Introduction
Intracranial stenosis may result in ischemic
infarction (1) and is associated
with a risk of ischemic stroke (2).
Extracranial and intracranial carotid artery stenosis is common
among symptomatic patients in China (3). Autopsy studies have proved that
cerebral vascular occlusion is the major cause of stroke (4). The most common location for
intracranial stenosis is the internal carotid artery (ICA)
(5) and it is accessed by evaluation
of the degree of luminal stenosis on angiography (6). Hemodynamic changes may provide
important information for clinical decision-making, but the degree
of ICA stenosis, which is responsible for hemodynamic changes, may
not be properly determined by using imaging modalities (7).
Application of suitable diagnostic methods for
intracranial stenosis remains challenging (8). The diagnostic methods currently used
for detection of intracranial stenosis are transcranial Doppler
ultrasound (9), digital subtraction
angiography, high-resolution magnetic resonance imaging (MRI)
(10), conventional catheter
angiography (9), CT angiography
(10) and magnetic resonance
angiography (MRA) (5). CT
angiography is less prone to movement artifacts within the blood
vessels and has a shorter signal-to-noise ratio than MRA, but has
the risk of degradation of image quality and limitations of
post-processing artifact interpretations (9). High-resolution MRI is suitable for
diagnosis of the C1, C3 and C5 segments only due to the inherent
signal-intensity loss of parallel imaging in the other segments
(5) but it cannot be applied for
patients with pacemakers (9). MRA
facilitates the determination of stenosis grade (5). Digital subtraction angiography is
usually performed after MRA (5).
Transcranial Doppler ultrasound is only effective when the blood
flow pattern is abnormal (5).
Overall, each diagnostic method has its own advantages and
disadvantages.
The purpose of the present prospective study was to
compare the sensitivities and accuracies of color-coded duplex
sonography with those of MRA for the detection of intracranial
stenosis while using conventional catheter angiography as a
reference standard in Chinese patients with coronary artery
disease.
Materials and methods
Inclusion/exclusion criteria
Patients aged ≥18 years with angiographic
confirmation of 3 vessels and/or left stem coronary artery disease,
as well as symptoms of a transient ischemic attack and cerebral
ischemia with/without neurologic deficits were included in the
study. Only patients with isolated intracranial stenosis were
included. Patients who had impairments of the brain, spinal cord or
nerve function, or diseases associated with functional
deterioration of organs (according to clinical diagnostic
parameters and MRI) were excluded from the study. Patients with
inadequate image quality for interpretation were also excluded from
the analysis. Prior to transcranial diagnosis, plaques
(atherosclerotic lesions) present in the extracranial vessels were
excluded by standard extracranial color-coded duplex
sonography.
Color-coded duplex sonography
All color-coded duplex sonographies were performed
using 19″ LED up and down 90° foldable color-duplex ultrasound
systems equipment (LOGIQ e; GE Healthcare) with a 2.4–10.0 MHz
linear array transducer (9L-D; GE Healthcare) for the extracranial
examination and a 4–10 MHz phased array (PA6-8 H46701J; GE
Healthcare) for the transtemporal examination.
Transcranial color-coded duplex sonographies were
performed with a 4 MHz center transmit frequency in color mode,
linear post-processing, highest transmit power, at intermediate
resolution and the pulse repetition frequency for the central focal
zone. The gain of color was maintained as per the acoustic bone
window of the proband to avoid colored speckles outside the borders
of vessels. The gate of Doppler was set at 5 mm and 0° angles in
all the measurements of blood flow. If the angle was <60°, it
was corrected in the segment of the arteries with a minimum of 20
mm. Transcranial color-coded duplex sonographies were started from
the axially-oriented transtemporal approach. The butterfly-shaped
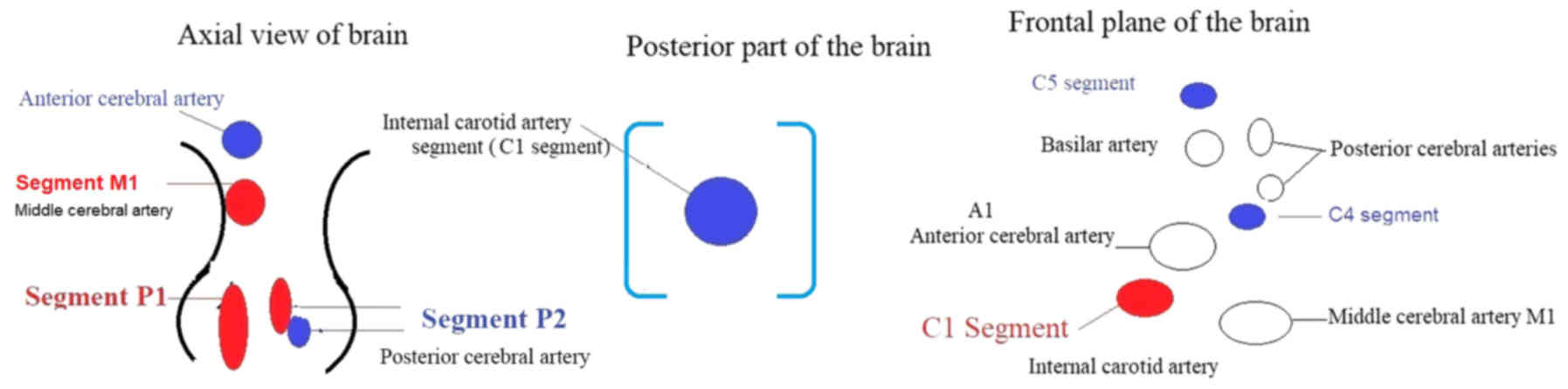
hypoechogenic mesencephalic brainstem was located. As illustrated
in Fig. 1, a P1 segment (indicated
in red) and P2 segment (red and blue) were identified for the
assessment of the posterior cerebral artery. The transducer was
moved slightly upwards, and the M1 segment (indicated in red) of
the middle cerebral artery and anterior cerebral artery (indicated
in blue) was visualized. Finally, the transducer was moved slightly
toward the posterior part of the brain and a cross-sectional view
of the terminal ICA (the C1 segment) was visualized. The transducer
was made perpendicular in an anti-clockwise direction towards the
frontal planes. During the anterior scanning, the C1 segment
(indicated in red) of the ICA, the A1 segment of the anterior
cerebral artery and the M1 segment of the middle cerebral artery
were visualized. Slightly frontal towards the posterior frontal
plane, the basilar artery was visualized at the top, and the
posterior cerebral arteries and C5 segment (blue color) near the
carotid canal were also visualized. Subsequently, in a slightly
lateral view, the C4 segment was identified (blue color; Fig. 1) (1).
