Introduction
Pulmonary thromboembolism (PTE) is characterized by
pulmonary circulation dysfunction and respiratory dysfunction,
which is caused by a thrombus from the venous system or right
heart, blocking the pulmonary artery or its branches. Due to its
high morbidity and mortality, it has become the third most common
cause of death among patients with cardiovascular disease (1).
Anti-coagulant treatment, including the use of
low-molecular-weight heparin or fondaparinux, has been suggested
for patients with a high or intermediate clinical probability of
acute PTE, unless contraindicated, e.g. by severe renal impairment,
high bleeding risk, arterial hypotension or extremes in body weight
and age (2). However, in cases of
high-risk pulmonary embolism, based on the evaluation of the risk
of early pulmonary embolism-associated death, treatments to break
down or remove the thrombus should be considered (3). For hemodynamically unstable patients
with confirmed high-risk PTE, the guidelines suggest immediate
thrombolysis treatment (2). Surgical
embolectomy or catheter-based thrombus removal are becoming more
common. However, even with improved safety and the development of
better techniques, its application remains restricted to patients
with thrombolysis treatment that is absolutely contraindicated or
has failed (3).
For patients with acute high-risk pulmonary
embolism, thrombolytic therapy may, relieve symptoms and reduce the
requirement for mechanical ventilation. Hence, thrombolytic therapy
improves patient outcomes by reducing right-ventricular injury,
improving exercise tolerance and preventing the recurrence of
pulmonary embolism, thereby improving the survival rate (4). The validated regimens for thrombolytic
therapy include urokinase, streptokinase and recombinant tissue
plasminogen activator (alteplase) (5). However, at present, there is no
preferred thrombolytic method, since these different regimens have
similar outcomes (5). Reteplase is a
third-generation thrombolytic drug that has yet to be fully
validated for treating PTE. Reteplase is also a derivative of
tissue-type plasminogen activator, which has been structurally
altered to retain a strong fibrin-selective thrombolytic effect
(6). This is useful, since fibrin is
one of the major components of a thrombus (7). Furthermore, reteplase has a reduced
binding force with the hepatic scavenger receptor and a prolonged
plasma half-life (6). In addition,
reteplase may be directly administered by intravenous injection,
making its use more convenient (8).
On the basis of the significantly improved blood clot penetration
and enhanced thrombolytic ability of reteplase in vivo
(6), various studies on acute
myocardial infarction have revealed that reteplase has a higher
vascular recanalization rate when compared to alteplase and
streptokinase (9–12).
However, evidence to justify its use in the
treatment of pulmonary embolism is currently limited and it has yet
to be validated for this type of treatment (13). The aim of the present study was to
compare the effectiveness of reteplase with urokinase in treating
high-risk pulmonary embolisms. Hence, the outcomes of patients with
high-risk PTE were treated with reteplase or urokinase were
retrospectively compared.
Materials and methods
Patients and data
All patients who were rescued by the Intensive Care
Unit (ICU) of Weinan Central Hospital of Shaanxi Province (Weinan,
China) between June 2013 and January 2017 were retrospectively
analyzed. A total of 37 patients with acute high-risk pulmonary
embolism were selected for analysis in the present study. Among
these patients, 17 were male and 20 were female. The age of the
reteplase group was 65.50 (age, 60.25–70.50), and the age of the
urokinase group was 65.50 (60.50–70.25). All patients complied with
the diagnostic criteria for high-risk pulmonary embolism of either
the American Heart Association (14)
or the Chinese Society of Cardiology of the Chinese Medical
Association on the diagnosis and management of acute pulmonary
embolism (15).
All patient data were retrieved from the hospital's
database. These data included information from the patient's
medical charts, including age, sex, medical history, treatment
outcome and complications. The criteria for inclusion in the
present study were as follows: i) Adult patients diagnosed with
high-risk PTE and ii) patients who underwent intravenous reteplase
or urokinase thrombolytic therapy. Patients were excluded if they
had any of the following: i) >2 weeks after disease onset; ii)
incomplete data set; iii) contraindications for thrombolytic
treatment (14); iv) other forms of
thrombolytic therapy, e.g. catheter-directed thrombolysis; v)
severe cardiovascular disease and cerebrovascular disease. The
present study conformed to the Declaration of Helsinki and was
approved by the Clinical Ethics Committee of Weinan Central
Hospital (Weinan, China). Patient consent was obtained at the time
of hospitalization.
