Introduction
Congenital melanocytic nevi (CMN) are present at
birth or arise within the first few weeks of life. Their size has
been used as the principal criterion for classification. Kopf et
al (1) proposed the
classification system currently in use according to which CMN are
classified into 3 groups: Small, medium and large/giant. Small CMN
are <1.5 cm; medium CMN measure 1.5–19.9 cm and large or giant
CMN are ≥20 cm in projected adult size (PAS).
Giant congenital melanocytic nevi (GCMN) often
present with an unsightly appearance that cannot be covered with
normal dressing; thus, GCMN places a considerable psychological
burden on the patients as well as their parents. The estimated
prevalence of CMN is 1–6% among all newborn infants (2), while GCMN (which are much rarer) are
estimated to affect 1 in 20,000 newborns (3,4).
It has been reported that the risk of melanoma in a
patient with CMN increases with the size of the nevus (5,6). In
patients with a GCMN, the lifetime melanoma transformation risk is
as high as 5–10%, much higher than that for CMN and the common
acquired melanocytic nevi (AMN) (7,8). This
higher malignant rate warrants increased attention of clinicians to
GCMN; however, relatively few studies are dedicated exclusively to
GCMN. Furthermore, in-depth studies on the histopathological
features of GCMN are currently lacking. Studies suggest that CMN
may have different genetic signatures; most cases of GCMN (97.4%)
have a mutation in the gene neuroblastoma RAS (9).
To date, the etiology and molecular mechanisms of
GCMN have remained largely elusive and an enhanced knowledge in
this field will facilitate the exploration of novel treatments. The
present study aimed to determine the histopathological properties
of GCMN in a series of 98 cases and to investigate how these are
associated with the clinical presentation of this skin
condition.
Materials and methods
Selection of cases
Patients diagnosed with GCMN and admitted to the
Plastic Surgery Department of Shanghai Ninth People's Hospital
(Shanghai, China) between January 2013 and December 2015 were
included in the present analysis. Only nevi present at birth and
with a PAS of ≥20 cm were included. For the diagnosis in children,
considering that the lesion increases as the body grows, DeDavid's
estimating curves were used, via which the diameter of the skin
surface was calculated for patients of both sexes in different age
groups (9). A total of 98 GCMN cases
were identified and specimens were obtained during surgical
intervention. Age, sex, nevus location, size and clinical
appearance were recorded. For comparison, 30 CMN specimens (13
males and 17 females between 2 and 39 years old) were obtained from
patients admitted to the Plastic Surgery Department of Shanghai
Ninth People's Hospital between January 2015 and December 2015.
Patients with neurofibroma associated with melanocytic nevi and
acquired nevi were excluded from the present study. All patients or
their parents gave their written informed consent for the use of
their tissue specimens in this study. The study was approved by the
Ethics Committee of Shanghai Ninth People's Hospital (Shanghai,
China).
Histochemical staining
The specimens were routinely fixed in 4%
paraformaldehyde and embedded in paraffin wax. Sections (4 µm) were
obtained and histochemical staining was performed with hematoxylin
and eosin as well as Masson trichrome (for collagen). For
hematoxylin and eosin staining, the sections were stained for
hematoxylin for 7 min, differentiate in 1% acid alcohol for 10 sec,
rinse in running water for 30 min, stain with eosin for 1 min. For
Masson trichrome staining, the specimens were stained with
hematoxylin stain solution for 1 min and acid Fuchsin stain
solution for 30–60 sec, differentiated in
phosphomolybdic-phosphotungstic acid solution for 6 min, and
finally incubated in aniline blue counterstain for 5 min. All the
staining procedures were done in room temperature.
Immunohistochemistry (IHC)
IHC studies were performed on 4-µm sections of
paraformaldehyde-fixed, paraffin-embedded tissue using standard
techniques. Antigen retrieval was performed by boiling in citrate
buffer (pH 6.0) for 10 min. Primary antibodies against Ki-67 (cat.
no. ab15580; 1:200 dilution; Abcam, Cambridge, UK), HMB-45 (cat.
no. M063429-2; 1:50 dilution; Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA), Melan-A (cat. no. sc20032; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), S100 (cat. no. sc52205;
1:200 dilution; Santa Cruz Biotechnology, Inc.) and Sox10 (cat. no.
ab221733; 1:200 dilution; Abcam) were incubated at 4°C overnight.
Endogenous peroxidase was quenched by incubating the sections for
10 min with 0.3% hydrogen peroxide in methanol. Then the sections
were incubated with horseradish peroxidase-conjugated goat
anti-mouse (cat. no. ab6789) or goat anti-rabbit (cat. no. ab6721;
both 1:1,000 dilution; Abcam) secondary antibodies for 1 h at room
temperature. 3-amino-9-ethylcarbazole was the chromogenic
substrate. Sections were counterstained with hematoxylin for 2 min
at room temperature. Melanoma tissue and normal skin tissue from
Pathology Department of Shanghai Ninth People's Hospital were used
for positive and negative controls, respectively. A total of 5
melanoma tissue specimens (3 males and 2 females between 58 and 72
years old) were collected between July 2012 and February 2014. A
total of normal skin tissue specimens (1 males and 4 females
between 21 and 46 years old) were collected from January 2013 to
May 2014. IHC reactivity was independently scored as positive or
negative by MW and QY. The two authors achieved the same results,
which is indicative of the ease of reproducibility.
