval of the version to be
published and agreed to be accountable for all aspects of the study
in ensuring that questions related to the accuracy or integrity of
any part of the study are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The experiments were approved by the Institutional
Ethics Committee of Faculty of Medicine, UMF Carol Davila
(Bucharest, Romania).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
5HT
|
serotonin
|
|
MAOIs
|
monoamine oxidase inhibitors
|
|
SSRIs
|
selective serotonin reuptake
inhibitors
|
References
|
1
|
Rammes G, Eisensamer B, Ferrari U, Shapa
M, Gimpl G, Gilling K, Parsons C, Riering K, Hapfelmeier G, et al:
Antipsychotic drugs antagonize human serotonin type 3 receptor
currents in a noncompetitive manner. Mol Psychiatry. 9:846–858,
818. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jensen AA, Davies PA, Bräuner-Osborne H
and Krzywkowski K: 3B but which 3B and that's just one of the
questions: The heterogeneity of human 5-HT3 receptors. Trends
Pharmacol Sci. 29:437–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies PA: Allosteric modulation of the
5-HT(3) receptor. Curr Opin Pharmacol. 11:75–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan P: Facilitation of
5-hydroxytryptamine3 receptor desensitization by fluoxetine.
Neuroscience. 62:515–522. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fantini J and Barrantes FJ:
Sphingolipid/cholesterol regulation of neurotransmitter receptor
conformation and function. Biochim Biophys Acta. 1788:2345–2361.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicolae I, Nicolae CD, Coman OA,
Stefănescu M, Coman L and Ardeleanu C: Serum total gangliosides
level: Clinical prognostic implication. Rom J Morphol Embryol.
52:1277–1281. 2011.PubMed/NCBI
|
|
7
|
Perez-Caballero L, Torres-Sanchez S, Bravo
L, Mico JA and Berrocoso E: Fluoxetine: A case history of its
discovery and preclinical development. Expert Opin Drug Discov.
9:567–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haj-Mirzaian A, Kordjazy N, Amiri S,
Haj-Mirzaian A, Amini-Khoei H, Ostadhadi S and Dehpour A:
Involvement of nitric oxide-cyclic guanosine monophosphate pathway
in the antidepressant-like effect of tropisetron and ondansetron in
mice forced swimming test and tail suspension test. Eur J
Pharmacol. 780:71–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta D, Radhakrishnan M, Kurhe Y,
Thangaraj D, Prabhakar V and Kanade P: Antidepressant-like effects
of a novel 5-HT3 receptor antagonist 6z in acute and chronic murine
models of depression. Acta Pharmacol Sin. 35:1493–1503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramamoorthy R, Radhakrishnan M and Borah
M: Antidepressant-like effects of serotonin type-3 antagonist,
ondansetron: An investigation in behaviour-based rodent models.
Behav Pharmacol. 19:29–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bravo G and Maswood S: Acute treatment
with 5-HT3 receptor antagonist, tropisetron, reduces immobility in
intact female rats exposed to the forced swim test. Pharmacol
Biochem Behav. 85:362–368. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devadoss T, Pandey DK, Mahesh R and Yadav
SK: Effect of acute and chronic treatment with QCF-3
(4-benzylpiperazin-1-yl) (quinoxalin-2-yl) methanone, a novel
5-HT(3) receptor antagonist, in animal models of depression.
Pharmacol Rep. 62:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kos T, Popik P, Pietraszek M, Schäfer D,
Danysz W, Dravolina O, Blokhina E, Galankin T and Bespalov AY:
Effect of 5-HT3 receptor antagonist MDL 72222 on behaviors induced
by ketamine in rats and mice. Eur Neuropsychopharmacol. 16:297–310.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahesh R, Rajkumar R, Minasri B and
Venkatesha Perumal R: Potential antidepressants: Pharmacology of
2-(4-methyl piperazin-1-yl)-1,8-naphthyridine-3-carbonitrile in
rodent behavioural models. Pharmazie. 62:919–924. 2007.PubMed/NCBI
|