Introduction
Inflammatory bowel disease (IBD) is a chronic
non-specific intestinal inflammatory disease, like ulcerative
colitis (UC) or Crohn's disease. Over the previous 2 decades, the
morbidity and prevalence of UC has increased, becoming one of the
modern incurable diseases, as determined by the World Health
Organization (1). The duration,
extent and severity of colonic mucosal inflammation is largely
associated with the risk of cancer formation and promotion in UC
(2). In order to cure UC, early
detection and studies examining its pathogenesis is crucial.
MicroRNAs (miRNAs) are small non-coding RNAs with
the length of 19–24 nucleotides that bind with the 3′-untranslated
regions (3′-UTR) of mRNA sequence to regulate the expression of its
target genes (3,4). At present, a number of studies have
demonstrated that various miRNAs are closely associated with the
process of UC, indicating that miRNAs may be a therapeutic target
for UC (5–9).
miR-21 has been suggested to serve a significant
role in the development of IBD. A previous study demonstrated that
the degree of intestinal inflammatory response, apoptosis of
intestinal epithelial cells and their pathological scores were
significantly decreased following the knockdown of miR-21 in Wistar
rats treated with dextran sulfate sodium (DSS), as compared with
the control group, which indicated that miR-21 may promote the
occurrence and development of intestinal inflammation (10). Similarly, increased levels of miR-21
were identified in patients with UC, as compared with the control
patients (11). However, the
understanding of the regulation, role and targets of miR-21-5p
remains limited.
Several studies have described the association
between miR-21-5p and signal transducer and activator of
transcription 3 (STAT3): Gryshkova et al (12) identified an association among
miR-21-5p, STAT3 and inflammatory responses in cardiac injury. In
patients with celiac disease, miR-21-5p upregulation may have been
caused by its target STAT3, indicating an increased activation of
miR-21-5p in patients with Marsh 3C stage disease (13). An additional study demonstrated that
STAT3 was upregulated in patients with UC and that the STAT3
expression increased with the severity of UC, suggesting that STAT3
may be an evaluation index of UC severity and prognosis and a new
target in UC therapy (14). In
addition, the expression levels of interleukin (IL)-6 and IL6
receptor (IL6R) in UC rats were significantly increased, as
compared with the control group (15). Wang et al (16) demonstrated that dandelion
polysaccharides decreased the expression of IL-6 in UC rats and the
protein expression of IL6Rα and gp130 in the IL6R/STAT3 pathway,
which decreased the transcriptional levels of STAT3 and IL6R mRNA
and alleviated the inflammatory state in the colonic tissues of
rats. Therefore, the IL6R/STAT3 pathway is associated with the
process of UC, but the mechanism in which miR-21-5p mediates UC
through the IL6R/STAT3 pathway remains to be elucidated.
In the present study, the role of miR-21-5p in UC
was explored, with a particular focus on the effect of miR-21-5p on
the IL6R/STAT3 signal pathway in UC and the regulation of
inflammatory pathways and apoptosis-associated proteins in RAW264.7
cells.
Materials and methods
Human sera specimens
The study was approved by the Human Ethics Committee
Review Board of Renmin Hospital of Wuhan University (Wuhan, China),
and informed consent was obtained from each patient. Sera specimens
were obtained from 45 patients with UC and 45 healthy individuals
in the Renmin Hospital of Wuhan University (Wuhan, China) between
May 2017 and June 2018. None of the patients had received prior
treatment. All patients recruited for the present study were
diagnosed with UC. The sera specimens were stored at −80°C until
further use. The study did not use patient names, initials,
hospital numbers, or in any manner give information by which the
individuals can be identified.
UC rat model
A total of 60 male Wistar rats (specific
pathogen-free grade, 6 weeks, weighing 180–220 g) were obtained
from Shanghai JiesiJie Experimental Animal Co., Ltd. Prior to the
experiments, rats were maintained in an environmentally controlled
room (22°C±2°C, 12:12 h light:dark cycle) with ad libitum
access to food and water for 7 days, in order to acclimate to their
new environment prior to initiation of the experiment. Animal
experiments were approved and supervised by the Animal Care and Use
and the Animal Ethics Committees at Renmin Hospital of Wuhan
University. All rats were randomly divided into two groups (n=30
per group); the control and model groups. Following fasting for 4
h, the rats in the model group were given ad libitum access
to 5% DSS solution for 7 days, while the rats in the control group
were given ad libitum access to purified water for 7 days.
During the experiment, all rats received ad libitum access
to food. At the time of sacrifice, the average weight of the
control rats was 263 g and the average weight of the model rats was
180 g. The rats received intraperitoneal anesthesia with 350 mg/kg
7% chloral hydrate prior to cervical dislocation. The rats
exhibited no sign of peritonitis during the animal model.
Evaluation of disease activity index
(DAI)
From the beginning of the experiment, the general
condition of rats was observed and recorded daily. The rat body
mass was measured and the fecal characteristics and hematochezia
were observed regularly every day. The DAI was assessed according
to DAI score (17,18).
Evaluation of colon macroscopic damage
index (CMDI)
All rats were anesthetized and sacrificed by
cervical dislocation. The distal colon tissues (~8 cm from the
anus) at the same horizontal segment were used for the preparation
of pathological tissue sections. The changes in the mucosa and
serosal surfaces of the colon tissue were observed with the naked
eye. The CMDI of colonic tissue was assessed according to the
criteria for the assessment of microscopic colonic damage (19).