For the siphon segments, the axial mesencephalic
image plane was preferred. For the diagnosis, the coronal planes
were used for the middle cerebral artery. The M1 segment in the
middle cerebral artery and the carotid siphon C1 and C5 segments
were diagnosed on the bilateral sides (1).
The end-diastolic blood flow velocities, peak
systolic blood flow velocities and mean blood flow velocities were
recorded. The pulsatility index, resistance index and C1/ICA index
were calculated for each vessel segment as per Equations i, ii and
iii (1).
Pulsatility index=Peak systolic blood flow velocity - End diastolic
blood flow velocityMean blood flow velocity
Resistance index=Peak systolic blood flow velocity - End diastolic
blood flow velocityPeak systolic blood flow velocity
C1ICAindex=Mean blood flow velocity of C1Mean blood flow velocity
of the ICA
MRA
3.0 Tesla MRI equipment (GE Healthcare) was used to
visualize the cervical intracranial artery, petrous intracranial
artery, cavernous intracranial artery, supraclinoid portions,
anterior cerebral artery, segment A1, segment A2, middle cerebral
artery, segment M1, segment M2, posterior cerebral artery, segment
P1, segment P2, intracranial vertebral artery, the proximal basilar
artery and distal basilar artery. The field of view was as small as
possible over the middle cerebral artery and 512×512 mm2
matrices. T1-weighted imaging (T1WI) was performed with a
repetition time/echo time (RT/ET) of 565/15.79 msec, T2WI with
fast-spin-echo array coil spatial sensitivity encoding and a RT/ET
of 2,884/50 msec, proton density-weighted imaging with a RT/ET of
6,241/32.6 msec and short T1 inversion recovery imaging with a
RT/ET of 3,701/56.3 msec were acquired for determination of the
middle cerebral artery lumen diameter. A total of 16 MR slices (2
mm slice thickness ×0.5 mm slice interval) with 6-fold signal
averaging including stenosis were acquired (11).
Invasive catheter angiography
The patients who exhibited stenosis on color-coded
duplex sonography and/or MRA were subjected to invasive catheter
angiography. Femoral punctures were given to patients by injection
of vehicles using Ultrasvist (Bayer Healthcare AG). In late venous
phase with standard anteroposterior, lateral and oblique images
were acquired with 1,024×1,024 matrix, pixel size of 0.21×0.21 and
a field of view of 22 cm with 5 ml/sec of the contrast agent inflow
rate (12).
Image analysis
All Digital Imaging and Communications in Medicine
files were uploaded onto a workstation (version 4.0; GE
Healthcare). The artery diameter of the maximal stenosis side and
non-stenosis region were measured. The proximal and distal views
were examined. The analysis was performed orthogonal to the long
axis of the artery and the stenosis was evaluated in segment M1 for
comparison (12). The degree of
stenosis was considered as per Equation iv, in accordance with the
Warfarin-Aspirin Symptomatic Intracranial Disease methodology
(9) under consultation of a
neuroradiologist (25 years of experience).
%Stenosis=[1-Artery diameter of the maximal stenosis sideArtery
diameter of the non - stenosis region]×100
A reduction in diameter of ≥50% of the artery was
considered as a positive obstructive lesion; otherwise, the
diagnosis of obstructive lesion was negative (13).
Advantage score analysis
The advantages of each of the modalities adopted
were evaluated by clinical decision-making analysis. The advantage
score of each diagnostic method adopted was evaluated as per
Equations v and vi (14):
Advantage score=Number of patients with a true positive obstructive
lesionNumber of patients subjected to diagnosis-(Number of patients
with false positive stenosisNumber of patients were subjected to
diagnosisxRisk of overdiagnosis)
Risk of overdiagnosis=Threshold probability1-Threshold
probability
The invasive catheter angiography was used as the
gold standard to determine the true- and false-positive rates of
the other methods.
