Introduction
Rathke cleft cysts (RCCs) originate from the Rathke
pouch, with a prevalence rate of 4% (1). In the sellar region, RCCs can be found
in any age group (2). Kim et
al (3) reported that the age
range of RCC patients was 11–68 years, with an average age of 37
years and an RCC size range of 5–40 mm (3). RCCs can be divided into symptomatic and
asymptomatic types. Common symptoms of symptomatic RCCs include
headache, vision loss, visual field defects and pituitary endocrine
dysfunction (4). Uncommon symptoms
include meningitis (5), pituitary
abscess (6) and intracranial
aneurysms (7). RCCs can be slow or
acute in onset (7). MRI is the
primary diagnostic technique for RCCs. Han et al (8) reported 10 cases of hypointense signals
on T1 weighted images (TlWIs) and hyperintense signals on T2
weighted images (T2WIs), 6 cases of isointense signals on T1WIs and
hyperintense signals on T2WIs, 8 cases of hyperintense signals on
T1WIs and hyperintense signals on T2WIs, and 3 cases of hypointense
signals on TlWIs and hypointense signals on T2WIs. The observation
of intracystic nodules on MRI is an important diagnostic marker for
RCCs (9). The incidence of
intracystic nodules reported in previous studies was 9–77%
(9–14). The cyst wall of RCCs is constructed
from simple cuboidal epithelium or ciliated columnar epithelium,
and the cyst contents include protein, cholesterol and
mucopolysaccharide components (3).
Benvensite et al (15)
revealed that RCCs were occasionally associated with inflammatory
responses.
The posterior pituitary exhibits hyperintense
signals on MRI-T1WIs and is designated the posterior pituitary
bright spot (PPBS) (13,14,16).
PPBS is the imaging feature of antidiuretic hormone (ADH) in the
posterior pituitary. Schreckinger et al (17) determined that postoperative RCC
patients were more prone to diabetes insipidus. The positive rate
of PPBS has also been reported in healthy populations (16) and patients with pituitary adenomas
(18). However, the relationship
between RCCs and PPBS has not yet been reported.
In the current study, the MRI features, including
PPBS, of RCCs and their relationship with histopathological
presentations were investigated. The relationship between the PPBS
and inflammatory responses was also analyzed.
Materials and methods
Patients
The current study was performed retrospectively. The
sample size can be estimated according to the following formula:
n=Z2 × [P × (1-P)]/E2, where n is the sample
size, Z is the statistical value and E is the error. When the
confidence is 95%, Z=1.96; P represents the incidence of PPBS- in
the normal population, which is 4.1% according to the latest
literature (19). E was set to 10%
in the current study. RCC patients from January 1st 2010 to January
1st 2016 were enrolled in this retrospective study. All patients
underwent RCC resection of the sellar region using the
transsphenoidal approach.
The inclusion criteria for the current study were as
follows: i) Patients pathologically diagnosed with RCC without
sellar region involvement or primary endocrine diseases (such as
primary hyperthyroidism and Cushing's disease caused by adrenal
tumors); ii) patients did not receive radiotherapy or chemotherapy;
iii) patients received surgery that was performed by the same
surgeon; iv) patients had complete pre-operative and post-operative
MRI data. Patients were excluded if treatment was discontinued or
if they did not attend follow-up after surgery. Written informed
consent was obtained from every patient, or their legal guardian
and the study was approved by the Ethics Review Board of Fujian
Medical University.
MRI parameters
Patients received pituitary plain and enhanced MRI
scans using the Siemens 3.0T (Siemens AG). T1WI (axial and
sagittal) and T2WI (the axial and coronal) scans were subsequently
performed. The following parameters were used for acquisition of
T1WI: TSE (turbo spin echo) sequence, TR (time of
repetiton)=400–500 millisec, TE (echo time)=8–15 millisec, 3
stimulations. The following parameters were used for acquisition of
T2WI: TSE sequence, TR=3000 millisec, TE=83–98 millisec, 2
stimulations. The scanning field of view was 180×180 mm; matrices
were 320–384×240-252; the thickness of the axial scanning layer was
3.0 mm and the layer distance was 3.0 mm; the thickness of the
coronal and sagittal layer was 2.5 mm and the layer distance was
2.5 mm. Three-dimensional enhanced MRI scanning was then performed.
Gd-DTPA (gadolinium-diethylenetriaminepentaacetate) was used at
dose of 0.2 mmol/kg body weight and the scanning parameters were
the same as the aforementioned scan.
MRI evaluation
Pre-operative MRI scans were reviewed by a professor
of neurosurgery, an associate professor of neurosurgery and an
associate professor of radiology from Fuzhou General Hospital. A
consensus was reached among the physicians on all diagnoses. The
MRI images at 1 week before and after the operation were analyzed.
The following parameters were assessed: i) Location and size of
RCC: The RCC was observed on MRI-T1WI sagittal, T2WI sagittal and
enhanced T1WI sagittal. The line connecting the tuberculum sellae
and the dorsum sellae was used as a reference to classify the RCCs
as intrasellar, intrasellar-suprasellar or purely suprasellar. In
the INFINITT PACS Medical Imaging System (syngo.via software VB10;
Siemens Healthineers), the maximum diameter was used to determine
the RCC size. Lesions with diameter <10 mm were considered small
RCCs and lesions with a diameter ≥10 mm were considered large RCCs.
ii) MRI signal and intracystic nodules in the RCC: The RCC signal
was observed using both the MRI-T1WI and MRI-T2WI sequence. The
nodules in the RCC (nodule number, position, size and signal
characteristics) were observed under multiple MRI sequences. iii)
PPBS presentations: PPBS was observed under sagittal MRI-T1WI and
defined as PPBS positive (+) and PPBS negative (−).
Surgical approach
Patients were placed into a supine position with a
neck extension of 15–20°. The right nasal approach was selected and
a nasal septum incision was performed under a surgical microscope.