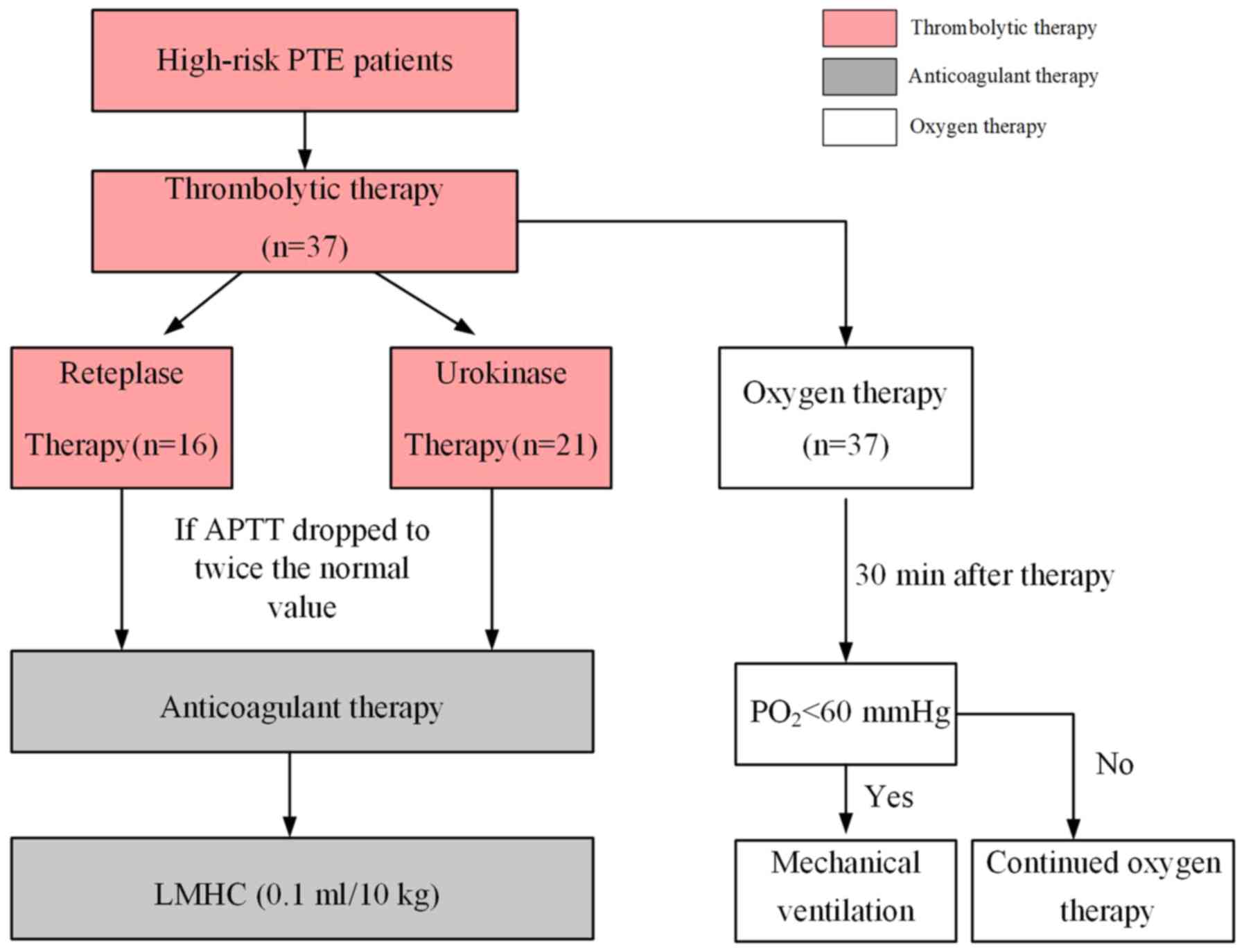
Therapeutic methods
According to the treatment with different
thrombolytic drugs, the subjects were divided into two groups: The
reteplase group and the urokinase group. For each group of
patients, the respective thrombolytic agent was applied according
to the following standard procedures (5,16): A
dose of 18 mg of reteplase (Shandong Ahua Biological Pharmaceutical
Co. Ltd.) was diluted in 20 ml saline and intravenously
administered to patients in the reteplase group for 10 min, while
another dose of 18 mg reteplase was administered to patients after
30 min, resulting in a total dose of 36 mg. Urokinase for injection
(Tianjin Biochem Pharmaceutical Co. Ltd.) was used at a dose of
20,000 U/kg for patients in the urokinase group. This was diluted
in 40 ml saline and intravenously pumped over 2 h.