Histopathological evaluation
Digital images of the specimens were captured and
analyzed with Image-Pro Plus software version 6.0 (Media
Cybernetics, Rockville, MD, USA). Histopathological features were
evaluated based on standard H&E-stained sections and Masson
trichrome-stained sections. The nevi cell network, depth of
invasion, involvement of cutaneous appendages, cytological atypia
and architectural features were then determined. The criteria of
architectural disorder and the grading of cytological atypia were
based on standard criteria mentioned in the literature (10,11). The
cell density was measured in lesions with deep or light color, in
1,000 µm depth below the epidermal basement membrane, and a square
measuring 500×500 µm was marked to calculate the cell density. Five
visual fields of each sample were randomly selected for
calculating, and the evaluation was performed at a magnification of
×100. In the specimens from non-surgical treatment (chemical
peeling or laser therapy), the depth from the epidermal basement
membrane to the dermal treatment plane was measured under a
microscope with Image-Pro Plus software.
Statistical analysis
Statistical analysis was performed using SPSS
version 11.5 software (SPSS, Inc., Chicago, IL, USA). All of the
quantitative values were expressed as the mean ± standard
deviation. Student's t-test was used to determine the significant
differences between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical features
A total of 98 patients diagnosed with GCMN,
comprising 57 males (58.2%) and 41 females (41.8%), aged from 3
months to 37 years (median age, 6 years), were included. The most
common localization of the nevi was the head and neck (44.9%),
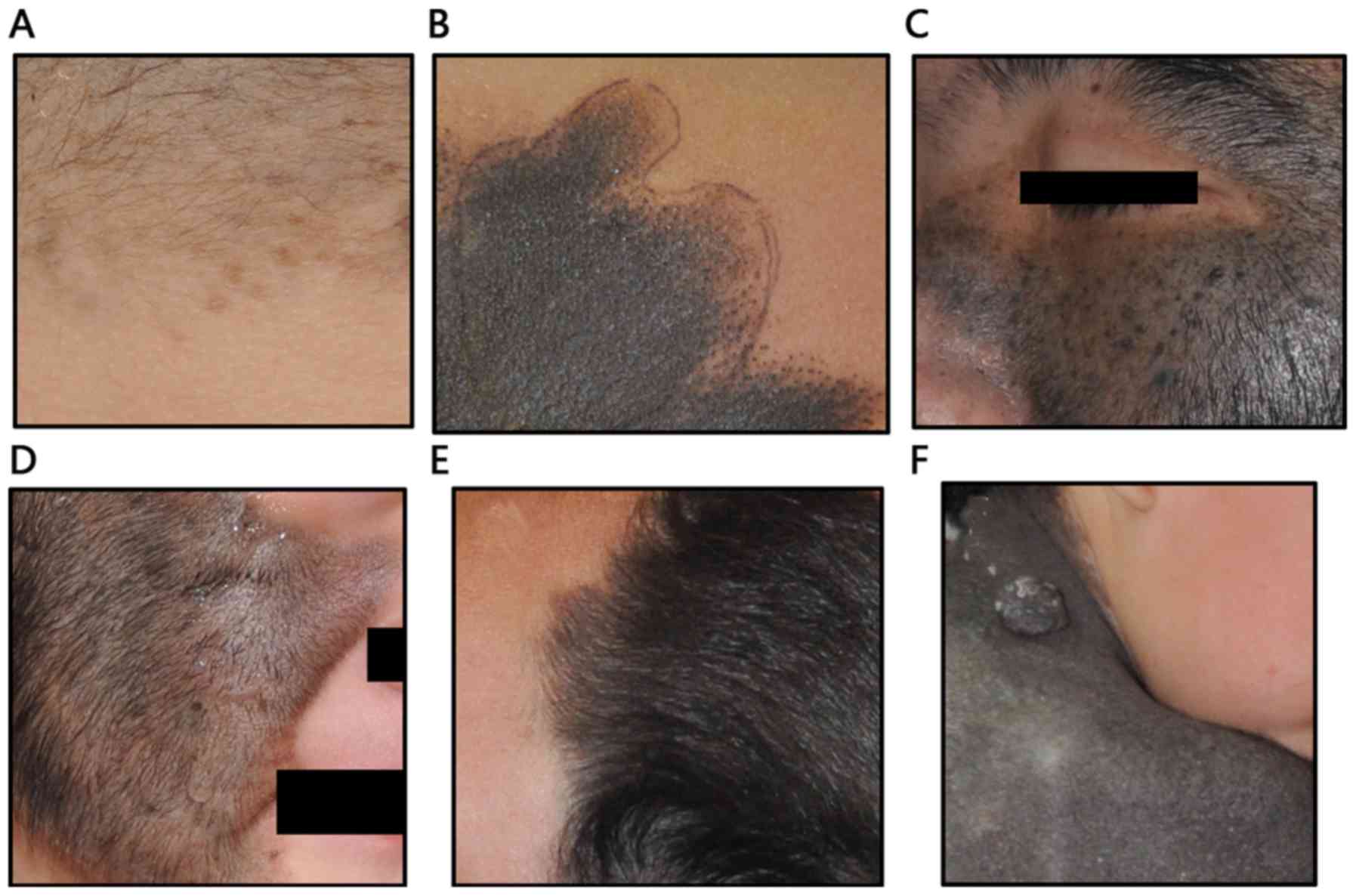
followed by the trunk (31.6%) and extremities (23.5%). Light black
was the most common nevi color (39.8%), followed by deep black
(32.7%) and brown (27.5%). Most of the lesions were covered with
dense hair (72.4%); the lesion color was not associated with this
feature (Fig. 1). Regarding prior
treatment, 10 patients (10.2%) had received partial non-surgical
therapy, 7 received laser therapy and 3 had been subjected to
chemical peeling. Table I presents
the clinical features of the lesions.