Cost
The cost of each diagnostic modality was
calculated.
Statistical analysis
InStat, version 3.0 for Windows (GraphPad Inc.) was
used for statistical analysis. Categorical data were compared using
the Wilcoxon matched-pairs test (12). All variables were considered
significant at a 99% confidence level. The mean reader difference
values were calculated for each diagnostic method adopted to assess
interobserver reliability (15).
Continuous data were compared using the Friedman test followed by
the Nemenyi test (considering a critical value q of >3.314 as
indicative of significance). The cost was analyzed by one-way
analysis of variance (16) followed
by the Tukey-Kramer multiple-comparisons test.
Results
Patient characteristics
Between January 2015 and December 2017, a total of
1,005 patients with 3 vessels and/or left stem coronary artery
disease were available at Luoyang Central Hospital Affiliated to
Zhengzhou University (Luoyang, China) and the referring hospitals.
All patients were subjected to interview (panel of a cardiologist,
a neurologist and a physician of the institute, all with a minimum
of 3 years of experience) and the cardiovascular risk of each
patient was estimated based on demographic, clinicopathological and
laboratory data (Table I). Among
those patients, three had impairments of the brain, one had
impairments of the spinal cord, one had impairments of nerve
function, one had diseases associated with functional deterioration
of organs and for one patient, the image quality of color-coded
duplex sonography was inadequate for interpretation. Therefore,
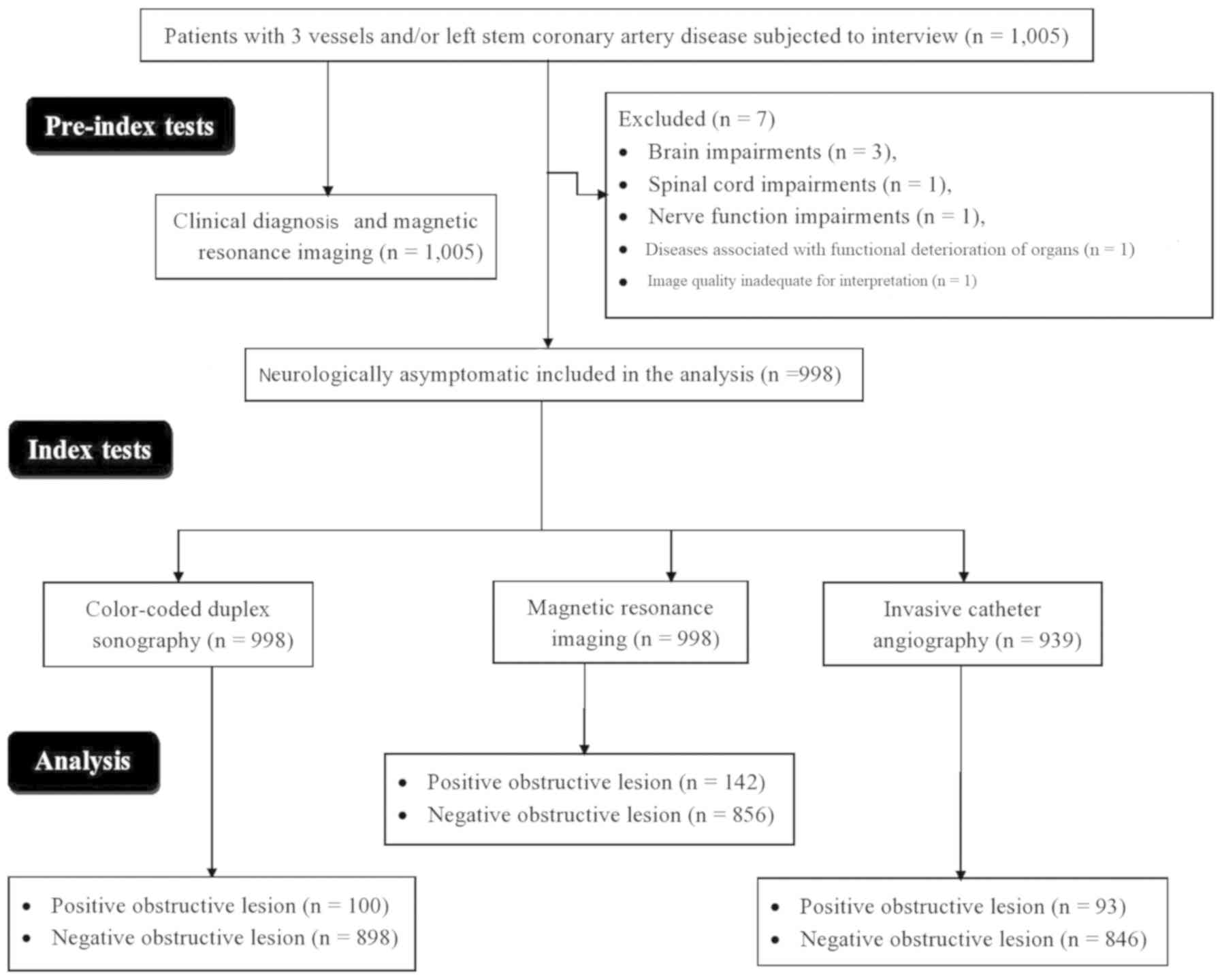
these patients were excluded from the analysis. A total of 998
neurologically asymptomatic patients were subjected to color-coded
duplex sonography and MRA. The flow diagram for inclusion of
patients in the present study is provided in Fig. 2.
 | Table I.Demographic and clinicopathological
characteristics and laboratory parameters of the patients enrolled
(n=998). |
Table I.
