Introduction
In Europe and in the US, prostate cancer is the most
common cancer type in males (1). The
occurrence of prostate cancer varies among different ethnicities
(2). In Chinese males, prostate
cancer has an incidence of 121 in 1,000,000 males (2) and it is the fifth leading cause of
cancer-associated mortality in males (3). Mortality due to prostate cancer may be
reduced by proper diagnosis (4).
Radiological images have a crucial role in the diagnosis of
prostate cancer (5). Additionally,
serum prostate-specific antigen testing is frequently performed
following biopsy due to the high frequency in the elevation of
prostate-specific antigen expression in patients with prostate
cancer (6).
Prostate-specific antigen is produced by the
prostate but is not a prostate cancer biomarker. It may also be
altered during inflammation or infection, as well as in benign
prostatic hyperplasia (6). In
Chinese males, it is also associated with obesity (7). Urologists in China have put rigorous
effort in improving the quality of screening and treatment of
cancer of the prostate gland with radiological methods, e.g. MRI,
CT, transrectal ultrasound (TRUS), and positron emission tomography
(8). Among the radiological methods,
TRUS provides more appropriate details than MRI and CT (5). Multiparametric MRI (mpMRI) has value in
the diagnosis of prostate cancer (8)
but it is only able to diagnose localized prostate cancer with a
volume of ≥0.2 ml (9). A prostate
biopsy is performed in order to discover a prostate cancer in the
case of persistent altered prostate-specific antigen level and/or
in the case of suspicion warranting digital rectal examination
(10). mpMRI fused with TRUS
(mpMRI/TRUS)-guided biopsy has the sensitivity of mpMRI (11) with the practicality of TRUS (12) and is a promising method (13) for prostate cancer diagnosis (14). However, to overcome inaccuracies
associated with the biopsy technique, mpMRI/TRUS fusion biopsies
require a high volume of samples (14). Subjects with undetected cancerous
lesion(s) on mpMRI still have a certain probability of having
prostate cancer (15) but mpMRI/TRUS
is not performed for those.
The utility of power Doppler with grayscale
ultrasound is used in the detection of prostate cancer (5). Endorectal power Doppler ultrasound may
detect capsular extension, visualize tumor vascularity and improve
the sensitivity of grayscale ultrasound-guided biopsies (16). All of these pre-operative data should
be utilized by the surgeon to perform the best radical
prostatectomy technique in order to obtain the best achievable
functional result (17,18).
The objective of the present prospective study was
to evaluate the beneficial score of endorectal power Doppler
combined with grayscale ultrasound-guided biopsy over that of
mpMRI/TRUS-guided biopsy for decision making regarding
prostatectomy in Chinese males with a high risk of prostate
cancer.
Materials and methods
Materials
Levofloxacin (Loxof) was purchased from Ranbaxy
Pharmaceuticals Pvt. Ltd (Sun Pharmaceutical Industries, Ltd.).
Lidocaine jelly (Xylocaine gel) was purchased from AstraZeneca
Pharmaceuticals Co. Ltd. Diclofenac (50 mg) with paracetamol (500
mg) tablet (Lederflam Forte) was purchased from Wyeth, Inc. The
loose enema was purchased from Torrent Pharmaceuticals, Ltd.
Inclusion criteria
Between January 2013 and February 2019, a total of
1,215 males aged ≥40 years with complaints including weak flow
during urination, difficulties to start urinating, weak flow of
urine and a sensation of improper urinary bladder emptying were
available at an outpatient setting of Dongguan People's Hospital
Affiliated to Southern Medical University (Dongguan, China) and the
First Affiliated Hospital of Anhui Medical University (Anhui,
China) were included in the present study (19). Males with elevated prostate-specific
antigen (normal range, ≤3.0 ng/ml for subjects <50 years, ≤3.5
ng/ml for 50–59 years, ≤4.5 ng/ml for 60–69 years and ≤5.5 ng/ml
for ≥70 years) (20) or abnormal
structure of the prostate on palpitation on rectal examination
(hard, lumpy or enlarged prostate) under digital rectal examination
(19) were included in the
study.
Exclusion criteria
Males with confirmed prostate cancer, negative
biopsies for prostate cancer in the past 6 months, normal
prostate-specific antigen levels, normal rectal examinations, men
with normal prostate-specific antigen levels and abnormal prostate
palpitations (considered inflammation of the prostate and not
prostate cancer), a Gleason score of 3+3 and age of <40 years
were excluded from the study. Although patients with age >70
years were not recommended for prostatectomy, they were also
subjected to biopsies.
mpMRI
Males were examined with an MRI 3.0 Tesla (AIRIS
Vento O5 0.3T; Hitachi Aloka, Medical, Ltd.) using phased-array
torso coils (16 elements, Hitachi Aloka Medical, Ltd.) and with an
endorectal coil. Dynamic contrast-enhanced imaging,
diffusion-weighted imaging with b-values of >1,500, apparent
diffusion coefficient value imaging and multiplanar T2-weighted
imaging (T2WI) scans were performed for all patients (21). MR images were analyzed with the
Prostate Imaging Reporting and Data System version 1 (22). Based on the overall impression of the
prostate in MR images, each lesion was assigned a Likert scale
score (five-point scale method: Unlikely benign, 1; most probably
benign, 2; equivocal prostate cancer, 3; probably malignant, 4; and
highly suspicious of malignancy, 5 (23). Males with lesions with a Likert scale
score of ≥3 were subjected to mpMRI/TRUS-guided biopsies (according
to the institutional guidelines for prostate cancer examinations)
(23). Five radiologists (with a
minimum of 3 years of experience) of the institute(s) performed
mpMRI and assigned the Likert scale score to each of the lesions.
All enrolled patients were subjected to both types of biopsies,
which were performed sequentially.
Endorectal power Doppler/grayscale
ultrasound-guided biopsy
The patients were instructed to lie down in the left
lateral decubitus position with flexed knees and hips. Rectal
lidocaine jelly was applied to the rectal probe and the latex cuff.
