Introduction
Talaromyces marneffei (T. marneffei)
is a rare pathogenic Talaromyces species in humans and is
the only temperature-dependent dimorphic fungus of the
Talaromyces genus. In 1973, DiSalvo et al (1) reported the first case of a natural
T. marneffei infection in humans. Currently, T.
marneffei infection is a common opportunistic infection in
patients with HIV and is prevalent in South Asian countries and
South China (2–7). In the past two decades, the incidence
of Talaromyces spp. infection has risen dramatically in
tandem with the HIV epidemic. T. marneffei infections
generally occur in patients with late-stage acquired immune
deficiency syndrome (AIDS) or immunodeficiency (2). Therefore, Talaromyces spp.
infection is more likely to occur concurrently with other
opportunistic infections (7–9). The simultaneous identification of T.
marneffei and Cryptococcus neoformans (C.
neoformans) from blood and bronchial mucosal biopsy cultures is
uncommon, and very few cases have been reported to date (7). The diagnosis and treatment of
concurrent infection with T. marneffei and C.
neoformans is challenging in the clinical setting (7). In the current case report, a concurrent
infection with T. marneffei and C. neoformans (based
on blood and bronchial mucosal biopsy cultures) is presented in a
Chinese patient without HIV infection, which was successfully
treated. A literature review was performed to provide new insight
into the treatment of this rare concurrent infection.
Case report
In January 2017, a 57-year-old Chinese woman
presented with a fever (maximum temperature, 39°C), cough (coughing
a moderate quantity of white sputum) and pharyngalgia, which began
two days prior to hospital admission (12th January 2017; Taizhou
Hospital of Wenzhou Medical University, China). The patient was
diagnosed with hemolytic anemia 8 years prior to admission, and
treated with dexamethasone 9 mg/d, which was gradually reduced to
2.25 mg/d after symptoms improved. The patient was still taking
dexamethasone at the time of admission. However, the patient could
not provide any other details about Dexamethasone treatment. The
patient had no recent or direct contact with specific plants