Demographic and clinicopathological
characteristics and laboratory parameters of the patients enrolled
(n=998).
| Item | Value |
|---|
| Ethnicity |
|
| Han
Chinese | 912 (92) |
|
Mongolian | 73 (7) |
|
Tibetan | 13 (1) |
| Age (years) |
|
|
Range | 19–85 |
| Mean ±
SD | 59.85±8.89 |
| Sex |
|
| Male | 633 (63) |
|
Female | 365 (37) |
| Blood pressure
(mmHg) |
|
|
Diastolic | 86.52±5.45 |
|
Systolic | 135.12±14.12 |
| Diabetes | 201 (20) |
| Time from onset of
transient | 41.12±5.45 |
| ischemic symptoms
(days) |
|
| Transient ischemic
symptoms |
|
| Mild
paralysis in side of body | 55 (6) |
| Garbled
speech | 101 (10) |
| Double
vision | 52 (5) |
|
Dizziness | 173 (17) |
|
Headache | 203 (20) |
| Dyslipidemia | 173 (17) |
| Body mass index
(kg/m2) |
|
|
18.5–24.9 (normal) | 308 (31) |
| 25-29.9
(overweight) | 545 (55) |
| ≥30
(obese) | 145 (14) |
| Smoking |
|
|
Never | 790 (79) |
|
Previously | 145 (15) |
|
Currently | 63 (6) |
| Alcohol intake |
|
|
Never | 888 (89) |
|
Previously | 65 (6) |
|
Currently | 45 (5) |
| Hyperuricemia | 38 (4) |
| Sleep apnea
syndrome | 21 (2) |
| Pulmonary artery
pressure (mmHg) | 23.12±1.25 |
| Claudication | 5 (1) |
| Painful cramping in
hips | 3 (1) |
| Leg numbness | 15 (2) |
| Coldness in lower
legs | 8 (1) |
| Sores on toes | 11 (1) |
| Hair loss on
feet | 17 (2) |
| Slower growth of
toenails (self-reported by patients) | 16 (2) |
| Shiny skin of
legs | 42 (4) |
| Erectile
dysfunction in males | 52 (5) |
| Complaints of
disrupted sleep | 15 (2) |
Obstructive lesions of the ICA
In the transcranial and extracranial portions,
stenosis was detected in 909 patients by color-coded duplex
sonography and in 939 patients by MRA. Therefore, a total of 939
patients were subjected to invasive catheter angiography. Invasive
catheter angiography was superior in the detection of stenosis
compared with color-coded duplex sonography (P<0.0001; q=4.144)
and MRA (P<0.0001; q=7.301). The results of the different
diagnostic modalities regarding evaluation of obstructive lesions
are provided in Table II. The
pulsatility index, resistance index and C1/ICA index were higher
for obstructive lesions than for normal lesions (P<0.0001 for
all; data not shown). The intracranial stenosis in the other
intracerebral arteries were mostly found in M1 and M2 segments of
middle cerebral arteries, the vertebral artery and the anterior
cerebral artery. The distribution of intracranial stenosis in the
other intracerebral arteries assessed is presented in Table III.
 | Table II.Comparison of evaluation of
obstructive lesions of the internal carotid artery using different
imaging modalities. |
Table II.
Comparison of evaluation of
obstructive lesions of the internal carotid artery using different
imaging modalities.
|
| Diagnostic modality
adopted |
|---|
|
|
|
|---|
|
|
| Color-coded duplex
sonography (n=998) | Magnetic resonance
angiography (n=998) |
|---|
|
|
|
|
|
|---|
| Obstructive lesion
parameters | Invasive catheter
angiography (n=939) | Value |
P-valuea |
q-valuea | Value |
P-valuea |
q-valuea |
|---|
| Normal (0%) | 66 (7) | 89 (9) | <0.0001 | 4.144 | 59 (6) | <0.0001 | 7.301 |
| <50%
stenosis | 780 (83) | 809 (81) |
|
| 797 (80) |
|
|
| 50–69%
stenosis | 65 (7) | 38 (4) |
|
| 77 (8) |
|
|
| 70–99%
stenosis | 9 (1) | 41 (4) |
|
| 40 (4) |
|
|
| Occlusion (no flow
detected; 100%) | 19 (2) | 21 (2) |
|
| 25 (2) |
|
|
 | Table III.Distribution of intracranial stenosis
in the other intracerebral arteries assessed. |
Table III.