After the thrombolytic therapy, the clotting ability
was examined every 4 h. For each of the two groups, sequential
anti-coagulant therapy with low-molecular-weight heparin calcium
(dosage, 0.1 ml/10 kg) was started when the activated partial
thromboplastin time dropped to twice the normal value. In addition
to the thrombolytic anti-coagulant therapy, all patients were
treated with oxygen therapy. Oxygen therapy or mechanical
ventilation was performed according to the hypoxia state: Those
patients with a partial pressure of oxygen (PO2) <60
mmHg, which improved to PO2 ≥60 mmHg after 30 min of
oxygen inhalation, were allowed to continue to inhale oxygen;
patients with PO2 <60 mmHg, which remained at
PO2 <60 mmHg after 30 min, were subjected to
mechanical ventilation to achieve sufficient oxygen inhalation. The
process is presented in Fig. 1.
Elevating blood pressure therapy and symptomatic treatment were
provided as required.
Routine blood test, blood clotting series,
assessment of liver and kidney function, D-dimer (D-D), troponin T
(TNT) and pro-B-type natriuretic peptide (pro-BNP), as well as
blood gas analysis were performed prior to thrombolysis. The blood
gas analysis was performed with arterial blood. The other
examinations were performed using venous blood for analysis. D-D
was assessed by latex-enhanced immunoturbidimetry (Beijing Strong
Biotechnologies Inc.), TNT and pro-BNP were assessed by
electrochemiluminescence (F Hoffmann-La Roche Ltd.) and blood gas
analysis was performed using an electrode.
Outcome variables
Arterial systolic blood pressure (SBP), heart rate
(HR) and change in respiratory rate (RR) were recorded prior to
thrombolysis, and at 2, 4, 24 and 48 h after thrombolytic therapy.
The occurrence of complications, including bleeding, was closely
monitored. Furthermore, blood gas, TNT, pro-BNP and D-D were
measured prior to thrombolysis and at 48 h after thrombolysis.
According to the criteria for efficacy determination
in the Chinese Expert Consensus on the Diagnosis and Management of
Acute Pulmonary Embolism 2010 (17),
the improvement of dyspnea was evaluated using six levels: i) Cure:
The dyspnea and other symptoms completely disappeared according to
CT pulmonary angiography, pulmonary perfusion/ventilation scan or
catheter-directed pulmonary angiography; ii) marked improvement:
The dyspnea and other symptoms were obviously relieved or patients
were successfully resuscitated (segments with defective perfusion
decreased by 7–9 or the area of the lung with defective perfusion
was reduced by >75%); iii) partial improvement: The dyspnea and
other symptoms were reduced (the number of segments with defective
