Introduction
Autoimmune events have been reported in both
pediatric and adult patients with chronic hepatitis B and C
(1–3). Even though autoimmune hepatitis and
viral hepatitis are completely different entities, both are
secondary to disturbances of the immune system (hepatitis viruses B
and C are poorly cytopathic) and its failure to properly adjust the
immune reaction towards hepatocytes (4,5).
Autoimmunity is defined as the loss of tolerance
towards self-antigens, and its etiopathogenesis is still unknown.
There are several factors considered to play a role in
autoimmunity, such as genetic and environmental ones. Other
factors, such as stress, are still being studied regarding their
relationship with autoimmunity. Stress has been reported to be
associated with onset and exacerbation of several autoimmune
diseases. This is a new point of research, considering that stress
has a major impact on modern lifestyle, and it is known to
influence homeostasis and health (6,7).
Persistence of hepatitis B or C viral infection
depends on the interaction between the host's immune system and the
virus. The destruction of the hepatocytes occurs when the immune
reaction directed towards the hepatitis virus, essential for
identifying the virus and subsequent viral clearance, generates an
inflammatory reaction of the liver tissue through various
mechanisms which up-regulate pro-inflammatory cytokines. Therefore,
infected hepatocytes are destroyed by means of effector T cells.
Concurrent, specific antibodies are generated by B cells in order
to neutralize the virus. Previous research has shown that both
viruses (hepatitis B virus and hepatitis C virus) possess the
ability to escape the innate immune system, but a higher rate of
persistence has been reported for the hepatitis C virus because it
has the ability to escape the adaptive immunity as well. The
adaptive immunity is responsible of recognizing antigenic
structures and is sustained by B and T cells. One of its most
important features is long-term memory, providing a quick secondary
response. The ability of the hepatitis C virus to escape adaptive
immunity resides in its high mutation rate (5).
Interferon treatment, which is widely used for
treating chronic viral hepatitis, has also been incriminated in
inducing autoimmunity (3).
Interferon-based treatment is more advantageous than new therapies
(nucleotide/nucleoside analogues) for chronic hepatitis B as it can
provide immune-mediated control in many cases after only one year.
Nucleotidic/nucleozidic analogues require long-term treatment
courses and even though virological suppression is achieved in
almost all patients, AgHBs clearance is low. Recently, a new
strategy was approached by adding pegylated interferon in patients
previously treated with nucleosidic/nucleotidic analogues in order
to speed the clearance of AgHBs (8).
Children with chronic viral hepatitis B are often in
an immune tolerant state, but there is not enough data on how or if
they should be treated is this phase of the disease. An association
of interferon and lamivudin was reported to improve the rates of
undetectable viremia and ‘e’ seroconversion in these patients
(9). Positive antinuclear
antibodies, anti-smooth muscle antibodies, circulating immune
complexes and rheumatoid factor were often reported in patients
with chronic viral hepatitis B and C, but occurrence of autoimmune
diseases did not appear to be correlated with the hepatitis B or C
virus infection (10).
The present study assessed the autoimmune phenomena
associated to pediatric chronic hepatitis B and C and whether the
risk for autoimmune events is higher in patients who have received
interferon treatment.
Patients and methods
An observational prospective study was conducted,
which included 114 pediatric patients previously diagnosed with
chronic viral hepatitis, 92 patients with chronic hepatitis B and
22 with chronic hepatitis C. The patients presented to the
Pediatrics Department of ‘Grigore Alexandrescu’ Emergency
Children's Hospital in Bucharest, Romania, over a period of 40
months. Legal guardians of the patients signed an informed consent
prior to inclusion in the study. The research was approved by the
Ethics Committee of ‘Grigore Alexandrescu’ Emergency Children's
Hospital - registration no. 8954/04.04.2018.
