Introduction
Neuropathic pain is caused by nerve dysfunction or
lesions (1), and is caused by the
response of the central and peripheral nervous systems to the nerve
injury (2). Neuropathic pain is
regarded as a serious public health concern worldwide and results
in poor quality of life for patients (3). Various factors, such as surgery,
trauma, toxicity and congenital disorders, can exacerbate
neuropathic pain (4). However, no
effective treatments have been established for neuropathic pain.
Therefore, it is of great importance to explore the molecular
mechanism of this condition.
MicroRNAs (miRNAs) are a class of small and
non-coding RNA molecules, which serve as post-transcriptional
regulators of gene expression by binding to the 3′-untranslated
region (UTR) of target mRNAs (5).
Numerous studies have suggested that miRNAs are involved in many
biological processes, including cell development, proliferation,
differentiation, apoptosis and the stress response (6–8). A
number of aberrantly expressed miRNAs have been identified in
various types of human cancer (9,10).
Moreover, several miRNAs have recently been reported to participate
in the development of neuropathic pain. For instance, miR-98
suppresses the progression of neuropathic pain by targeting high
mobility group A2 (11). miR-206-3p
acts as an inhibitor of chronic constriction injury-induced
neuropathic pain by regulating the expression of histone
deacetylase 4 (12). miR-28-5p
attenuates the development of neuropathic pain by binding to the
3′-UTR of zinc finger E-box-binding homeobox in vivo
(13). Notably, Lu et al
(14) reported that miR-448 was
differentially expressed in rat cortex following anesthesia with
sevoflurane, suggesting a role for miR-448 in neurological
function. On basis of these results, the present study investigated
the role of miR-448 in the progression of neuropathic pain.
Materials and methods
Animal studies
A total of 62 adult female Sprague-Dawley rats with
weights ranging from 180 to 210 g were purchased from the Shanghai
Animal Laboratory Center. All rats were housed in individual cages
with a constant temperature of 25°C and had free access to food and
water. The health and behavior of the rats were monitored every
day. A rat model of neuropathic pain was established by chronic
constriction injury (CCI) as previously described (15). A total of 30 rats were randomly
divided into the sham group and CCI group (n=15 rats per group).
Rats were anesthetized by the intraperitoneal injection of 40 mg/kg
sodium pentobarbital. The sciatic nerves on both sides were exposed
and separated from the surrounding tissues. Sham-operated rats were
subjected to sciatic nerve exposure and isolation without ligation.
The rats were euthanized on days 0, 3, 7, 14 and 21 following
surgery by the intravenous administration of 140 mg/kg
pentobarbital. Total cardiac arrest, pupil dilation, muscle
flaccidity and absence of reflexes were used to confirm euthanasia.
As the segmental nerves L4-L6 contribute to the sciatic nerve
(16), the dorsal spinal cords of
these nerves were collected immediately following euthanasia.
The experimental procedures for care and use of
animals were approved by the Medical Ethics Committee of Shengli
Oilfield Central Hospital (reference number, 20170016), and were
performed strictly under the requirements of the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health.
Humane endpoints were followed in accordance with the OECD Guidance
Document on the Recognition, Assessment and Use of Clinical Signs
as Humane Endpoints for Experimental Animals Used in Safety
Evaluation (2000), and measures were taken to minimize pain and
discomfort, such as a cut-off time for the pain threshold
assessment.
Cell culture
Rat microglial cells (HAPI) were obtained from the
American Type Culture Collection. All cells were cultured in DMEM
(Lonza Group Ltd.) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin. Cells were incubated in a 5%
CO2 incubator at 37°C. The miR-448 mimic, miR-448
inhibitor and scrambled controls were purchased from Shanghai
GenePharma Co., Ltd. The sequences were as follows: miR-448 mimic,
5′-UUGCAUAUGUAGGAUGUCCCAU-3′; miR-448 mimic NC,
5′-UUCUCCGAACGUGUCACGUTT-3′; miR-448 inhibitor,
5′-AUGGGACAUCCUACAUAUGCAA-3′; miR-448 inhibitor NC,
5′-CAGUACUUUUGUGUAGUACAA-3′. Lipofectamine™ 2000 (Thermo Fisher
Scientific, Inc.) was used to transfect the miRNAs (100 nM) into
HAPI cells according to the manufacturer's protocol. At 48 h
post-transfection, the cells were harvested for the subsequent
experiments.
Lentivirus (LV) production and
infection
Lentiviruses delivering short hairpin (sh) RNAs
against miR-448 [LV-miR-448 knockdown (KD)], sirtuin 1 [SIRT1
(LV-SIRT1 KD)] and their respective scrambled sequences
(LV-miR-scrambled) were purchased from Shanghai GenePharma Co.,
Ltd.
A total of 32 rats were randomly assigned into four
groups: Sham group, CCI + LV-miR-scrambled, CCI + LV-miR-448 KD
group and CCI + LV-miR-KD + SIRT1 KD group (n=8 per group). A PE-10
polyethylene catheter was inserted into the rats to allow the
delivery of the lentiviral vectors. A volume of 10 µl of 0.5%
lidocaine (0.9–1.1 mg/kg) was injected into rats through an
intrathecal catheter. The catheter was confirmed to be successfully
inserted when paralysis of the bilateral hind limbs arose
immediately after anesthesia. Then intrathecal catheter was also
used for lentivirus injection (1×108 PFU/ml, 10 µl). All
rats were euthanized 7 days postoperatively.