 | Table I.Clinical features of patients with
giant congenital melanocytic nevi (n=98 patients). |
Table I.
Clinical features of patients with
giant congenital melanocytic nevi (n=98 patients).
| Feature | N (%) |
|---|
| Sex |
|
| Male | 57 (58.2) |
|
Female | 41 (41.8) |
| Location of
lesion |
|
| Head and
neck | 44 (44.9) |
|
Trunk | 31 (31.6) |
|
Extremities | 23 (23.5) |
| Color of lesion |
|
| Light
black | 39 (39.8) |
| Deep
black | 32 (32.7) |
|
Brown | 27 (27.5) |
| Hair on lesion |
|
| Yes | 71 (72.4) |
| No | 27 (27.6) |
IHC characteristics
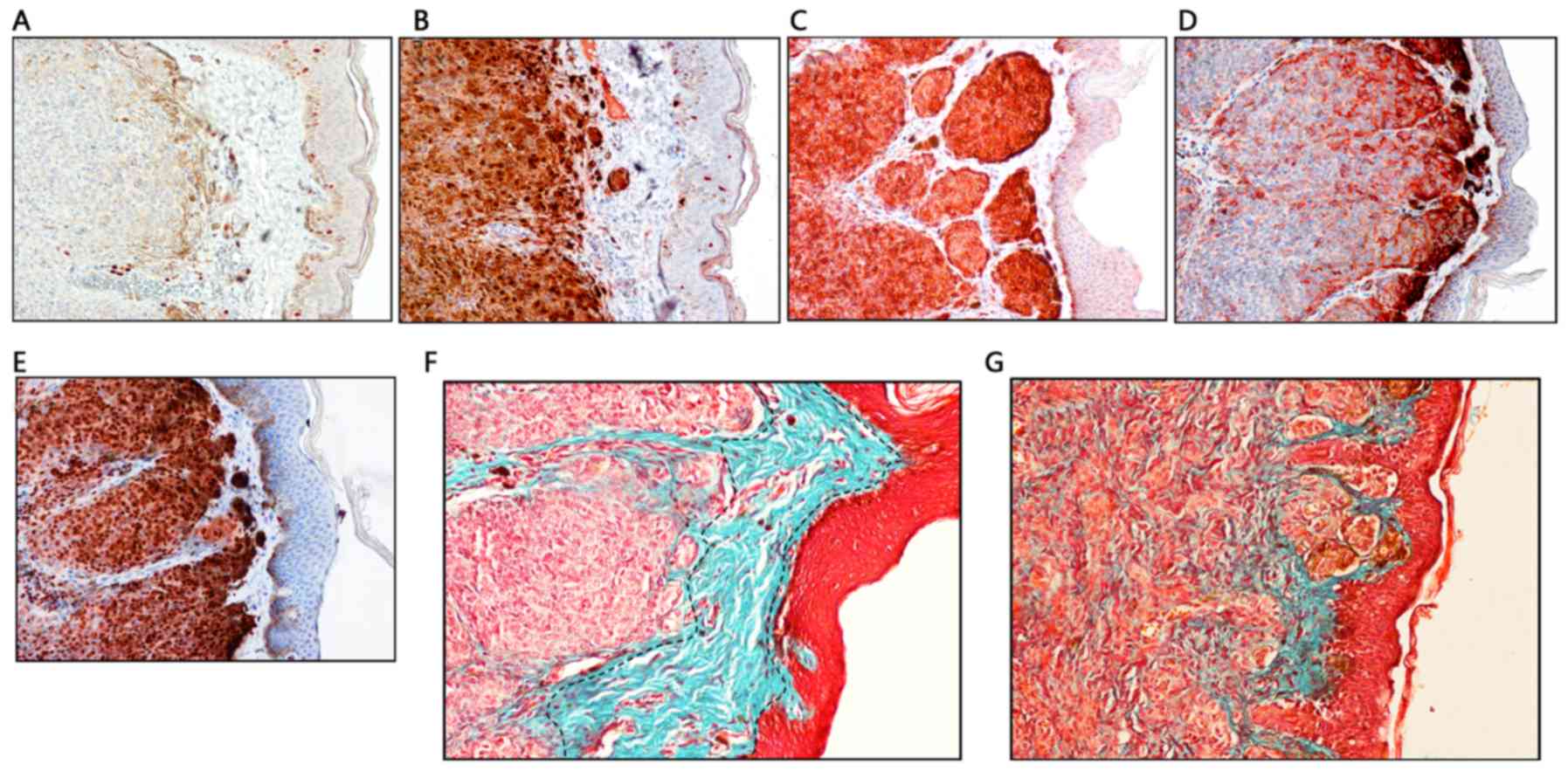
Nuclear immunoreactivity of dermal nevi cells was
noted for Ki-67, but the overall positive rate of dermal nevi cells
was low, ranging from 1–9% (Fig.
