Introduction
Pulmonary arterial hypertension (PAH) is a
progressive disease associated with a massive increase in pulmonary
vascular resistance and pulmonary artery pressure (PAP). PAH is a
rare disease with an incidence of approximately 2.4–7.6 cases per
million (1), which may lead to fatal
right heart failure in the absence of appropriate treatment. The
pathogenesis of PAH has not been fully elucidated; however,
dysfunction of three metabolic/physiological pathways, including
the endothelin pathway, the prostacyclin pathway and the nitric
oxide pathway, have been attributed to the pathogenesis of PAH
(2,3). Targeting of these pathways had been
rationally exploited for the discovery of chemotherapeutics against
PAH. For example, prostacyclin analogues, phosphodiesterase type 5
(PDE-5) inhibitors and endothelin receptor antagonists (ERAs) are
drugs that are commonly used for the treatment of PAH (4). These drugs relieve symptoms, raise
exercise capacity and improve hemodynamics. However, the efficacies
of these commonly used drugs in delaying the progression of the
disease are limited. Owing to these limitations, the current
treatment options for PAH are not satisfactory. Combination
therapies exist, where two or three drugs aimed at different
pathways, such as ERAs, prostacyclin analogues and PDE-5 inhibitors
are simultaneously used (5).
Previous studies had indicated that combination therapy
significantly improved activity tolerance, hemodynamic parameters,
clinical deterioration time and quality of life for patients with
PAH (6–8). Results from a previous meta-analysis
suggest that combination therapies only offered a modest increase
in exercise ability (9). The
evidence to support these treatment options is limited. Bosentan,
an ERA, serves a crucial role in proliferation inhibition,
improvement of endothelial function and expansion of pulmonary
vessels (10). ERA treatment
significantly improved the activity tolerance and exercise capacity
of PAH patients as well as prolonging the survival time (11); however, PAH remains a progressive
disease with a high mortality rate (12–15). The
mortality rate of pulmonary hypertension in the United States was
about 4.5–12.3/10 million in 2015 (16). In order to achieve long-term
efficacy, combined therapy has been widely used in clinical
practice. However, only a few randomized controlled trials are
available regarding the efficacy and safety of combining bosentan
with prostacyclin analogues or PDE-5 inhibitors, and there is
limited evidence to support the superior effects of bosentan
combination therapy over monotherapy (17). The present meta-analysis focused on
providing an improved analysis of bosentan combination for PAH
treatment, and laying a theoretical foundation for the development
of other treatment strategies in the future. Bosentan was the first
oral PAH targeted drug in 2002 (18). Subsequently, a number of
multi-center, randomized controlled clinical trials have been
published to confirm its efficacy in controlling pulmonary
hypertension (19,20). Bosentan was approved in China for the
treatment of PAH in 2006 and was permitted for use as class I drug
in 2015, according to European Society of Cardiology-European
Respiratory Society Guidelines on Pulmonary Hypertension (21). The evidence-based medicine of
bosentan combined with prostacyclin analogues or PDE-5 inhibitors
is lacking. Therefore, it is necessary to conduct meta-analyses of
randomized controlled trials to evaluate the effects of bosentan
combined with prostacyclin analogues or PDE-5 inhibitors for the
treatment of PAH. The present meta-analysis may provide evidence of
the efficacy and safety of bosentan therapy combined with
prostacyclin analogues or PDE-5 inhibitors.
Materials and methods
Study inclusion and exclusion
criteria
The following criteria was used for selecting
previous studies to analyse: i) Only randomized controlled trials
(RCT) that combined bosentan with prostacyclin analogues or PDE-5
inhibitors for the treatment of PAH were included; ii) studies in
which the control group was treated with bosentan or placebo were
included (bosentan monotherapy), and follow-up time in the study
was ≥12 weeks.; iii) studies of the bosentan treatment within 3
months prior to randomization were included. Efficacy indicator of
primary endpoint was indicated as six-minute walk distance (6MWD),
and adverse events were examined to evaluate safety.
Literature search
RCT of bosentan combination therapy vs. bosentan
monotherapy for treatment of PAH were searched from PubMed
(www.ncbi.nlm.nih.gov/pubmed), Embase
(www.embase.com), and the Cochrane library
(www.cochranelibrary.com). The following
keywords were used for searching the relevant trial studies
included in this meta-analysis: ‘Phosphodiesterase type 5
inhibitor’ or ‘PDE-5 inhibitor’ or ‘sildenafil’ or ‘tadalafil’ or
‘vardenafil’ or ‘prostacyclin analogs’ or ‘epoprostenol’ or
‘iloprost’ or ‘treprostinil’ paired with ‘pulmonary arterial
hypertension’ or ‘PAH’ and ‘bosentan’.
Quality assessment
The selected studies were also assessed for the
quality of trials using the Cochrane Collaboration recommended tool
for assessing risk of bias (22).
This tool included the domains of selection bias (random sequence
generation and allocation concealment), performance bias (blinding
of participants and personnel), detection bias (blinding of outcome
assessment), attrition bias (incomplete outcome data), reporting
bias (selective reporting) and other sources of bias. The ‘risk of
bias’ assessment tool was used to further review bias among
individual studies (22).