RNA extraction and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated from tissues or cells using a
miRNeasy mini kit (Qiagen) according to the manufacturer's
protocol. RT was performed using TaqMan miRNA reverse-transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. qPCR was conducted to detect the
expression levels of miR-448 and SIRT1 using a SYBR Premix Ex Taq™
II commercial kit (Takara Biotechnology Co. Ltd.) and the Applied
Biosystems 7900 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The PCR conditions were as follows: 95°C for 5 min, followed by 40
cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 40 sec and
then a final extension at 72°C for 5 min. mRNA levels were
quantified using the 2−ΔΔCq method and normalized to the
internal reference gene GAPDH or U6 (17). Primers used in this study are as
follows: miR-448, forward, 5′-GCCGAGTTGCATATGTAGGA-3′ and reverse,
5′-ATGCATGCCACGGGCATATACACT-3′; SIRT1, forward,
5′-ATGAAGCACCAACCGTATC-3′ and reverse, 5′-CTGAATTGACCTTGACTGATG-3′;
U6, forward, 5′-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3′ and reverse,
5′-GCTTCACGAATTTGCGTGTCATCCTTGC-3′; GAPDH: forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Pain threshold assessment
Behavioral testing was conducted between 9:00 a.m.
and 4:00 p.m on days 0, 3, 7, 14 and 21 following surgery. The paw
withdrawal threshold (PWT) was used to calculate mechanical
allodynia using a calibrated electronic von Frey filament (0.5×0.5
cm2; cat. no. 2393; IITC Inc. Life Science) on and the
plantar surface of each hind paw. The time taken to elicit the paw
withdrawal response was evaluated. Paw withdrawal latency (PWL) was
used to evaluate the thermal hyperalgesia response to radiant heat
using a thermal paw stimulation system (BME-410C; Institute of
Biomedical Engineering, Chinese Academy of Medical Sciences). The
animals were exposed to heat for 10 sec and the experiment had a
cut-off time of 20 sec regardless of whether the animals reacted or
not. The duration between the start of the stimulus and paw
withdrawal was recorded.
ELISA
The frozen spinal cord tissues (30 mg) were
dissolved in 300 µl RIPA lysis buffer (Beyotime Institute of
Biotechnology) at 4°C for 3 h and the homogenate was then
centrifuged at 12,000 × g for 10 min at 4°C to remove debris. The
protein expression levels of interleukin (IL)-6 (cat. no. R6000B),
IL-1β (cat. no. RLB00) and tumor necrosis factor-α (TNF-α, cat. no.
RTA00) in the tissue homogenate was then dermined using ELISA kits
according to the manufacturer's protocols (R&D Systems China
Co., Ltd.).
Luciferase activity assay
The target gene of miR-448 was predicted using
TargetScan 7.1 bioinformatics software (www.targetscan.org/vert_71). The wild-type (WT) or
mutant (Mut) SIRT1 binding site in miR-448 was amplified and
sub-cloned into the pGL3 basic vector (Promega Corporation). Next,
50 ng of pGL3 vectors and 200 ng of miR-448 mimics or miR-448
inhibitor were co-transfected into rat microglial cells using
Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Cells transfected with only Lipofectamine™ 2000 reagent were
used as the mock transfection group. After 48 h, Luciferase
activity was measured using a Dual-Luciferase Reporter Assay system
(E1910; Promega Corporation) according to the manufacturer's
instructions. Renilla luciferase activity was used as the
internal control.
Statistical analysis
The data are presented as mean ± standard deviation.
Differences between two groups were compared using Student's
t-test. One-way ANOVA analysis followed by Tukey's post hoc test
was used for the comparison of two or more groups. All data
analyses were conducted using SPSS software (version 18.0; SPSS
Inc.) and GraphPad Prism software (version 5.0; GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
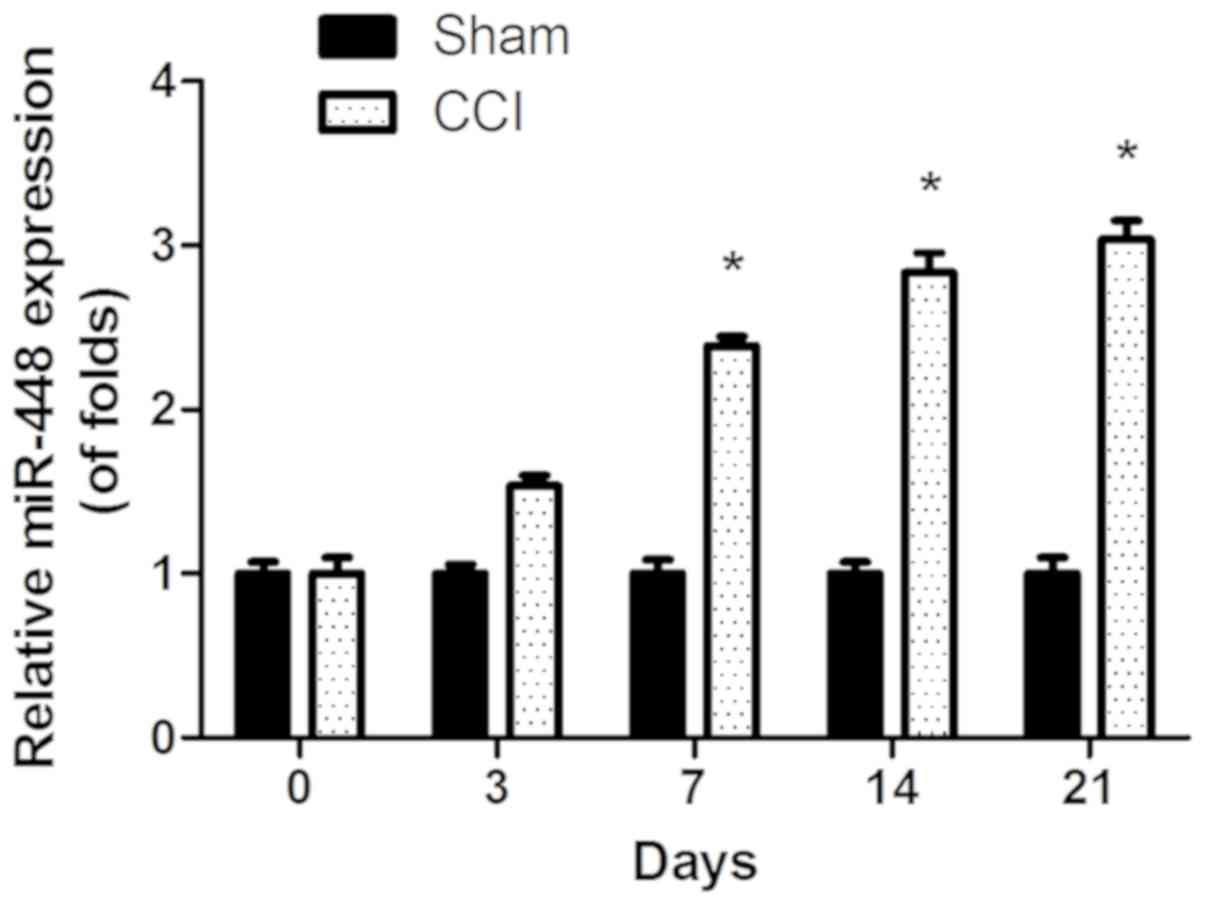
Expression of miR-448 is significantly
increased in a rat model of CCI
To investigate the role of miR-448 in neuropathic
pain development, the expression level of miR-448 was first
measured in the spinal cords of CCI and sham-operated rats. As
shown in Fig. 1, the expression
level of miR-448 increased significantly in a time-dependent manner
in CCI compared with sham-operated rats (P<0.05).