The nasal septum mucosa was retracted and the nasal septum bone was
pushed to the left side. The surgical corridor was achieved between
the right mucosal membrane lining nasal septum and ethmoid vertical
plate to the front of the sphenoid sinus using a nasal speculum. A
grinding drill was used to remove the anterior wall of the sphenoid
sinus and the sellar face, and the sellar dura was opened in an ‘X’
or ‘T’ shape. A circular scraper, tumor tweezers and an aspirator
were used to gradually remove cyst tissue, using minimal force to
strip the cyst wall. When the resection was satisfactory, the
lesion bed was washed repeatedly.
Histopathological examinations
The histopathological features of 31 RCC specimens
were observed. The specimens were fixed in 10% neutral formalin
solution at room temperature for 12 h, sliced (3 µm) and paraffin
embedded before staining with hematoxylin and eosin (H&E) at
room temperature for 5 min for each stain. Examinations were
performed under a light microscope (BX51; Olympus Corporation),
including analyzing the type and the extent of fibrosis of the wall
epithelium, as well as the presence of inflammatory infiltrates.
The number of inflammatory cells (including neutrophils,
lymphocytes and macrophages) in the slide with the largest extent
of infiltration was counted. Inflammation was defined as ≥10
inflammatory cells/5 fields (magnification, ×100) and <10
inflammatory cells/5 fields was determined to be negative for
inflammation (magnification, ×100).
Statistical analysis
Data were analyzed using SPSS 16.0 statistical
software (SPSS, Inc.). Quantitative data were expressed as the mean
± standard deviation. Categorical data was expressed as a
percentage and analyzed using χ2 tests (including the
Fisher's exactness test). Measurement data (including age and cyst
size) were tested for normality and were compared using independent
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient demographics
A total of 45 cases were included in the current
study, including 15 males and 30 females. The average age was
43.18±14.08 years, ranging from 13–68 years. Among the 45 cases, 18
had pre-operative headache (40.00%), 13 experienced dizziness
(28.89%), 12 demonstrated visual impairment (26.67%), 3 had
polydipsia (6.67%), 1 was going through menopause (2.22%), 1 was
lactating (2.22%), 3 experienced fatigue (6.67%) and 8 had no
symptoms, but were diagnosed based on the result of their annual
medical imaging examination (17.78%). Cyst diameter ranged from
5.60–25.47 mm (mean, 13.80±4.99 mm). No cases of multiple RCCs were
observed.
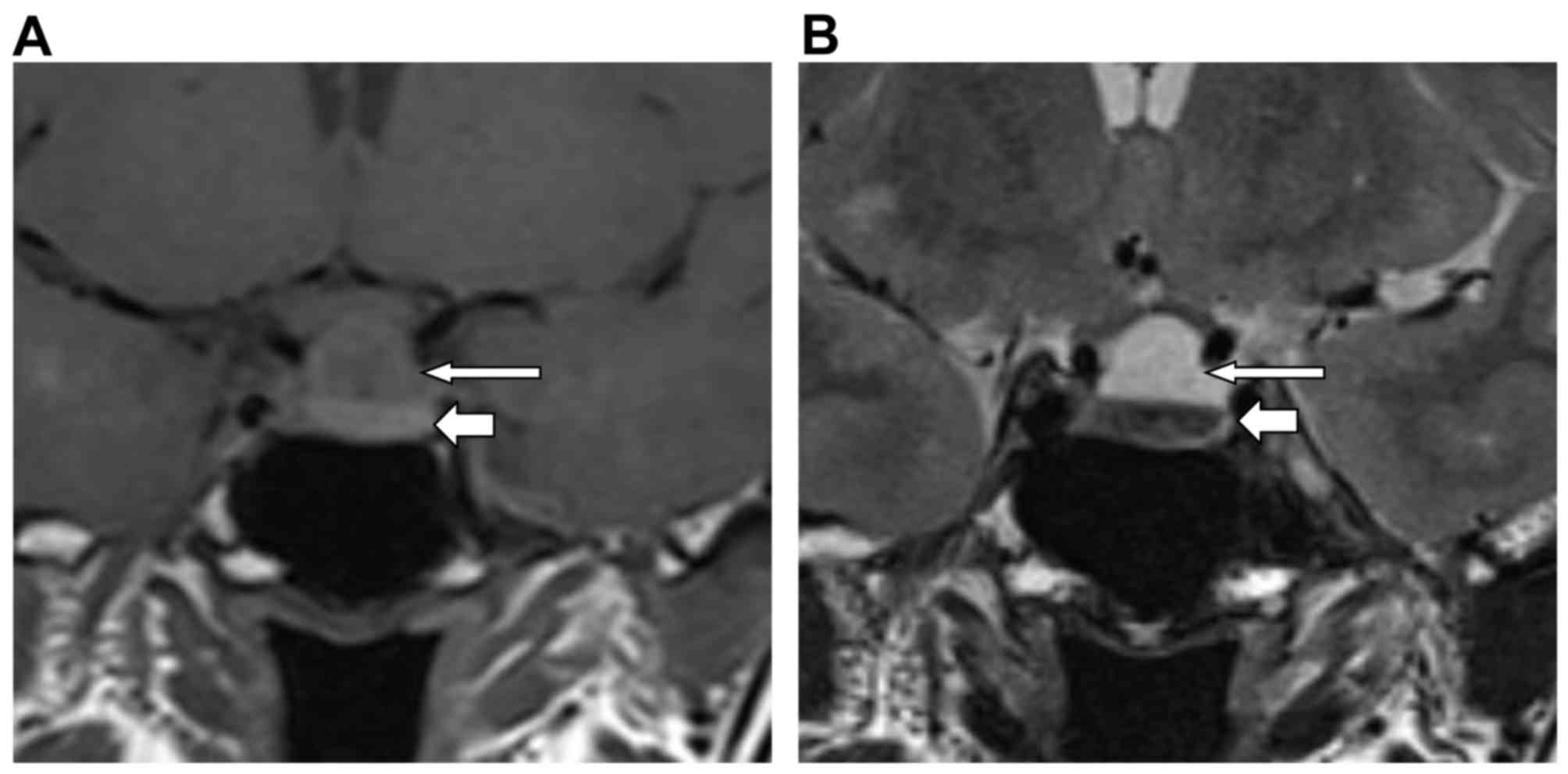
RCC features on MRI
RCC features were analyzed using MRI. There were 12
intrasellar RCC cases, 33 intrasellar-suprasellar cases and no
purely suprasellar cases. A total of 12 cases were classified as
small RCC and 33 cases were classified as large RCC. MRI-T1WI
revealed 18 cases with an isointense signal, 16 cases with a
hyperintense signal, 9 cases with a hypointense signal, 1 case with
a heterogeneous signal and 1 case with a stratification effect
[where the upper part of the RCC had an isointense signal (long
arrow) and the lower part had a hyperintense signal (short arrow),
with a clear border between them; Fig.
