|
1
|
Peacock AJ, Murphy NF, Mcmurray JJ,
Caballero L and Stewart S: An epidemiological study of pulmonary
arterial hypertension. Eur Respir J. 30:104–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43 (12 Suppl
S):13S–24S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Budhiraja R, Tuder RM and Hassoun PM:
Endothelial dysfunction in pulmonary hypertension. Circulation.
109:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badesch DB, Abman SH, Simonneau G, Rubin
LJ and McLaughlin VV: Medical therapy for pulmonary arterial
hypertension: Updated ACCP evidence-based clinical practice
guidelines. Chest. 131:1917–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoeper MM, Faulenbach C, Golpon H, Winkler
J, Welte T and Niedermeyer J: Combination therapy with bosentan and
sildenafil in idiopathic pulmonary arterial hypertension. Eur
Respir J. 24:1007–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seyfarth HJ, Pankau H, Hammerschmidt S,
Schauer J, Wirtz H and Winkler J: Bosentan improves exercise
tolerance and Tei index in patients with pulmonary hypertension and
prostanoid therapy. Chest. 128:709–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Channick RN, Olschewski H, Seeger W, Staub
T, Voswinckel R and Rubin LJ: Safety and efficacy of inhaled
treprostinil as add-on therapy to bosentan in pulmonary arterial
hypertension. J Am Coll Cardiol. 48:1433–1437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galie N, Manes A, Negro L, Palazzini M,
Bacchi-Reggiani ML and Branzi A: A meta-analysis of randomized
controlled trials in pulmonary arterial hypertension. Eur Heart J.
30:394–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z, Krasnici N and Lüscher TF:
Endothelin-1 potentiates human smooth muscle cell growth to PDGF:
Effects of ETA and ETB receptor blockade. Circulation. 100:5–8.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Price LC and Howard LS: Endothelin
receptor antagonists for pulmonary arterial hypertension: Rationale
and place in therapy. Am J Cardiovasc Drugs. 8:171–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaughlin VV, Shillington A and Rich S:
Survival in primary pulmonary hypertension: The impact of
epoprostenol therapy. Circulation. 106:1477–1482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barst RJ, Rubin LJ, McGoon MD, Caldwell
EJ, Long WA and Levy PS: Survival in primary pulmonary hypertension
with long-term continuous intravenous prostacyclin. Ann Intern Med.
121:409–415. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Provencher S, Sitbon O, Humbert M, Cabrol
S, Jaïs X and Simonneau G: Long-term outcome with first-line
bosentan therapy in idiopathic pulmonary arterial hypertension. Eur
Heart J. 27:589–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sitbon O, Humbert M, Nunes H, Parent F,
Garcia G, Hervé P, Rainisio M and Simonneau G: Long-term
intravenous epoprostenol infusion in primary pulmonary
hypertension: Prognostic factors and survival. J Am Coll Cardiol.
40:780–788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galie N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Respir J. 46:903–975. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Channick RN: Combination therapy in
pulmonary arterial hypertension. Am J Cardiol. 111 (8
Suppl):16C–20C. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haworth S and Rabinovitch M: Pulmonary
circulationPaediatric Cardiology. 3rd. Anderson RH, Baker EJ, Penny
DJ, et al: Churchill Livingstone; Philadelphia: pp. 117–141. 2010,
View Article : Google Scholar
|
|
19
|
Channick RN, Simonneau G, Sitbon O,
Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M,
Bodin F and Rubin LJ: Effects of the dual endothelin-receptor
antagonist bosentan in patients with pulmonary hypertension: A
randomised placebo-controlled study. Lancet. 358:1119–1123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rubin LJ, Badesch DB, Barst RJ, Galie N,
Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al:
Bosentan therapy for pulmonary arterial hypertension. N Engl J Med.
346:896–903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension. Kardiol Pol. 73:1127–1206.