The prostate gland was examined with a Color Doppler System (F31;
Hitachi Aloka Medical Ltd.) and 7.5-MHz endorectal probes (Hitachi
Aloka Medical Ltd.) in the axial and sagittal sections. Ultrasound
equipment was set at the level of background noise. In total, seven
sonographers (blinded regarding the mpMRI results; minimum 3 years
of experience) of the institute(s) performed endorectal power
Doppler with grayscale ultrasound examinations. After the
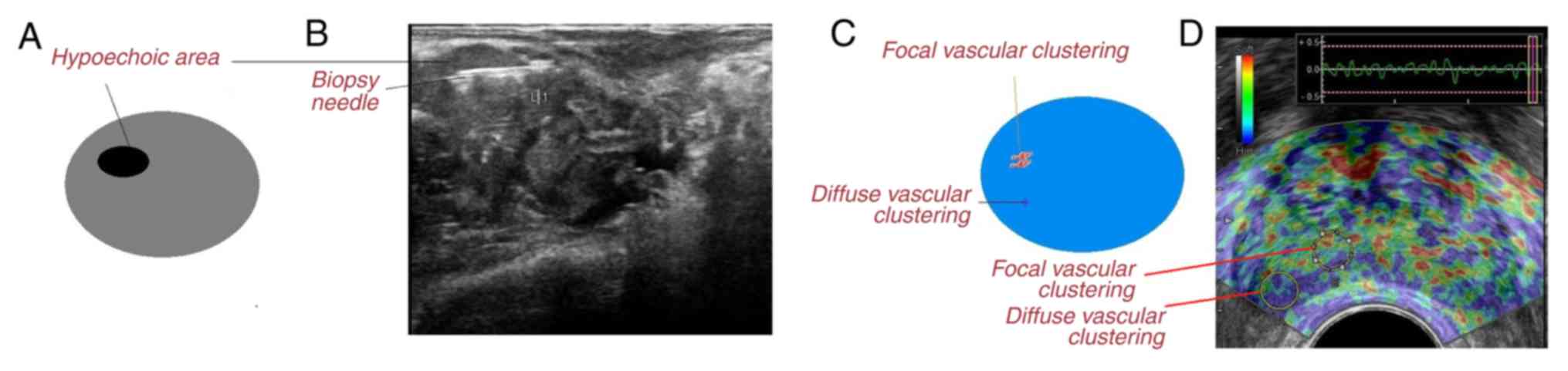
endorectal power Doppler/grayscale ultrasound examinations, a 18 G
biopsy needle (BD Surgical, Inc.) was inserted in the location
where the most intensive signal had been detected and two cores
from the transitional zone, six cores from the peripheral zone, two
cores from the hypoechoic region (darker area in the grayscale
ultrasound image) and two cores from vascular clustering area
(increased number of vessels in power Doppler ultrasound image)
(Fig. 1) were collected (5). A total of 15 physicians (blinded
regarding the mpMRI results, minimum of three years of experience)
of the institute(s) performed the biopsies. Endorectal power
Doppler/grayscale ultrasound-guided biopsies adhered to the
Standards for Reporting of Diagnostic accuracy studies guidelines
(24). To overcome the deformation
of ultrasound images obtained by transrectal ultrasound, the
ultrasound probe was held against the skin without pressure.
mpMRI/TRUS-guided biopsy
In the same setting as for mpMRI, biopsies were
performed using 18G needle Tru-cut Biopsy Guns (cat. no. 661830,
Biocore II BR; Histo, S.A.) under guidance of ultrasound (F31;
Hitachi Aloka Medical Ltd.) with a 7.5-MHz endorectal probe
(Hitachi Aloka Medical Ltd.). A total of 14 cores were randomly
collected from the base, the middle third part, apex (medial and
lateral parts) and each side of the transition zone of the
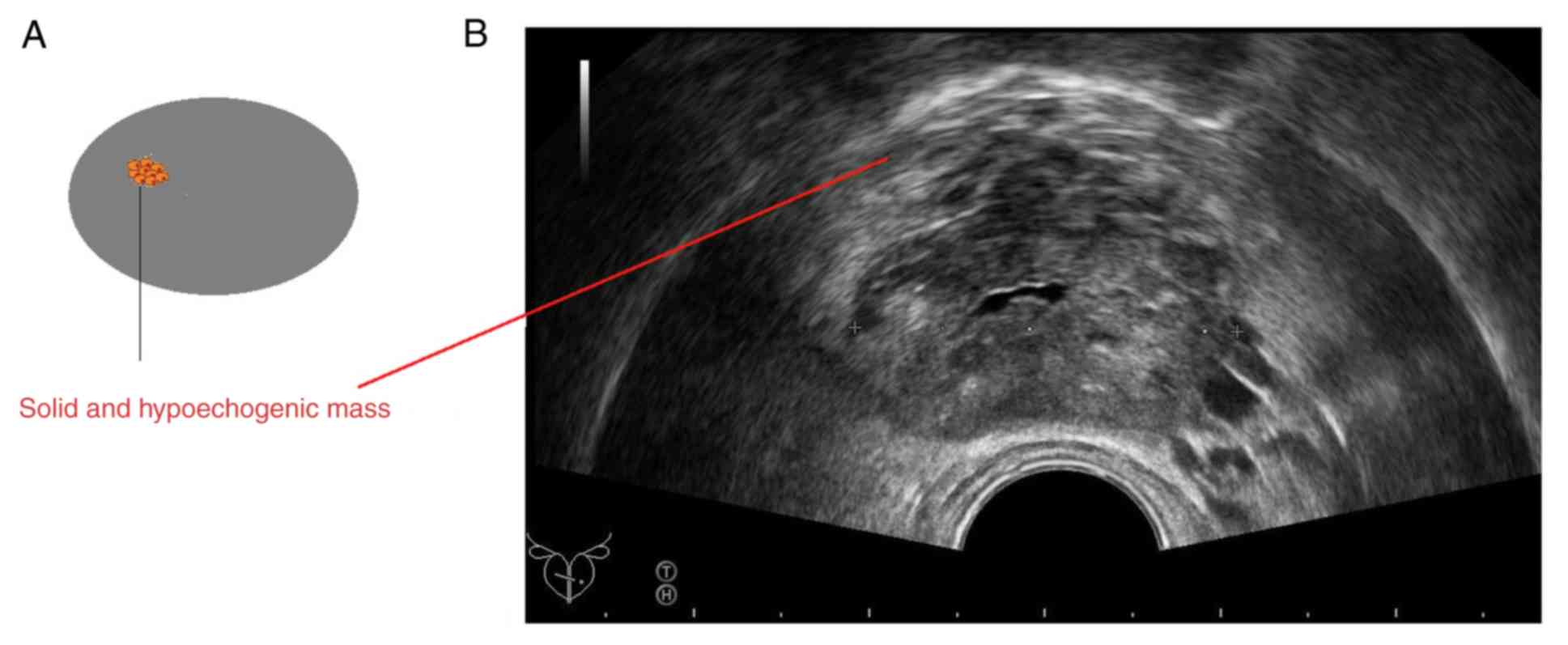
prostate. In addition, two cores were collected from solid and
hypoechogenic areas (denser mass than usual on the grayscale
ultrasound image; Fig. 2) (21). A total of 15 physicians (minimum
three years of experience, blinded regarding endorectal power
Doppler/grayscale ultrasound-guided biopsies) of the institute(s)
performed the biopsies. The mpMRI/TRUS guided biopsies adhered to
the guidelines of the Standards of Reporting for MRI-Targeted
biopsy studies consortium (25).