including rotten sugar canes or animals such as bamboo rats and had
not traveled to any endemic areas such as South Asian countries and
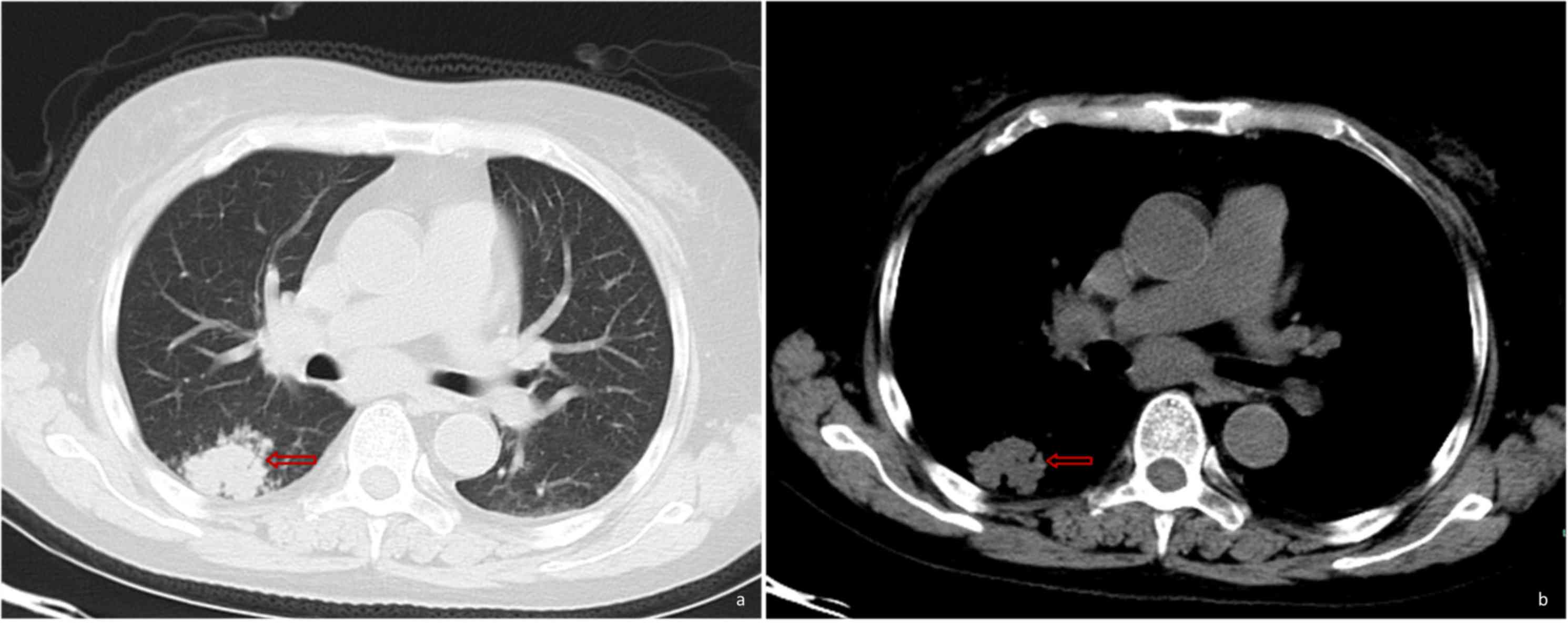
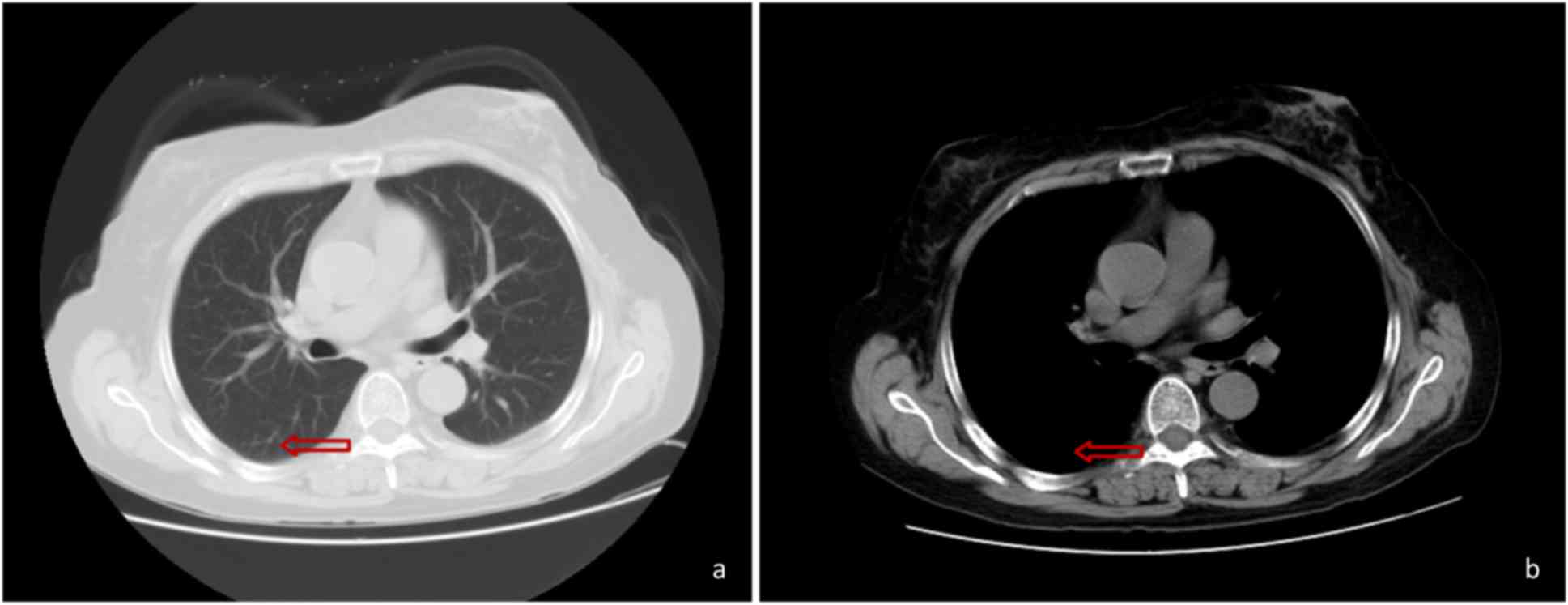
South China. The CT scan performed at Xianju County People's
Hospital (China) revealed the presence of a hyperdense mass in the
right lower lung (Fig. 1). The
patient was subsequently treated with antibiotics but exhibited a
poor response.
The patient was then referred to Taizhou Hospital of
Wenzhou Medical University, China on 12th January 2017 with a body
temperature of 37.3°C, which increased thereafter, a pulse rate of
115 beats/min, a respiratory rate of 17 breaths/min, a blood
pressure of 107/94 mmHg and an oxygen saturation of 99%. A physical
examination was performed upon admission and demonstrated palpable
lymph nodes that were 1–3 cm in size, and tenderness over the left
cervical and supraclavicular areas. On the second day of hospital
admission, laboratory tests using whole blood specimen (BC-6800
plus; Mindray Medical International Limited) at 25°C for 1 min
revealed a white blood cell count of 3,300 cells/ml (normal range,
4.0–10×109 cells/l), neutrophils 93.3% (normal range,
40–75%), lymphocytecount 200 cells/ml (normal range,
1.1–3.2×109 cells/l), hemoglobin 61 g/dl (normal range,
115–150 g/l) and platelet count 22,000 cells/ml (normal ranges:
125–350×109 cells/l). C-reactive protein level
(CRP-M100; Mindray Medical International Limited) was 311 mg/dl
(normal range, <5.0 mg/l) using whole blood specimen at 25°C for
2 min and a negative result for HIV antibodies (Microelisa
Stripplate; Bejing Wantai Biological) using serum at 37°C for 120
min was determined. Serum chemistry (Beckman AU5800) using serum at
37°C for 40 min revealed that alanine transaminases was 22 U/l
(normal range: 7–40 U/l) and aspartate transaminases was 32 U/l
(normal range: 13–35 U/l), these levels were normal. A type-B
ultrasound examination revealed left cervical, left
supraclavicular, bilateral inguinal and retroperitoneal
lymphadenopathy. Since the chest CT scan failed to reveal any
enlarged pulmonary hilar or mediastinal lymph nodes, the patient
did not undergo an endobronchial ultrasound bronchoscopy.