2015.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
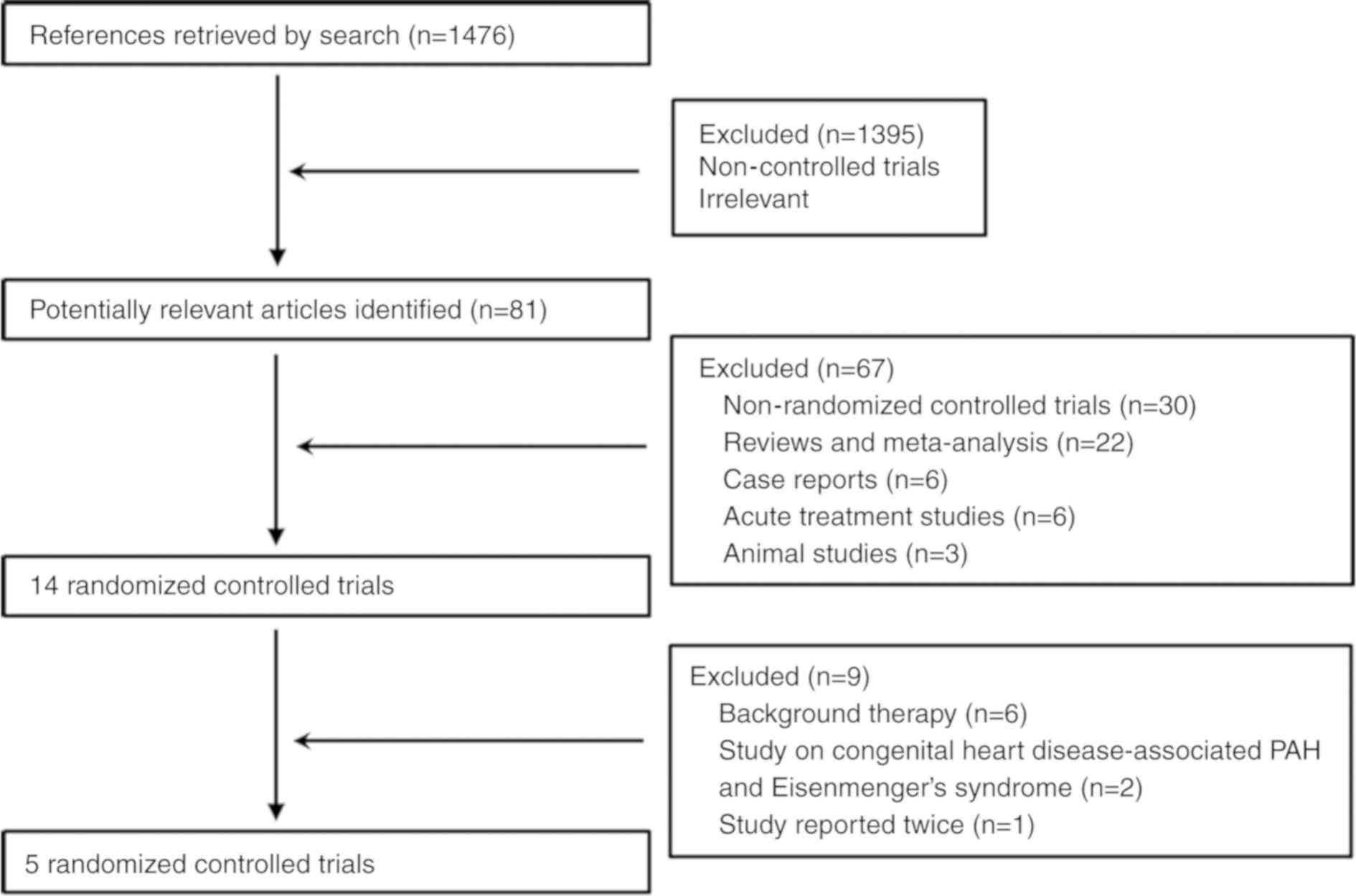
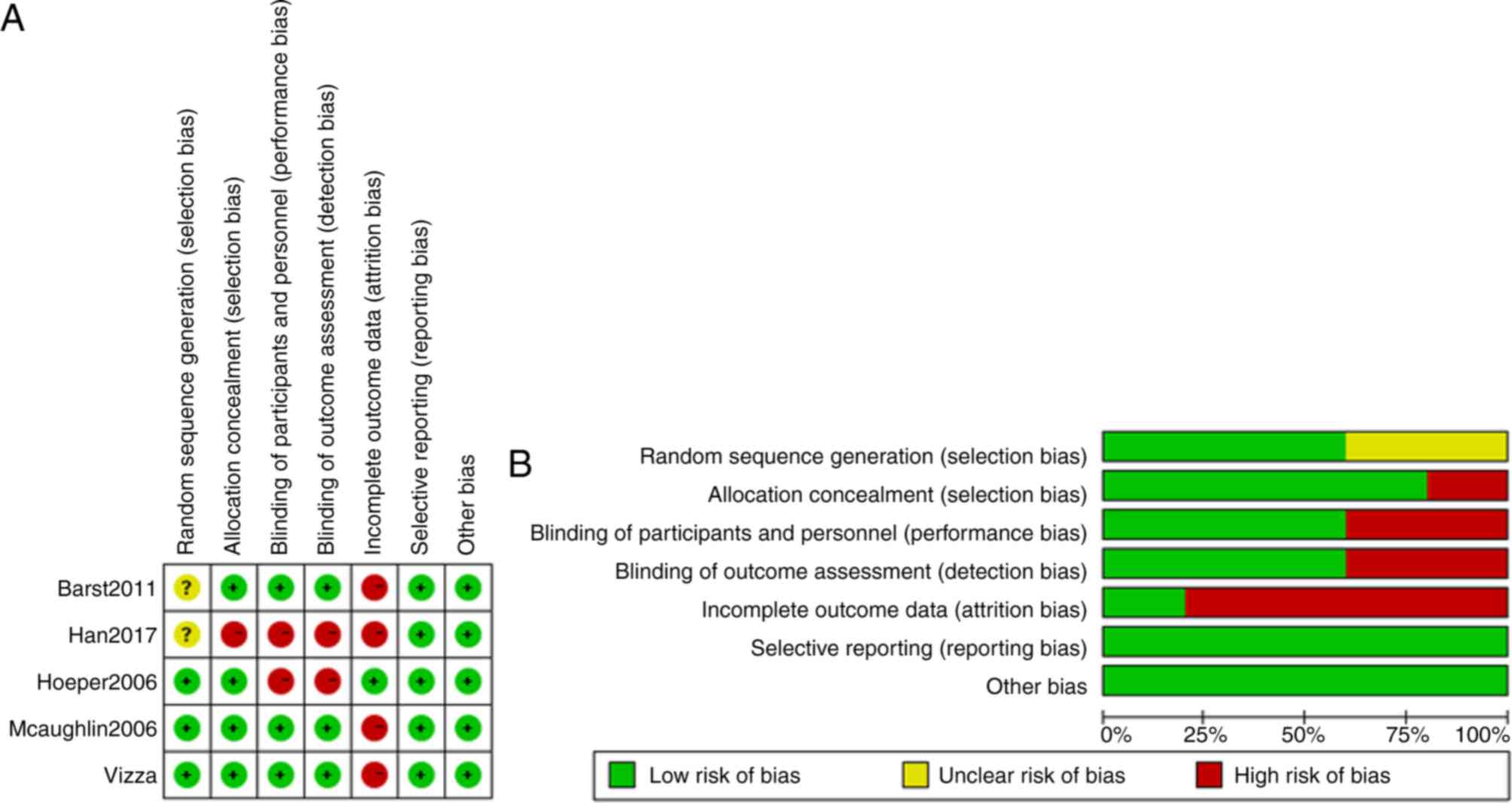
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
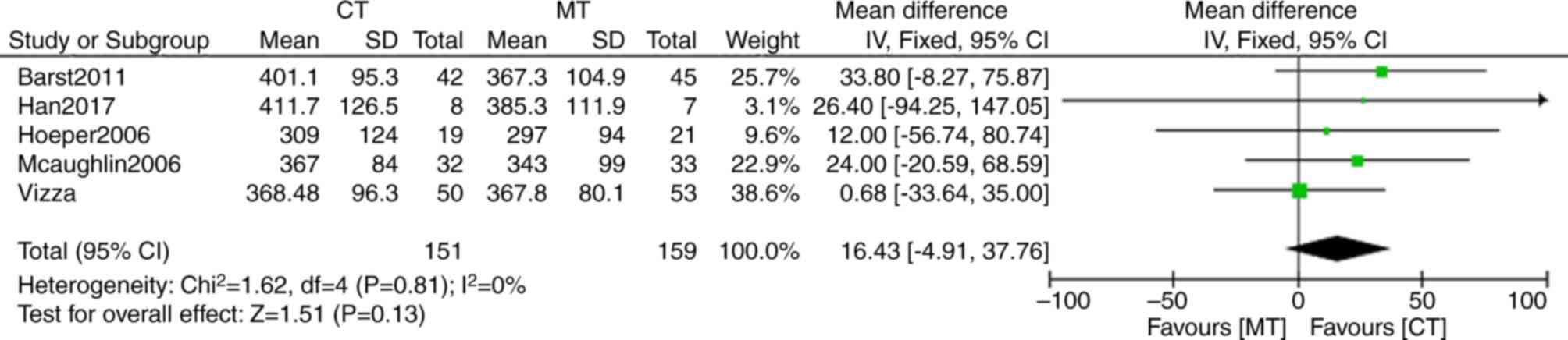
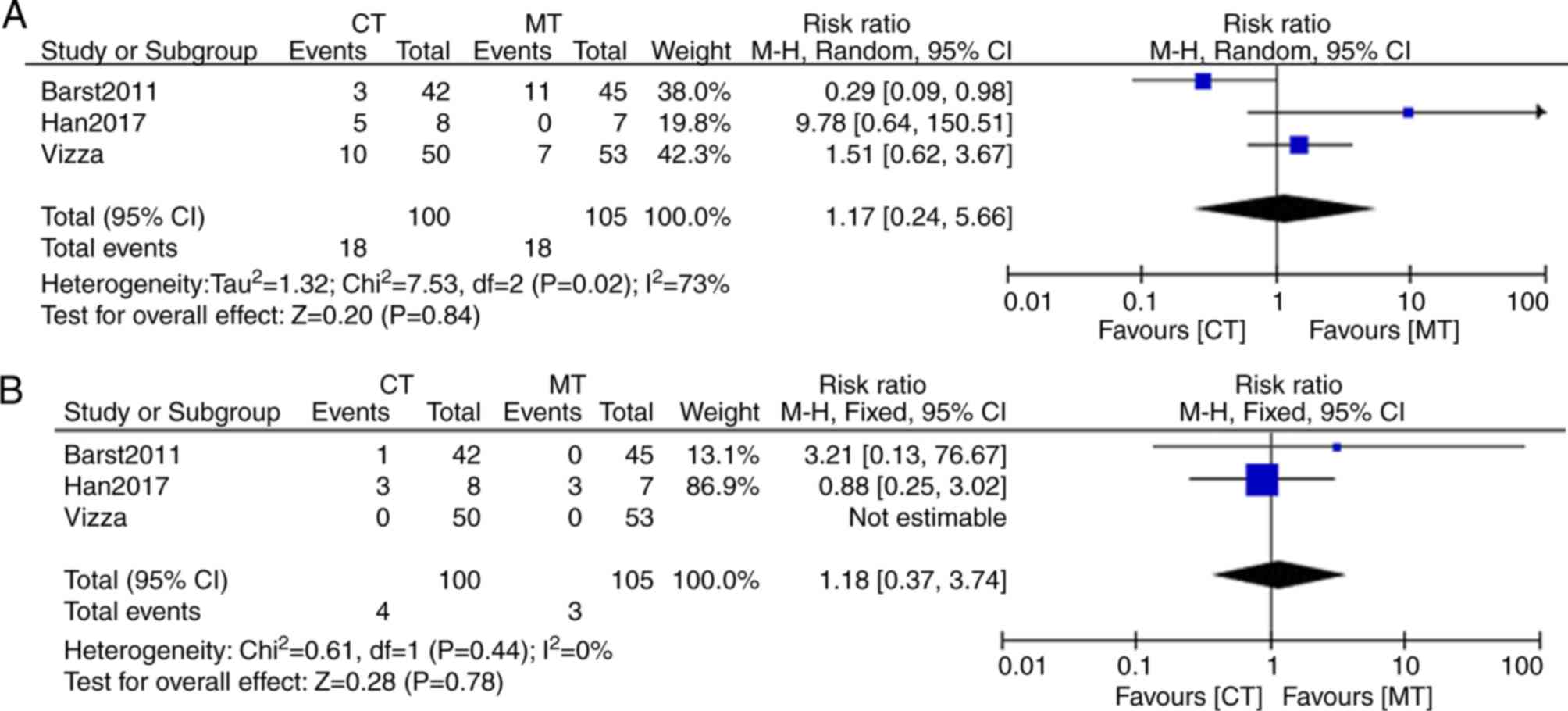
Barst RJ, Oudiz RJ, Beardsworth A,
Brundage BH, Simonneau G, Ghofrani HA, Sundin DP and Galiè N;
Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST)
Study Group, : Tadalafil monotherapy and as add-on to background
bosentan in patients with pulmonary arterial hypertension. J Heart
Lung Transplant. 30:632–643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galie N, Brundage BH, Ghofrani HA, Oudiz
RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth
A, et al: Tadalafil therapy for pulmonary arterial hypertension.
Circulation. 119:2894–2903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Alto M, Romeo E, Argiento P, Sarubbi B,
Santoro G, Grimaldi N, Correra A, Scognamiglio G, Russo MG and
Calabrò R: Bosentan-sildenafil association in patients with
congenital heart disease-related pulmonary arterial hypertension
and Eisenmenger physiology. Int J Cardiol. 155:378–382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iversen K, Jensen AS, Jensen TV, Vejlstrup
NG and Sondergaard L: Combination therapy with bosentan and
sildenafil in Eisenmenger syndrome: A randomized,
placebo-controlled, double-blinded trial. Eur Heart J.
31:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McLaughlin VV, Benza RL, Rubin LJ,
Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H,
Rubenfire M, Rubenfire M, et al: Addition of inhaled treprostinil
to oral therapy for pulmonary arterial hypertension: A randomized
controlled clinical trial. J Am Coll Cardiol. 55:1915–1922. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tapson VF, Jing ZC, Xu KF, Pan L, Feldman
J, Kiely DG, Kotlyar E, McSwain CS, Laliberte K, Arneson C, et al:
Oral treprostinil for the treatment of pulmonary arterial
hypertension in patients receiving background endothelin receptor
antagonist and phosphodiesterase type 5 inhibitor therapy (the
FREEDOM-C2 study): A randomized controlled trial. Chest.
144:952–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sitbon O, Channick R, Chin KM, Frey A,
Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, et al:
Selexipag for the treatment of pulmonary arterial hypertension. N
Engl J Med. 373:2522–2533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghofrani HA, Galiè N, Grimminger F, Grünig
E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A,
et al: Riociguat for the treatment of pulmonary arterial
hypertension. N Engl J Med. 369:330–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tapson VF, Torres F, Kermeen F, Keogh AM,
Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K,
et al: Oral treprostinil for the treatment of pulmonary arterial
hypertension in patients on background endothelin receptor
antagonist and/or phosphodiesterase type 5 inhibitor therapy (the
FREEDOM-C study): A randomized controlled trial. Chest.
142:1383–1390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simonneau G, Torbicki A, Hoeper MM,
Delcroix M, Karlócai K, Galiè N, Degano B, Bonderman D, Kurzyna M,
Efficace M, et al: Selexipag: An oral, selective prostacyclin
receptor agonist for the treatment of pulmonary arterial
hypertension. Eur Respir J. 40:874–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McLaughlin VV, Oudiz RJ, Frost A, Tapson
VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH and Rubin
LJ: Randomized study of adding inhaled iloprost to existing
bosentan in pulmonary arterial hypertension. Am J Respir Crit Care
Med. 174:1257–1263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoeper MM, Leuchte H, Halank M, Wilkens H,
Meyer FJ, Seyfarth HJ, Wensel R, Ripken F, Bremer H, Kluge S, et
al: Combining inhaled iloprost with bosentan in patients with
idiopathic pulmonary arterial hypertension. Eur Respir J.
28:691–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
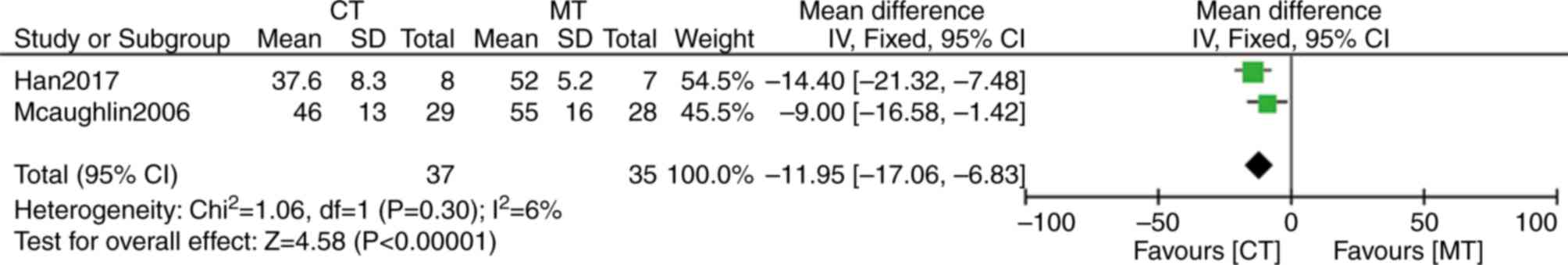
Han X, Zhang Y, Dong L, Fang L, Chai Y,
Niu M, Yu Y, Liu L, Yang X, Qu S and Li S: Treatment of pulmonary
arterial hypertension using initial combination therapy of bosentan
and iloprost. Respir Care. 62:489–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vizza CD, Jansa P, Teal S, Dombi T and
Zhou D: Sildenafil dosed concomitantly with bosentan for adult
pulmonary arterial hypertension in a randomized controlled trial.
BMC Cardiovasc Disord. 17:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dingemanse J and van Giersbergen PL:
Clinical pharmacology of bosentan, a dual endothelin receptor
antagonist. Clin Pharmacokinet. 43:1089–1115. 2004. View Article : Google Scholar : PubMed/NCBI
|