For prophylactic purposes, all patients had been
prescribed levofloxacin 500 mg twice a day for three days prior to
the biopsies and all patients had received a cleansing enema prior
to the examinations (26). To
control pain, a rectal lidocaine jelly was applied 30 min prior to
the biopsies and afterwards, oral 50 mg diclofenac with 500 mg
paracetamol was prescribed twice a day for two days (5). Anticholinergic drugs (oxybutynin, 5 mg
orally 2–3 times a day) were given to the patients prior to the
biopsies to overcome difficulties in diagnosis due to reactions
including pelvic muscle pressure and pelvic muscle contraction.
Pathological analysis
The biopsy lesions were preserved in 10% formalin
and sent to a laboratory for histopathology purposes. All samples
were paraffin-embedded and slides were prepared. The slides were
stained with hematoxylin and eosin. Each slide was examined under a
light microscope (Olympus) by urologic pathologists. A total of 13
pathologists (minimum three years of experience) of the
institute(s) examined the histopathological results. Each
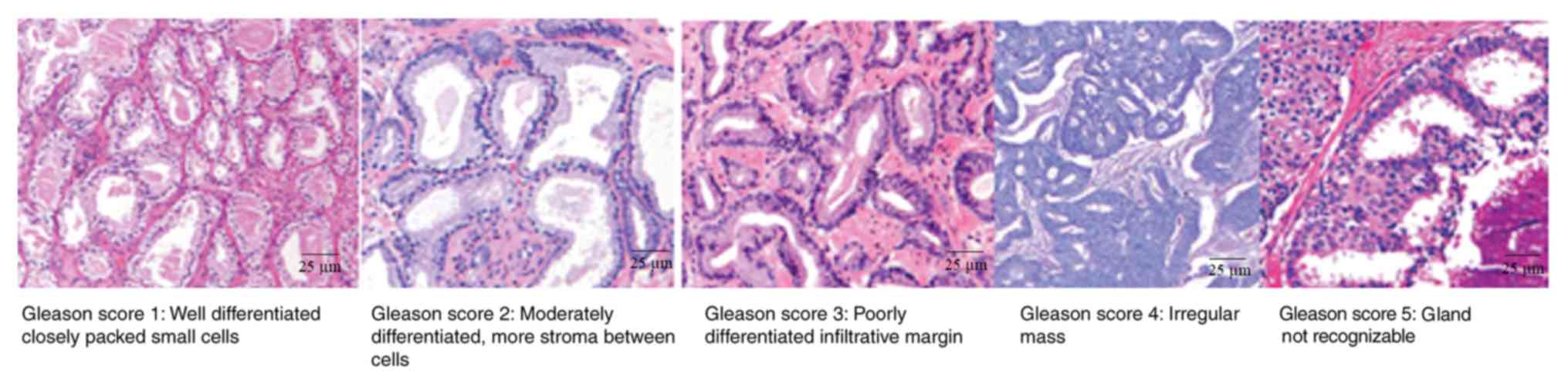
histopathological specimen was assigned primary and secondary
Gleason scores (Fig. 3), numbers of
cores positive for cancer and the percentage of cores with cancer
(21,27). Samples with Gleason scores ≥3+4
(defined as most of the tumor being of grade 3 and the next-largest
section of the tumor being grade 4) were considered as prostate
cancer (21,28).
Robotic-assisted laparoscopic radical
prostatectomy
After obtaining histopathological results of the two
types of biopsies, the urologists performed robotic-assisted
laparoscopic radical prostatectomy in males aged <70 years with
Gleason scores of ≥3+4 in any one of the biopsy reports (29). During the surgical procedure, the
prostate and surrounding tissues were removed (9). A total of 17 urologists (minimum three
years of experience) of the institute(s) performed the
prostatectomy. A single-surgeon approach was used. Details on
prostatectomy were reported in line with the Strengthening the
Reporting of Cohort Studies in Surgery criteria (30).
Histopathology of the surgical
specimen
Sampling of the resected prostate was performed
using the Stanford technique. Transverse (4–5 mm) and sagittal (6–7
mm) sections from apex to base were taken and subjected to
pathological examination (9).
Gleason scores were recorded (21,27).
Specimens with Gleason scores of ≥3+4 were considered as prostate
cancer (21,28). A total of 10 pathologists (minimum
three years of experience; blinded regarding radiological
examinations) of the institute(s) examined the histopathological
results.
Prostatectomy decision analysis
Decision curve analysis was performed to evaluate
the prostatectomy decision in the subjects enrolled as per Eq. i
(31):
LesionswithaccurateGleasoncores≥3+4presentMenincludedinthepathology-(LesionswithfalseGleasoncores≥3+4presentMenincludedinthepathology×LevelofdiagnosticconfidenceabovewhichGleasoncores≥3+4present1-LevofdiagnosticconfidenceabovewhichGleasoncores≥3+4present)(i)
Statistical analysis
InStat Software for Windows (version 3.0; GraphPad
Inc.) was used for statistical analysis. Differences between groups
in discrete variables were analyzed using Fisher's exact test
(21). Continuous data are presented
as the mean ± standard deviation. Linear weighted k coefficients
were determined to evaluate interobserver agreement (poor
agreement, k ≤0.40; moderate agreement, 0.4> k ≤0.60; and
substantial agreement, k >0.60) (22). All results were considered
significant at a confidence level of 99%.