Statistical analysis
Results are represented as risk ratios for
dichotomous data and mean differences for continuous data with 95%
confidence intervals (CI). Statistical heterogeneity across studies
was tested using Cochran's Q test. The fixed effects model was
selected for analysis when no significant heterogeneity between the
studies was found (P>0.10; I2 ≤50%). Alternatively,
the random effects model was used in case heterogeneity among
studies. The RevMan software package (version 5.2; Cochrane) was
used for all statistical analyses.
Results
Study characteristics
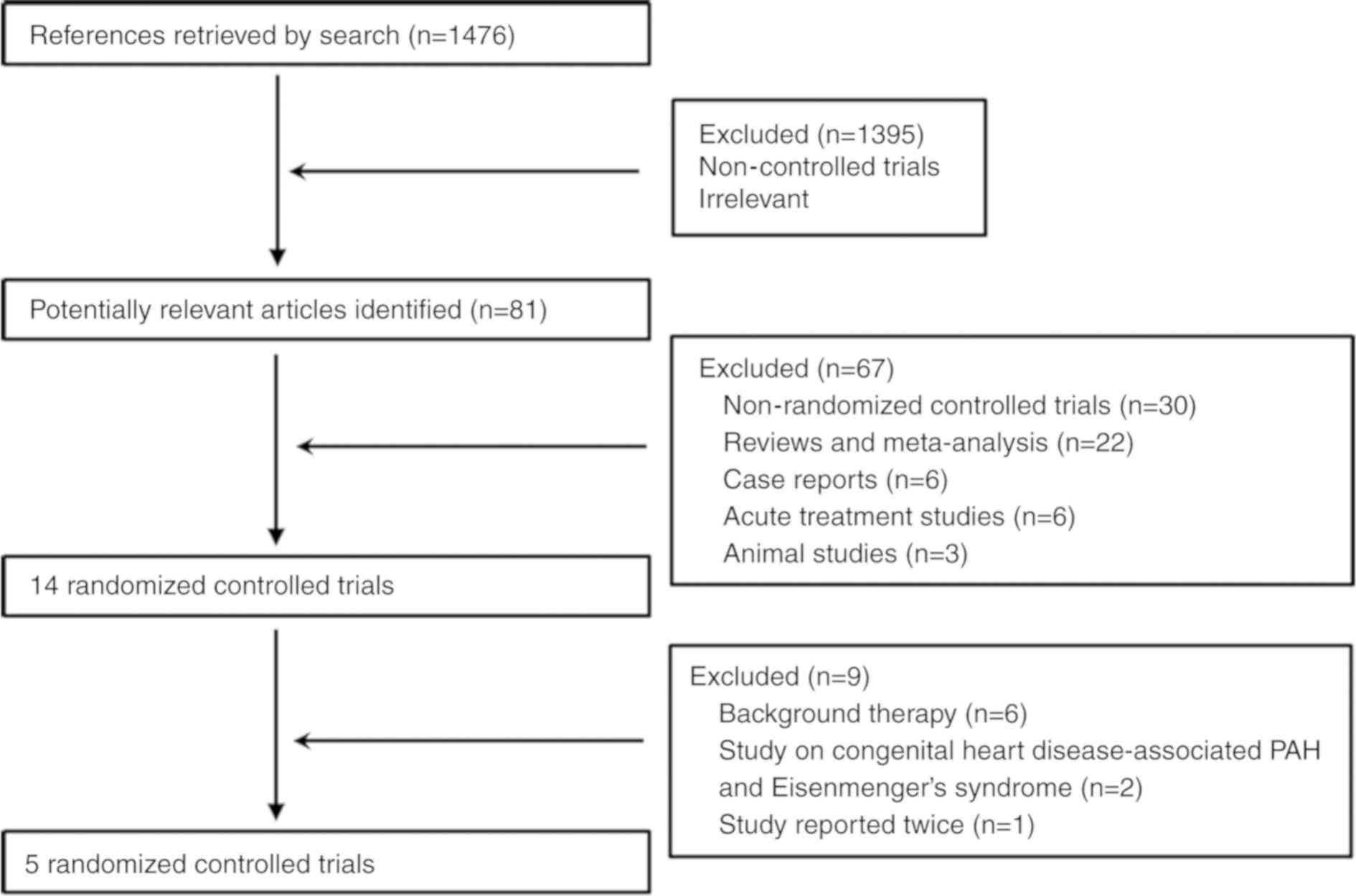
In the present meta-analysis, one RCT (PHIRST-1:
Tadalafil in the Treatment of Pulmonary Arterial Hypertension) was
reported twice (23,24). The trials consisted of patients with
congenital heart disease-associated PAH (25) and Eisenmenger's syndrome (26). The subjects of six studies were
treated with ERA or PDE-5 inhibitor as background treatment
(27–32); therefore, these were excluded from
the analysis. Overall, a total of 310 subjects in 5 RCTs were
included in this analysis (Fig. 1
and Table I).