1A]. MRI-T2WI identified 5 cases with an isointense signal, 27
cases with a hyperintense signal, 11 cases with a hypointense
signal, 1 case with a heterogeneous signals and 1 case of
stratification (isointense signal, long arrow; hyperintense signal,
short arrow; Fig. 1B).
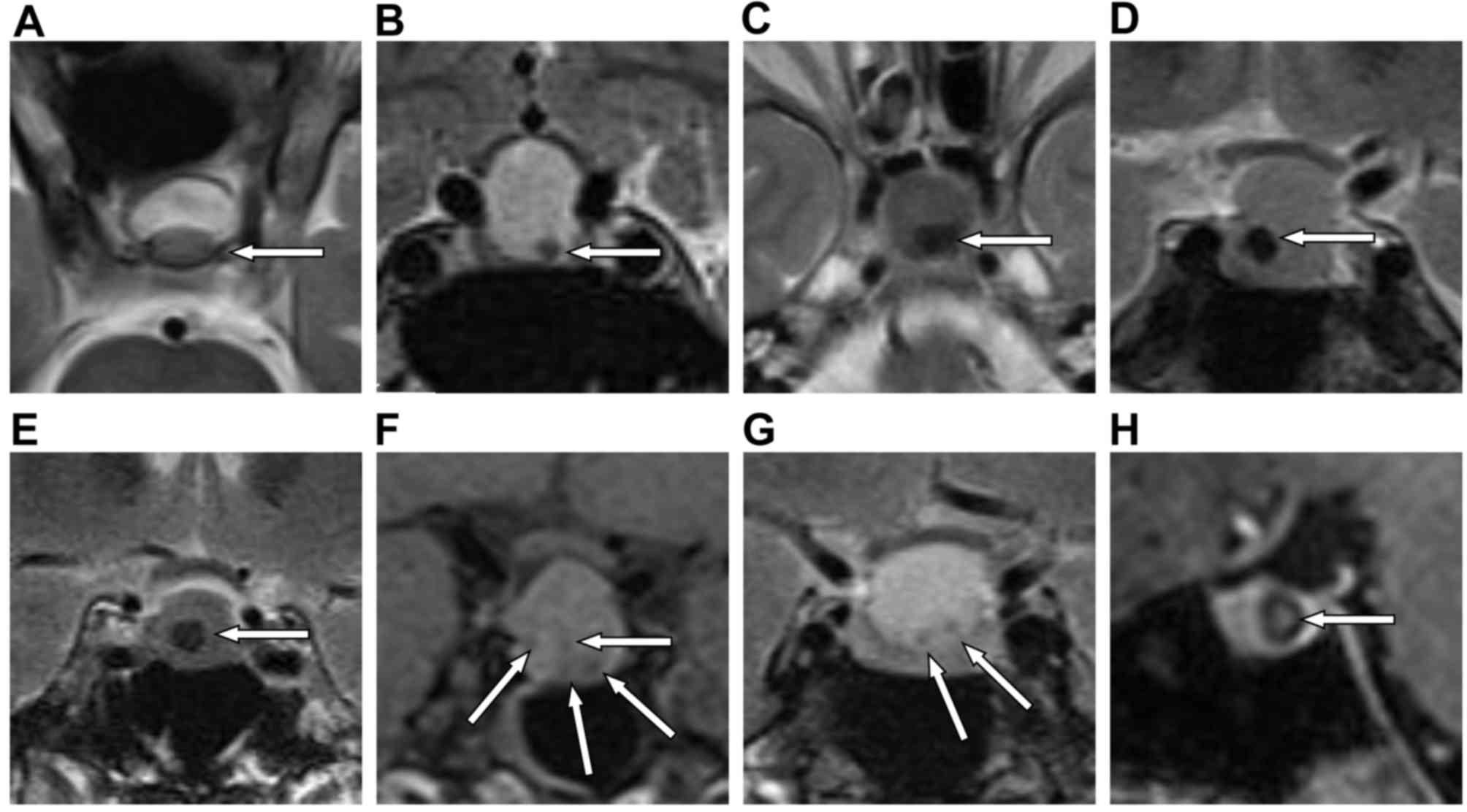
Of the 45 cases, 20 exhibited intracystic nodules in
T1WI, T2WI and T1 contrast enhanced sequences, as presented in
Table I and Fig. 2. The range in nodule diameter was
1.67–9.76 (5.19±2.57) mm and the corresponding RCC diameter was
7.06–20.12 (12.65±3.07) mm, with the figures in brackets presenting
the mean ± standard deviation. Of the intracystic nodules of RCCs,
3 cases exhibited hyperintense signals on T1WI, 5 cases had
hypointense signals on T1WI, 13 cases had hypointense signals on
T2WI, 4 cases had hypointense signal in the enhanced sequence and 2
cases had multiple nodules (as indicated by white arrows in
Fig. 2F and G). There were no
nodules with hyperintense signals on T2WI. The nodules were mostly