perfusion decreased by 1–6 or the area of the lung with defective
perfusion was reduced by 50–75%); iv) no improvement: The dyspnea
and other symptoms exhibited no significant changes; v)
deterioration: The dyspnea and other symptoms were aggravated; vi)
death. The outcomes were independently evaluated by three chief
physicians and one deputy chief physician with more than 10 years
of experience, and discrepancies were resolved by consensus.
Effective thrombolysis was defined as the sum of the cure, marked
improvement and partial improvement rates.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp.). The normality of distribution was evaluated
using the Kolmogorov-Smirnov test. Variables with a normal
distribution were compared using Student's t-test and their values
are expressed as the mean ± standard deviation. Categorical
variables were presented as the frequency (percentage). The data in
Table I were analyzed between groups
using the Chi-square and the t-test. The data in Table II were analyzed between groups using
the Chi-square test. The data in Table
III were compared by two-way analysis of variance (ANOVA),
followed by Sidak's multiple-comparisons test. Two-sided P-values
<0.05 were considered to indicate statistical significance. The
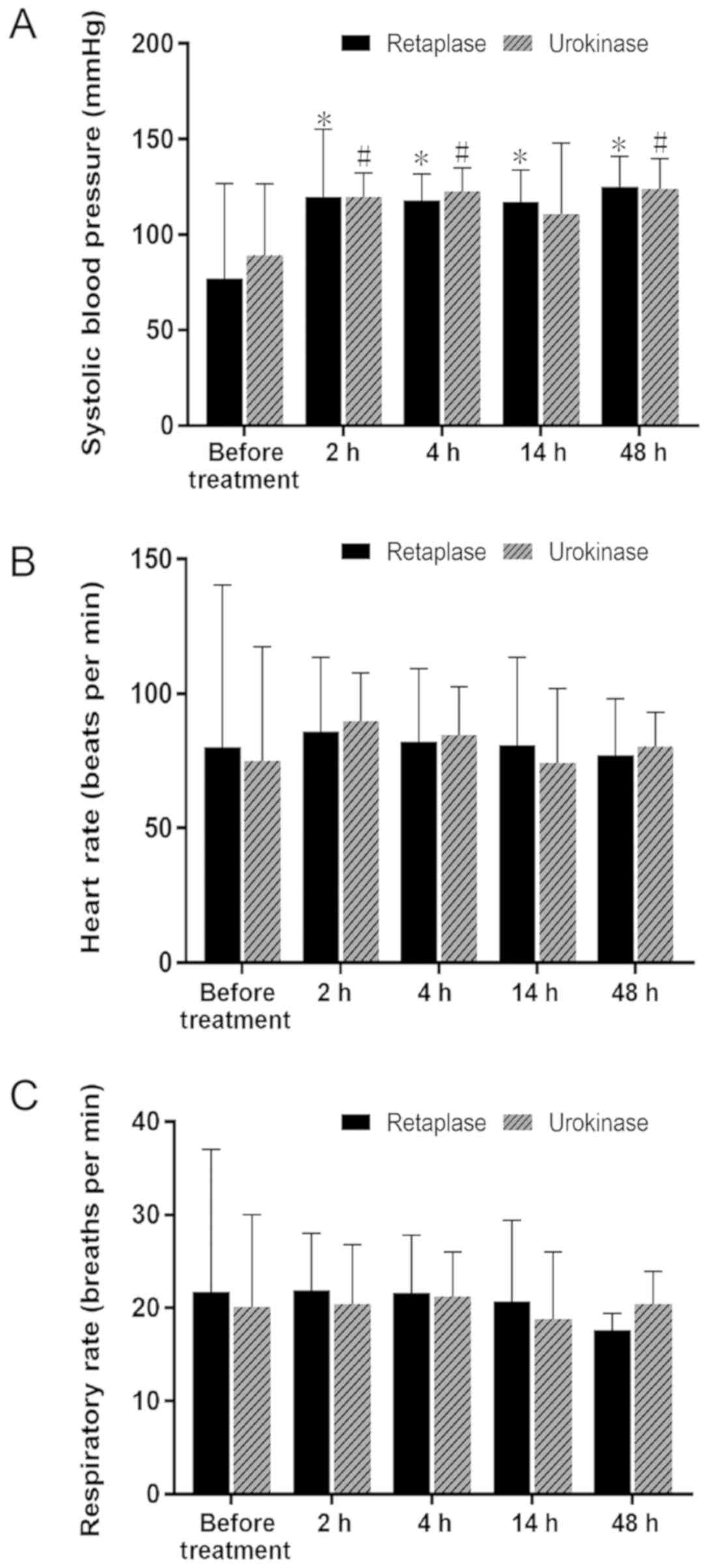
data in Fig. 2 were analyzed using
repeated-measures ANOVA with the time being the within factor and
treatment (reteplase and urokinase) as the between factor.
Bonferroni-adjusted post-hoc comparison was used to identify
differences between paired data.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Parameter | Reteplase (n=16) | Urokinase (n=21) | P-value |
|---|
| Age (years) | 66.8±5.5 | 64.5±12.2 | 0.268 |
| Sex
(male/female) | 6/10 | 12/9 | 0.236 |
| BMI
(kg/m2) | 24.5±1.9 | 24.3±1.9 | 0.391 |
| Previous surgery
prior to the event | 10 (62.5) | 13 (61.9) | 0.970 |
|
Orthopedic surgery | 5 (31.2) | 7 (33.3) |
|
| Cerebral
surgery | 0 | 3 (14.3) |
|
| Chest
surgery | 2 (12.5) | 2 (9.52) |
|
|
Hepatobiliary surgery | 2 (12.5) | 0 |
|
| Breast
surgery | 1 (6.25) | 0 |
|
| Thyroid
surgery | 0 | 1 (4.76) |
|
| SBP (mmHg) | 77.0±49.7 | 89.3±37.2 | 0.264 |
| HR (bpm) | 80.2±60.2 | 75.0±42.4 | 0.410 |
| RR (bpm) | 21.7±15.3 | 20.1±9.9 | 0.389 |
|
PO2/FiO2 (mmHg) | 178.3±62.0 | 167.3±97.0 | 0.388 |
| TNT (ng/l) | 0.11±0.12 | 0.23±0.28 | 0.124 |
| D-D (ng/l) | 13,931±24,282 | 28,129±32,519 | 0.161 |
| Pro-BNP (pg/ml) | 2,488±2,571 | 3,617±4,388 | 0.280 |
| Uric acid
(µmol/l) | 299±100 | 352±106 | 0.085 |
| Blood glucose
(mmol/l) | 6.40±1.70 | 5.91±1.23 | 0.185 |
 | Table II.Comparison of thrombolytic effects
between the reteplase and urokinase groups. |
Table II.