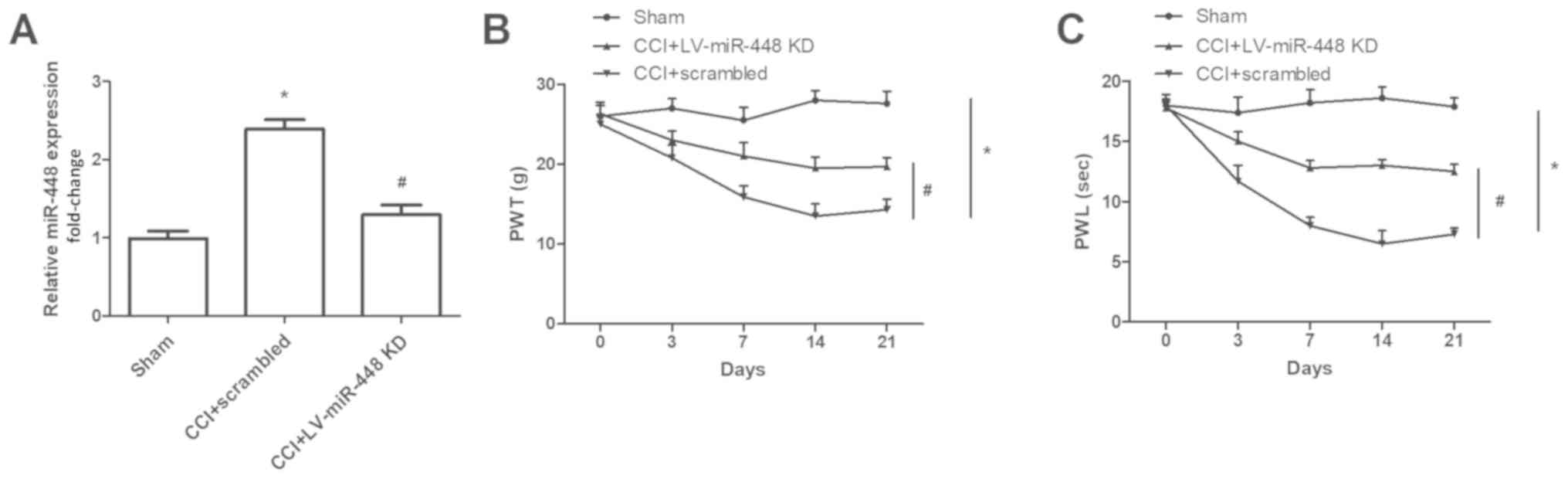
miR-448 downregulation inhibits the
development of neuropathic pain
As miR-448 was persistently expressed in CCI rats,
the downregulation of miR-448 may attenuate the development of
neuropathic pain. To test this hypothesis, an miR-448 inhibitor was
intrathecally administered to the rats. Compared with that in the
sham-operated group, miR-448 was significantly upregulated in CCI
rats, and both the PWT and PWL were decreased significantly in rats
with CCI (all P<0.05; Fig. 2).
Compared with CCI + scrambled group, miR-448 was significantly
downregulated in CCI rats treated with the miR-488 inhibitor
(P<0.05; Fig. 2A). Additionally,
the PWT and PWL were lower in CCI rats compared with sham-operated
group, and these effects were abrogated by miR-448 downregulation
(P<0.05; Fig. 2B-C).