The patient was treated with Ceftazidime (2.0 g
intravenous infusion per 12 h) and Levofloxacin (0.5 g iv infusion
once a day) on the day of admission. On day 5 of treatment,
hyperpyrexia occurred again similar to two days before admission
and blood cultures were subsequently performed. Endoscopic
esophageal ultrasound-guided fine-needle aspiration of the left
supraclavicular lymph nodes was performed and the aspirates were
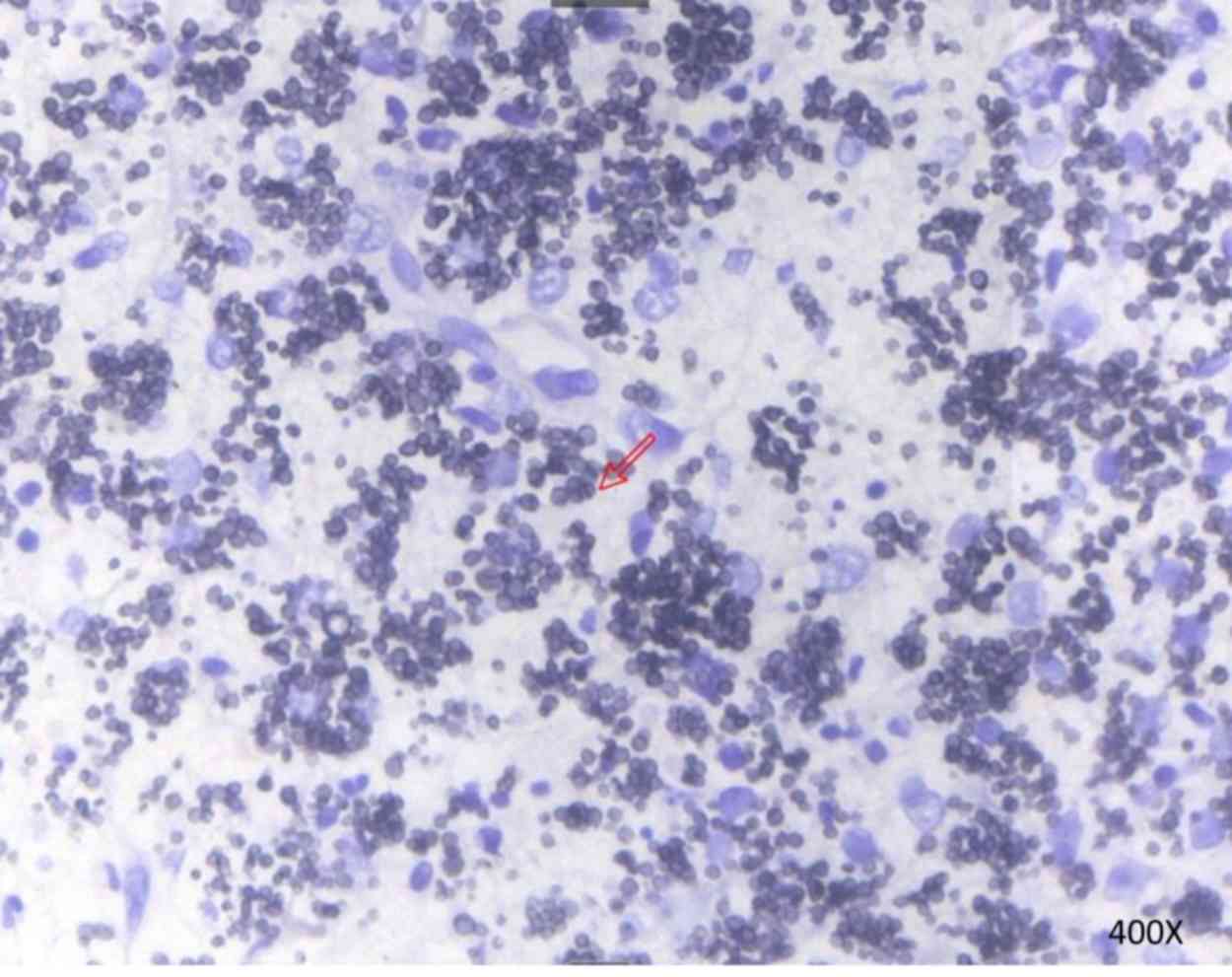
submitted for histopathology. Blood cultures grew T.
marneffei after 5 days of incubation at 25°C, and the aspirate
from a supraclavicular lymph node stained with Gomeri methenamine
silver (25°C constant temperature water bath for 50 min) revealed
yeast-like fungi with transverse septa, which confirmed the
presence of T. marneffei (Fig.
2). Subsequently, on hospital day 11, treatment with
intravenous voriconazole (6 mg/kg per 12 h for the first 24 h,
followed by 4 mg/kg per 12 h) began. The fever subsided after 4
days of treatment, and the patients' cough and pharyngalgia also
improved. The patient was treated with intravenous voriconazole for
2 weeks, and received oral voriconazole therapy of 200 mg twice a
day. Blood cultures were performed on hospital admission day 23 for
the detection of T. marneffei. Blood cultures on day 5 did
not exhibit T. marneffei growth, but did exhibit C.
neoformans. Antimicrobial susceptibility testing of C.