2A). All of the lesions exhibited diffuse dermal reactivity to
S100 and Melan-A antibodies (Fig. 2B and
C). HMB-45 expression was observed in the superficial dermis
(Fig. 2D). In addition, all nevi
cells exhibited marked and clear nuclear staining for Sox10
(Fig. 2E).
Histological evaluation
All of the GCM had epidermal flattening with loss of
the rete ridge pattern. Most of the GCMN samples were composed of
intradermal nevi (95.9%)-nevi cells infiltrating the whole dermis
and even into the subcutaneous tissue. Visible pigment granules
were identified around superficial nevus cells. The distribution
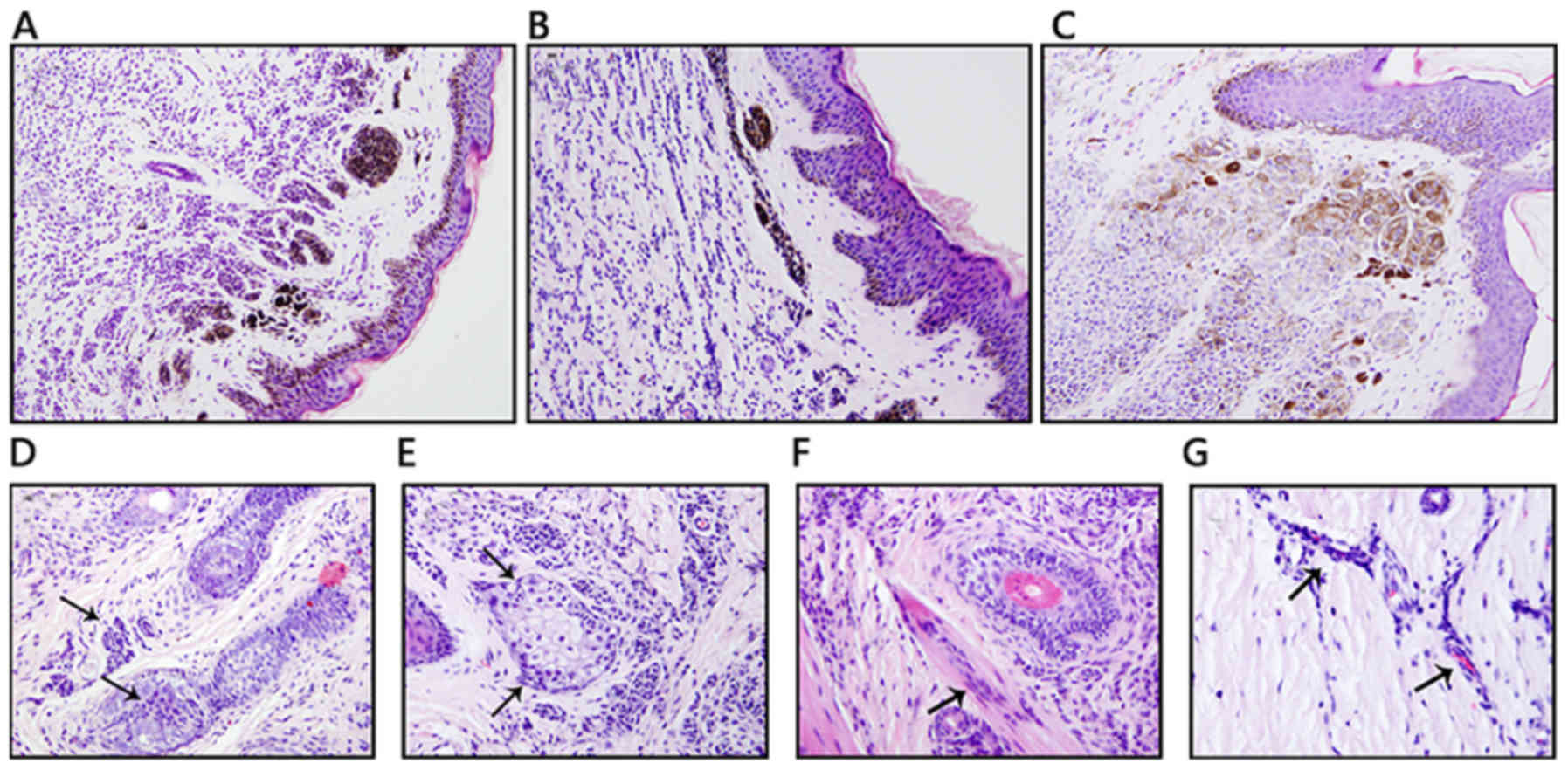
patterns of nevus cells in the dermis were diverse; nevus cells may
distribute evenly in dermis (62 out of 98; 63.3%), or be cord-like
between collagen bundles (23 out of 98; 23.5%); certain lesions
presented with nevus cells gathered in nests (14 out of 98; 14.3%;
Fig. 3A-C).