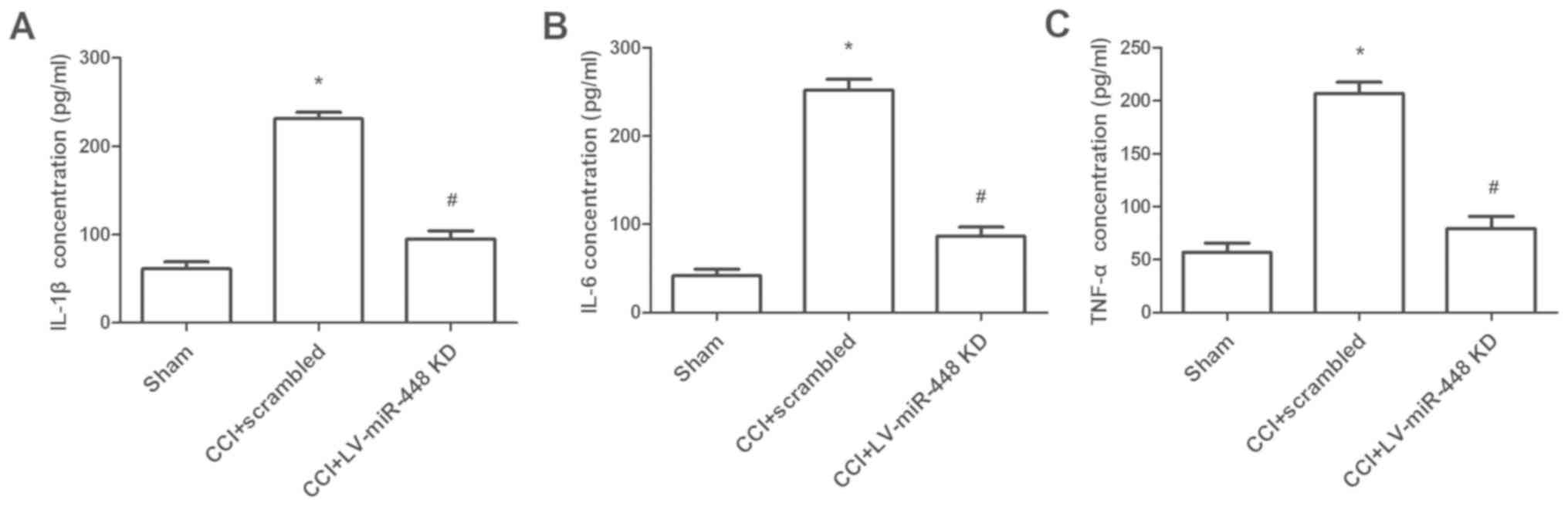
miR-448 downregulation depresses
inflammatory cytokines levels in vivo
To further investigate the biological effect of
miR-448 on neuropathic pain, the expression levels of inflammatory
cytokines were measured in CCI rats when miR-448 was downregulated.
The protein levels of IL-1β, IL-6 and TNF-α were significantly
increased in rats with CCI compared with those in the sham-operated
group, and the levels were significantly decreased following
treatment with the miR-448 inhibitor (all P<0.05; Fig. 3).
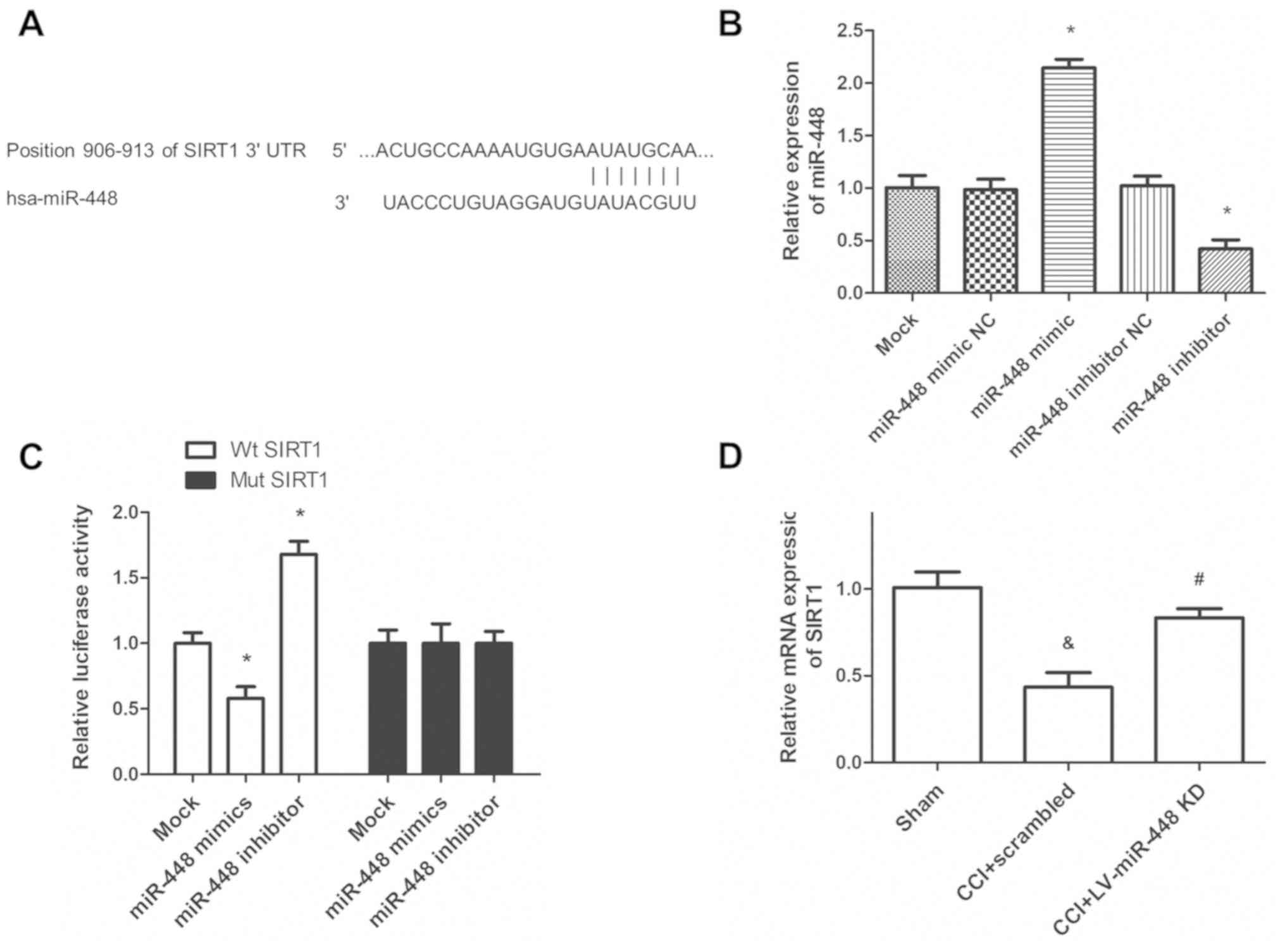
miR-448 directly targets the 3′UTR of
SIRT1
The potential mechanism of miR-448 in regulating
neuropathic pain was further explored, and the potential target
gene of miR-448 was investigated. The TargetScan analysis suggested
that the 3′-UTR of SIRT1 contained a binding region for miR-448
(Fig. 4A). To confirm the
transfection efficiency of miR-448 mimic or inhibitor, the relative
expression of miR-448 was quantified using RT-qPCR. It was noted
that miR-448 mimic transfection significantly increased miR-448
level in HAPI cells compared with the miR scrambled control,
whereas miR-448 inhibitor transfection significantly decreased the
miR-448 expression level (P<0.05; Fig. 4B). The luciferase reporter assay
suggested that treatment with the miR-448 mimic significantly
attenuated the luciferase activity in WT SIRT1 3′UTR transfected
cells, compared with treatment with miR-scrambled control, while
the inhibition of miR-448 promoted luciferase activity (Fig. 4C). However, the miR-448 mimic did not
affect the luciferase activity in Mut SIRT1 3′UTR transfected
cells. Additionally, the RT-qPCR analysis results showed that the
reduced SIRT1 mRNA expression in rats with CCI was significantly
upregulated following miR-448 inhibitor treatment (P<0.05;
Fig. 4D).