The subjects were divided in two groups - the first
group included patients who had received treatment while the second
group were treatment-naive. The patients who had been treated for
chronic viral hepatitis were included in the study group at least 6
months after the medication was stopped (none of them was receiving
treatment at the time of evaluation). The national treatment guide
was followed: chronic viral hepatitis B patients were treated with
α-2b interferon three times a week, while chronic viral hepatitis C
patients were treated with pegylated-interferon once a week and
daily ribavirin.
Several laboratory tests were performed for each
patient. Autoimmune markers were pursued annually, including
cryoglobulins, circulating immune complexes, complement
abnormalities, rheumatoid factor and non-organ specific
autoantibodies - antinuclear antibodies (ANA), anti-smooth muscle
antibodies (ASMA), anti-liver kidney microsomal antibodies
(LKM1).
Microsoft Excel was used to organize the data, while
SPSS Kaplan-Meier survival curves were applied to evaluate the
influence of treatment and period of time needed for the occurrence
of the autoimmune phenomenon in these patients.
Results
One hundred and fourteen patients were enrolled, all
of them previously diagnosed with hepatitis B or C viral infection.
The age of the patients ranged from 18 months to 18 years, mean age
9 years with a standard deviation of 4.9. In all patients we
identified the mother to be chronically infected with hepatitis B
or C virus, so we considered that the infection was transmitted
vertically. Regarding gender, male to female ratio was 1.23.
No clinically overt autoimmune manifestations were
identified, but serological evidence of autoimmunity was found in
50% of the patients at some point during follow-up. Forty-six
percent of patients included in the study group had previously
received treatment, of them 50% were subsequently identified with
autoimmune phenomena. Fifty-four percent of the patients were
treatment-naive, and 50% of them presented autoimmune
manifestations.
Regarding the markers that defined the autoimmune
phenomenon, 31 patients were defined by only one positive marker,
24 had two simultaneous positive autoimmune markers, and further 2
had three simultaneous positive markers. The markers that
determined the autoimmune phenomenon are listed in Table I.
 | Table I.Autoimmune markers to determine the
autoimmune phenomena. |
Table I.
Autoimmune markers to determine the
autoimmune phenomena.
|
| Patients (number and
percent) |
|---|
|
|
|
|---|
| Autoimmune
markers | N | % |
|---|
| No autoimmune
markers | 57 | 50 |
| Autoimmune
phenomena | 57 | 50 |
| Positive markers |
|
Circulating immune
complexes | 5 | 4.39 |
|
Cryoglobulins | 10 | 8.77 |
|
Cryoglobulins, C4 complement
fraction | 1 | 0.88 |
|
Cryoglobulins, circulating
immune complexes | 4 | 3.51 |
| ANA | 3 | 2.63 |
| ANA,
circulating immune complexes | 2 | 1.75 |
|
Rheumatoid factor | 12 | 10.53 |
|
Rheumatoid factor, circulating
immune complexes | 3 | 2.63 |
|
Rheumatoid factor,
cryoglobulins | 8 | 7.02 |
|
Rheumatoid factor,
cryoglobulins, circulating immune complexes | 2 | 1.75 |
| LKM1, C4
complement fraction | 1 | 0.88 |
| LKM1,
ANA | 1 | 0.88 |
| ASMA | 1 | 0.88 |
| ASMA,
circulating immune complexes | 2 | 1.75 |
| ASMA,
cryoglobulins | 2 | 1.75 |
| Total number of
patients | 114 | 100 |
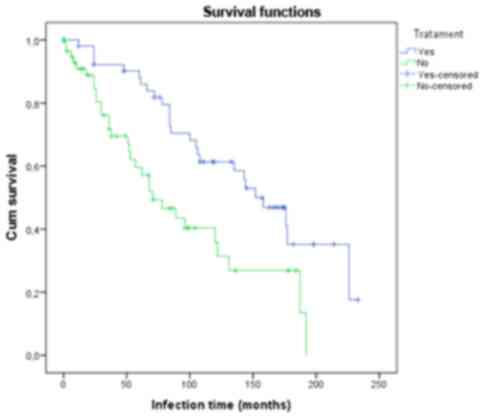
We found that the probability of identifying
autoimmune manifestations increased the longer the patient was
chronically infected. For patients who had received treatment the
autoimmune markers were identified after a longer period of time
when compared to the group who had not been treated. At ~190
months, in the treatment group the probability of the occurrence of
an autoimmune event was ~65%, while at the same time in the
non-treatment group all the patients presented with autoimmune
phenomena (Fig. 1).