Distribution of intracranial stenosis
in the other intracerebral arteries assessed.
| Artery | Invasive catheter
angiography (n=939) | Color-coded duplex
sonography (n=998) | Magnetic resonance
angiography (n=998) |
|---|
| Internal carotid
artery |
|
|
|
| Petrous
segment | 7 (1) | 8 (1) | 9 (1) |
|
Cavernous segment | 7 (1) | 8 (1) | 9 (1) |
|
Cerebral segment | 4 (0.4) | 5 (0.5) | 3 (0.3) |
|
Vertebral artery | 15 (1.5) | 14 (1) | 13 (1) |
|
Anterior cerebral artery | 13 (1) | 15 (1.5) | 14 (1) |
| Middle cerebral
artery |
|
|
|
| M1
segment | 45 (5) | 41 (4) | 40 (4) |
| M2
segment | 39 (4) | 40 (4) | 41 (4) |
| Basilar
artery | 5 (0.5) | 4 (0.4) | 4 (0.4) |
|
Posterior communicating
artery | 1 (0.1) | 0 (0) | 0 (0) |
|
Posterior cerebral artery | 1 (0.1) | 1 (0.1) | 1 (0.1) |
Interobserver reliability
The quality of the acoustic window was categorized
as excellent (1,550–1,300 HU), intermediate (1,299–1,150 HU) and
poor (≤1,149 HU), and <1,000 HU was considered to indicate
transtemporal window insufficiency. Color-coded duplex sonography
had fewer readers' errors than invasive catheter angiography
(P<0.0001; Table IV).
 | Table IV.Mean reader differences. |
Table IV.
Mean reader differences.
|
|
| Color-coded duplex
sonography (n=998) | Magnetic resonance
angiography (n=998) |
|---|
|
|
|
|
|
|---|
| Parameter | Invasive catheter
angiography (n=939) | Value |
P-valuea | Value |
P-valuea |
|---|
| Number of
readers | 8 | 7 | N/A | 5 | N/A |
| Number of readers'
errors | 47 (5) | 9 (1) | <0.0001 | 31 (3) | 0.037 |
Diagnostic parameters
MRA (P=0.390) and color-coded duplex sonography
(P=0.484) detected the same number of true-positive obstructive