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| Author, year | Study | n | Sex
(male/female) | MT | CT | Follow-up
(weeks) | Primary
endpoint | (Refs.) |
|---|
| McLaughlin et
al, 2006 | STEP | 67 | 14/53 | Placebo +
bosentan | Bosentan + Iloprost
(5 ug) | 12 | 6MWD | (33) |
| Hoeper et
al, 2006 | COMBI | 40 | 9/31 | Bosentan | Bosentan + Iloprost
(5 ug) | 12 | 6MWD | (34) |
| Barst et al,
2011 | PHIRST | 87 | 19/68 | Placebo +
bosentan | Bosentan + tadafil
(40 mg) | 16 | 6MWD | (23) |
| Han et al,
2017 | BIPH | 15 | 5/10 | Bosentan | Bosentan + Iloprost
(10 ug) | 12 | 6MWD | (35) |
| Vizza et al,
2017 |
| 103 | 25/79 | Bosentan | Bosentan +
Sildenafil (20 mg) | 12 | 6MWD | (36) |
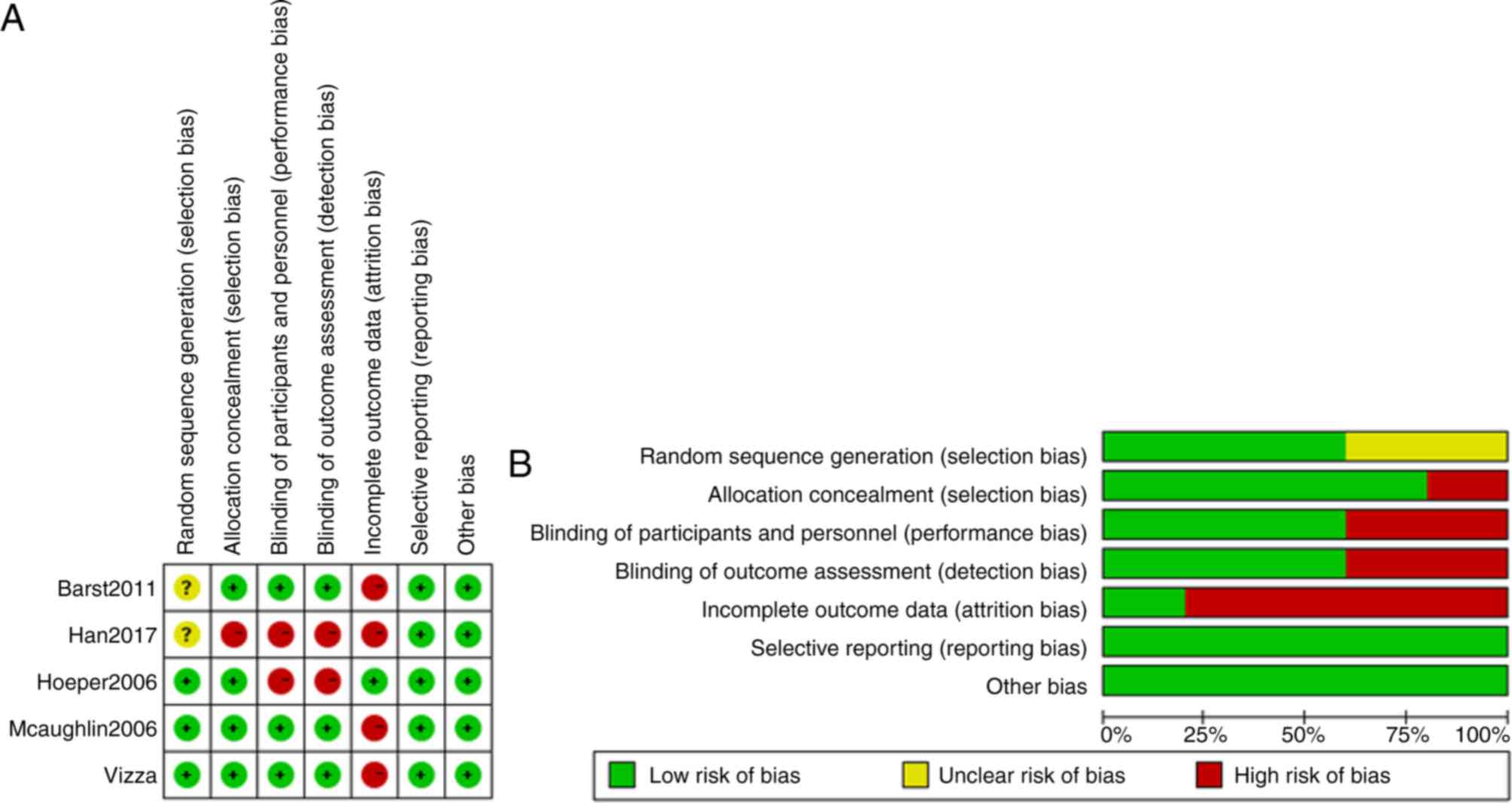
Data quality
The quality of the five studies was assessed and the
risk of bias was estimated by Cochrane Collaboration's tool. The
results were shown in Fig. 2. The
majority of the included studies had a low risk of bias according
to the following criteria: Selection bias, performance bias,
detection bias, attrition bias, reporting bias and other sources of
bias.