Results
Patients
Among included patients, 52 patients had an age of
<40 years, 18 had confirmed prostate cancer, 26 had negative
biopsy reports in the past 6 months, 11 had normal
prostate-specific antigen values and 14 had normal rectal
examinations, and were therefore excluded from the analysis.
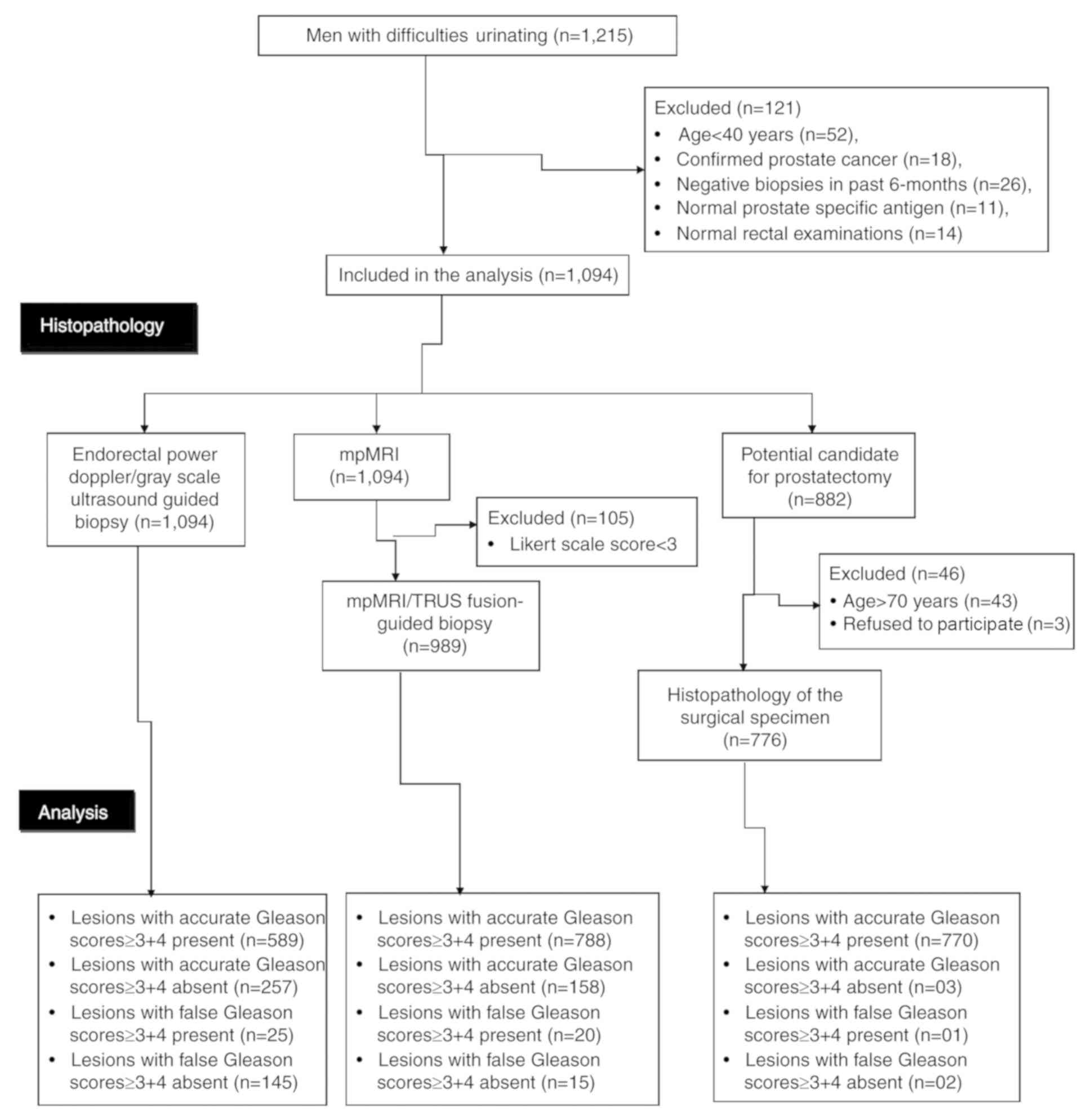
Finally, the data of 1,094 subjects were included in the study. The
flow diagram of the study is presented in Fig. 4.
Medical history and demographics
The demographic parameters of the cohort are
provided in Table I. Among the
patients (age range, 41–91 years; mean age: 69.45±8.47 years)
enrolled, 90% were Han Chinese, 9% were Mongolians and 1% were
Tibetans. Furthermore, 48% of the subjects had a body mass index of
<25 kg/m2, 33% of 25–30 kg/m2 and 19% of
>30 kg/m2. A total of 293 patients were obese
(definition, waist circumference >490 cm). In addition, 32% of
the subjects had diabetes and 18% had hypertension.
 | Table I.Demographical characteristics of the
male patients enrolled (n=1,094). |
Table I.
Demographical characteristics of the
male patients enrolled (n=1,094).
| Characteristic | Value |
|---|
| Age (years) |
|
|
Range | 41–91 |
| Mean ±
SD | 69.45±8.47 |
| Ethnicity |
|
| Han
Chinese | 983 (90) |
|
Mongolian | 102 (9) |
|
Tibetan | 9 (1) |
| Serum
prostate-specific antigen (ng/ml) |
|
| 40–49
years | 7.12±1.01 |
| 50–59
years | 9.01±1.22 |
| 60–69
years | 10.22±1.55 |
| ≥70
years | 11.19±1.89 |
| Body mass index
(kg/m2) |
|
|
<25 | 523 (48) |
|
25–30 | 359 (33) |
|
>30 | 212 (19) |
|
Diabetesa | 347 (32) |
|
Hypertensionb | 201 (18) |
| Manual rectal
examination |
|
|
Hardness | 414 (38) |
|
Lumps | 343 (31) |
|
Enlarged prostate | 337 (31) |
| Central
obesity |
|
| No | 801 (73) |
|
Yes | 293 (27) |
| Alcohol abuse | 45 (4) |
| Chronic urinary
tract infection | 12 (1) |
Pathological analysis
By using endorectal power Doppler/grayscale
ultrasound-guided biopsies following pathological analysis, Gleason
scores of ≥3+4 were determined in 589 patients. On mpMRI, lesions
were identified in 105 subjects with a Likert scale score of <3.