The median for the time until the autoimmune
manifestations occurred was 158 months in patients who had received
treatment (with a 95% confidence interval between 123.27 and 192.72
months) and 71 months in treatment-naive patients (with a 95%
confidence interval between 41.34 and 100.65 months) (Table II). The fact that there is no
overlap between the confidence intervals for the two compared
groups shows that the reported difference is clinically
significant.
 | Table II.Median time until the autoimmune
manifestations occurred. |
Table II.
Median time until the autoimmune
manifestations occurred.
|
| Median for survival
time |
|---|
|
|
|
|---|
|
| Median | 95% Confidence
interval |
|---|
|
|
|
|
|---|
| Interferon
(standard/pegylated) treatment | Estimate | Standard error | Lower bound | Upper bound |
|---|
| Yes | 158,000 | 17,717 | 123,275 | 192,725 |
| No |
71,000 | 15,131 |
41,343 | 100,657 |
| Overall | 120,000 | 18,107 |
84,510 | 155,490 |
The identified difference was found to be
statistically significant - log-rank (χ2 = 9.397, df =
1, P=0.002), Breslow (χ2 = 10.454, df = 1, P=0.001),
Tarone-Ware (χ2 = 10.157, df = 1, P=0.001) (Table III). The log-rank test tends to
focus more on the significance of the difference of the curves
later in time. The Breslow test tends to look at what happens to
the curves earlier in the time course, while the Tarone-Ware tests
focuses on what happens at the middle of the time course. We report
a statistically significant difference between the two compared
curves at each tested time (Table
III).
 | Table III.Comparison between the two curves. |
Table III.
Comparison between the two curves.
|
| Overall
comparisons |
|---|
|
|
|
|---|
|
| Chi-square | df | Sig. |
|---|
| Log-rank
(Mantel-Cox) |
9,397 | 1 | 0.002 |
| Breslow (Generalized
Wilcoxon) | 10,454 | 1 | 0.001 |
| Tarone-Ware | 10,157 | 1 | 0.001 |
Discussion
Previous studies have linked interferon treatment to
the occurrence of autoimmune markers. A research study conducted in
2014 by Pop et al (11)
showed that antinuclear antibodies occurred in 13.79% of the
pediatric patients with chronic viral hepatitis B who had received
interferon therapy and had previously tested negative. For patients
who had not received treatment the incidence for antinuclear
antibodies was only 2.56%. The difference was statistically
significant (P=0.042). The same study states that the presence of
these antibodies does not interfere with treatment response.
Other studies, which included adult patients, have
shown that autoimmune markers were identified in 85% of the cases
with chronic viral hepatitis C and 89% of the cases with chronic
viral hepatitis B. The serologic autoimmune phenomenon was reported
in 74% of patients who had received short-term treatment (<30
weeks) vs. 85% of the patients with long-time treatment (>50
weeks) (10). In our study, which
included patients with both hepatitis B and hepatitis C, autoimmune
markers were identified in 50% of the cases, less than reported in
previous research conducted on adult subjects, possibly secondary
to the shorter time of evolution in children. According to our
study, patients with chronic viral infection who have not received
treatment will most likely develop autoimmune serological
phenomenon earlier when compared to those who received
interferon-based therapy.