of a round shape; however some displayed oval and irregular shapes
and 1 case exhibited concentric rings (indicated by a white arrow
in Fig. 2H). Three cases displayed
intracystic nodules adherent to the cyst wall and the intracystic
nodules in 17 cases were non-adherent. Several intracystic nodules
were located at the center of the RCC (as indicated by white arrows
in Fig. 2E and H), or in the lower,
posterior or anterior parts of the RCC (as indicated by white
arrows in Fig. 2A-D, F and G). These
results indicated that RCCs have various signal intensities on MRI
and that intracystic nodules also have various shapes and signal
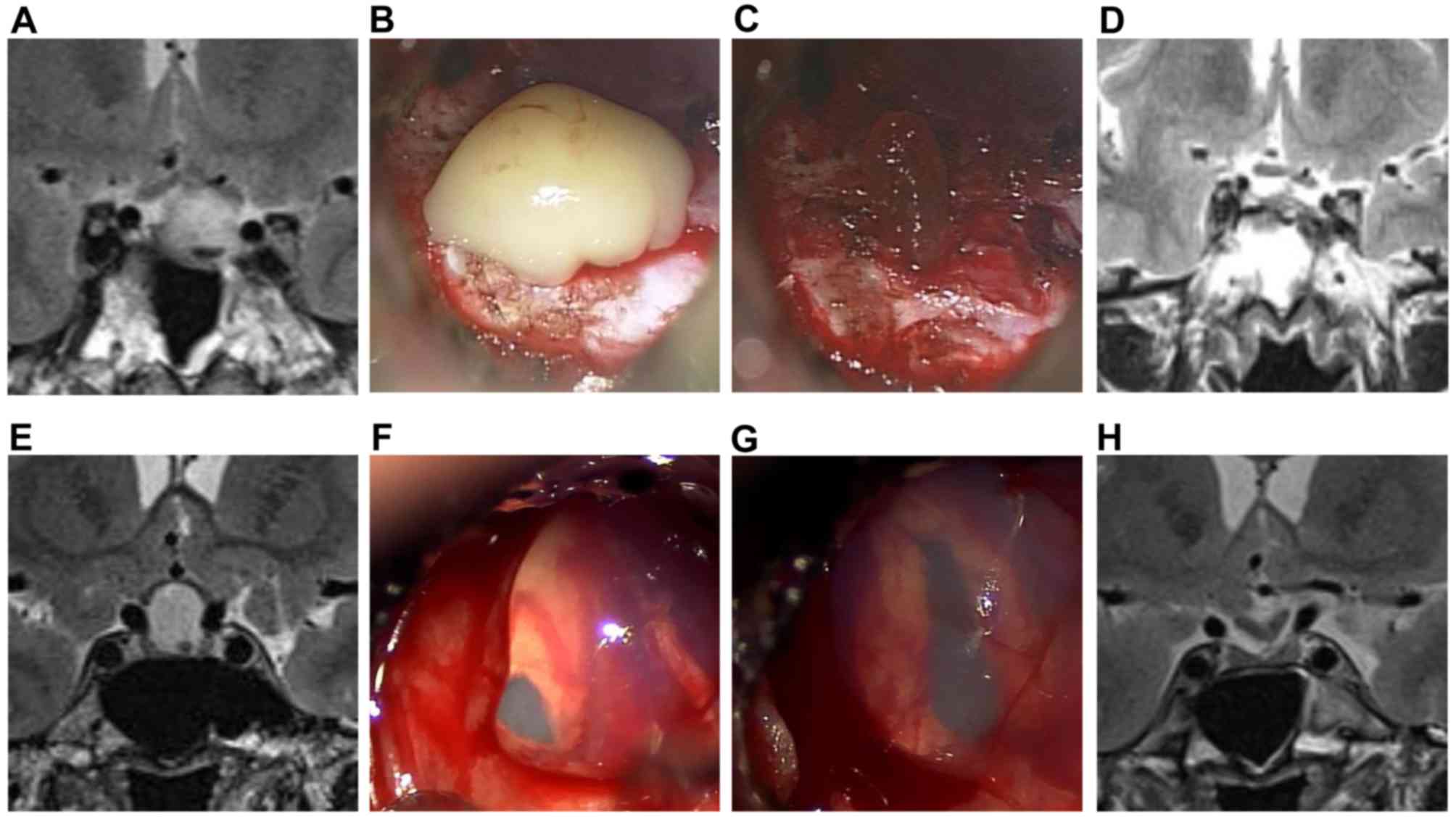
intensities on MRI. It is worth noting that the nodules observed
through MRI were not always identified during surgery. This implies
that some of the observed nodules could be real or could be
artifact created by the MRI (Fig.
3A-H).
 | Table I.Intracystic nodules and RCC content
from surgery in 20 RCC cases. |
Table I.
Intracystic nodules and RCC content
from surgery in 20 RCC cases.