Therefore, the other 989 patients were subjected to
mpMRI/TRUS-guided biopsies. mpMRI/TRUS-guided biopsies followed by
pathological analysis revealed Gleason scores of ≥3+4 in 808
patients. Among those subjects with a Likert scale score of <3
(n=105) who were not subjected to mpMRI/TRUS-guided biopsy, 14 had
reported Gleason scores of ≥3+4 determined from endorectal power
Doppler/grayscale ultrasound-guided biopsies. Therefore, a total of
822 patients had Gleason scores of ≥3+4 in either of the biopsy
reports. Among them, 43 patients had an age of ≥70 years (as per
institutional guidelines for surgery). Therefore, the urologists
did not offer them any prostatectomy and 3 further patients refused
to undergo surgery. Finally, a total of 776 subjects underwent
prostatectomy and all resected prostates were subjected to
pathological analysis (Fig. 5).
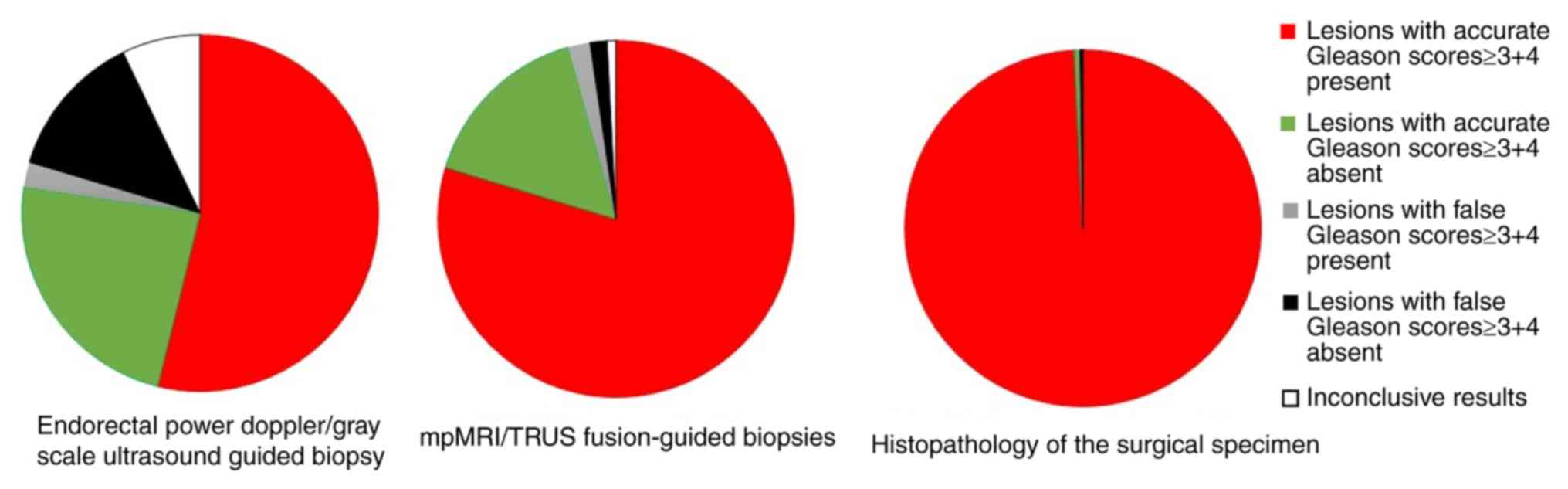
Diagnostic parameters
Endorectal power Doppler/grayscale ultrasound-guided
biopsy had a lower sensitivity than histopathology of the surgical
specimen (0.93 vs. 1.00, P<0.0001) but mpMRI/TRUS-guided biopsy
had the same sensitivity as that of the histopathology of the
surgical specimen (0.99 vs. 1.00, P=0.02) and negligible
inconclusive results (8 vs. 0, P=0.024). Accuracy was in the order
of histopathology of the surgical specimen
(1.00)>mpMRI/TRUS-guided biopsy (0.944)>endorectal power
Doppler/grayscale ultrasound-guided biopsy (0.783). However,
compared with the histopathological examination of the surgical
specimen, the combined results of the two biopsies exhibited
insignificant false-negative results (P=0.012), inconclusive
results (P=0.024) and sensitivity (P=0.024) but higher accuracy
(Table II).
 | Table II.Diagnostic parameters determined with
various pathological methods. |
Table II.
Diagnostic parameters determined with
various pathological methods.
| Parameters | Either biopsy
results (n=1,094 subjects) | Histopathology of
the surgical specimen (n=776) | P-value |
|---|
| Lesions with
accurate Gleason scores ≥3+4 present | 808 (74) | 770 (99.2) | <0.0001 |
| Lesions with
accurate Gleason scores ≥3+4 absent | 249 (22) | 03 (0.4) | <0.0001 |
| Lesions with false
Gleason scores ≥3+4 present | 14 (1) | 01 (0.1) | 0.006 |
| Lesions with false
Gleason scores ≥3+4 absent | 15 (2)a | 02 (0.3) | 0.012 |
| Inconclusive
results | 8 (1)a | 00 (0) | 0.024 |
| Accuracy | 0.964 | 1 | <0.0001 |
| Sensitivity | 0.99a | 1 | 0.024 |
Interobserver agreement
Compared with final results adopted, all evaluations
had a moderate linear-weighted agreement (0.4> k ≤0.60; Table III).
 | Table III.Interobserver agreement for the
different evaluation methods. |
Table III.