Effect of miR-448 on neuropathic pain
is reversed by SIRT1
To detect whether miR-448 affects the development of
neuropathic pain by regulating SIRT1 expression, rescue experiments
were performed. SIRT1 mRNA expression levels were detected in rats
subjected to CCI co-infected with miR-448 inhibitor and shSIRT1
lentiviruses. The increased SIRT1 mRNA expression level induced by
miR-448 inhibitor treatment was significantly decreased following
shSIRT1 infection (P<0.05; Fig.
5A). Furthermore, the suppressive effects of the miR-448
inhibitor on mechanical allodynia and thermal hyperalgesia were
substantially reversed by shSIRT1 infection (Fig. 5B-C; all P<0.05). Additionally, the
decreased expression of inflammatory cytokines, including IL-1β,
IL-6 and TNF-α, caused by the miR-448 inhibitor, was also reversed
by shSIRT1 (all P<0.05; Fig.
5D-F). The data indicated that miR-448 enhanced neuropathic
pain by targeting SIRT1.
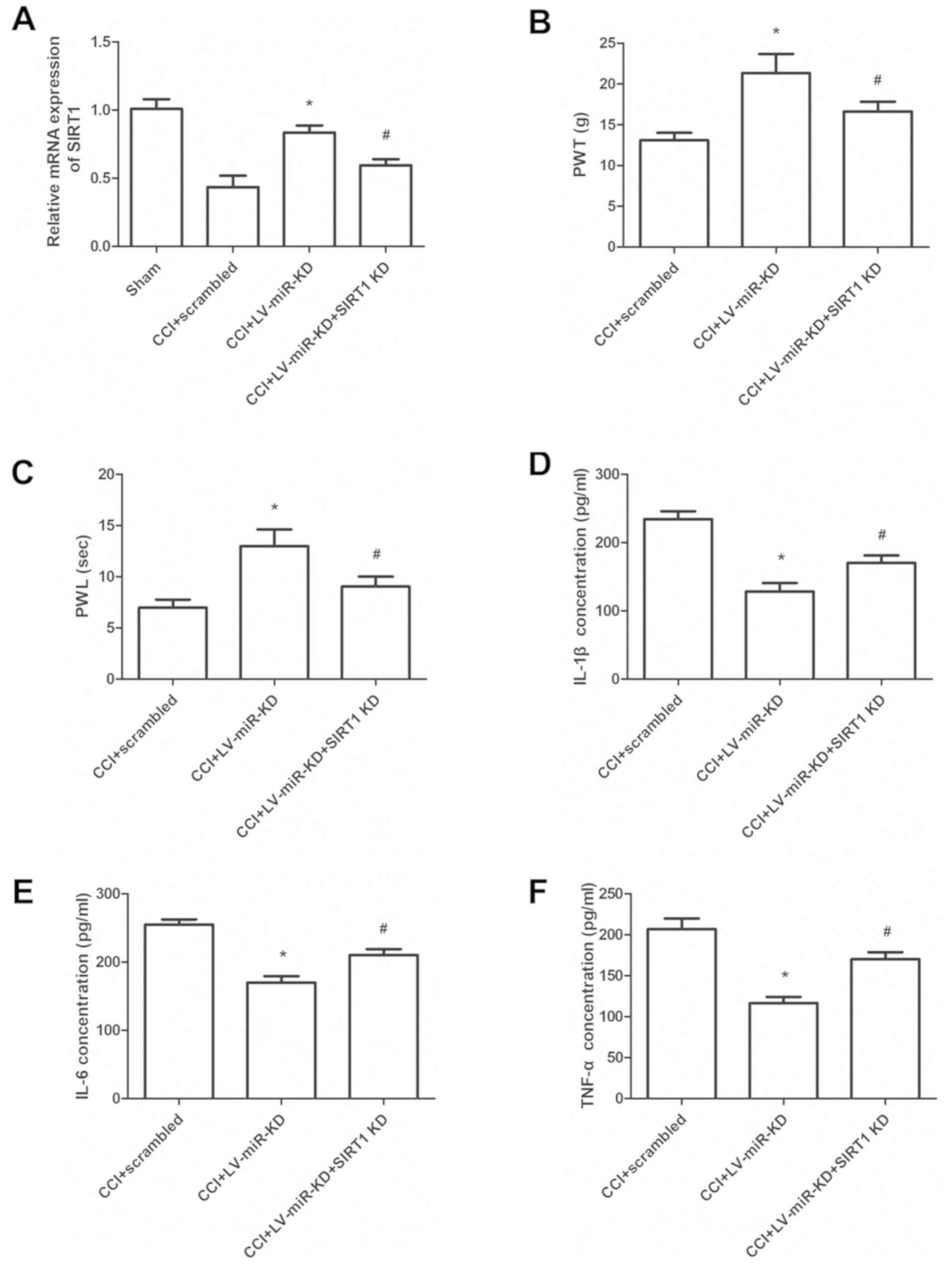 | Figure 5.SIRT1 abrogated the effect of miR-448
on neuropathic pain development. miR-448 inhibitor lentivirus, or
miR-448 inhibitor and shSIRT1 lentivirus, were co-infected into CCI
rats. (A) SIRT1 mRNA expression in CCI rats. (B) Mechanical
allodynia as assessed by the PWT. (C) Thermal hyperalgesia as
assessed by the PWL. The protein levels of (D) IL-1β, (E) IL-6 and
(F) TNF-α in the L4-L6 spinal cords of rats were measured by ELISA
on postoperative day 7. *P<0.05 vs. the CCI + scrambled group;
#P<0.05 vs. the CCI + LV-miR-KD group. SIRT1, sirtuin
1; miR, microRNA, sh, short hairpin; CCI, chronic constriction
injury; PWT, paw withdrawal threshold; PWL, paw withdrawal latency;
IL, interleukin; TNF-α, tumor necrosis factor-α; LV, lentivirus;
KD, knockdown. |
Discussion
Neuropathic pain can be caused by different
neurological diseases resulting from injury of the peripheral
nerves. Patients with cancer, diabetes and acquired
immunodeficiency syndrome are likely to suffer from neuropathic
pain (18–20). It has been noted that the expression
of IL-1β, IL-6 and TNF-α can be induced by nerve surgery (21), indicating that inflammation could
play a crucial role in the progression of neuropathic pain
(22).
miRNAs are a class of non-coding RNAs 18–24
nucleotides in length. A number of miRNAs have been shown to be
aberrantly expressed in various types of diseases, including
inflammatory diseases, heart diseases and several types of human
cancer (23,24). Increasing evidence has reported that
miRNAs may be involved in the development of neuropathic pain
development (11,12). Altered expression of miR-448 has been
detected in various human diseases. For instance, miR-448 was
expressed at low levels in glioma cells, and suppressed cell
proliferation and promoted apoptosis (25). Zhang et al (26) suggested that miR-448 was highly
expressed in vascular smooth muscle cells (VSMCs) from coronary
atherosclerotic plaques, and overexpression of miR-448 promoted
VSMC migration. Hong et al (27) indicated that miR-448 is upregulated
in gastric cancer tissues compared with adjacent non-tumor samples,
and can enhance the glycolytic metabolism of gastric cancer cells
by downregulating lysine demethylase 2B (KDM2B). All evidence
suggests the various roles of miR-448 in different diseases. In the