Generally, nevus cells in the superficial portion
are rounded-to-polygonal cells with uniform nuclei, i.e., type-A
cells. With increasing depth of the location, cells transform to
type-C spindle or fibroblast-like nevomelanocytes, although lesions
with only spindle- or fibroblast-like nevus cell distribution were
also present in certain cases. Arrector pili muscles, hair
follicles and sebaceous gland were occasionally involved (Fig. 3D-G). Nevus cells were also observed
around capillaries. Cell atypia is commonly identified in such
lesions, but mitosis is rarely present.
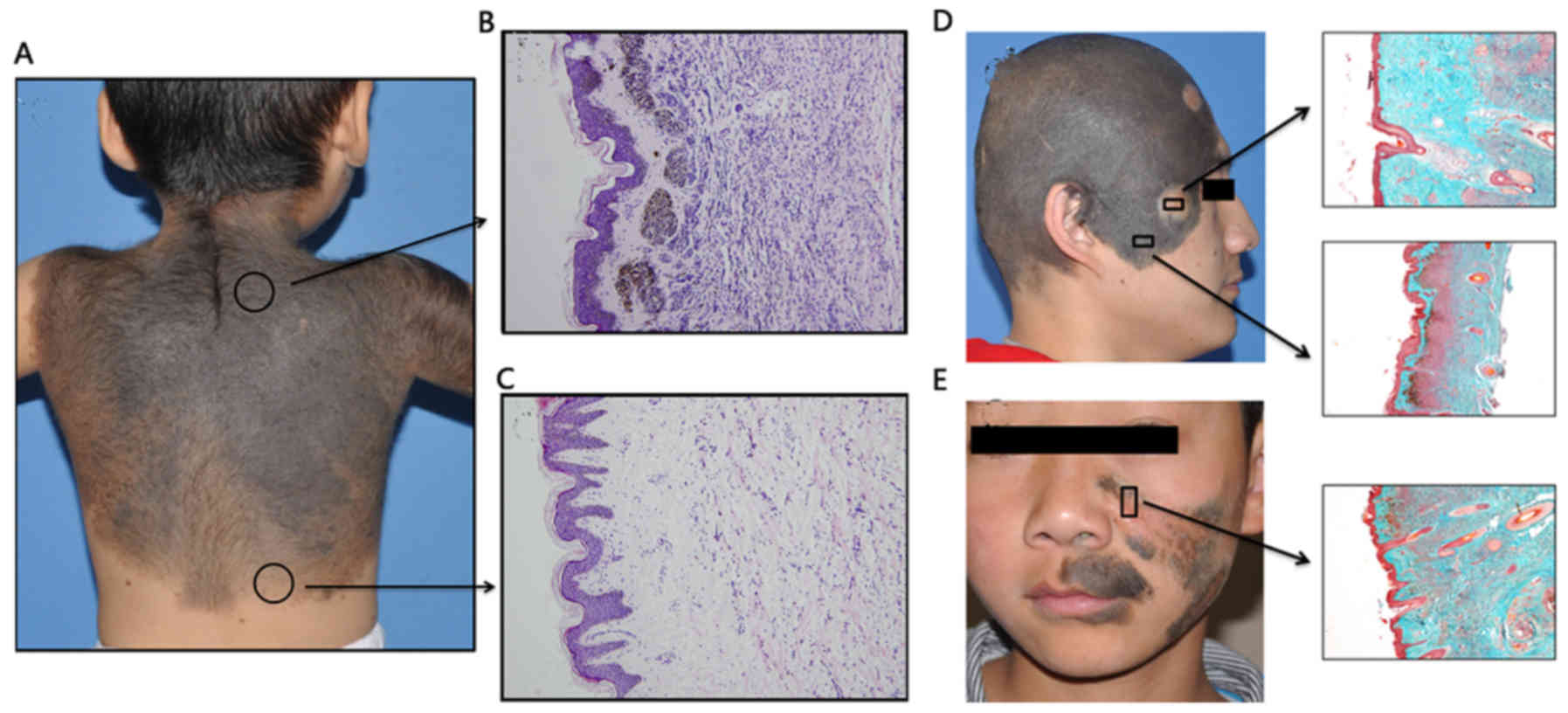
When texture was taken into account, it was noted
that the more dermal infiltration and collagen destruction
occurred, the poorer the skin texture was. Lesions of different
color were compared, and the cell density among different lesions
was measured at the same depth (1,000 µm below the epidermal
basement membrane) under a microscope. The results demonstrated
that deep-colored lesions had a higher nevus cell density compared
with light-colored lesions (257.8±23.9 vs. 114.6±20.9 cells per
250,000 µm2; P<0.01; Fig.