Interobserver agreement for the
different evaluation methods.
| Parameter | Endorectal power
doppler/grayscale ultrasound-guided biopsies | mpMRI | mpMRI/TRUS-guided
biopsies | Pathological
analysis |
|---|
| Adhered
guideline | STARD | Likert scale
score | START | Stanford
technique |
| Evaluators (n) | 15 | 5 | 15 | 13 |
| k value | 0.51 | 0.53 | 0.56 | 0.49 |
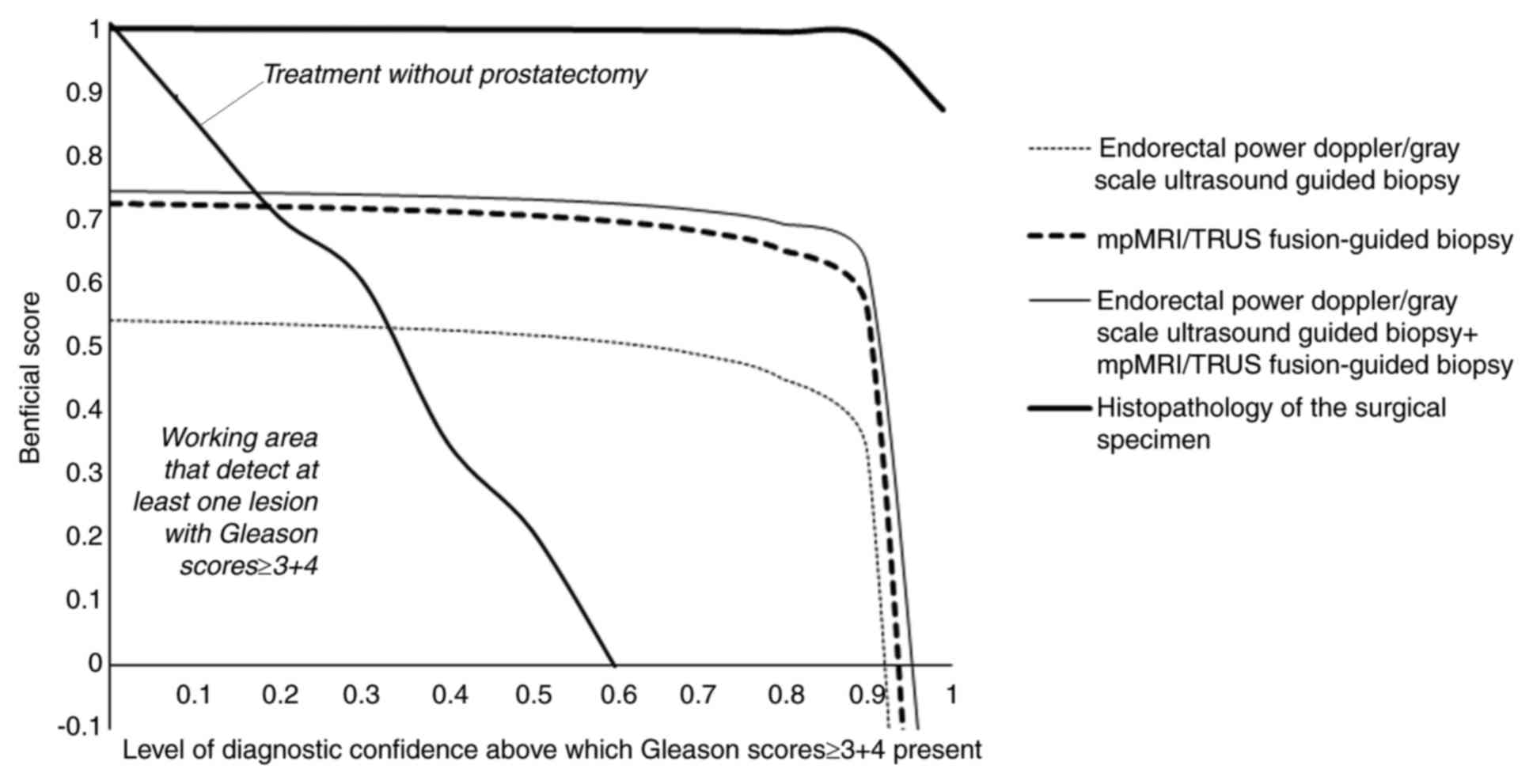
Prostatectomy decision analysis
The working area that detects at least one lesion
with Gleason scores ≥3+4 for endorectal power Doppler/grayscale
ultrasound-guided biopsies, mpMRI/TRUS-guided biopsies, and
combinations of endorectal power Doppler/grayscale
ultrasound-guided biopsies and mpMRI/TRUS-guided biopsies was
0.330–0.920, 0.180–0.935 and 0.170–0.965, respectively. Above
0.920, 0.935 and 0.965, endorectal power Doppler/grayscale
ultrasound-guided biopsies, mpMRI/TRUS-guided biopsies and
combinations of endorectal power Doppler/grayscale
ultrasound-guided biopsies and mpMRI/TRUS-guided biopsies had the
risk of overdiagnosis (Fig. 6).
Discussion
The present study indicated that mpMRI/TRUS-guided
biopsies had a higher working area that detects Gleason scores ≥3+4
at least one time in the collected lesion. In addition, the same
sensitivity, negligible inconclusive results and high accuracy to
those of the histopathology analysis of the surgical specimen were
obtained. The results of the study were in line with those of
previous studies (8,9,11–13,15,19,21,23).
mpMRI/TRUS-guided biopsy promises the detection of clinically
significant prostate cancer(s).
The negative predictive value of mpMRI/TRUS-guided
biopsy was then assessed. During the study, 105 (10%) of the
patients had a Likert scale score of <3 determined by mpMRI and
mpMRI/TRUS-guided biopsies identified lesions with Gleason scores
<3+4 in 173 (16%) of patients but they had abnormal rectal
examinations and elevated prostate-specific antigen. In addition,
among the patients with a Likert scale score of <3, 14 had
Gleason scores of ≥3+4 determined by endorectal power
Doppler/grayscale ultrasound-guided biopsies. Furthermore, analysis
of the mpMRI/TRUS-guided biopsies of the lesions of 20 patients
gave a false-positive result, as no prostate cancer was detected by
histopathological analysis of the surgical specimen. Seminal
vesicle invasion may be more precisely detected by histopathology
compared with mpMRI (32). Low-grade
tumors are isointense and not detected on T2WI. In addition, tumors
in the transition zone are more difficult to detect than those in
the peripheral zone (33).
Post-irradiation tissue damage, scars, atrophy and prostatitis may
also be assumed to be prostate cancer on mpMRI (32). The results of the present study
indicate a moderate performance of mpMRI/TRUS-guided biopsy in the
detection of prostate cancer.
Compared to histopathology of the surgical specimen,
endorectal power Doppler/grayscale ultrasound-guided biopsies had a
moderate working area that detects Gleason scores ≥3+4 at least one
time in the collected lesions, lower sensitivity and accuracy, and
a higher number of inconclusive results (78; 7%) and false-negative
results (145; 13%). The results of the present study were in line
with those of previous studies (5,16,34,35).