lesions with invasive catheter angiography set as the gold
standard. As compared to invasive catheter angiography, the
sensitivities of color-coded duplex sonography and MRA were 0.935
and 0.957 and the accuracies were 0.920 and 0.974, respectively
(Table V).
 | Table V.Diagnostic parameters. |
Table V.
Diagnostic parameters.
|
|
| Color-coded duplex
sonography (n=998) | Magnetic resonance
angiography (n=998) |
|---|
|
|
|
|
|
|---|
| Item | Invasive catheter
angiography (n=939) | Value |
P-valuea | Value |
P-valuea |
|---|
| True-positive
obstructive lesion | 93 (10) | 87 (9) | 0.3900 | 89 (9) | 0.4840 |
| True-negative
obstructive lesion | 846 (90) | 778 (78) | <0.0001 | 824 (83) | <0.0001 |
| False-positive
obstructive lesion | 0 (0) | 13 (1) | 0.0003 | 53 (5) | <0.0001 |
| False-negative
obstructive lesion | 0 (0) | 120 (12) | <0.0001 | 32 (3) | <0.0001 |
| Sensitivity | 1 | 0.935 | <0.0001 | 0.957 | <0.0001 |
| Accuracy | 1 | 0.920 | <0.0001 | 0.974 | <0.0001 |
Clinical decision-making analysis
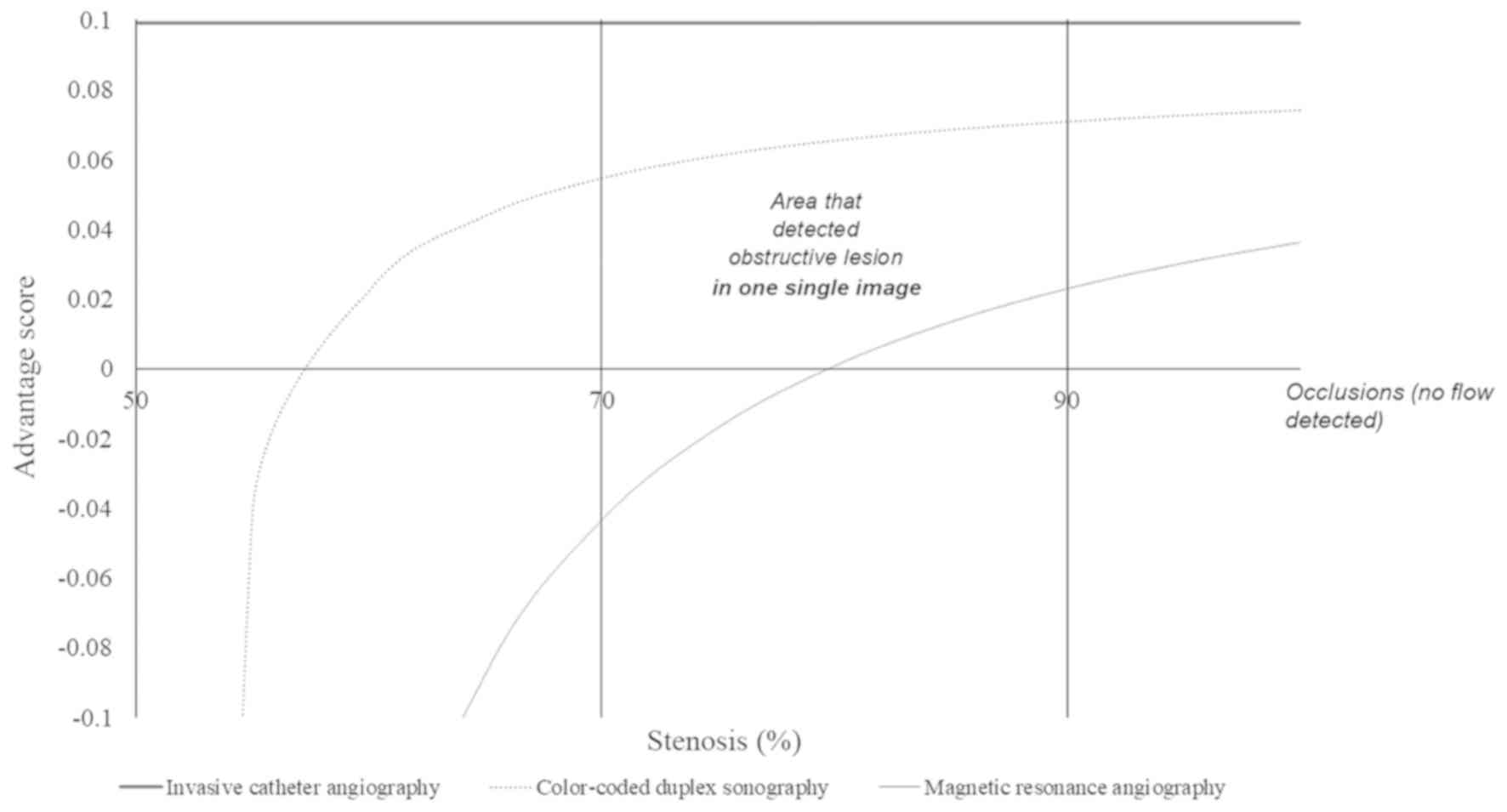
Color-coded duplex sonography was able to detect an
obstructive lesion in one single image for ICAs with ≥57% stenosis,
while MRA was capable of detecting an obstructive lesion in one
single image for ICAs with ≥80% stenosis. For ICAs that had <57%
of stenosis, color-coded duplex sonography had a risk of
overdiagnosis and for ICAs that had <80% of stenosis, MRA had a
risk of overdiagnosis (Fig. 3).
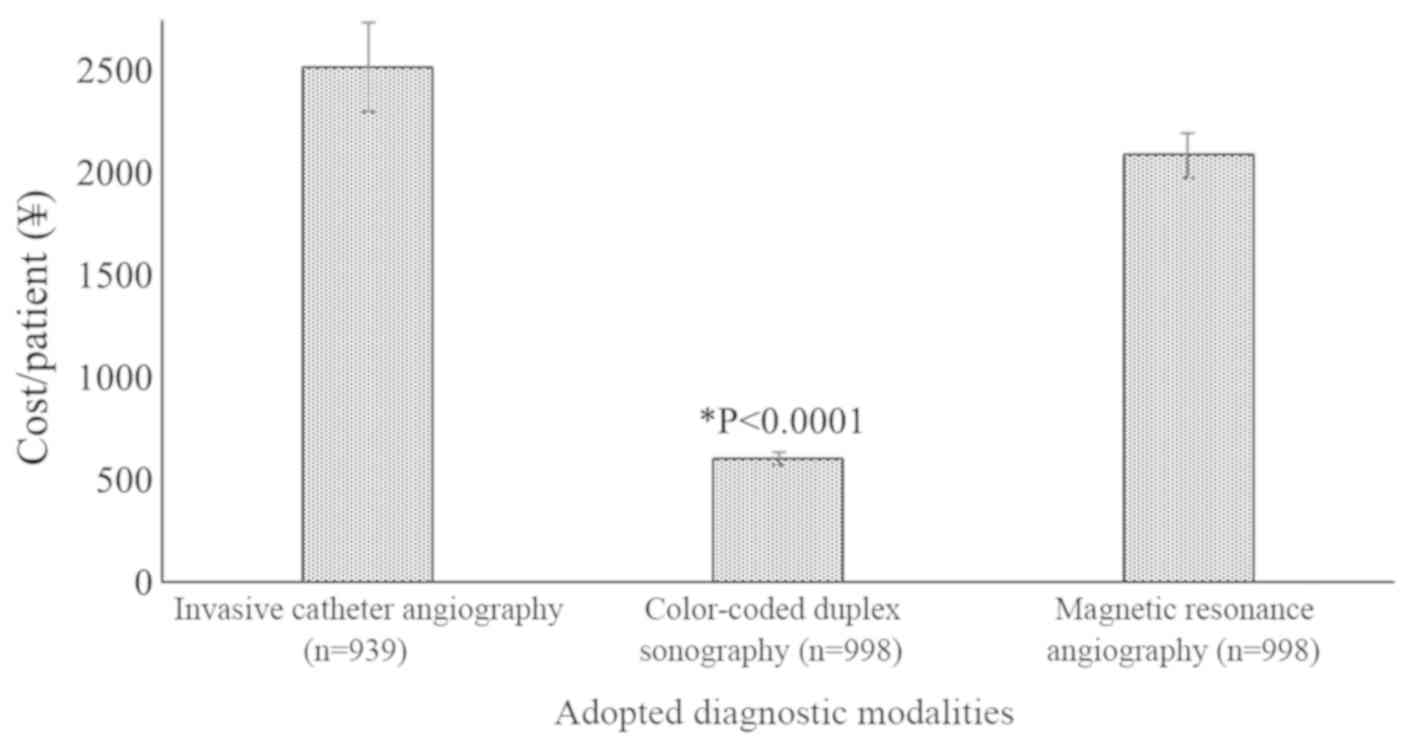
Cost
Color-coded duplex sonography was the cheapest of
the 3 methods applied, and the cost per patient was significantly
lower than that of invasive catheter angiography (P<0.0001,
q=419.81) and MRA (P<0.0001, q=330.21; Fig. 4).