neoformans using the broth dilution method (10) (bioMerieux, Ltd.) was conducted and
the rank order of potency, which was based on minimum inhibitory
concentration (MIC) values, were itraconazole (0.06), voriconazole
(0.125), amphotericin B (0.5), fluconazole (1) and 5-fluorocytosine (4). A period of 2 weeks into treatment with
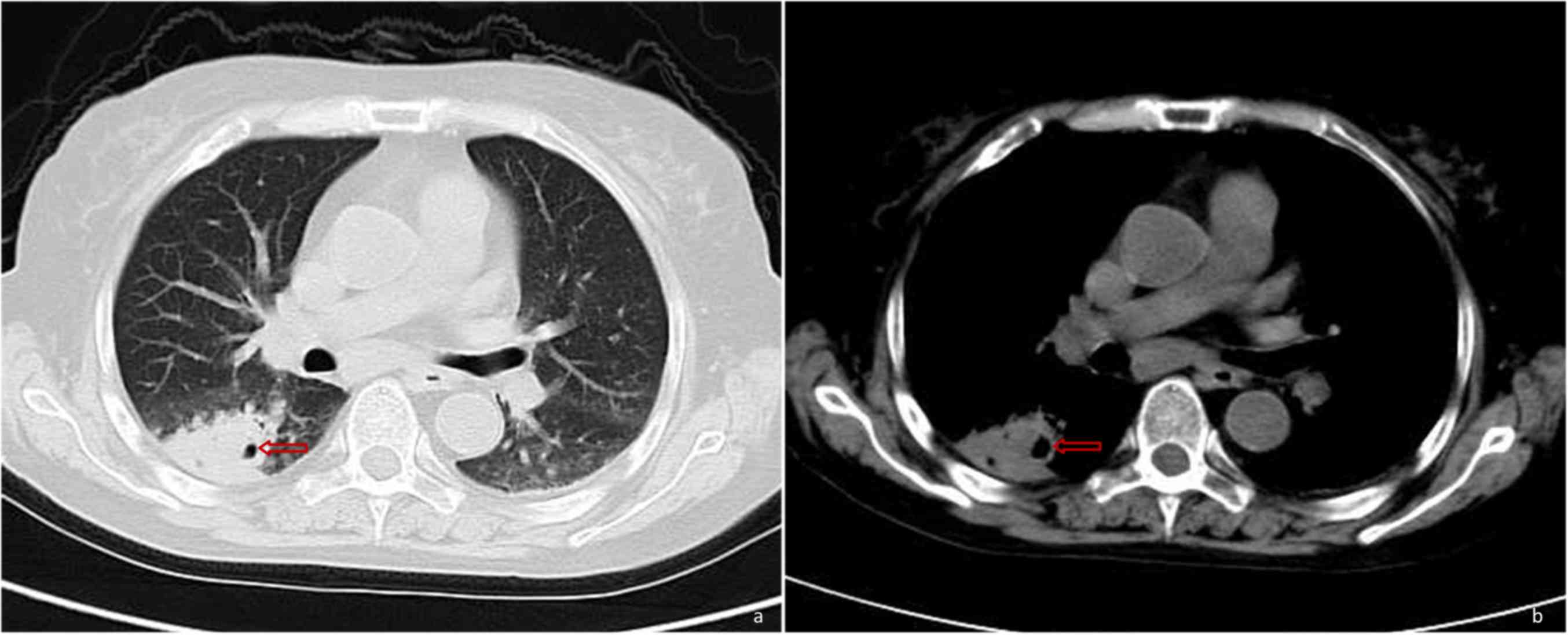
oral voriconazole, the patient underwent a CT scan of the chest on
hospital day 37, which revealed deterioration compared with the
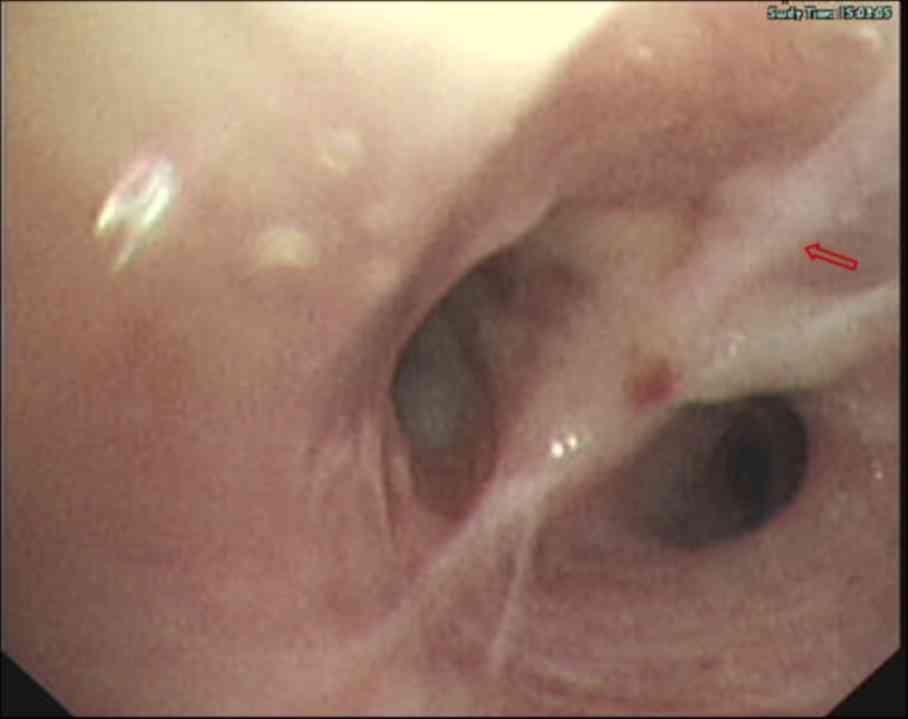
previous scans (Fig. 3). On the same
day, a bronchoscopy was performed, which revealed hyperplasia and
edema of the airway mucosa of the right lower lobe bronchus
(Fig. 4). Although the patient's
symptoms had been significantly relieved, on day 38, the patient
was treated with intravenous voriconazole of 4 mg/kg per 12 h for
10 additional days based on the chest CT scan and bronchoscopy
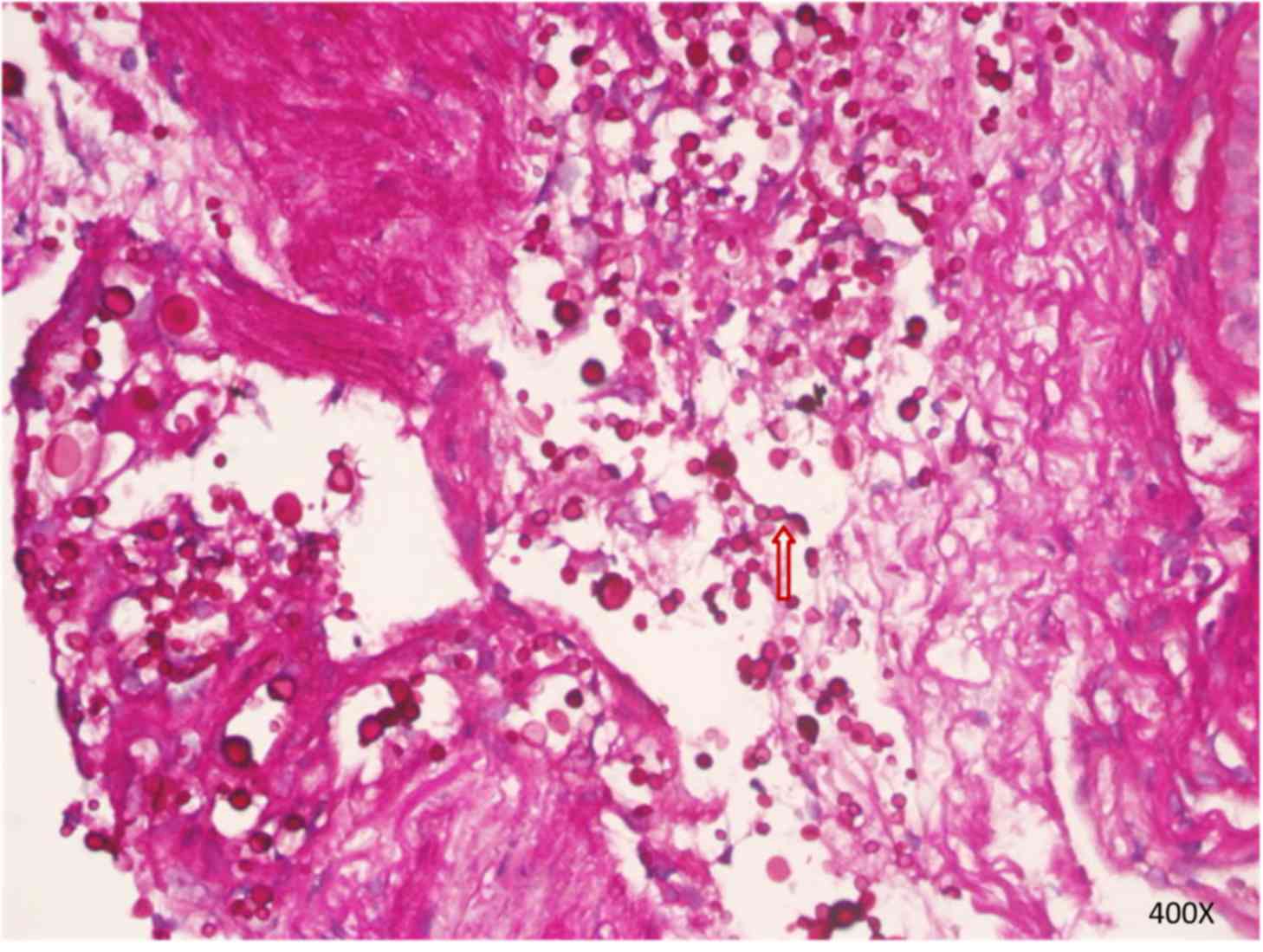
results. A period of 3 days later, the periodic acid-Schiff-stained
(25°C; 15 min) preparation of bronchial mucosal mesenchyme
confirmed the presence of C. neoformans (Fig. 5). The patient achieved dramatic
clinical improvement and was discharged home on oral voriconazole
(200 mg twice a day) for a period of 2 months. At the 2 month
follow up following the completion of therapy, there was no
evidence of ongoing infection and a repeat CT scan of the chest
revealed significant improvement in the right lower lung lesion
(Fig. 6).
Discussion
T. marneffei causes a disseminated and lethal
fungal disease, which is known as talaromycosis. In South Asian
countries, talaromycosis has become the third most common disease
in patients infected with HIV, following tuberculosis and
cryptococcosis (2–5). Talaromycosis is becoming increasingly
prevalent in patients who are HIV-negative and exhibit no apparent
risk factors or underlying immunodeficiency, including organ
transplant recipients, patients with hematologic malignancies or
patients receiving glucocorticoids or immunosuppressants (1,5,11–14). The
majority of T. marneffei infections occur in patients with
AIDS or an immunodeficiency disorder, whose CD4+ T cell count is
typically <100 cell/pl (15).