Power Doppler US is not able to differentiate cancerous vascular
clustering lesions from hypervascular inflammation (5). Endorectal power Doppler/grayscale
ultrasound-guided biopsy appears to have a barely sufficient
diagnostic performance in the detection of prostate cancer.
According to the present results, combination of the
two biopsies provided a sensitivity of 0.99, accuracy of 0.964, the
highest working area that detects at least one lesion with Gleason
scores ≥3+4, as well as insignificant false-negative lesions and
inconclusive results compared with the results of the
histopathology of the surgical specimen. However, the Chinese
Guidelines on Urologic Diseases in the newest 2014 version do not
recommend this combination of these two biopsies for detection of
prostate cancer (36). The next
version of the Chinese Guidelines on Urologic Diseases requires to
be updated.
Of note, the present study has several limitations.
For instance, survival data during the follow-up were not reported.
Furthermore, there was a lack of randomization. In addition, the
treatment strategies for 46 patients, to whom the urologists had
not offered any prostatectomy (n=43), those who refused to undergo
surgery (n=3), and those with a low and intermediate risk, were not
included. Two types of biopsy were performed, which was not
recommended in a clinical setting. In addition, the results of
Chinese populations may not be comparable to those from other
regions of the world.
In conclusion, mpMRI/TRUS-guided biopsy was
indicated to have a moderate performance and endorectal power
Doppler/grayscale ultrasound-guided biopsy had a scant performance
for decision-making regarding prostatectomy. It is recommended that
the health department of the P.R. China releases a new algorithm
for the detection of prostate cancer in Chinese males in its new
Guidelines on Urologic Diseases.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have reviewed and approved the
manuscript submitted for publication. ZH was project administrator
and contributed to the data curation, literature review and
supervision of the study. ZY contributed to the conceptualization,
literature review and resources of the study. LL contributed to the
conceptualization, validation and literature review included in the
study. XX contributed to the resources, data curation, formal
analysis, literature review, and editing of the manuscript for
intellectual content. JY contributed to the formal analysis,
software processing and literature review of the study. WH
contributed to the investigation, literature review, software and
supervision of the study. JC contributed to the supervision,
validation, formal analysis and literature review included in the
study. YK contributed to the formal analysis and literature review,
as well as drafting reviewing and editing of the manuscript for
intellectual content. The authors agreed to be accountable for all
aspects of work ensuring integrity and accuracy.
Ethical approval and consent to
participate
The protocol of the study (DPH/CL/04/15 dated 12
January 2013) was approved by the review board of Southern Medical
University. The study adhered to China, Strengthening the Reporting
of Observational studies in Epidemiology statement and the 2008
Helsinki Declaration. An informed consent form had been signed by
each of the patients enrolled prior to participation regarding
pathology, radiology, biopsies, surgeries (if required), and to
undergo an additional procedure for research purposes only.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Egidi MG, Cochetti G, Serva MR, Guelfi G,
Zampini D, Mechelli L and Mearini E: Circulating microRNAs and
kallikreins before and after radical prostatectomy: Are they really
prostate cancer markers? Biomed Res Int. 2013:2417802013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan J, Xue W, Sha J, Yang H, Xu F, Xuan H,
Li D and Huang Y: Incidental prostate cancer at the time of
cystectomy: The incidence and clinicopathological features in
Chinese patients. PLoS One. 9:e944902014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong MC, Goggins WB, Wang HH, Fung FD,
Leung C, Wong SY, Ng CF and Sung JJ: Global incidence and mortality
for prostate cancer: Analysis of temporal patterns and trends in 36
countries. Eur Urol. 70:862–874. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kahraman T, Cubuk R, Sinanoglu O, Tasalı
N, Ozarar M and Saydam B: Comparison of power Doppler ultrasound
with gray scale transrectal ultrasound in predicting cancer
positive prostate biopsy cores. Eurasian J Med. 42:81–85. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guelfi G, Cochetti G, Stefanetti V,
Zampini D, Diverio S, Boni A and Mearini E: Next generation
sequencing of urine exfoliated cells: An approach of prostate
cancer microRNAs research. Sci Rep. 8:71112018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Ma M, Nan X and Sheng B: Obesity
inversely correlates with prostate-specific antigen levels in a
population with normal screening results of prostate cancer in
northwestern China. Braz J Med Biol Res. 49(pii):
S0100–879X2016000800704. 2016.
|
|
8
|
Kaufmann S, Kruck S, Kramer U, Gatidis S,
Stenzl A, Roethke M, Scharpf M and Schilling D: Direct comparison
of targeted MRI-guided biopsy with systematic transrectal
ultrasound-guided biopsy in patients with previous negative
prostate biopsies. Urol Int. 94:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baco E, Ukimura O, Rud E, Vlatkovic L,
Svindland A, Aron M, Palmer S, Matsugasumi T, Marien A, Bernhard
JC, et al: Magnetic resonance imaging-transectal ultrasound
image-fusion biopsies accurately characterize the index tumor:
Correlation with step-sectioned radical prostatectomy specimens in
135 patients. Eur Urol. 67:787–794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cochetti G, Poli G, Guelfi G, Boni A,
Egidi MG and Mearini E: Different levels of serum microRNAs in
prostate cancer and benign prostatic hyperplasia: Evaluation of
potential diagnostic and prognostic role. Onco Targets Ther.
9:7545–7553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Wang W, Zhang B, Shi J, Fu Y, Li
D, Guo S, Zhang S, Huang H, Jiang X, et al: Comparison of free-hand
transperineal mpMRI/TRUS fusion-guided biopsy with transperineal
12-core systematic biopsy for the diagnosis of prostate cancer: A
single-center prospective study in China. Int Urol Nephrol.
49:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siddiqui MM, Rais-Bahrami S, Turkbey B,
George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL,
Linehan WM, et al: Comparison of MR/ultrasound fusion-guided biopsy
with ultrasound-guided biopsy for the diagnosis of prostate cancer.