4A-C). As to the depth of nevus cell infiltration, it was
observed that in all of the GCMN lesions, the nevus cells defused
deep into the whole dermis and even infiltrated into subdermal
adipose tissue (Table II). Of note,
most of the GCMN cases (95.9%) had a sub-epidermal non-involvement
zone, which is quite similar to a Grenze zone, and a normal
collagen band was observed by Masson staining (Fig. 2F). The width of this sub-epidermal
non-involvement zone was measured in all specimens, and the average
value was 292.8±74.4 µm. Small CMN specimens featured nevus cells
involving all of the superficial dermis and partially the epidermis
in a junctional nevi pattern (Fig.
2G).
 | Table II.Histopathological characteristics of
light- and deep-color lesions. |
Table II.
Histopathological characteristics of
light- and deep-color lesions.
| Item | Light color | Deep color |
|---|
| Cell
densitya (n/250,000
µm2) | 114.6±20.9 |
257.8±23.9b |
| Depth of cell
infiltration | Whole dermis | Whole dermis |
Non-surgical therapy
In the present study, 10 patients were subjected to
non-surgical therapy. The histopathological changes induced by
these therapies were evaluated. Laser treatment specimens indicated
that laser treatment only removed melanin in the superficial dermal
plane (Fig. 4E); hence, slight
collagen hyperplasia remained and the intradermal nevus cells were
not involved. Indeed, the whole skin structure appeared relatively
unchanged.
In chemical peeling specimens, the upper third of
the dermal nevus cells and melanin were cleaned and replaced by
thick collagen (Fig. 4D); the depth
of the chemical peeling treatment was measured in 3 specimens and
the average depth of this treatment was 1,031.2±158.9 µm. High
counts of inflammatory cells infiltrated into the remaining nevi
cells in the deep dermis, and remaining nevi cells exhibited no
obvious morphological changes. The gross appearance after chemical
peeling therapy indicated visible improvement of the pigment
appearance, albeit with scarring.
Discussion
The current knowledge regarding GCMN is poor, and
previous studies mainly focus on clinical and histopathological
characteristics of CMN (12–15). Although GCMN is a sub-group of CMN,
these nevi have a different mutation spectrum and a higher
malignant transformation rate. Chung et al (16) reviewed giant nevi, describing the
epidemiological characteristics and treatment of GCMN. Furthermore,
Arneja and Gosain (17) reported on
their experience treating GCMN. However, studies on the
histopathological features of GCMN are lacking and are urgently
required.
When considering the anatomic distribution of nevi,
Mark et al (18) reported
that CMN was distributed as follows: 38% on the trunk, 38% on the
legs and arms, 14% on the head and neck, and 10% on the feet and
hands. Sahin et al (19)
investigated only medium-sized CMN, and indicated that the most
common localization was on the head and neck, followed by anterior
and posterior trunk. In the present cohort of GCMN cases, the most
common localization was on the head and neck (44.9%), followed by
trunk (31.6%) and extremities (23.5%).
The distribution pattern in the present study was
similar to that reported by Sahin et al (19), which suggests large-sized nevi may be
different from small CMN regarding their distribution. Mark et
al (18) indicated that nearly
all CMN are brown, but according to the present results, light and
deep black were the most common color of GCMN. This color
difference between studies may be explained by more dense nevi
cells infiltration and melanin synthesis in GCMN. The color depth
is correlated with nevus cell infiltration, and thus, the deeper
the color, the more nevus cell infiltration and superficial melanin
deposition are present. Skin texture is also associated with the
infiltration of nevus cells; infiltrating cells destroy dermal
collagen, and thus, the skin texture is affected. It may therefore
be recommended that nevi cell infiltration is estimated by noting
the texture and color of the nevus.
In the present study, the IHC characteristics of
GCMN were determined by evaluation of Ki67, Melan-A, S-100, HMB-45
and SOX-10 immunopositivity in GCMN lesions. The nuclear
proliferation marker Ki67 assumes an important role in the
distinction between melanocytic lesions; benign nevi have
proliferation indices of <5%, while the average index of
melanomas is 27% (range, 5–50%) (20,21). The
present study indicated that the overall positive rate was low
(ranging from 1–6%), which is quite close to the rate reported in
CMN by Lebe et al (22). The
result implied that the size of the lesion is not associated with
the proliferative activity. The overall low Ki-67 labeling observed
in the present study is consistent with the benign nature of GCMN.
HMB45 was discovered in 1986 from an extract of melanoma, and the
antibody detecting the cytoplasmic pre-melanosomal glycoprotein was
named gp100 (23). In CMN, HMB45
staining is usually restricted to the junction; in the present
cohort of GCMN cases, even upper-layer nevus cells exhibited HMB45
staining, which proves that nevus cells in the superficial layer of
GCMN are more active than those of CMN. This may explain for the
higher malignant transformation rate of GMCN.
SOX10 is a key molecule in the embryonic development
of melanocytes. Haploinsufficiency of SOX10 causes aganglionosis of
the colon and pigmentation defects. Extensive research has revealed
that SOX10 has an important role in GCMN and melanoma (24,25). The
present study demonstrated that nevus cells in GCMN exhibited
marked nuclear staining for SOX10. However, the underlying
molecular mechanisms via which SOX10 exerts its effects remain
elusive.