Meta-analysis results
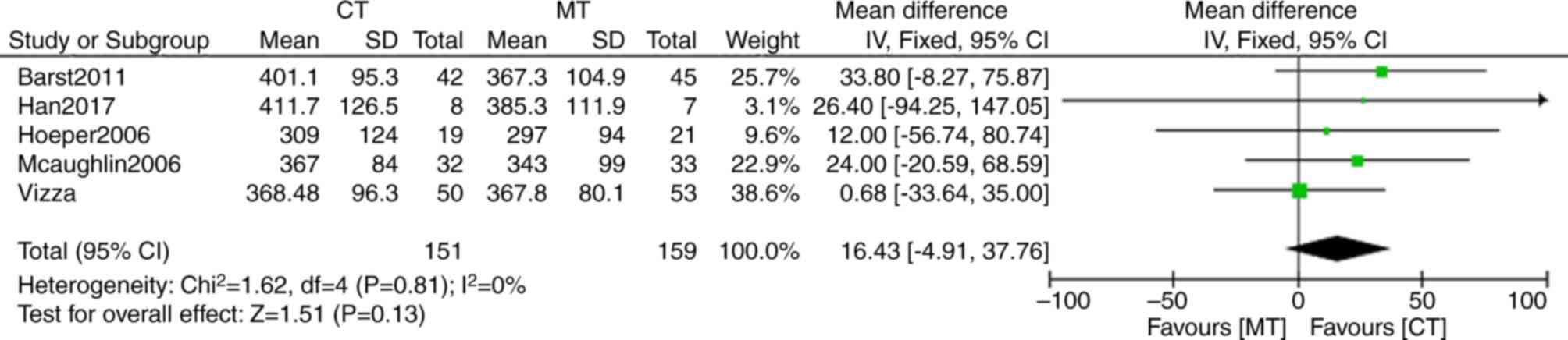
6MWD was used as an indicator of exercise ability in
all the five trials included in the present study. Compared with
bosentan monotherapy, four of the five studies in bosentan
combination therapy reported a significant improvement in walking
distance (Fig. 3). The mean
difference of 6MWD in bosentan combination therapy was 16.43 m (95%
CI: −4.91, 37.76), but there was no statistical significance
between bosentan combination and bosentan monotherapy (P=0.13). No
significant heterogeneity (I2=0%; P=0.81) was detected
in bosentan combination therapy compared with bosentan
monotherapy.
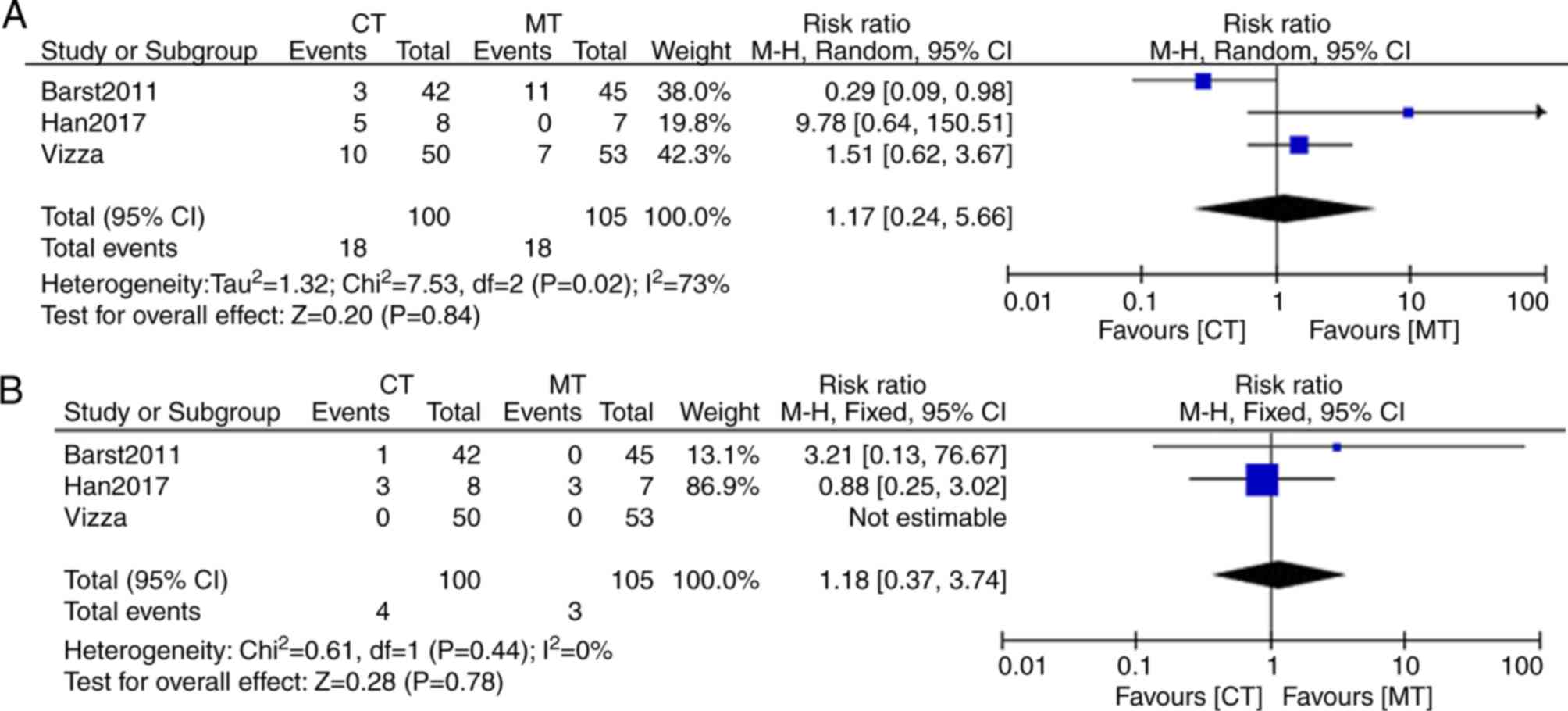
Cardiac functional improvement was also one of the
efficacy indicators of this meta-analysis. The New York Heart
Association (NYHA) and the World Health Organization (WHO)
functional classification systems were used to identify functional
impairment in PAH. McLaughlin et al (33) and Hoeper et al (34) performed their studies using the NYHA
functional classification, the remaining three studies were
performed using the WHO functional classification (23,35,36).