The autoimmune phenomenon in chronic viral hepatitis
still carries a high degree of uncertainty, especially in the
pediatric field. Long-term studies have concluded that patients
with chronic viral hepatitis B who do not receive treatment
developed an antibody titer similar to that of patients who have
received interferon treatment, but after a longer period of time
(12). This contradicts the results
of the present study. Our results stand by the idea that even
though interferon-based therapies may induce autoimmunity, viruses
themselves are more likely to induce the appearance of autoimmune
markers over time in patients who do not receive treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
DP contributed to designing the study,
interpretation of data and revised the manuscript for important
intellectual content. ID drafted the manuscript, acquired and
contributed to interpretation of data. AD and MA contributed to
drafting the manuscript, analyzed and interpreted the data. MP
acquired the data and contributed to drafting the manuscript. CB
contributed to designing the study and revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Legal guardians of the patients signed an informed
consent prior to inclusion in the study. The research was approved
by the Ethics Committee of ‘Grigore Alexandrescu’ Emergency
Children's Hospital (Bucharest, Romania) - registration no.
8954/04.04.2018.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsieh MY, Dai CY, Lee LP, Huang JF, Chuang
WL, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, et al: Antinuclear
antibody titer and treatment response to peginterferon plus
ribavirin for chronic hepatitis C patients. Kaohsiung J Med Sci.
28:86–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muratori P, Muratori L, Verucchi G, Attard
L, Bianchi FB and Lenzi M: Non-organ-specific autoantibodies in
children with chronic hepatitis C: Clinical significance and impact
on interferon treatment. Clin Infect Dis. 37:1320–1326. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kansu A, Kuloğlu Z, Demirçeken F and
Girgin N: Autoantibodies in children with chronic hepatitis B
infection and the influence of interferon alpha. Turk J
Gastroenterol. 15:213–218. 2004.PubMed/NCBI
|
|
4
|
Lapierre P and Lamarre A: Regulatory T
cells in autoimmune and viral chronic hepatitis. J Immunol Res.
2015:4797032015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piconese S, Cammarata I and Barnaba V:
Viral hepatitis, inflammation, and cancer: A lesson for
autoimmunity. J Autoimmun. 95:58–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharif K, Watad A, Coplan L, Lichtbroun B,
Krosser A, Lichtbroun M, Bragazzi NL, Amital H, Afek A and
Shoenfeld Y: The role of stress in the mosaic of autoimmunity: An
overlooked association. Autoimmun Rev. 17:967–983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grigore O, Mihailescu AI, Solomon I, Boda
D and Caruntu C: Role of stress in modulation of skin neurogenic
inflammation. Exp Ther Med. 17:997–1003. 2019.PubMed/NCBI
|
|
8
|
Viganò M, Grossi G, Loglio A and
Lampertico P: Treatment of hepatitis B: Is there still a role for
interferon? Liver Int. 38 (Suppl 1):79–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu
W, Gan Y, Tang H, Chen D, Wang F, et al: Antiviral therapy in
hepatitis B virus-infected children with immune-tolerant
characteristics: A pilot open-label randomized study. J Hepatol.
68:1123–1128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orságová I, RoŽnovský L, Petroušová L,
Konečná M, Kabieszová L, Martinek J, Kloudová A and Pavliska L:
Investigation of autoimmunity markers during interferon alpha
therapy of chronic hepatitis B and C - twenty years of experience.
Klin Mikrobiol Infekc Lek. 22:61–67. 2016.(In Czech). PubMed/NCBI
|
|
11
|
Pop TL, Stefănescu A, Samaşca G and Miu N:
Clinical significance of the antinuclear antibodies in chronic
viral hepatitis B in children. Clin Lab. 60:931–939. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vajro P and Veropalumbo C: Treating
children with HBeAg-positive chronic hepatitis B: No small
accomplishment. Dig Liver Dis. 46:1064–1065. 2014. View Article : Google Scholar : PubMed/NCBI
|