Previous studies summarized the pathological
characteristics of CMN and concluded that nevus cells extend
between collagen bundles of the reticular dermis as single cells or
cords of cells (26). The present
study indicated that in certain cases of dermal GCMN, nevus cell
infiltration is relatively dense and the phenomenon of single or
cords of cells extending between collagen may not appear.
In the present study, 95.9% of the GCMN featured a
sub-epidermal non-involvement zone, which is different in small
CMN, while numerous CMN cases had a junctional and compound
pattern. This is a noteworthy phenomenon, which reflects the
derivation of the nevus. Mutation of melanoblasts during migration
in the embryonic stage leads to development into a nevus, and
mutations at different time-points cause different clinical and
histopathological characteristics. Earlier mutations in migration
stages result in a larger nevus size with a deeper infiltration,
since a certain distance is present between the proliferated nevus
cells and epidermis, so that a nevus cell-poor zone is formed under
the epidermis. If a mutation occurs in a late stage, the resulting
nevus is smaller in size and proliferating nevus cells are close to
the epidermis or even involved in the basal layer of the
epidermis.
Non-surgical techniques, including chemical peeling
and laser therapy, are occasionally used to treat GCMN. Although it
has been suggested that laser therapy is effective in removing
small melanocytic nevi (27), the
present study suggests that it only attacks the superficial,
pigment-bearing portion of the nevus and sub-cutaneous nevus cells
are left behind and covered with a layer of superficial scar
tissue. Indeed, the remaining nevus cells in the deep dermis still
possess a malignant transformation capability, as melanoma
formation in GCMN was reported after dermabrasion (28,29).
With this regard, caution is therefore warranted when selecting a
non-surgical therapy for GCMN.
The current knowledge on GCMN is limited, and the
mechanisms underlying its genesis and development have remained to
be fully elucidated. In the present study, a large cohort of GCMN
cases demonstrated diverse clinical appearances and
histopathological characteristics. Different distribution patterns
of nevus cells were identified among GCMN cases, which were also
quite different from CMN. The proliferation rate of nevus cells was
similar, and HMB-45 staining was more intense in the upper portion
of nevi. A sub-epidermal non-involvement zone was identified in
most of the GCMN, which is indicative of their different origin
from that of CMN, and is in accordance with the differences in
mutation spectrum between GCMN and small CMN.
In summary, GCMN have unique morphological and IHC
features compared with small CMN or AMN. Due to their higher
malignant transformation rate and poorer cosmetic appearance, GCMN
requires a different and urgent therapeutic strategy. The present
study performed an in-depth summary of the histopathological
features of GCMN, and provides a deeper understanding of this
disease, which will provide a foundation for exploring novel
treatment options.
Acknowledgements
The authors would like to thank Dr Hainan Zhu
(Department of Plastic and Reconstructive Surgery, Shanghai Ninth
People's Hospital, Shanghai Jiao Tong University, School of
Medicine, Shanghai, China) for data collection and insightful
discussion.
Funding
The present study was funded by the Shanghai Pujiang
Talents Program [grant no. PJ(2015)0002993] and National Natural
Science Foundation of China (grant no. 81871595).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FX, QL and QY designed the study. BG and LS
collected the clinical data. QY, MW and LS performed the
experiments and analyzed the data. MW and QY wrote the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients or their parents gave their written
informed consent for the use of their tissue specimens in this
study. The study was approved by the Ethics Committee of Shanghai
Ninth People's Hospital (Shanghai, China).
Patient consent for publication
All patients or their parents gave their written
informed consent for the use of their tissue specimens and images
in this study.
Competing interests
The authors declare no competing interests.
References
|
1
|
Kopf AW, Bart RS and Hennessey P:
Congenital nevocytic nevi and malignant melanomas. J Am Acad
Dermatol. 1:123–130. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hashmi GS, Ahmed SS and Khan S: Congenital
giant melanocytic nevi. Rare Tumors. 1:e92009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyon VB: Congenital melanocytic nevi.
Pediatr Clin North Am. 57:1155–1176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arad E and Zuker RM: The shifting paradigm
in the management of giant congenital melanocytic nevi: Review and
clincal applications. Plast Reconstr Surg. 133:367–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watt AJ, Kotsis SV and Chung KC: Risk of
melanoma arising in large congenital melanocytic nevi: A systematic
review. Plast Reconstr Surg. 113:1968–1974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krengel S, Hauschild A and Schafer T:
Melanoma risk in congenital melanocytic naevi: A systematic review.
Br J Dermatol. 155:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bett BJ: Large or multiple congenital
melanocytic nevi: Occurrence of cutaneous melanoma in 1008 persons.