Comparison of thrombolytic effects
between the reteplase and urokinase groups.
| Item | Reteplase
(n=16) | Urokinase
(n=21) | P-value |
|---|
| Thrombolytic
effect |
|
| 0.474 |
|
Cure | 0 | 0 |
|
| Marked
improvement | 11 (68.8) | 13 (61.9) |
|
| Partial
improvement | 4 (25.0) | 6 (28.6) |
|
| No
improvement | 0 | 0 |
|
|
Deterioration | 0 | 0 |
|
|
Death | 1 (6.2) | 2 (9.5) |
|
| Major
bleeding complications | 0 | 1 (4.8) |
|
| Minor
bleeding complications | 6 (37.5) | 2 (9.5) |
|
| Overall effective
rate (%) | 93.8 | 90.5 | >0.999 |
 | Table III.Comparison of
PO2/FiO2, TNT, D-D and pro-BNP prior to and
after treatment (48 h) in the two groups. |
Table III.
Comparison of
PO2/FiO2, TNT, D-D and pro-BNP prior to and
after treatment (48 h) in the two groups.
| Variable | Reteplase
(n=16) | Urokinase
(n=2) | P-value |
|---|
|
PO2/FiO2 (mmHg) |
|
|
|
|
Baseline | 178.3±62.0 | 167.3±97.0 | 0.914 |
| 48
h | 242.6±104.9 |
269.8±80.7a | 0.583 |
| Pro-BNP
(pg/ml) |
|
|
|
|
Baseline | 2,488±2,571 | 3,617±4,388 | 0.473 |
| 48
h | 967±1,067 | 2,673±2,877 | 0.190 |
| TNT (µg/l) |
|
|
|
|
Baseline | 0.111±0.116 | 0.226±0.277 | 0.963 |
| 48
h |
0.016±0.009a | 0.991±2.610 | 0.078 |
| D-D (µg/l) |
|
|
|
|
Baseline | 13,931±24,282 | 28,129±32,519 | 0.080 |
| 48
h | 2,342±1,224 |
5,313±3,547a | 0.889 |
Results
Baseline characteristics
A total of 37 patients were available for analysis
in the present study and the age of these patients ranged from 28
to 86 years (average age, 65.5±11.5 years). Among those patients,
16 were treated with reteplase, while 21 patients were treated with
urokinase. Trauma and bed rest after surgery were the major causes
of pulmonary embolism, accounting for 62.2% of cases. The other
causes were hypertension (8.1%), diabetes (8.1%), cerebral
infarction (5.4%), coronary heart disease (5.4%) and lower
extremity varicose veins (10.8%; data not shown). However, there
was no significant difference in the baseline characteristics
between the two groups (Table
I).
Patient outcomes
As presented in Table
II, the proportion of patients with a marked and partial
improvement was comparable between the two groups. The overall
effective thrombolysis rate was 93.8% (15/16) and 90.5% (19/21) for
the reteplase and urokinase group, respectively, but the difference
was not statistically significant (P>0.999).
The treatment significantly improved the SBP in each
of the two groups, but there was no significant difference between
these two groups prior to and after thrombolytic therapy (Fig. 2A). Furthermore, the difference in HR
and RR of patients in each of these two groups was not
statistically significant prior to and after thrombolytic therapy,
and there was no significant difference between these two groups
(Fig. 2B and C).
There was no significant difference in
PO2/fraction of inspired oxygen (FiO2),
pro-BNP and D-D prior to and after treatment in the reteplase
group, but TNT significantly decreased after treatment (P=0.046).
There was a significant difference in
PO2/FiO2 and D-D prior to vs. after
thrombolysis in the urokinase group (P=0.007 and 0.001,
respectively), but there was no significant difference in TNT and
pro-BNP. Furthermore, there was no significant difference in
PO2/FiO2, pro-BNP, TNT and D-D between the
two groups after thrombolysis. TNT exhibited a downward trend after
thrombolysis in the reteplase group, while TNT exhibited an upward
trend after thrombolysis in the urokinase group (Table III). The difference in the other
indicators was not statistically significant.