| Case | Nodular signals | Nodular shape and
position | Description of RCC
content |
|---|
| 1 | T2 low signal | Posterior lower
region of the RCC, oval, adherent to the cyst wall | Milky white mucus
containing brown granules |
| 2 | T2 low signal | Right lower region of
the RCC, oval, not adherent to the cyst wall | White jelly |
| 3 | High signal nodules
& scattered low signal small nodules in T1 | Small nodules
scattered; large nodules located in the rear of the RCC, not
adherent to the cyst wall, irregularly shaped | Gray and white
jelly |
| 4 | Circular low signal
small nodule in enhanced coronal T1WI | Centered | Thin transparent
mucus |
| 5 | T1 low signal | Circular and
centered | White jelly |
| 6 | Enhanced sagittal low
signal | Circular and
centered | Milky white
jelly |
| 7 | T2 low signal of
small nodules | 2 circular nodules in
the lower region of the RCC | Milky white
jelly |
| 8 | T1 low signal, T2 low
signal | Circular non-adherent
nodules in the right lower region of the RCC | Milky white sticky
mucus |
| 9 | T2 low signal
nodules | Circular non-adherent
nodules in the back region of the RCC | Gray mucus |
| 10 | T1 and T1 enhance low
signal strip nodules | Irregular, front | Transparent thin
mucus |
| 11 | T2 low signal nodules
in the lower part | Oval,
non-adherent | Yellow jelly |
| 12 | T1 high signal, T2
low signal nodules | Irregular, bottom,
adherent to the cyst wall | Clear liquid and
yellow solid matter |
| 13 | T2 low signal
nodules | Irregular, bottom,
non-adherent | Yellow-green sticky
mucus |
| 14 | T1 low signal
nodules | Irregular, bottom,
non-adherent | Transparent liquid
and jelly-like coagulum |
| 15 | T2 low signal
nodules | Oval, back | Gray mucus and brown
jelly-like granules |
| 16 | T2 low signal
nodules | Circular,
centered | Gray jelly-like
material containing granules |
| 17 | T2 low signal nodules
and T1 | T2 round in coronal
section, T1 | Egg white mucus
containing brown |
|
| enhanced low
signal | target-like in
midline sagittal section | semi-solid
granules |
| 18 | T1 high signal, T2
low signal of irregular nodules | Irregular | Egg white mucus
containing brown solid granules |
| 19 | T1 low signal
nodules | Irregular,
scattered | Viscous liquid
containing granules |
| 20 | T2 low signal
nodules | Round, bottom right
region of the RCC | Egg white mucus |
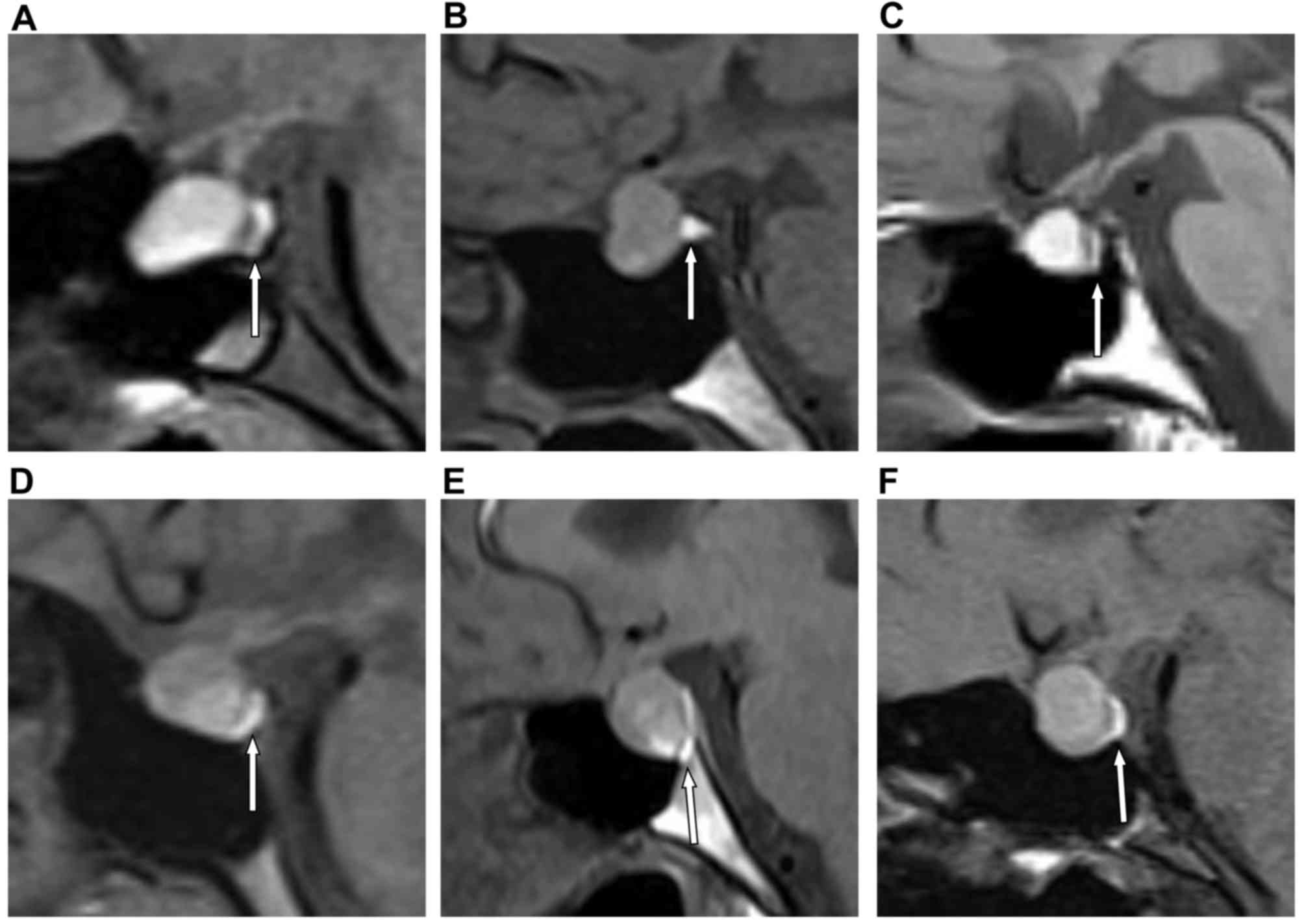
PPBS
To determine the PPBS positive incidence in RCC
patients, the MRI-T1WI of the 45 RCC patients were analyzed. Among
the 45 RCC cases, PPBS was identified in only 10 cases. PPBS
appeared in linear, triangular, crescent and bilinear shapes in the
sagittal view and were located at the posterior part of the sella
turcica, in contact with the dorsum sellae (Fig. 4A-F). These results indicated that the
incidence of positive PPBS in RCC patients was low.