Within these patients, concurrent cryptococcosis,
tuberculosis, Pneumocystis jirovecii pneumonia or concurrent
cytomegalovirus and salmonella infections, are common
(6–9). However, concurrent infection with T.
marneffei and C. neoformans are rarely reported. In the
current case report, the literature was reviewed and 8 cases of
concurrent infection with T. marneffei and C.
neoformans were presented in patients who were HIV-positive
(7). To the best of our knowledge,
this is the first case report describing a patient with concurrent
infection with T. marneffei and C. neoformans who was
also HIV-negative. The two types of fungi were simultaneously
identified from blood culture (T. marneffei and C.
neoformans) and bronchial mucosal biopsy cultures (C.
neoformans).
Immunodeficiency represents a major risk factor for
T. marneffei infection (5).
Ma et al (16) impaired the
immune state of monocytes via the addition of dexamethasone and
declared that immunosuppressants, including dexamethasone, exhibit
inhibitory effects on monocytes and macrophages, and can increase
patient susceptibility to T. marneffei infection. CD4+ T
cell-induced immunodeficiency may also serve a pathogenic role in
HIV-negative patients with immunodeficiency (2,13,17). A
number of reports have indicated that anti-cytokine and
anti-interferon-γ autoantibodies are associated with adult-onset
immunodeficiency (1,14,17,18).
These results indicated another possible reason for cellular
immunodeficiency in patients who are HIV-negative, which
contributes to host susceptibility to T. marneffei
infection. The patient presented in the current case study received
long-term glucocorticoid treatment (oral dexamethasone) for
erythroblastic anemia for 8 years. The resulting decrease in white
blood cell count further led to immunosuppression and T-cell
immunodeficiency. Therefore, the patient exhibited an increased
susceptibility to opportunistic infections. Unfortunately, the
detection techniques were not available in the hospital this
patient was admitted to, so these autoantibodies (including
anti-cytokine and anti-interferon-γ autoantibodies) were not
detected, which is a limitation of the current case study.
Talaromycosis is associated with non-specific
manifestations, including fever, weight loss, systemic lymph node
enlargement, hepatosplenomegaly, skin lesions and anemia (4,19).
Evidence has indicated that symptoms of T. marneffei
infection in patients who are HIV-negative differ from those in
patients who are HIV-positive due to the disparities in the causes
and features of immunosuppression (5,15). The
patient of the current case study presented with fever, cough,
lymph node enlargement, anemia and thrombocytopenia, all of which
were non-specific, and may lead to a misdiagnosis of tuberculosis
or other fungal infections. The clinical implication of a
misdiagnosis is that fungal infections are likely in patients with
persistent fever following routine antibacterial therapy, with
long-term glucocorticoid treatment or with an immunodeficiency
disorder, and in these patients, concurrent opportunistic
infections should be considered. Clinically, T. marneffei
infection shares similar manifestations with tuberculosis or
infections that are caused by C. neoformans, Histoplasma
capsulatum and Nocadias. Concurrent infections with two
or more pathogens should therefore be considered and confirmed by
further tests. Furthermore, rare forms of these diseases deserve
additional attention. The diagnosis and treatment of disseminated
T. marneffei is difficult due to the existence of mixed
infections, particularly in patients with concurrent fungal
infections. Kawila et al (5)
reported that T. marneffei infection-associated mortality in
patients who were HIV-negative was unexpectedly higher than that
inpatients infected with HIV. This result may be due to the fact
that T. marneffei infection develops more quickly and a skin
rash usually appears in patients who are HIV-positive, which makes
diagnosis easier. In contrast, the symptoms of T. marneffei
infection are more complex and more likely to be confused with
other conditions in patients without HIV infection. Furthermore,
mixed infections are common in patients without HIV infection,
which contributes to a higher mortality (16). The patients presented in the current
case study received intravenous voriconazole immediately following
the diagnosis of T. marneffei infection. The symptoms of
fever, cough, pharyngalgia and hemoptysis were improved gradually
following treatment. The patient was changed to oral therapy 14
days after the treatment began. Blood cultures taken on day 5
indicated no T. marneffei growth, but indicated the growth
of C. neoformans. These results demonstrated that the
treatment was effective for T. marneffei infection, but
unfortunately, it was undetermined as to whether C.
neoformans infection occurred before or after the patient
started receiving voriconazole. This was due to the fact no
assessment of C. neoformans antigens or bronchoscopy was
performed at the early stage of the disease and the repeat chest CT
scan was not performed early enough. C. neoformans was
simultaneously identified from serial blood cultures and confirmed
using bronchial mucosal biopsy. The C. neoformans infection
in the patient of the case study was sensitive to voriconazole,
which was used to treat the T. marneffei infection, making
it easier to effectively treat the patient and preventing the
condition from becoming life threatening. The valuable clinical
information demonstrated from the present case study is that the
symptoms induced by the two pathogens in a concurrent infection may
be similar. If a patient responds poorly to initial monotherapy,
the possibility of coinfection should be considered. When
coinfection occurs, it is necessary to determine if initial therapy
is effective for treating all concurrent infections. If not, new
treatment options should be considered. The patient of the current
report did not exhibit any symptoms of central nervous system
disorders and refused to receive a lumbar puncture. Due to this,
whether the patient had C. neoformans meningitis could not
be determined and this is a limitation of the current case
report.