Complications
After invasive catheter angiography, three patients
suffered injuries to the catheterized artery, one patient had an
irregular heart rhythm, two patients reported allergic reactions to
the medications used during the procedure, one patient had
increased bleeding and one patient suffered an infection.
Discussion
In the present study, color-coded duplex sonography,
MRA and invasive catheter angiography were used to assess the
degree of stenosis in patients with coronary artery disease with
satisfactory sensitivity and accuracy, as well as manageable
readers' errors and diagnostic costs. The results were consistent
with those of previous prospective studies (1,5).
Catheter angiography provides excellent visualization but it is
risky, invasive, expensive (15) and
requires contrast agent injection (7). In addition, invasive catheter
angiography has a risk of false-negative predictions in the chronic
stage of the disease or segmental stenosis in young patients
(16). For MRA, the use of 3.0 and
1.5 Tesla been debated, e.g. 1.5 Tesla MRA has higher sensitivity
and accuracy than 3.0 Tesla MRA but 3.0 Tesla has a higher spatial
resolution and signal-to-noise ratio (17). All in all, the present study was
successful in the pre-operative evaluation of risk factors for
coronary artery bypass grafting surgery.
Compared to invasive catheter angiography, the
color-coded duplex sonography detected a similar number of
obstructive lesions (93 vs. 100, P=0.363), but MRA reported higher
numbers of obstructive lesions (142 vs. 100, P=0.019). 3.0 Tesla
MRA imaging has limitations in the detection of decreased velocity
of inflowing blood (17). Therefore,
MRA should be applied to detect occluded lesions, while detection
of the degree of stenosis in lesions using this technique remains
challenging. The higher numbers of positive obstructive lesions
detected indicated a reduced accuracy of 3.0 Tesla MRA.
The present study reported significantly higher
numbers of false-positive obstructive lesions for MRA than
color-coded duplex sonography (53 vs. 13, P<0.0001). These
results were not in line with those of one previous study (17) but were consistent with those of
retrospective studies (15,18). 3.0 Tesla MRA image resolution or
image artifacts are responsible for the false-positive results
(19), particularly for vasculitis
(16). The present study reported
that MRA overestimates the prevalence of incidental aneurysms in
patients with coronary artery disease.
In the present study, a clinical decision-making
curve indicated that color-coded duplex sonography has a higher
working area and a lower risk of overdiagnosis than MRA. These
results study were in line with those of a previous prospective
study (5). Color-coded duplex
sonography is a more suitable approach for the evaluation of
cerebrovascular diseases than MRA.
Of note, the present study had several limitations,
for instance, all patients included were Chinese. Due to certain
diseases, the condition is more prevalent in Asians and the results
may not be completely generalized to Caucasian patients. The
sensitivity (0.935) of color-coded duplex sonography was lower than
that of MRA (0.957). Insufficient transcranial acoustic bone
windows (20) and tandem stenosis
(5) were responsible for the lower
sensitivity of color-coded duplex sonography in the present study,
while MRA provided clearer images with lower blood fluctuation of
arteries (17).
In conclusion, invasive catheter angiography, MRA
and color-coded duplex sonography were used to assess the risk for
coronary artery bypass grafting surgery. Invasive catheter
angiography is risky, inaccurate for segmental stenosis in young
patients (≤45 years) and expensive. Color-coded duplex sonography
was able to detect an obstructive lesion in one single image for
ICAs with ≥57% stenosis, while MRA was only capable of detecting an
obstructive lesion in one single image for ICAs with ≥80% stenosis.
Color-coded duplex sonography is a reliable method for the
detection of intracranial stenosis in patients with coronary artery
disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study available from the corresponding author on reasonable
request.
Authors' contributions
All authors had read and approved the manuscript
prior to submission for publication. LX was the project
administrator and contributed to the conceptualization, formal
analysis and literature review of the study. WC contributed to
resources, software and literature review of the study and drafted
and edited the manuscript for intellectual content. HW contributed
to resources, formal analysis, data curation and literature review
of the study. The authors agree to be accountable for all aspects
of the work, ensuring integrity and accuracy.
Ethics approval and consent to
participate
The protocol (no. LCH/CL/11/15 dated 1 January 2015)
of the study was approved by the Luoyang Central Hospital
Affiliated to Zhengzhou University human ethics committee (Luoyang,
China). Informed consent forms were signed by all participants,
which included consent for biopsy, radiology and to an additional
procedure for research purposes only. The study adhered to the law
of China, the STrengthening the Reporting of OBservational studies
in Epidemiology statement and the Declaration of Helsinki (version
from 2008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Valaikiene J, Ryliskyte L, Valaika A,
Puronaite R, Dementaviciene J, Vaitkevicius A, Badariene J,
Butkuviene I, Kalinauskas G and Laucevicius A: A high prevalence of
intracranial stenosis in patients with coronary artery disease and
the diagnostic value of transcranial duplex sonography. J Stroke
Cerebrovasc Dis. 28:1015–1021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bos D, van der Rijk MJ, Geeraedts TE,
Hofman A, Krestin GP, Witteman JC, van der Lugt A, Ikram MA and
Vernooij MW: Intracranial carotid artery atherosclerosis:
Prevalence and risk factors in the general population. Stroke.