After meta-analysis, the result showed that there was significant
heterogeneity (I2 =73%; P=0.02) in WHO functional class
improvement I between bosentan combination therapy and bosentan
monotherapy (Fig. 4). The random
effects model was used for the analysis. Functional class
improvement I from baseline to endpoint of study was indicated to
be 18% (18/100) in bosentan combination therapy and 17% (18/105) in
bosentan monotherapy (Fig. 4A). The
WHO functional class improvement II from baseline to endpoint of
study was 4% (4/100) in bosentan combination therapy and 2.9%
(3/105) in bosentan monotherapy, without significant heterogeneity
(I2=0%; P=0.44) (Fig.
4B). Therefore, functional class improvements I and II
exhibited no significant difference between the bosentan
combination and monotherapy groups (P>0.05).
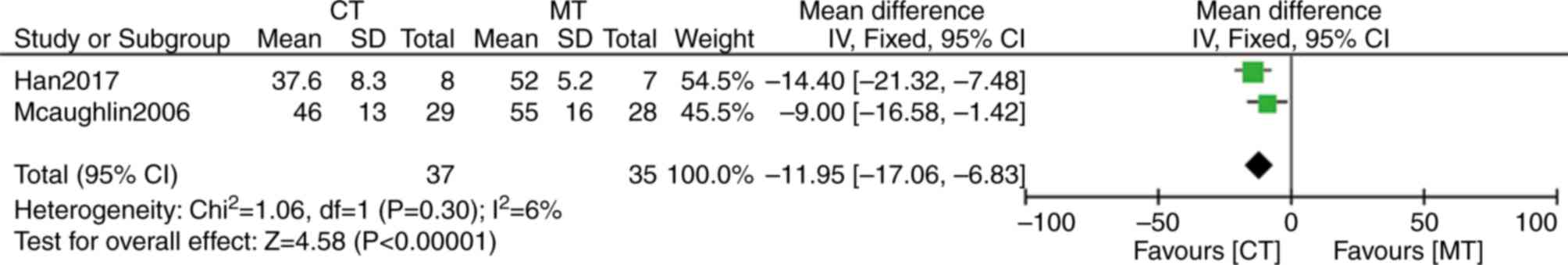
Two of the five trial studies reported the effects
of bosentan combination therapy on mean PAP (mPAP; Fig. 5) (33,35). The
difference of mPAP demonstrated an average of only 11.95 mmHg (95%
CI: −17.06, −6.83; P<0.00001) between bosentan combination
therapy and monotherapy, and there was no heterogeneity between the
groups (I2=6%; P=0.30). These data suggested that
combination therapy may significantly reduce mPAP.
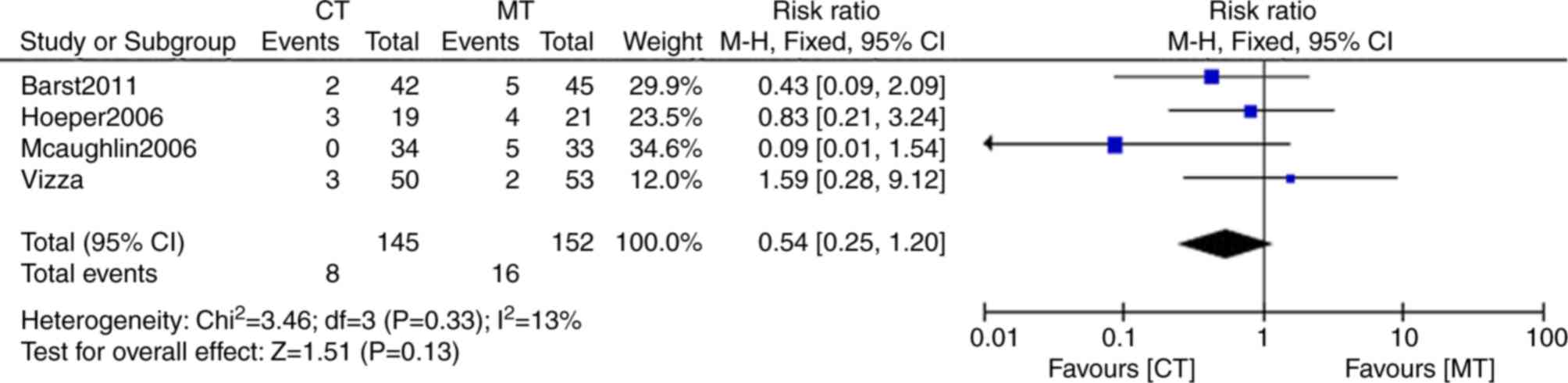
One study did not include any data of clinical
worsening (35) The clinical
worsening rate in combination therapy was 5.5% (8/145) compared
with that of monotherapy of 10.5% (16/152). The heterogeneity
between the groups was found to be non-significant
(I2=13%; P=0.33). Clinical worsening incidence in the
combination therapy was below that of monotherapy (risk ratio,
0.54; 95% CI: 0.25, 1.20), but without statistical significance
(P=0.13; Fig. 6).