J Am Acad Dermatol. 52:793–797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hale EK, Stein J, Ben-Porat L, Panageas
KS, Eichenbaum MS, Marghoob AA, Osman I, Kopf AW and Polsky D:
Association of melanoma and neurocutaneous melanocytosis with large
congenital melanocytic naevi-results from the NYU-LCMN registry. Br
J Dermatol. 152:512–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charbel C, Fontaine RH, Malouf GG, Picard
A, Kadlub N, El-Murr N, How-Kit A, Su X, Coulomb-L'Hermine A, Tost
J, et al: NRAS mutation is the sole recurrent somatic mutation in
large congenital melanocytic nevi. J Invest Dermatol.
134:1067–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy GF and Mihm MC: Recognition and
evaluation of cytological dysplasia in acquired melanocytic nevi.
Hum Pathol. 30:506–512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weinstock MA, Barnhill RL, Rhodes AR and
Brodsky GL: Reliability of the histopathologic diagnosis of
melanocytic dysplasia the dysplastic nevus panel. Arch Dermatol.
133:953–958. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeDavid M, Orlow SJ, Provost N, Marghoob
AA, Rao BK, Wasti Q, Huang CL, Kopf AW and Bart RS: Neurocutaneous
melanosis: Clinical features of large congenital melanocytic nevi
in patients with manifest central nervous system melanosis. J Am
Acad Dermatol. 35:529–538. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Price HN and Schaffer JV: Congenital
melanocytic nevi-when to worry and how to treat: Facts and
controversies. Clin Dermatol. 28:293–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alikhan A, Ibrahimi OA and Eisen DB:
Congenital melanocytic nevi: Where are we now? Part i. clinical
presentation, epidemiology, pathogenesis, histology, malignant
transformation, and neurocutaneous melanosis. J Am Acad Dermatol.
67:495 e1–e17. 2012.
|
|
15
|
Gallus S and Naldi J; Oncology Study Group
of the Italian Group for Epidemiologic Research in Dermatology, :
Distribution of congenital melanocytic naevi and congenital
naevus-like naevi in a survey of 3406 Italian school children. Br J
Dermatol. 159:433–438. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chung C, Forte AJ, Narayan D and Persing
J: Giant nevi: A review. J Craniofac Surg. 17:1210–1215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arneja JS and Gosain AK: Giant congenital
melanocytic nevi. Plast Reconstr Surg. 124 (Suppl 1):e1–e13. 2009.
View Article : Google Scholar
|
|
18
|
Mark GJ, Mihm MC, Liteplo MG, Reed RJ and
Clark WH: Congenital melanocytic nevi of the small and garment
type. Clinical, histologic, and ultrastructural studies. Hum
Pathol. 4:395–418. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahin S, Levin L, Kopf AW, Rao BK, Triola
M, Koenig K, Huang C and Bart R: Risk of melanoma in medium-sized
congenital melanocytic nevi: A follow-up study. J Am Acad Dermatol.
39:428–433. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nasr MR and El-Zammar O: Comparison of
pHH3, Ki-67, and survivin immunoreactivity in benign and malignant
melanocytic lesions. Am J Dermatopathol. 30:117–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohsie SJ, Sarantopoulos GP, Cochran AJ and
Binder SW: Immunohistochemical characteristics of melanoma. J Cutan
Pathol. 35:433–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebe B, Pabuccuoglu U and Ozer E: The
significance of Ki-67 proliferative index and cyclin D1 expression
of dysplastic nevi in the biologic spectrum of melanocytic lesions.
Appl Immunohistochem Mol Morphol. 15:160–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shakhova O, Zingg D, Schaefer SM, Hari L,
Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F,
Mihic-Probst D, et al: Sox10 promotes the formation and maintenance
of giant congenital naevi and melanoma. Nat Cell Biol. 14:882–890.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rothberg BE, Moeder CB, Kluger H, Halaban
R, Elder DE, Murphy GF, Lazar A, Prieto V, Duncan LM and Rimm DL:
Nuclear to non-nuclear Pmel17/gp100 expression (HMB45 staining) as
a discriminator between benign and malignant melanocytic lesions.
Mod Pathol. 21:1121–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bakos RM, Maier T, Besch R, Mestel DS,
Ruzicka T, Sturm RA and Berking C: Nestin and SOX9 and SOX10
transcription factors are coexpressed in melanoma. Exp Dermatol.
19:e89–e94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tannous ZS, Mihm MC Jr, Sober AJ and
Duncan LM: Congenital melanocytic nevi: Clinical and
histopathologic features, risk of melanoma, and clinical
management. J Am Acad Dermatol. 52:197–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SE, Choi JY, Hong KT and Lee KR:
Treatment of acquired and small congenital melanocytic nevi with
combined Er: YAG laser and long-pulsed alexandrite laser in Asian
skin. Dermatol Surg. 41:473–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zutt M, Kretschmer L, Emmert S, Haenssle
H, Neumann C and Bertsch HP: Multicentric malignant melanoma in a
giant melanocytic congenital nevus 20 years after dermabrasion in
adulthood. Dermatol Surg. 29:99–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishi K, Matsuda N, Kubota Y, Katsube KI,
Imanishi N and Nakajima T: Rapid, severe repigmentation of
congenital melanocytic naevi after curettage and dermabrasion:
Histological features. Br J Dermatol. 156:1251–1257. 2007.
View Article : Google Scholar : PubMed/NCBI
|