No standard antifungal treatment for T.
Marneffei infection has been established and currently certain
studies (20,21) have identified that amphotericin B
liposomal was effective in the treatment of T. Marneffei
infection and recommended as initial therapy for the disease.
However, amphotericin B's common side effects include electrolyte
disturbance, renal compromise and hepatic impairment, which limits
its clinical use (22). Voriconazole
is an antifungal medication. It is in the triazole family of
medications. Voriconazole has been revealed to exhibit potent in
vitro activity and is used to treat multiple aggressive fungal
infections with adequate safety and efficacy profiles (23). A recent retrospective study indicated
that the intravenous administration of voriconazole as initial
therapy for T. Marneffei infection was effective and well
tolerated (24). The continuous
administration of voriconazole for 12 weeks was demonstrated to be
effective for the treatment of T. marneffei infection in
patients with advanced HIV disease (25). However, no recommendation has been
published on the duration of voriconazole administration for the
treatment of disseminated T. marneffei infection in patients
who are HIV-negative. A previous retrospective study indicated that
the duration of therapy for T. marneffei infection was
prolonged in patients who are HIV-negative compared with the
treatment duration in patients who are HIV-positive (5). Voriconazole was used instead of
amphotericin B for the current case report, following the
confirmation of T. marneffei infection as Voriconazole has
fewer side effects, is cheaper, was readily available at the
hospital of admittance, is easier to purchase and has been
determined to be effective in the treatment for talaromycosis
(22,24). A number of studies have indicated
that C. neoformans was sensitive to voriconazole treatment
(26,27) and that its MIC value was low,
indicating that voriconazole may be appropriate for the treatment
of C. neoformans infection. Therefore, the patient continued
to receive voriconazole following the confirmation of C.
neoformans infection. Symptoms subsequently improved and the
pulmonary lesion almost disappeared following treatment. For T.
marneffei infection in patients who are HIV-negative, further
studies are required to understand the duration that voriconazole
should be administered, particularly in cases of concurrent fungal
infections. The current case report should serve to indicate the
importance of monitoring the condition of patients closely, to
determine the most appropriate treatment duration of voriconazole.
If no improvement is observed despite treatment at a dosage
determined via drug susceptibility testing, prolonging initial
treatment may be the most appropriate option.
In conclusion, the current case study indicated that
despite being rare, concurrent septicemia and bronchopulmonary
infection caused by T. marneffei and C. neoformans
can occur. The risk of concurrent infection with T.
marneffei and C. neoformans is higher in patients with a
severely compromised immune system. When coinfection occurs, one
pathogen may outgrow the other in blood cultures, masking the
presence of the other therefore the diagnosis and treatment of
concurrent infections with T. marneffei and C.
neoformans are difficult. Clinicians should therefore be fully
aware of the possibility of concurrent infections with T.
marneffei and other opportunistic pathogens. For
immunosuppressed patients, early diagnosis and treatment are
crucial for improving patient prognosis. Further studies are
warranted to better understand why an increasing number of patients
who are HIV-negative are infected with T. marneffei or other
opportunistic pathogens.
Acknowledgements
Not applicable.
Funding
Funding was received from The Science and Technology
Foundation of Taizhou (grant no. 1801KY18) and the Projects of
Medical and Health Technology Program in Zhejiang Province (grant
no. 2015KYB439).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and XW conceived and designed the review. DL and
YX prepared the patient data and figures. LL analyzed and
interpreted the data. All authors agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Taizhou Hospital of Wenzhou
Medical University passed the ethical review (approval nο:
K20190214). The Ethics Committee of Taizhou Hospital of Wenzhou
Medical University waived the need for consent for the publication
of patient information in the present manuscript due to the reason
that the patient had passed away.
Patient consent for publication
Due to the death of the patient, no consent for
publication was gained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DiSalvo AF, Fickling AM and Ajello L:
Infection caused by Penicillium marneffei: Description of first
natural infection in man. Am J Clin Pathol. 60:259–263. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vanittanakom N, Cooper CR Jr, Fisher MC
and Sirisanthana T: Penicillium marneffei infection and recent
advances in the epidemiology and molecular biology aspects. Clin
Microbiol Rev. 19:95–110. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ustianowski AP, Sieu TP and Day JN:
Penicillium marneffei Infection in HIV. Curr Opin Infect Dis.