Complications during treatment
The hospital stay in the reteplase group was 7.00
(5.25–8.75) days, and the hospital stay in the urokinase group was
8.00 (6.25–10.00) days. In the reteplase group, three patients had
subcutaneous ecchymosis at the puncture site, which was absorbed
without any special treatment. Furthermore, one patient had
epistaxis and errhysis at the puncture site, one patient had
increased bleeding from their drainage tube on the second day after
the operation and one patient had pseudoaneurysm due to a short
compression time after elbow artery puncture, which was performed
prior to thrombolytic therapy. These three complications improved
after enhanced hemostasis by compression. Furthermore, one patient
in the reteplase group had an unsuccessful resuscitation and died.
In the urokinase group, all initial resuscitations were successful.
One patient had epistaxis, while one patient had errhysis at the
puncture site, and these two patients improved after hemostasis by
compression. Furthermore, one patient died of gastric hemorrhage
after 22 h of thrombolysis and one patient died of brain failure
after 11 days of resuscitation. Overall, the occurrence of
complications in the reteplase group was higher when compared with
that in the urokinase group, but no life-threatening bleeding
occurred. However, life-threatening bleeding occurred in the
urokinase group.
Discussion
Pulmonary embolism occurs when a detached thrombus
(embolism) from any part of the venous territory becomes lodged in
the pulmonary artery. Although the origin of the embolism may be
venous thrombosis in any location (upper extremities, prostatic,
uterine and renal veins and right heart chambers), it is a lower
extremity deep vein thrombosis in most cases (90–95%), which is
frequently asymptomatic. High-risk pulmonary embolism, previously
referred to as massive pulmonary embolism, is characterized by
hypotension or shock, accounts for ~5% of cases and is associated
with early mortality in at least 15% of cases. Thrombolytic
treatment is usually recommended for these patients (18).
In recent years, due to the in-depth understanding
and improvement in diagnostic methods for PTE, the number of
diagnoses have rapidly increased. For patients with high-risk
pulmonary embolism, thrombolytic therapy may restore lung perfusion
early, relieve symptoms, reduce the requirement for mechanical
ventilation, reduce right-ventricular injury and improve exercise
tolerance, which effectively prevents the recurrence of pulmonary
embolism and improves the survival rate (1,2,15,18). In
the present study, reteplase and urokinase were used for the
treatment of high-risk pulmonary embolism, and each of the two
drugs achieved good effects by rapidly improving the patient's
subjective symptoms and stabilizing their hemodynamics. Although
the results were similar for the two groups of patients, it was
suggested that the use of reteplase may reduce myocardial damage
and prevent myocardial damage when compared with urokinase, with
fewer major bleeding episodes. Therefore, the present study
provides clinical experience for the further application of
reteplase in patients with acute high-risk pulmonary embolism and
suggests that it may be appropriate to broaden the application
range of reteplase.
The results of the present study indicated that in
terms of effectiveness, there was no significant difference between
reteplase and urokinase. The two groups exhibited high levels of
effectiveness and these results were supported by previous evidence
suggesting that thrombolytic therapy is beneficial for high-risk
PTE patients (4,5) and guidelines that support the use of
thrombolysis in high-risk patients (18,19).
To date, certain studies have revealed significant
differences in outcome among different thrombolytic agents
(5,20–24). In
the present study, biomarkers and clinical parameters were compared
in order to determine whether a difference exists between the
reteplase and urokinase treatment groups. The results revealed only
few significant differences between these two groups in certain
biomarkers and clinical parameters. The improvement of TNT after
thrombolysis was significant in the reteplase group, while the
improvement of PO2/FiO2 and D-D was
significant in the urokinase group, but the other indicators were
not significantly different between the two drug treatment groups.
TNT is a common marker of myocardial damage (25), PO2/FiO2
indicates the level of respiratory distress and D-D is a product of
fibrolysis (26). In general, these
similar results may be due to the small number of patients enrolled
in the present study, preventing the differences from reaching a
significant level. However, it is noteworthy that TNT continued to
exhibit an upward trend after the thrombolytic therapy with
urokinase, while TNT declined in the reteplase group, suggesting
that reteplase may be better for improving myocardial damage.
The major concern with thrombolysis is the
occurrence of complications, the most common of which is major
bleeding (5). In the present study,
complications of bleeding increased after treatment in the
reteplase group, but no fatal bleeding occurred. However, one
patient died of respiratory cardiopulmonary arrest due to the
pulmonary embolism itself, which was not associated with reteplase.