Histopathological findings
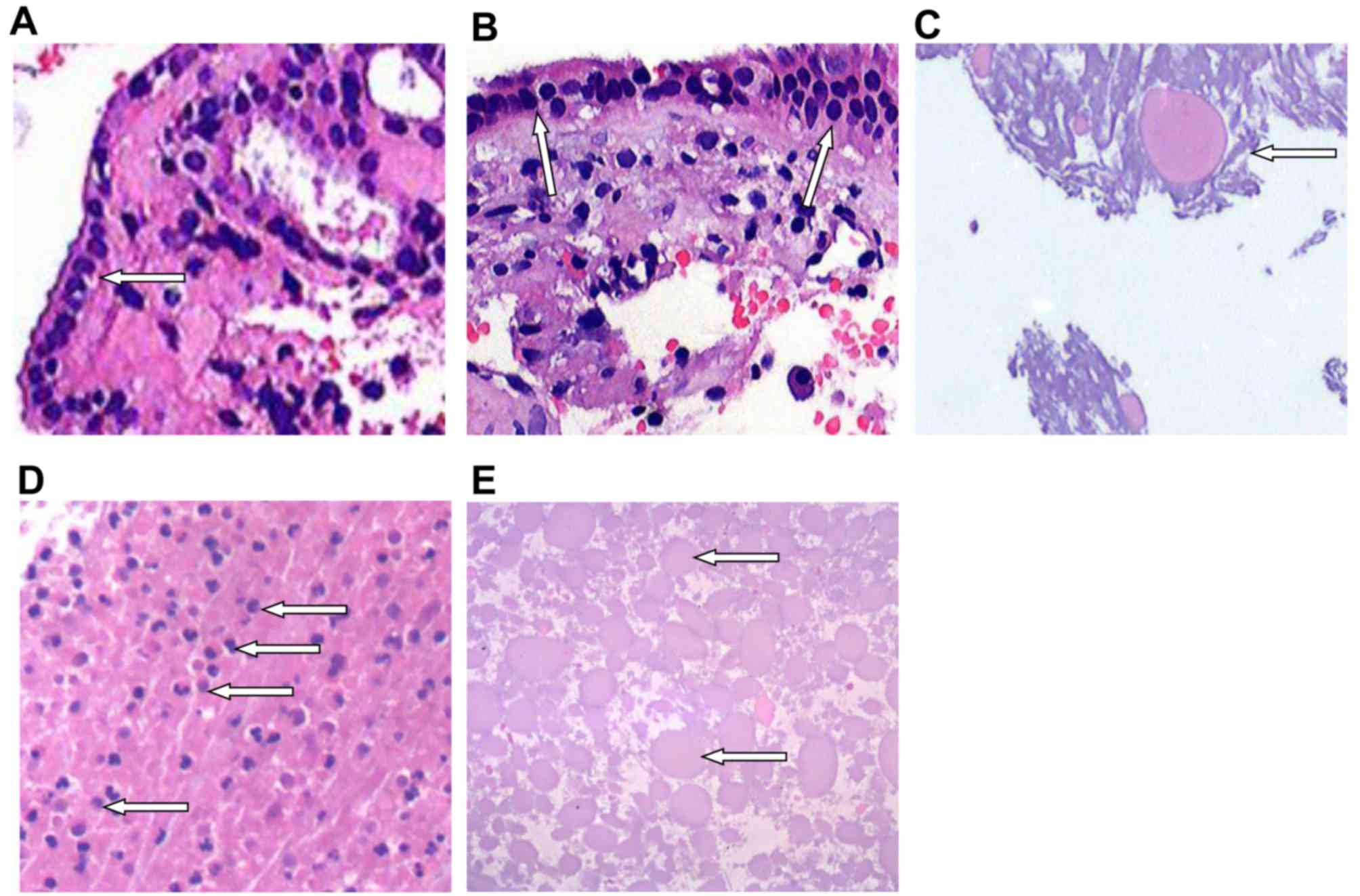
To observe the histopathological features of RCC,
H&E staining was performed. Among the 31 pathological
specimens, 12 cases exhibited cyst wall epithelium, including 4
cases of monolayer cube epithelium (as indicated by the white arrow
in Fig. 5A), 7 cases displayed
pseudostratified ciliated columnar epithelium and 1 case displayed
ciliated columnar epithelium and squamous metaplasia (as indicated
by the white arrows in Fig. 5B). Of
the 25 cases with cyst content, 17 cases presented pink staining on
H&E, 4 cases exhibited blue staining (as indicated by the white
arrow in Fig. 5C) and 4 cases had
both pink and blue staining (Fig.
5D). The arrows in Fig. 5D
indicate inflammatory cell infiltrates (mainly mononuclear cells).
In the tissue sections of RCC specimens with blue staining, the
contents of the cysts were diverse. The size and coloration of
protein bodies were highly variable and some even exhibited hyaline
degeneration. No cholesterol crystals were identified. A total of
14 cases displayed inflammatory responses (Fig. 5E). The arrows in Fig. 5E show numerous pink stained protein
globules in the cyst fluid. There were numerous lymphoid cells and
some eosinophils in the anterior pituitary gland.
Relationship of PPBS with age, sex,
RCC location and size
According to the presence of PPBS on MRI, patients
were divided into the PPBS+ group (n=10) and the PPBS- group
(n=35). The differences in age, sex, RCC location and size of cysts
between the two groups were analyzed. As presented in Table II, of the 45 RCC cases, there were
15 males with 2 cases of PPBS+ and 13 cases of PPBS-. Of the 30
female cases, 8 cases of PPBS+ and 24 cases of PPBS- were
identified. No significant differences were identified between the
sex of the PPBS+ and PPBS- patients. In the PPBS+ and PPBS- groups,
the average ages were 42.00±16.65 years and 43.51±13.51 years,
respectively. No significant differences between the ages of the
two groups were identified. Similarly, there were no significant
differences in RCC location or size of cysts between the two
groups. These results suggested that the presence of PPBS was not
associated with sex, age, RCC size or RCC location.
 | Table II.Association between PPBS and age, sex,
cyst location and RCC size. |
Table II.