present study, miR-448 was significantly increased in CCI rat
compared with the sham-operated group, and downregulation of
miR-448 markedly attenuated the progression of neuropathic pain. A
previous study suggested that miR-448 was upregulated in the spinal
cord tissues of spinal cord ischemia/reperfusion injury model rats,
as well as in cells treated with hypoxia and that downregulation of
miR-448 inhibited apoptosis in nerve cells and improved
neurological function (28).
Additionally, Lu et al (14)
reported that the expression level of miR-448 was significantly
upregulated in rats treated with sevoflurane compared with controls
(14). An et al indicated
that lead exposure resulted in the upregulation of miR-448 in rat
models (29). The aforementioned
studies support the results obtained in the present study.
Previous evidence has suggested that
neuroinflammation plays a crucial role in the development of
neuropathic pain (30). IL-1β, IL-6
and TNF-α are commonly implicated as inflammation-associated
cytokines (31,32). In the present study, the ELISA
results suggested that the expression levels of IL-1β, IL-6, and
TNF-α were decreased in the spinal cords of rats following
treatment with the miR-448 inhibitor. This suggested that the
downregulation of miR-448 markedly decreased the progression of
neuropathic pain through by attenuating neuroinflammation.
miRNAs exert their functions by binding to the
target genes at a post-transcriptional level (33,34).
Previous evidence has suggested that miR-448 exhibits differing
functions in human diseases. For example, Su et al (25) revealed that miR-448 suppresses cell
proliferation and promotes apoptosis in glioma by downregulating
cortactin. Yang et al (35)
reported that miR-448 contributes to the development of
osteoarthritis by downregulating matrilin-3 expression. Zhang et
al (26) suggested that
overexpression of miR-448 enhanced cell proliferation and migration
in vascular smooth muscle cells (VSMCs) by targeting MEF2C.
Previous studies reported that several genes are target genes of
miR-448 (29,36); however, only one of those target
genes, SIRT1, was investigated in the present study. For example,
Hong et al (27) reported
that miR-448 can promote glycolytic metabolism in gastric cancer,
and KDM2B was revealed to be the target gene that can repress
glycolysis. Insulin like growth factor 1 receptor (IGF1R) was
reported to be the target gene of miR-448 in colorectal cancer
cells and IGF1R gene overexpression reversed the suppressive effect
of miR-448 on tumor progression in vitro (36). Additionally, Wang et al
(28) suggested that downregulation
of miR-448 can relieve SCII by upregulating SIRT1. In the present
study, luciferase reporter assay results confirmed that SIRT1 was
the target of miR-448. Additionally, rescue experiments were
performed to detect whether miR-448 affects the development of
neuropathic pain by regulating SIRT1 expression. Overexpression of
SIRT1 reversed the effect of miR-448 on neuropathic pain
development. Therefore, miR-448 may enhance neuropathic pain by
targeting SIRT1. A major study reported that downregulation of
miR-448 relieved SCII, inhibited apoptosis of nerve cells and
improved neurological function by upregulating SIRT1 (28), which was in accordance with the
results obtained in the present study.