In the urokinase group, complications of fatal bleeding increased
after the treatment. Furthermore, one patient treated with
urokinase developed gastrointestinal hemorrhage and died, while
another patient died of the pulmonary embolism itself (death from
brain failure after respiratory arrest). In the present study, the
complications increased following application of reteplase, which
is similar to previous results (14), but different from the results
reported by Liu et al (27).
The possible reason may be that the present study is a
single-center retrospective study and the sample size was
small.
The present study has certain limitations. First,
the small sample size resulted from the single-center nature of the
study. Data from multiple centers may increase the power of
evidence of the results. In order to reach exact conclusions, the
sample size requires to be increased in future studies. In
addition, the present study was retrospective in nature. Hence, the
patients were not allocated to groups in a randomized manner.
Therefore, this may have introduced a certain level of bias.
Furthermore, due to lack of bedside echocardiography equipment at
the ICU department in the early years, no echocardiographic data
were available for all patients. In future prospective studies by
our group, inclusion of these parameters will be considered.
Furthermore, the patients were not followed up after discharge.
Hence, it was not possible to establish the long-term outcomes for
these patients.
In conclusion, reteplase, a third-generation
thrombolytic drug, has not been previously validated for the
treatment of pulmonary embolism. The results of the present study
suggest that, although reteplase is similar to urokinase in terms
of thrombolytic effectiveness for patients with high-risk pulmonary
embolism, it may also reduce myocardial damage.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LM conceived and designed the study and prepared the
manuscript. YZ, QF and TZ made substantial contributions to
acquisition and interpretation of data. R-YY and XS analyzed the
data. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Ethics Committee of Weinan Central Hospital (Weinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meyer G: Effective diagnosis and treatment
of pulmonary embolism: Improving patient outcomes. Arch Cardiovasc
Dis. 107:406–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalikias G and Konstantinides S: Acute
phase treatment of pulmonary embolism. Curr Vasc Pharmacol.
12:393–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Streiff MB, Agnelli G, Connors JM,
Crowther M, Eichinger S, Lopes R, McBane RD, Moll S and Ansell J:
Guidance for the treatment of deep vein thrombosis and pulmonary
embolism. J Thromb Thrombolysis. 41:32–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marti C, John G, Konstantinides S,
Combescure C, Sanchez O, Lankeit M, Meyer G and Perrier A: Systemic
thrombolytic therapy for acute pulmonary embolism: A systematic
review and meta-analysis. Eur Heart J. 36:605–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone J, Hangge P, Albadawi H, Wallace A,
Shamoun F, Knuttien MG, Naidu S and Oklu R: Deep vein thrombosis:
Pathogenesis, diagnosis, and medical management. Cardiovasc Diagn
Ther. 7 (Suppl 3):S276–S284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marcos-Contreras OA, Ganguly K, Yamamoto
A, Shlansky-Goldberg R, Cines DB, Muzykantov VR and Murciano JC:
Clot penetration and retention by plasminogen activators promote
fibrinolysis. Biochem Pharmacol. 85:216–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadowski M, Ząbczyk M and Undas A:
Coronary thrombus composition: Links with inflammation, platelet
and endothelial markers. Atherosclerosis. 237:555–561. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smalling RW, Bode C, Kalbfleisch J, Sen S,
Limbourg P, Forycki F, Habib G, Feldman R, Hohnloser S and Seals A:
More rapid, complete, and stable coronary thrombolysis with bolus
administration of reteplase compared with alteplase infusion in
acute myocardial infarction. RAPID Investigators. Circulation.
91:2725–2732. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg DG, Levin E, Lausell A, Brown A,
Gardner J, Perez E, Veenendaal M, Ong YS and Gunn M: Feasibility
and timing of prehospital administration of reteplase in patients
with acute myocardial infarction. J Thromb Thrombolysis.
13:147–153. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morrow DA, Antman EM, Sayah A, Schuhwerk
KC, Giugliano RP, deLemos JA, Waller M, Cohen SA, Rosenberg DG,
Cutler SS, et al: Evaluation of the time saved by prehospital
initiation of reteplase for ST-elevation myocardial infarction:
Results of the early retavase-thrombolysis in myocardial infarction
(ER-TIMI) 19 trial. J Am Coll Cardiol. 40:71–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luiz T, Wilhelms A, Madler C, Pollach G,
Haaff B, Grüttner J and Viergutz T: Outcome of out-of-hospital
cardiac arrest after fibrinolysis with reteplase in comparison to
the return of spontaneous circulation after cardiac arrest score in
a geographic region without emergency coronary intervention. Exp
Ther Med. 13:1598–1603. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adivitiy a and Khasa YP: The evolution of
recombinant thrombolytics: Current status and future directions.