Association between PPBS and age, sex,
cyst location and RCC size.
| Variable | PPBS+ (n=10) | PPBS- (n=35) | P-value |
|---|
| Age, years | 42±16.65 | 43.51±13.51 | 0.363 |
| Sex |
|
| 0.456 |
|
Male | 2 | 13 |
|
|
Female | 8 | 22 |
|
| Size of cysts,
mm | 11.18±2.63 | 14.55±5.27 | 0.137 |
| Cyst locations |
|
| 1.000 |
|
Intrasellar | 3 | 9 |
|
|
Intrasellar-suprasellar | 7 | 26 |
|
Relationship of PPBS and inflammatory
responses
The relationship between PPBS status and the
inflammatory response was analyzed. Among the 31 patients with
histopathological specimens, 10 had PPBS+. Among the 10 cases with
PPBS+, 2 (20%) had inflammatory responses. There were 21 cases of
PPBS- and among them, 14 (66.7%) had inflammatory responses. The
PPBS+ cases had significantly lower inflammatory responses than the
PPBS- cases (P<0.05; Table
III). Thus, the data demonstrated that the inflammatory
response may be associated with the presence of PPBS.
 | Table III.Association between PPBS and
inflammation status in RCCs. |
Table III.
Association between PPBS and
inflammation status in RCCs.
| Pathology
groups | PPBS+ | PPBS- | P-value (two
sided) | P-value (single
sided) |
|---|
| Inflammatory
response | 2 | 14 | 0.023 | 0.019 |
| No inflammatory
response | 8 | 7 |
|
|
Discussion
In the present study, it was demonstrated that most
RCCs have T1-hyperintense signal with varying signal intensity.
Different patients may exhibit hyper-, iso- or hypo-intense signals
on the same sequence of MRI. A stratification effect is observed in
a rare number of cases. In the present study, one case presented
with the stratification effect, revealing a fake ‘liquid-liquid
level’. This may be due to material deposition within the
intracystic contents. Different components and materials may
exhibit different signals on MRI. To the best of our knowledge, the
present study is the first to analyze the rate of PPBS on MRI in
RCC patients. The low rate of PPBS on MRI scans was also revealed
to have a potential relationship with the inflammatory response
exhibited by RCC patients.
According to sample size calculation, at least 16
RCC cases (n=1.962×0.041×0.959/0.12=16) are required to obtain
solid conclusions regarding PPBS- in RCC patients. In the present
study, the 45 cases included were sufficient to reach a conclusion.
Of the 45 cases, 20 had intracystic nodules, accounting for 44.44%
of all RCCs. Most of the nodules were not adherent to the cyst
wall. Whether the nodules are adherent to the cyst wall may be
associated with the maturity of the nodules. It is hypothesized
that when a nodule matures and solidifies, it easily sinks and
adheres to the posterior or lower wall. In the present study, it
was determined that multiple nodules may occur in one RCC, and the
nodular shape may be round, oval or irregular. In the current
study, there was a target-like nodule, which may be of specific
value for preoperative diagnosis. It is also worth commenting that
among the 20 nodular cases identified by MRI, surgery could only
distinguish solid nodules in 6 of the cases and most were
mucus-like or jelly-like nodules. The formation of intracystic
nodules may be due to the accumulation and solidification of
protein material within the cyst. Different MRI pulse sequences
(repeats of the same scans or different scans), may reveal immature
nodules. In the present study, nodules with hypointensity signals
on T2WI were the most frequently observed, followed by nodules with
hypointensity signals on T1WI. Hyperintensity signals on T1WI were
rare and no hyperintensity signals on T2WI were identified.
Of the 45 patients, 10 were PPBS+, with a positive
rate of 22.22%. It has been reported that the PPBS positive rate is
52–100% in the general population (16) and 79.7% in patients with pituitary
adenoma (18). Furthermore, the PPBS
positive rate has been demonstrated to be significantly lower in
RCC patients compared with that in normal individuals (16), suggesting that the cyst has an effect
on the posterior pituitary and pituitary stalk. Additionally, it
has been reported that inflammatory responses are present within
certain cases of RCC (6,20). The current study revealed that the
PPBS+ rate was significantly lower in RCC patients with
inflammatory responses than those without. The low PPBS positive
rate in RCC may be associated with RCC-induced inflammatory
responses. It is speculated that the inflammatory responses to RCCs
can affect the pituitary stalk and the posterior pituitary, leading
to a decrease in the transport and storage of ADH. This results in
insufficient storage of ADH in the posterior pituitary, which may
cause irreversible damage.
According to the characteristics of RCC images, RCCs
can be differentiated from craniopharyngiomas, arachnoid cysts,
abscesses and mucoceles. Although craniopharyngioma can exhibit
significant cystic changes, parenchyma tissue is always present.
The cystic wall and parenchyma reveal a significant enhancement
effect on enhanced MRI. Furthermore, the vast majority of
craniopharyngiomas are located above the sella turcica, rather than
in the pituitary fossa (21). The
MRI signal of an arachnoid cyst in the sellar region is consistent
with that of cerebrospinal fluid. Arachnoid cysts are located
outside the pituitary, which differentiates them from RCCs
(22). Pituitary abscesses are rare
and on MRI, enhancement effects are observed in the pituitary
stalk, the dura mater around the pituitary and the sphenoid sinus
mucosa (23). Mucoceles of the
sphenoid sinus are located in the sphenoid sinus, rather than in
the pituitary fossa, which are easily identified.
Despite the results obtained, the current study has
limitations. Firstly, the present study was limited by the small
sample size. Furthermore, the surgical procedure was written by
surgeons without the support of audio and video data, therefore the
intracystic contents were described subjectively. As such, further
studies are warranted.
In conclusion, the signal intensity and shape of RCC
is varied on MRI. However, the intracystic nodules are featured
characteristics of RCCs when investigated by MRI, which are
observed in nearly 50% of RCC patients. In rare cases, RCCs
demonstrated a target-like/concentric ring pattern. This shape may
indicate that intracystic nodules are maturing, which is of great
importance for RCC diagnosis. Inflammatory responses were observed
in some RCC cases, which may affect the appearance of the PPBS on
T1WI. The mechanism underlying the appearance of PPBS on T1WI with
inflammatory responses is unclear and needs further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fujian
Provincial Key Project of Science and Technology Plan (grant no.