43:1878–1884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin H, Peng Q, Nan D, Lv P, Liu R, Sun W,
Teng Y, Liu Y, Fan C, Xing H, et al: Prevalence and risk factors of
intracranial and extracranial artery stenosis in asymptomatic rural
residents of 13 villages in China. BMC Neurol. 17:1362017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazighi M, Labreuche J, Gongora-Rivera F,
Duyckaerts C, Hauw JJ and Amarenco P: Autopsy prevalence of
intracranial atherosclerosis in patients with fatal stroke. Stroke.
39:1142–1147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valaikiene J, Schuierer G, Ziemus B,
Dietrich J, Bogdahn U and Schlachetzki F: Transcranial color-coded
duplex sonography for detection of distal internal carotid artery
stenosis. Am J Neuroradiol. 29:347–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baradaran H, Patel P, Gialdini G,
Al-Dasuqi K, Giambrone A, Kamel H and Gupta A: Quantifying
intracranial internal carotid artery stenosis on MR Angiography. Am
J Neuroradiol. 38:986–990. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Zhao B, Bu C and Xie G:
Relationship between the hemodynamic changes on multi-Td pulsed
arterial spin labeling images and the degrees of cerebral artery
stenosis. Magn Reson Imaging. 32:1277–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pu Y, Liu L and Wang Y, Zou X, Pan Y, Soo
Y, Leung T, Zhao X, Wong KS and Wang Y; Chinese IntraCranial
AtheroSclerosis (CICAS) Study Group, : Geographic and sex
difference in the distribution of intracranial atherosclerosis in
China. Stroke. 44:2109–2114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liebeskind DS, Kosinski AS, Saver JL and
Feldmann E; SONIA Investigators, : Computed tomography angiography
in the stroke outcomes and neuroimaging of intracranial
atherosclerosis (SONIA) study. Interv Neurol. 2:153–159. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffis EJ, Jethwa P, Gupta G, Bonello K,
Gandhi CD and Prestigiacomo CJ: Accuracy of computed tomographic
angiography compared to digital subtraction angiography in the
diagnosis of intracranial stenosis and its impact on clinical
decision-making. J Stroke Cerebrovasc Dis. 22:1013–1017. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryoo S, Lee MJ, Cha J, Jeon P and Bang OY:
Differential vascular pathophysiologic types of intracranial
atherosclerotic stroke: A high-resolution wall magnetic resonance
imaging study. Stroke. 46:2815–2821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Huang J, Degnan AJ, Chen S, Gillard
JH, Teng Z and Lu J: Comparison of high-resolution MRI with CT
angiography and digital subtraction angiography for the evaluation
of middle cerebral artery atherosclerotic steno-occlusive disease.
Int J Cardiovasc Imaging. 29:1491–1498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kochar M and Min JK: Physiologic
assessment of coronary artery disease by cardiac computed
tomography. Korean Circ J. 43:435–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fitzgerald M, Saville BR and Lewis RJ:
Decision curve analysis. JAMA. 13:409–410. 2015. View Article : Google Scholar
|
|
15
|
Bash S, Villablanca JP, Jahan R, Duckwiler
G, Tillis M, Kidwell C, Saver J and Sayre J: Intracranial vascular
stenosis and occlusive disease: Evaluation with CT angiography, MR
angiography, and digital subtraction angiography. Am J Neuroradiol.
26:1012–1021. 2005.PubMed/NCBI
|
|
16
|
Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D,
Jung SC, Kang DW and Kim JS: Isolated MCA disease in patients
without significant atherosclerotic risk factors: A high-resolution
magnetic resonance imaging study. Stroke. 46:697–703. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirooka R, Ogasawara K, Inoue T, Fujiwara
S, Sasaki M, Chida K, Ishigaki D, Kobayashi M, Nishimoto H, Otawara
Y, et al: Simple assessment of cerebral hemodynamics using
single-slab 3D time-of-flight MR angiography in patients with
cervical internal carotid artery steno-occlusive diseases:
Comparison with quantitative perfusion single-photon emission CT.
AJNR Am J Neuroradiol. 30:559–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho YD, Lee JY, Kwon BJ, Kang HS and Han
MH: False-positive diagnosis of cerebral aneurysms using MR
angiography: Location, anatomic cause, and added value of source
image data. Clin Radiol. 66:726–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Lee DH, Ryu C-W, Pyun HW, Choi CG,
Kim SJ and Suh DC: Incidental saccular aneurysms on head MR
angiography: 5 years' experience at a single large-volume center. J
Stroke. 16:189–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JD, Ryu SJ, Chang YJ, Hsu KC, Chen YC,
Huang YC, Lee M, Hsiao MC and Lee TH: Carotid ultrasound criteria
for detecting intracranial carotid stenosis. Eur Neurol.
57:156–160. 2007. View Article : Google Scholar : PubMed/NCBI
|