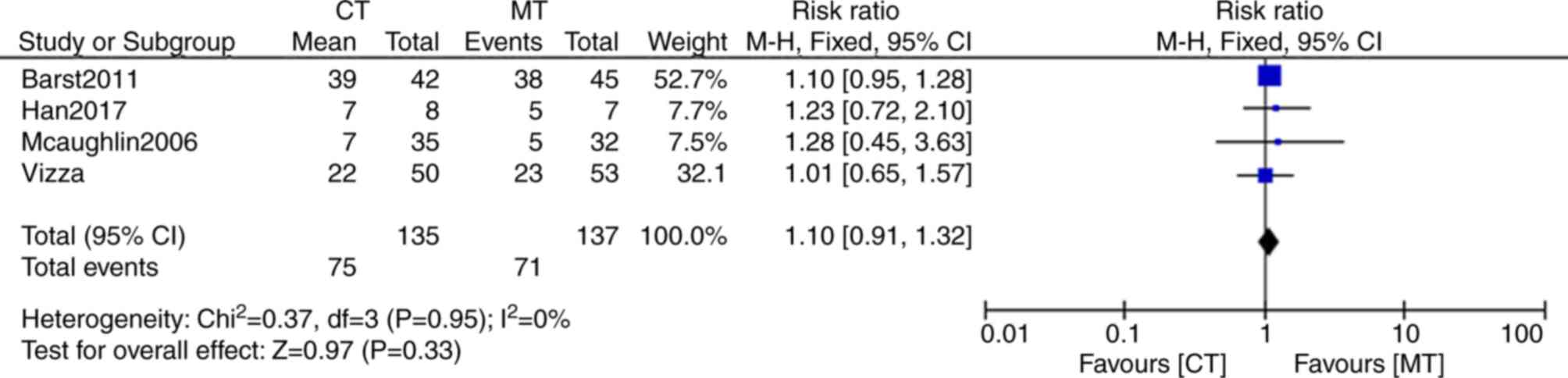
All of the five trials described adverse events, but
in one study, detailed data on adverse events was not provided
(23). These adverse events mainly
included headaches, coughing, flushing, chest pains, nausea,
dizziness and diarrhea. A total of 71 events (51.8%; n=137) were
reported in the monotherapy group, whereas 75 adverse events
(55.6%, n=135) were reported in the combination therapy group
(Fig. 7). The risk ratio of adverse
events between combination and monotherapy was 1.1 (95% CI: 0.91,
1.32). However, the difference between the groups was not
statistically significant (P=0.33). Thus, the incidence of adverse
events was not significantly different between the bosentan
combination therapy and the monotherapy groups.
Discussion
For the present meta-analysis, rigorous selection
criteria were applied. Studies of the bosentan treatment within 3
months prior to randomization, and studies in which the control
group was treated with bosentan or placebo were included. These
criteria resulted in only five studies that were included in this
analysis, which comprised a total of 310 subjects. The present
meta-analysis referred to the outcomes of previous studies of
combination therapy, and also formed the basis on the safety and
efficacy of combining bosentan with prostacyclin analogues or PDE-5
inhibitors.
The results from the present meta-analysis
demonstrated that bosentan combined with prostacyclin analogues or
PDE-5 inhibitors was superior to the bosentan monotherapy in
reducing mPAP by 11.95 mmHg. However, compared with bosentan
monotherapy, bosentan combined with prostacyclin analogues or PDE-5
inhibitors did not improve exercise capacity, cardiac function or
clinical worsening in PAH. Notably 5.5% of the patients in the
combination therapy developed clinical worsening compared with
10.5% in monotherapy. The clinical worsening rate was significantly
reduced in the bosentan combination therapy group although the
curative effect of treatment was not significant. These data
indicated that although bosentan therapy relieved the patient of
the symptoms and clinical worsening, it still failed to prevent and
slow the progression of PAH. The incidence of adverse events in
bosentan combination therapy was similar to that of monotherapy,
which suggested that bosentan combination therapy was safe for PAH
patients.
Drug interaction is a problem that cannot be ignored
in combination drug therapies (37).
Although the combination of bosentan with sildenafil or tadalafil
could lead to a decrease in plasma concentration of sildenafil and
tadalafil, bosentan concentration was increased by inducing
cytochrome P450 3A4 isoenzyme. However, the clinical significance
of this interaction has not been well established. Currently, there
is no evidence that interactions between bosentan and sildenafil
decrease drug safety.
Since the treatment regimens among the five studies
were different, the effect of bosentan combination therapy could
not be harmonized. In some studies, treatment with prostacyclin
analogues or PDE-5 inhibitors was initiated prior to treatment with
bosentan, whereas in other studies patients were treated with
bosentan for some time and then given prostacyclin analogues or
PDE-5 inhibitors (27–32). It was difficult to determine which
combination therapy regimen was most effective, and current
published guidelines do not offer a specific recommended regimen.