2018Y0067).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW conceptualized the study. QN, ZW and JZ collected
the cases and the subsequent data. LW analyzed the data. SW and QN
wrote the original manuscript. SW reviewed and edited the
manuscript as well as acquired funding for the project.
Ethics approval and consent to
participate
All subjects gave their written informed consent for
inclusion before they participated in the study. The study was
conducted in accordance with the Declaration of Helsinki, and the
protocol was approved by the ethics review board of Fujian Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuan EC, Yoo F, Chyu J, Bergsneider M and
Wang MB: Treatment outcomes of Rathke's cleft cysts managed with
marsupialization. J Neurol Surg B Skull Base. 78:112–115. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin M, Wedemeyer MA, Bradley D, Donoho DA,
Fredrickson VL, Weiss MH, Carmichael JD and Zada G: Long-term
surgical outcomes following transsphenoidal surgery in patients
with Rathke's cleft cysts. J Neurosurg. 130:831–837. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JE, Kim JH, Kim OL, Paek SH, Kim DG,
Chi JG and Jung HW: Surgical treatment of symptomatic Rathke cleft
cysts: Clinical features and results with special attention to
recurrence. J Neurosurg. 100:33–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jahangiri A, Molinaro AM, Tarapore PE,
Blevins L Jr, Auguste KI, Gupta N, Kunwar S and Aghi MK: Rathke
cleft cysts in pediatric patients: Presentation, surgical
management and postoperative outcomes. Neurosurg Focus. 31:E32011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mrelashvili A, Braksick SA, Murphy LL,
Morparia NP, Neena N and Neeraj K: Chemical meningitis: A rare
presentation of Rathke's cleft cyst. J Clin Neurosci. 21:692–694.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naama O, Gazzaz M, Boulahroud O and
Elmoustarchid B: Infection of a Rathke cleft cyst: A rare cause of
pituitary abscess. Surg Infect (Larchmt). 15:358–360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jimbo H, Ichikawa M, Fukami S, Otsuka K,
Tsurukiri J, Sunaga S and Lkeda Y: Rapid De Novo aneurysm formation
after Rathke cleft cyst rupture. World Neurosurg.
88:690.e11–690.e16. 2016. View Article : Google Scholar
|
|
8
|
Han X and Zhao DJ: Imaging diagnosis of
Rathke's cleft cyst. J Med Imag. 20:782–784. 2010.
|
|
9
|
Wang SS, Xiao DY, Yu YH, Jing JJ, Zhao L
and Wang RM: Diagnostic significance of intracystic nodules on MRI
in Rathke's cleft cyst. Int J Endocrinol. 2012:9587322012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Binning MJ, Gottfried ON, Osborn AG and
Couldwell WT: Rathke cleft cyst intracystic nodule: A
characteristic magnetic resonance imaging finding. J Neurosurg.
103:837–840. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byun WM, Kim OL and Kim D: MR imaging
findings of Rathke's cleft cysts: Significance of intracystic
nodules. AJNR Am J Neuroradiol. 21:485–488. 2000.PubMed/NCBI
|
|
12
|
QI C and Wang N: Value of intracystic
nodules on MRI to diagnosis of Rathke's cleft cyst. Chin J Clin
Neurosurg. 19:212–214. 2014.
|
|
13
|
Kilday JP, Laughlin S, Urbach S, Bouffet E
and Bartels U: Diabetes insipidus in pediatric germinomas of the
suprasellar region: Characteristic features and significance of the
pituitary bright spot. J Neurooncol. 121:167–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saeki N, Hoshi S, Sunada S, Sunami K,
Murai H, Kubota M, Tatsuno I, Iuchi T and Yamaura A: Correlation of
high signal intensity of the pituitary stalk in macroadenoma and
postoperative diabetes insipidus. AJNRAm J Neuroradiol. 23:822–827.
2002.
|
|
15
|
Benveniste RJ, King WA, Walsh J, Lee JS,
Naidich TP and Post KD: Surgery for Rathke cleft cysts: Technical
considerations and outcomes. J Neurosurg. 101:577–584. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Côté M, Salzman KL, Sorour M and Couldwell
WT: Normal dimensions of the posterior pituitary bright spot on
magnetic resonance imaging. J Neurosurg. 120:357–362. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schreckinger M, Szerlip N and Mittal S:
Diabetes insipidus following resection of pituitary tumors. Clin
Neurol Neurosurg. 115:121–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Lin K, Xiao D, Zhao L, Qin Y and
Wei L: MR imaging analysis of posterior pituitary in patients with
pituitary adenoma. Int J Clin Exp Med. 8:7634–7640. 2015.PubMed/NCBI
|
|
19
|
Klyn V, Dekeyzer S, Van Eetvelde R, Roels
P, Vergauwen O, Devolder P, Wiesmann M, Achten E and Nikoubashman
O: Presence of the posterior pituitary bright spot sign on MRI in
the general population: A comparison between 1.5 and 3T MRI and
between 2D-T1 spin-echo- and 3D-T1 gradient-echo sequences.
Pituitary. 21:379–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogawa Y, Watanabe M and Tominaga T:
Intraparenchymal infiltration of Rathke's cleft cysts manifesting
as severe neurological deficits and hypopituitarism: 2 Case
reports. BMC Res Notes. 9:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Locatelli D, Pozzi F, Agresta G, Padovan
S, Karligkiltis A and Castelnuovo P: Extended endoscopic endonasal
approach for suprasellar craniopharyngioma. J Neurol Surg B Skull
Base. 79:S196–S198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abbas M, Khairy S, AlWohaibi M, Aloraidi A
and AlQurrashi WW: Bilateral temporal extradural hematoma on top of
bilateral temporal arachnoid cyst: First case report and extensive
literature review. World Neurosurg. 115:134–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ifergan H, Cazeneuve N, Merenda P and
Magni C: MR imaging features of a pituitary abscess: A case report.
Ann Endocrinol (Paris). 80:62–63. 2019. View Article : Google Scholar : PubMed/NCBI
|