Bioengineered. 8:331–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lippi G, Mattiuzzi C and Favaloro EJ:
Novel and emerging therapies: Thrombus-targeted fibrinolysis. Semin
Thromb Hemost. 39:48–58. 2013.PubMed/NCBI
|
|
14
|
Jaff MR, McMurtry MS, Archer SL, Cushman
M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD,
Thistlethwaite P, et al: Management of massive and submassive
pulmonary embolism, iliofemoral deep vein thrombosis, and chronic
thromboembolic pulmonary hypertension: A scientific statement from
the American Heart Association. Circulation. 123:1788–1830. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pulmonary Circulation and Right
Ventricular Function Assembly of Chinese Society of Cardiology of
Chinese Medical Association: Chinese expert consensus on the
diagnosis and management of acute pulmonary embolism (2015).
Zhonghua Xin Xue Guan Bing Za Zhi. 44:197–211. 2016.(In Chinese).
PubMed/NCBI
|
|
16
|
Simpson D, Siddiqui MA, Scott LJ and
Hilleman DE: Reteplase: A review of its use in the management of
thrombotic occlusive disorders. Am J Cardiovasc Drugs. 6:265–285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Department of Pulmonary Vascular Diseases,
Chinese Medical Association Cardiovascular Disease: Chinese expert
consensus on the diagnosis and management of acute pulmonary
embolism. Chin J Intern Med. 49:74–81. 2010.(In Chinese).
|
|
18
|
Konstantinides SV, Torbicki A, Agnelli G,
Danchin N, Fitzmaurice D, Galiè N, Gibbs J, Huisman MV, Humbert M,
Kucher N, et al: Authors/task force members. corrigendum to: 2014
ESC Guidelines on the diagnosis and management of acute pulmonary
embolism. Eur Heart J. 36:26422015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Torbicki A, Perrier A, Konstantinides S,
Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D,
Janssens U, et al: ESC committee for practice guidelines (CPG).
Guidelines on the diagnosis and management of acute pulmonary
embolism: The task force for the diagnosis and management of acute
pulmonary embolism of the European society of cardiology (ESC). Eur
Heart J. 29:2276–2315. 2008.PubMed/NCBI
|
|
20
|
Bloomer TL, El-Hayek GE, McDaniel MC,
Sandvall BC, Liberman HA, Devireddy CM, Kumar G, Fong PP and Jaber
WA: Safety of catheter-directed thrombolysis for massive and
submassive pulmonary embolism: Results of a multicenter registry
and meta-analysis. Catheter Cardiovasc Interv. 89:754–760. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daley MJ, Murthy MS and Peterson EJ:
Bleeding risk with systemic thrombolytic therapy for pulmonary
embolism: Scope of the problem. Ther Adv Drug Saf. 6:57–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long B and Koyfman A: Current
controversies in thrombolytic use in acute pulmonary embolism. J
Emerg Med. 51:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagamalesh UM, Prakash VS, Naidu KCK,
Sarthak S, Hegde AV and Abhinay T: Acute pulmonary thromboembolism:
Epidemiology, predictors, and long-term outcome-A single center
experience. Indian Heart J. 69:160–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldhaber SZ, Kessler CM, Heit J, Markis
J, Sharma GV, Dawley D, Nagel JS, Meyerovitz M, Kim D and Vaughan
DE: Randomised controlled trial of recombinant tissue plasminogen
activator versus urokinase in the treatment of acute pulmonary
embolism. Lancet. 2:293–298. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mannu GS: The non-cardiac use and
significance of cardiac troponins. Scott Med J. 59:172–178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olson JD: D-dimer: An overview of
hemostasis and fibrinolysis, assays, and clinical applications. Adv
Clin Chem. 69:1–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XY, Zhang Y, Li MW, Wang XP, Qi DD,
Hao PY, Zhang H, Cheng QQ, Zhao LS, Gao CY and Hu DY: Efficacy of
thrombolytic therapy using reteplase in cases with acute ST-segment
elevation myocardial infarction: Results from a multicenter
clinical trial. Zhonghua Xin Xue Guan Bing Za Zhi. 44:766–770.
2016.(In Chinese). PubMed/NCBI
|