The neuroprotective effect of SIRT1 has been widely
investigated in previous studies. Fujita and Yamashita (37) suggested that SIRT1 is involved in the
protection of neurons from degeneration in models of neurological
diseases, including traumatic brain injury and Alzheimer's disease
(37). Zhao et al (38) suggested that the expression level of
SIRT1 was increased in resveratrol-treated animals, and exerted
neuroprotective effects on spinal cord injury. Additionally, Zhang
et al (39) reported that
SIRT1 alleviates neuropathic pain in rats with type 2 diabetes
mellitus. Furthermore, a recent study reported that downregulation
of miR-377 could decrease proinflammatory cytokine release through
upregulation of SIRT1 (40). SIRT1
has also been reported to regulate the secretion of inflammatory
factors induced by high glucose or hypoxia (41), suggesting the crucial role of SIRT1
in host inflammatory responses. The aforementioned studies indicate
that SIRT1 exhibits a neuroprotective role by suppressing
neuroinflammation, which was consistent to the results obtained in
the present study.
In conclusion, the present study demonstrated that
miR-448 promoted the development of neuropathic pain in a rat model
of CCI by regulating neuroinflammation via SIRT1. Therefore,
miR-448 may serve a novel target for neuropathic pain
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and YC designed the study, and contributed to the
writing of the manuscript. YC and WG performed all the experiments,
analyzed the data, and contributed significantly to the revision of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experimental procedures for care and use of
animals were approved by the Medical Ethics Committee of Shengli
Oilfield Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao Y and Yang Z: Effect of Wnt signaling
pathway on pathogenesis and intervention of neuropathic pain. Exp
Ther Med. 16:3082–3088. 2018.PubMed/NCBI
|
|
2
|
Sommer C, Leinders M and Üçeyler N:
Inflammation in the pathophysiology of neuropathic pain. Pain.
159:595–602. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bouhassira D and Attal N: Emerging
therapies for neuropathic pain: New molecules or new indications
for old treatments? Pain. 159:576–582. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lema MJ, Foley KM and Hausheer FH: Types
and epidemiology of cancer-related neuropathic pain: The
intersection of cancer pain and neuropathic pain. Oncologist. 15
(Suppl 2):S3–S8. 2010. View Article : Google Scholar
|
|
5
|
Jiang YR, Du JY, Wang DD and Yang X:
miRNA-130a improves cardiac function by down-regulating TNF-α
expression in a rat model of heart failure. Eur Rev Med Pharmacol
Sci. 22:8454–8461. 2018.PubMed/NCBI
|
|
6
|
Mao Q, Chen C, Liang H, Zhong S, Cheng X
and Li L: Astragaloside IV inhibits excessive mesangial cell
proliferation and renal fibrosis caused by diabetic nephropathy via
modulation of the TGF-β1/Smad/miR-192 signaling pathway. Exp Ther
Med. 18:3053–3061. 2019.PubMed/NCBI
|
|
7
|
Meng Y, Shang F and Zhu Y: miR-124
participates in the proliferation and differentiation of brain
glioma stem cells through regulating Nogo/NgR expression. Exp Ther
Med. 18:2783–2788. 2019.PubMed/NCBI
|
|
8
|
Wang JR, Liu B, Zhou L and Huang YX:
MicroRNA-124-3p suppresses cell migration and invasion by targeting
ITGA3 signaling in bladder cancer. Cancer Biomark. 24:159–172.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Chu Y and Xu M, Zhang X, Zhou Y
and Xu M: miR-21 promotes cell migration and invasion of
hepatocellular carcinoma by targeting KLF5. Oncol Lett.
17:2221–2227. 2019.PubMed/NCBI
|
|
10
|
Qiu T, Wang K, Li X and Jin J: miR-671-5p
inhibits gastric cancer cell proliferation and promotes cell
apoptosis by targeting URGCP. Exp Ther Med. 16:4753–4758.
2018.PubMed/NCBI
|
|
11
|
Zhang Y, Su Z, An LJ, Li L, Wei M, Ge DJ
and Liu HL: miR-98 acts as an inhibitor in chronic constriction
injury-induced neuropathic pain via downregulation of high-mobility
group AT-hook 2. J Cell Biochem. 120:10363–10369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen J, He T, Qi F and Chen H: MiR-206-3p
alleviates chronic constriction injury-induced neuropathic pain
through targeting HDAC4. Exp Anim. 68:213–220. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao Y, Wang S, Xie Y, Jin K, Bai Y and
Shan S: MiR-28-5p relieves neuropathic pain by targeting Zeb1 in
CCI rat models. J Cell Biochem. 119:8555–8563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu Y, Jian MY, Ouyang YB and Han RQ:
Changes in rat brain MicroRNA expression profiles following
sevoflurane and propofol anesthesia. Chin Med J (Engl).
128:1510–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bennett GJ and Xie YK: A peripheral
mononeuropathy in rat that produces disorders of pain sensation
like those seen in man. Pain. 33:87–107. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmalbruch H: Fiber composition of the
rat sciatic nerve. Anat Rec. 215:71–81. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni HD, Xu LS, Wang Y, Li H, An K, Liu M,
Liu Q, Deng H, He Q, Huang B, et al: Astrocyte activation in the
periaqueductal gray promotes descending facilitation to
cancer-induced bone pain through the JNK MAPK signaling pathway.