21:31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Supparatpinyo K, Khamwan C, Baosoung V,
Nelson KE and Sirisanthana T: Disseminated Penicillium marneffei
infection in Southeast Asia. Lancet. 344:110–113. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawila R, Chaiwarith R and Supparatpinyo
K: Clinical and laboratory characteristics of penicilliosis
marneffei among patients with and without HIV infection in northern
Thailand: A retrospective study. BMC Infect Dis. 13:4642013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng J, Gui X, Cao Q, Yang R, Yan Y, Deng
L and Lio J: A clinical study of acquired immunodeficiency syndrome
associated Penicillium mameffei infection from a non-endemic area
in China. PLOS One. 10:e01303762015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le T, Hong Chau TT, Kim Cuc NT, Si Lam P,
Manh Sieu TP, Shikuma CM and Day JN: AIDS-Associated Cryptococcus
neoformans and Penicillium marneffei Coinfection: A therapeutic
dilemma in resource-limited settings. Clin Infect Dis. 51:e65–e68.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larsson M, Nguyen LH, Wertheim HF, Dao TT,
Taylor W, Horby P, Nguyen TV, Nguyen MH, Le T and Nguyen KV:
Clinical characteristics and outcome of Penicillium mameffei
infection among HIV-infected patients in northern Vietnam. AIDS Res
Ther. 9:242012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatakeyama S, Yamashita T, Sakai T and
Kamei K: Case Report: Disseminated Talaromyces (Penicillium)
marneffei and mycobacterium tuberculosis coinfection in
a japanese patient with acquired immunodeficiency syndrome. Am J
Trop Med Hyg. 97:38–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nascimento E, Vitali LH, Kress MRVZ and
Martinez R: Cryptococcus neoformans and C. Gattii isolates from
both HIV-infected and uninfected patients: Antifungal
susceptibility and outcome of cryptococcal disease. Rev Inst Med
Trop Sao Paulo. 59:e492017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin JN, Lin HH, Lai CH, Wang JL and Yu TJ:
Renal transplant recipient infected with Penicillium marneffei.
Lancet Infect Dis. 10:1382010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chong YB, Tan LP, Robinson S, Lim SK, Ng
KP, Keng TC and Kamarulzaman A: Penicilliosis in lupus patients
presenting with unresolved fever: A report of 2 cases and
literature review. Trop Biomed. 29:270–276. 2012.PubMed/NCBI
|
|
13
|
Lee PP, Chan KW, Lee TL, Ho MH, Chen XY,
Li CH, Chu KM, Zeng HS and Lau YL: Penicilliosis in children
without HIV infection-are they immunodeficient? Clin Infect Dis.
54:e8–e19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chitasombat M and Supparatpinyo K:
Penicillium marneffei infection in immunocompromised host.
Curr Fungal Infect Rep. 7:44–50. 2013. View Article : Google Scholar
|
|
15
|
Wong SY and Wong KF: Penicillium mameffei
infection in AIDS. Pathol Res Int. 2011:7642932011. View Article : Google Scholar
|
|
16
|
Ma T, Chen R, Li X, Lu C and Xi L: The in
vitro fungicidal activity of human macrophages against Penicillium
marneffei is suppressed by dexamethasone. Microb Pathog. 86:26–31.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Browne SK, Burbelo PD, Chetchotisakd P,
Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool
K, Zaman R, Ding L, et al: Adult-onset immunodeficiency in thailand
and taiwan. N Engl J Med. 367:725–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee PP, Mao H, Yang W, Chan KW, Ho MH, Lee
TL, Chan JF, Woo PC, Tu W and Lau YL: Penicillium marneffei
infection and impaired IFN-γ immunity in humans with
autosomal-dominant gain-of-phosphorylation STAT1 mutations. J
Allergy Clin Immunol. 133:894–896.e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vanittanakom N and Sirisanthana T:
Penicillium marneffei infection in patients infected with human
immunodeficiency virus. Curr Top Med Mycol. 8:35–42.
1997.PubMed/NCBI
|
|
20
|
Sirisanthana T, Supparatpinyo K, Perriens
J and Nelson KE: Amphotericin B and itraconazole for treatment of
disseminated Penicillium marneffei infection in human
immunodeficiency virus-infected patients. Clin Infect Dis.
26:1107–1110. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JL, Hung CC, Chang SC, Chueh SC and
La MK: Disseminated Penicillium marneffei infection in a
renal-transplant recipient successfully treated with liposomal
amphotericin B. Transplantation. 76:1136–1137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walsh TJ, Pappas P, Winston DJ, Lazarus
HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R,
Donowitz G, et al: Voriconazole compared with liposomal
amphotericin B for empirical antifungal therapy in patients with
neutropenia and persistent fever. N Engl J Med. 346:225–234. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zmeili OS and Soubani AO: Pulmonary
aspergillosis: A clinical update. QJM. 100:317–334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouyang Y, Cai S, Liang H and Cao C:
Administration of voriconazole in disseminated talaromyces
(Penicillium) Marneffei Infection: A Retrospective Study.
Mycopathologia. 182:569–575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perfect JR, Marr KA, Walsh TJ, Greenberg
RN, DuPont B, de la Torre-Cisneros J, Just-Nübling G, Schlamm HT,
Lutsar I, Espinel-Ingroff A and Johnson E: Voriconazole treatment
for less-common, emerging, or refractory fungal infections. Clin
Infect Dis. 36:1122–1131. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alves IA, Staudt KJ, Silva CM, Lock GA,
Dalla Costa T and de Araujo BV: Influence of experimental
cryptococcal meningitis in wistar rats on voriconazole brain
penetration assessed by microdialysis. Antimicrob Agents Chemother.
61(pii): e00321–e00417. 2017.PubMed/NCBI
|
|
27
|
Herkert PF, Hagen F, de Oliveira Salvador
GL, Gomes RR, Ferreira MS, Vicente VA, Muro MD, Pinheiro RL, Meis
JF and Queiroz-Telles F: Molecular characterisation and antifungal
susceptibility of clinical Cryptococcus deuterogattii (AFLP6/VGII)
isolates from Southern Brazil. Eur J Clin Microbiol Infect Dis.
35:1803–1810. 2016. View Article : Google Scholar : PubMed/NCBI
|