As the current findings may be limited by the relatively short
duration (12 weeks) of the trials, it was not possible to determine
the long-term efficacy and safety of bosentan combination therapy.
Therefore, the true clinical features and progression of the
disease in patients could not be determined. Given the limited
number of studies included in the present analysis, the results
should be confirmed through future research. A larger randomized
controlled trial should be designed for future studies to
adequately assess the efficacy and safety of bosentan combination
therapy.
In conclusion, results from the present
meta-analysis suggested that bosentan combined with prostacyclin
analogues or PDE-5 inhibitors do not impart additional advantages
for the improvement of the 6MWD, cardiac function, clinical
worsening, and incidence of adverse events. However, bosentan
combined with prostacyclin analogues or PDE-5 inhibitors may
significantly reduce mPAP compared with bosentan monotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of China under Grant (grant nos. 81660308 and
81460663).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from PubMed, Embase, the Cochrane Library and
the www.clinicaltrials.gov website.
Authors' contributions
ZCD, ZQL and DXL contributed to study conception and
design; drafted the submitted article, revised it critically for
important intellectual content. ZCD and BL independently appraised
study quality of the included trials and contributed to the
analysis and interpretation of data. RLG contributed to study
conception and design. ZCD and BT contributed to acquisition,
analysis, and interpretation of data. ZCD and SL contributed to the
literature search, acquisition of data, and drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
6MWD
|
six-minute walk distance
|
|
ERA
|
endothelin receptor antagonist
|
|
mPAP
|
mean pulmonary artery pressure
|
|
PAH
|
pulmonary arterial hypertension
|
|
PDE-5
|
phosphodiesterase type 5
|
References
|
1
|
Peacock AJ, Murphy NF, Mcmurray JJ,
Caballero L and Stewart S: An epidemiological study of pulmonary
arterial hypertension. Eur Respir J. 30:104–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Humbert M, Morrell NW, Archer SL, Stenmark
KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O,
Voelkel NF and Rabinovitch M: Cellular and molecular pathobiology
of pulmonary arterial hypertension. J Am Coll Cardiol. 43 (12 Suppl
S):13S–24S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Budhiraja R, Tuder RM and Hassoun PM:
Endothelial dysfunction in pulmonary hypertension. Circulation.
109:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humbert M, Sitbon O and Simonneau G:
Treatment of pulmonary arterial hypertension. N Engl J Med.
351:1425–1436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badesch DB, Abman SH, Simonneau G, Rubin
LJ and McLaughlin VV: Medical therapy for pulmonary arterial
hypertension: Updated ACCP evidence-based clinical practice
guidelines. Chest. 131:1917–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoeper MM, Faulenbach C, Golpon H, Winkler
J, Welte T and Niedermeyer J: Combination therapy with bosentan and
sildenafil in idiopathic pulmonary arterial hypertension. Eur
Respir J. 24:1007–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seyfarth HJ, Pankau H, Hammerschmidt S,
Schauer J, Wirtz H and Winkler J: Bosentan improves exercise
tolerance and Tei index in patients with pulmonary hypertension and
prostanoid therapy. Chest. 128:709–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Channick RN, Olschewski H, Seeger W, Staub
T, Voswinckel R and Rubin LJ: Safety and efficacy of inhaled
treprostinil as add-on therapy to bosentan in pulmonary arterial
hypertension. J Am Coll Cardiol. 48:1433–1437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galie N, Manes A, Negro L, Palazzini M,
Bacchi-Reggiani ML and Branzi A: A meta-analysis of randomized
controlled trials in pulmonary arterial hypertension. Eur Heart J.
30:394–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z, Krasnici N and Lüscher TF:
Endothelin-1 potentiates human smooth muscle cell growth to PDGF:
Effects of ETA and ETB receptor blockade. Circulation. 100:5–8.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Price LC and Howard LS: Endothelin
receptor antagonists for pulmonary arterial hypertension: Rationale
and place in therapy. Am J Cardiovasc Drugs. 8:171–185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaughlin VV, Shillington A and Rich S:
Survival in primary pulmonary hypertension: The impact of
epoprostenol therapy. Circulation. 106:1477–1482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barst RJ, Rubin LJ, McGoon MD, Caldwell
EJ, Long WA and Levy PS: Survival in primary pulmonary hypertension
with long-term continuous intravenous prostacyclin. Ann Intern Med.
121:409–415. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Provencher S, Sitbon O, Humbert M, Cabrol
S, Jaïs X and Simonneau G: Long-term outcome with first-line
bosentan therapy in idiopathic pulmonary arterial hypertension. Eur
Heart J. 27:589–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sitbon O, Humbert M, Nunes H, Parent F,
Garcia G, Hervé P, Rainisio M and Simonneau G: Long-term
intravenous epoprostenol infusion in primary pulmonary
hypertension: Prognostic factors and survival. J Am Coll Cardiol.