Mol Pain. 15:17448069198319092019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Degu H, Wondimagegnehu A, Yifru YM and
Belachew A: Is health related quality of life influenced by
diabetic neuropathic pain among type II diabetes mellitus patients
in Ethiopia? PLoS One. 14:e02114492019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanda H, Liu S, Kanao M, Yi H, Iida T,
Huang W, Kunisawa T, Lubarsky DA and Hao S: Gene therapy with HSV
encoding p55TNFR gene for HIV neuropathic pain: An evidence-based
mini-review. Transl Perioper Pain Med. 2:24–32. 2017.PubMed/NCBI
|
|
21
|
Tilley DM, Cedeño DL, Kelley CA, DeMaegd
M, Benyamin R and Vallejo R: Changes in dorsal root ganglion gene
expression in response to spinal cord stimulation. Reg Anesth Pain
Med. 42:246–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Safieh-Garabedian B, Nomikos M and Saadé
N: Targeting inflammatory components in neuropathic pain: The
analgesic effect of thymulin related peptide. Neurosci Lett.
702:61–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Li Y, Li L, Yu Q, Chao K, Zhou G,
Qiu Y, Feng R, Huang S, He Y, et al: Circulating microRNA146b-5p is
superior to C-reactive protein as a novel biomarker for monitoring
inflammatory bowel disease. Aliment Pharmacol Ther. 49:733–743.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia QW, Chen ZH, Ding XQ, Liu JY, Ge PC,
An FH, Li LH, Wang LS, Ma WZ, Yang ZJ and Jia EZ: Predictive
effects of circulating miR-221, miR-130a and miR-155 for coronary
heart disease: A multi-ethnic study in China. Cell Physiol Biochem.
42:808–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su HY, Lin ZY, Peng WC, Guan F, Zhu GT,
Mao BB, Dai B, Huang H and Hu ZQ: MiR-448 downregulates CTTN to
inhibit cell proliferation and promote apoptosis in glioma. Eur Rev
Med Pharmacol Sci. 22:3847–3854. 2018.PubMed/NCBI
|
|
26
|
Zhang R, Sui L, Hong X, Yang M and Li W:
MiR-448 promotes vascular smooth muscle cell proliferation and
migration in through directly targeting MEF2C. Environ Sci Pollut
Res Int. 24:22294–22300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong X, Xu Y, Qiu X, Zhu Y, Feng X, Ding
Z, Zhang S, Zhong L, Zhuang Y, Su C, et al: MiR-448 promotes
glycolytic metabolism of gastric cancer by downregulating KDM2B.
Oncotarget. 7:22092–22102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Pang QJ, Liu JT, Wu HH and Tao DY:
Down-regulated miR-448 relieves spinal cord ischemia/reperfusion
injury by up-regulating SIRT1. Braz J Med Biol Res. 51:e73192018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
An J, Cai T, Che H, Yu T, Cao Z, Liu X,
Zhao F, Jing J, Shen X, Liu M, et al: The changes of miRNA
expression in rat hippocampus following chronic lead exposure.
Toxicol Lett. 229:158–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Myers RR, Campana WM and Shubayev VI: The
role of neuroinflammation in neuropathic pain: Mechanisms and
therapeutic targets. Drug Discov Today. 11:8–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fei H, Shi M, Chen L, Wang Z and Suo L:
MicroRNA-18 promotes apoptosis of islet β-cells via targeting NAV1.
Exp Ther Med. 18:389–396. 2019.PubMed/NCBI
|
|
32
|
Li QY, Xu HY and Yang HJ: Effect of
proinflammatory factors TNF-α,IL-1β, IL-6 on neuropathic pain.
Zhongguo Zhong Yao Za Zhi. 42:3709–3712. 2017.(In Chinese).
PubMed/NCBI
|
|
33
|
Li HL, Sun JJ, Ma H, Liu SJ, Li N, Guo SJ,
Shi Y, Xu YY, Qi ZY, Wang YQ, et al: MicroRNA-23a inhibits
endometrial cancer cell development by targeting SIX1. Oncol Lett.
18:3792–3802. 2019.PubMed/NCBI
|
|
34
|
Wang S, Li C, Yu Y and Qiao J: Decreased
expression of microRNA-145 promotes the biological functions of
fibroblasts in hypertrophic scar tissues by upregulating the
expression of transcription factor SOX-9. Exp Ther Med.
18:3450–3460. 2019.PubMed/NCBI
|
|
35
|
Yang H, Wu D, Li H, Chen N and Shang Y:
Downregulation of microRNA-448 inhibits IL-1β-induced cartilage
degradation in human chondrocytes via upregulation of matrilin-3.
Cell Mol Biol Lett. 23:72018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li B, Ge L, Li M, Wang L and Li Z: miR-448
suppresses proliferation and invasion by regulating IGF1R in
colorectal cancer cells. Am J Transl Res. 8:3013–3022.
2016.PubMed/NCBI
|
|
37
|
Fujita Y and Yamashita T: Sirtuins in
neuroendocrine regulation and neurological diseases. Front
Neurosci. 12:7782018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Chen S, Gao K, Zhou Z, Wang C,
Shen Z, Guo Y, Li Z, Wan Z, Liu C and Mei X: Resveratrol protects
against spinal cord injury by activating autophagy and inhibiting
apoptosis mediated by the SIRT1/AMPK signaling pathway.
Neuroscience. 348:241–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Z, Ding X, Zhou Z, Qiu Z, Shi N,
Zhou S, Du L, Zhu X, Wu Y, Yin X and Zhou C: SIRT1 alleviates
diabetic neuropathic pain by regulating synaptic plasticity of
spinal dorsal horn neurons. Pain. 160:1082–1092. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui C, Li Y and Liu Y: Down-regulation of
miR-377 suppresses high glucose and hypoxia-induced angiogenesis
and inflammation in human retinal endothelial cells by direct
up-regulation of target gene SIRT1. Hum Cell. 32:260–274. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang YC, Lin CW, Hsieh MC, Wu HJ, Wu WS,
Wu WC and Kao YH: High mobility group B1 up-regulates angiogenic
and fibrogenic factors in human retinal pigment epithelial ARPE-19
cells. Cell Signal. 40:248–257. 2017. View Article : Google Scholar : PubMed/NCBI
|