JAMA. 313:390–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borkowetz A, Platzek I, Toma M, Laniado M,
Baretton G, Froehner M, Koch R, Wirth M and Zastrow S: Comparison
of systematic transrectal biopsy to transperineal magnetic
resonance imaging/ultrasound-fusion biopsy for the diagnosis of
prostate cance. BJU Int. 116:873–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pepe P, Garufi A, Priolo G and Pennisi M:
Can MRI/TRUS fusion targeted biopsy replace saturation prostate
biopsy in the re-evaluation of men in active surveillance? World J
Urol. 34:1249–1253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lian H, Zhuang J, Wang W, Zhang B, Shi J,
Li D, Fu Y, Jiang X, Zhou W and Guo H: Assessment of free-hand
transperineal targeted prostate biopsy using multiparametric
magnetic resonance imaging-transrectal ultrasound fusion in Chinese
men with prior negative biopsy and elevated prostate-specific
antigen. BMC Urol. 17:522017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ezquer A, Ortega Hrescak MC, Sanagua C,
Roggia-Rebullida P, López R, Cenice F, Veglia FH, Veglia F and
Fernández A: Transrectal doppler ultrasound during prostate biopsy:
Clinical utility and limitations. Actas Urol Esp. 39:13–19.
2015.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cochetti G, Boni A, Barillaro F, Pohja S,
Cirocchi R and Mearini E: Full neurovascular sparing
extraperitoneal robotic radical prostatectomy: Our experience with
PERUSIA technique. J Endourol. 31:32–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boni A, Cochetti G, Del Zingaro M,
Paladini A, Turco M, Rossi de Vermandois JA and Mearini E: Uroflow
stop test with electromyography: A novel index of urinary
continence recovery after RARP. Int Urol Nephrol. 51:609–615. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu G and Wang Q: Comparisons between
magnetic resonance/ultrasound fusion-guided biopsy and standard
biopsy in the diagnosis of prostate cancer: A prospective cohort
study. Medicine (Baltimore). 97:e119622018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang M, Lin Y, Xu A, Uhlman M, Deng X,
Lin X, Wu S, Diao P, Xie K and Tang P: Percent free
prostate-specific antigen does not improve the effectiveness of
prostate cancer detection in Chinese men with a prostate-specific
antigen of 2.5–20.0 ng/ml: A multicenter study. Med Oncol.
31:9252014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mariotti GC, Falsarella PM, Garcia RG,
Queiroz MRG, Lemos GC and Baroni RH: Incremental diagnostic value
of targeted biopsy using mpMRI-TRUS fusion versus 14-fragments
prostatic biopsy: A prospective controlled study. Eur Radiol.
28:11–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Equator Network, . The Standards for
Reporting of Diagnostic accuracy studies guidelines. http://www.equator-network.org/reporting-guidelines/stard/September
6–2019
|
|
23
|
Barentsz JO, Richenberg J, Clements R,
Choyke P, Verma S, Villeirs G, Rouviere O, Logager V and Fütterer
JJ; European Society of Urogenital Radiology, : ESUR prostate MR
guidelines 2012. Eur Radiol. 22:746–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosenkrantz AB, Kim S, Lim RP, Hindman N,
Deng FM, Babb JS and Taneja SS: Prostate cancer localization using
multiparametric MR imaging: Comparison of prostate imaging
reporting and data system (PI-RADS) and Likert scales. Radiology.
269:482–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Costa DN, Lotan Y, Rofsky NM, Roehrborn C,
Liu A, Hornberger B, Xi Y, Francis F and Pedrosa I: Assessment of
prospectively assigned Likert scores for targeted magnetic
resonance imaging-transrectal ultrasound fusion biopsies in
patients with suspected prostate cancer. J Urol. 195:80–87. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moore CM, Kasivisvanathan V, Eggener S,
Emberton M, Fütterer JJ, Gill IS, Grubb Iii RL, Hadaschik B, Klotz
L, Margolis DJ, et al: Standards of reporting for MRI-targeted
biopsy studies (START) of the prostate: Recommendations from an
International working group. Eur Urol. 64:544–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dell'atti L: Prostatic abscess after
transrectal ultrasound-guided prostate biopsy. Case report. G Chir.
39:260–262. 2013.
|
|
28
|
Ozok HU, Sagnak L, Tuygun C, Oktay M,
Karakoyunlu N, Ersoy H and Alper M: Will the modification of the
Gleason grading system affect the urology practice? Int J Surg
Pathol. 18:248–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Labanaris AP, Witt JH and Zugor V:
Robotic-assisted radical prostatectomy in men ≥75 years of age.
Surgical, oncological and functional outcomes. Anticancer Res.
32:2085–2089. 2012.PubMed/NCBI
|
|
30
|
Agha RA, Borrelli MR, Vella-Baldacchino M,
Thavayogan R and Orgill DP; STROCSS Group, : The STROCSS statement:
Strengthening the reporting of Cohort studies in surgery. Int J
Surg. 46:198–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fitzgerald M, Saville BR and Lewis RJ:
Decision curve analysis. JAMA. 13:409–410. 2015. View Article : Google Scholar
|
|
32
|
Bjurlin MA, Mendhiratta N, Wysock JS and
Taneja SS: Multiparametric MRI and targeted prostate biopsy:
Improvements in cancer detection, localization, and risk
assessment. Cent European J Urol. 69:9–18. 2016.PubMed/NCBI
|
|
33
|
Tan CH, Hobbs BP, Wei W and Kundra V:
Dynamic contrast-enhanced MRI for the detection of prostate cancer:
Meta-analysis. Am J Roentgenol. 204:W439–W448. 2015. View Article : Google Scholar
|
|
34
|
Sohail SK, Sarfraz R, Imran M, Khan NA and
Yusuf NW: Power doppler ultrasonography guided and random prostate
biopsy in prostate cancer diagnosis-a comparative study. J Pak Med
Assoc. 65:65–68. 2015.PubMed/NCBI
|
|
35
|
Delgado Oliva F, Arlandis Guzman S,
Bonillo García M, Broseta Rico E and Boronat Tormo F: Diagnostic
performance of power doppler and ultrasound contrast agents in
early imaging-based diagnosis of organ-confined prostate cancer: Is
it possible to spare cores with contrast-guided biopsy? Eur J
Radiol. 85:1778–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Na YQ, Ye ZQ, Sun YH and Sun G: Chinese
Guidelines on Urologic DiseasesPR China Helath Department. People's
Medical Publishing House; Beijing: 2015, (In Chinese).
|