40:780–788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galie N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Respir J. 46:903–975. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Channick RN: Combination therapy in
pulmonary arterial hypertension. Am J Cardiol. 111 (8
Suppl):16C–20C. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haworth S and Rabinovitch M: Pulmonary
circulationPaediatric Cardiology. 3rd. Anderson RH, Baker EJ, Penny
DJ, et al: Churchill Livingstone; Philadelphia: pp. 117–141. 2010,
View Article : Google Scholar
|
|
19
|
Channick RN, Simonneau G, Sitbon O,
Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M,
Bodin F and Rubin LJ: Effects of the dual endothelin-receptor
antagonist bosentan in patients with pulmonary hypertension: A
randomised placebo-controlled study. Lancet. 358:1119–1123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rubin LJ, Badesch DB, Barst RJ, Galie N,
Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al:
Bosentan therapy for pulmonary arterial hypertension. N Engl J Med.
346:896–903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension. Kardiol Pol. 73:1127–1206.
2015.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moher D, Pham B, Jones A, Cook DJ, Jadad
AR, Moher M, Tugwell P and Klassen TP: Does quality of reports of
randomised trials affect estimates of intervention efficacy
reported in meta-analyses? Lancet. 352:609–613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barst RJ, Oudiz RJ, Beardsworth A,
Brundage BH, Simonneau G, Ghofrani HA, Sundin DP and Galiè N;
Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST)
Study Group, : Tadalafil monotherapy and as add-on to background
bosentan in patients with pulmonary arterial hypertension. J Heart
Lung Transplant. 30:632–643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galie N, Brundage BH, Ghofrani HA, Oudiz
RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth
A, et al: Tadalafil therapy for pulmonary arterial hypertension.
Circulation. 119:2894–2903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Alto M, Romeo E, Argiento P, Sarubbi B,
Santoro G, Grimaldi N, Correra A, Scognamiglio G, Russo MG and
Calabrò R: Bosentan-sildenafil association in patients with
congenital heart disease-related pulmonary arterial hypertension
and Eisenmenger physiology. Int J Cardiol. 155:378–382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iversen K, Jensen AS, Jensen TV, Vejlstrup
NG and Sondergaard L: Combination therapy with bosentan and
sildenafil in Eisenmenger syndrome: A randomized,
placebo-controlled, double-blinded trial. Eur Heart J.
31:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McLaughlin VV, Benza RL, Rubin LJ,
Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H,
Rubenfire M, Rubenfire M, et al: Addition of inhaled treprostinil
to oral therapy for pulmonary arterial hypertension: A randomized
controlled clinical trial. J Am Coll Cardiol. 55:1915–1922. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tapson VF, Jing ZC, Xu KF, Pan L, Feldman
J, Kiely DG, Kotlyar E, McSwain CS, Laliberte K, Arneson C, et al:
Oral treprostinil for the treatment of pulmonary arterial
hypertension in patients receiving background endothelin receptor
antagonist and phosphodiesterase type 5 inhibitor therapy (the
FREEDOM-C2 study): A randomized controlled trial. Chest.
144:952–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sitbon O, Channick R, Chin KM, Frey A,
Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, et al:
Selexipag for the treatment of pulmonary arterial hypertension. N
Engl J Med. 373:2522–2533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghofrani HA, Galiè N, Grimminger F, Grünig
E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A,
et al: Riociguat for the treatment of pulmonary arterial
hypertension. N Engl J Med. 369:330–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tapson VF, Torres F, Kermeen F, Keogh AM,
Allen RP, Frantz RP, Badesch DB, Frost AE, Shapiro SM, Laliberte K,
et al: Oral treprostinil for the treatment of pulmonary arterial
hypertension in patients on background endothelin receptor
antagonist and/or phosphodiesterase type 5 inhibitor therapy (the
FREEDOM-C study): A randomized controlled trial. Chest.
142:1383–1390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simonneau G, Torbicki A, Hoeper MM,
Delcroix M, Karlócai K, Galiè N, Degano B, Bonderman D, Kurzyna M,
Efficace M, et al: Selexipag: An oral, selective prostacyclin
receptor agonist for the treatment of pulmonary arterial
hypertension. Eur Respir J. 40:874–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McLaughlin VV, Oudiz RJ, Frost A, Tapson
VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH and Rubin
LJ: Randomized study of adding inhaled iloprost to existing
bosentan in pulmonary arterial hypertension. Am J Respir Crit Care
Med. 174:1257–1263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoeper MM, Leuchte H, Halank M, Wilkens H,
Meyer FJ, Seyfarth HJ, Wensel R, Ripken F, Bremer H, Kluge S, et
al: Combining inhaled iloprost with bosentan in patients with
idiopathic pulmonary arterial hypertension. Eur Respir J.
28:691–694. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han X, Zhang Y, Dong L, Fang L, Chai Y,
Niu M, Yu Y, Liu L, Yang X, Qu S and Li S: Treatment of pulmonary
arterial hypertension using initial combination therapy of bosentan
and iloprost. Respir Care. 62:489–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vizza CD, Jansa P, Teal S, Dombi T and
Zhou D: Sildenafil dosed concomitantly with bosentan for adult
pulmonary arterial hypertension in a randomized controlled trial.
BMC Cardiovasc Disord. 17:2392017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dingemanse J and van Giersbergen PL:
Clinical pharmacology of bosentan, a dual endothelin receptor
antagonist. Clin Pharmacokinet. 43:1089–1115. 2004. View Article : Google Scholar : PubMed/NCBI
|