Evaluation of tissue damage index
(TDI)
A piece of fresh colonic tissue (1.0×1.0×0.2 cm) was
collected and fixed in 4% paraformaldehyde at 4°C for 24 h in an
embedding box, and then embedded in paraffin. Paraffin sections
(3–4 µm) were dewaxed with xylene for 10 min, dehydrated in 75 and
85% alcohol for 10 min and stained with hematoxylin for 2–3 min and
0.5% eosin for 1 min at room temperature. HE-stained samples were
observed using a TS100 inverted optical microscope (CFI60 optical
system; Nikon Corporation) at the magnification of 100. TDI of the
colonic tissue was assessed according to the histological grading
of colitis (20).
Cell transfection
RAW264.7 cells were purchased from American Type
Culture Collection. RAW 264.7 cells (1×105/ml) were
seeded in 96-well plates at a density of 1×105/ml.
RAW264.7 cells were induced by 1 mg/l LPS (Sigma-Aldrich; Merck
KGaA) at room temperature for 24 h. The miR-21-5p inhibitor and
inhibitor control from Shanghai GenePharma Co., Ltd. were
transfected into LPS-induced RAW264.7 cells using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2 for 48 h.
miR-21-5p inhibitor was 5′-UCAACAUCAGUCUGAUAAG and inhibitor
control was 5′-CAGUACUUUUGUGUAGUAG. After 24 h, the transfected
RAW264.7 cells were used for subsequent experiments. The RAW264.7
cells were divided into three groups: Untransfected (control);
cells transfected with inhibitor control (NC), and cells
transfected with miR-21-5p inhibitor. The success of the
transfection was examined by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
Dual-luciferase reporter assay
TargetScan software 7.2 (http://www.targetscan.org) identified that STAT3 was a
potential target of miR-21-5p and a luciferase activity assay was
performed to verify this prediction (21). The RAW264.7 cells were co-transfected
with STAT3 3′UTR pmirGLO plasmid [containing wild-type (WT) STAT3
3′-UTR or mutant (MUT) STAT3 3′-UTR; Promega Corporation] and
miR-21-5p inhibitor or NC vector using Lipofectamine®
2000 reagent. After 24 h, the fluorescence activity was measured by
the Renilla-Lumi™ Plus Luciferase Assay kit (Beyotime Institute of
Biotechnology) with the Dual-Luciferase Reporter System (Promega
Corporation). The method of normalization was basing on the
comparison with Renilla luciferase activity.
ELISA
The level of IL-6 and tumor necrosis factor-α
(TNF-α) in rat sera and RAW264.7 cells was respectively determined
by rat IL-6 ELISA kit (cat. no. SBJ-R0064) and rat TNF-α ELISA kit
(cat. no. SBJ-R0040) (both, Nanjing SenBeiJia Biological Technology
Co., Ltd.) according to the manufacturer's protocol.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
The paraffin sections were dewaxed with xylene twice
for 10 min and then cleaned with absolute alcohol for 5 min, 90%
alcohol for 2 min and 70% alcohol for 2 min, followed by the
washing of distilled water for 2 min. Then, tissue slides were
treated with 20 µg/ml proteinase K and incubated at 20–37°C for
15–30 min, followed by incubation with 50 µl TUNEL solution at 37°C
for 60 min in the dark. Finally, a drop of antifade mounting medium
(Beyotime Institute of Biotechnology) was added for 5 min and
samples observed by fluorescence microscopy (Invitrogen; Thermo
Fisher Scientific, Inc.) at an excitation wavelength of 450–500 nm
and an emission wavelength of 515–565 nm. Five fields of view were
observed by fluorescent microscope.
MTT assay
Followed by transfection for 48 h, cell viability
was determined using Cell Proliferation and Cytotoxicity Assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The optical density value was measured at
490 nm with a Synergy™ 2 Multi-function Microplate Reader (BioTek
Instruments, Inc.).
Flow cytometry
Following transfection for 48 h, an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit (BD Biosciences) was used to analyze the apoptotic
level of RAW264.7 cells. The RAW264.7 cells were collected with
trypsin and washed with PBS, and then resuspended in 500 µl HEPES
buffer solution (Sigma-Aldrich; Merck KGaA) and incubated with 5 µl
Annexin V-FITC and 5 µl PI at room temperature for 15 min in the
dark. Finally, a FACSVerse™ flow cytometer with the FACSCanto II
FACP Array™ software (v.3.0; BD Biosciences) was used to analyze
the RAW264.7 cell apoptosis data.
Western blot analysis
Total proteins from colonic tissues and RAW264.7
cells were lysed using a radioimmunoprecipitation assay lysis
buffer (Cell Signaling Technology, Inc.). A BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to determine the
protein concentration. Equal amounts of protein (30 µg/lane) were
loaded on a 12% SDS-PAGE gradient gel to be separated and
transferred to polyvinylidene fluoride membranes (EMD Millipore).
Following blocking with 5% non-fat milk at 4°C for 2 h, membranes
were incubated overnight at 4°C with the following primary
antibodies: Anti-IL6R (1:500, cat. no., ab128008); anti-STAT3
(1:1,000; cat. no., ab68153); anti-intracellular adhesion molecule
1 (ICAM-1; 1:1,000; cat. no., ab171123); anti-NF-κB (1:1,000; cat.
no., ab32360); anti-caspase-3 (1:500; cat. no., ab4051);
anti-cleaved caspase-3 (1:500; cat. no., ab2302); anti-caspase-9
(1:1,000; cat. no., ab52298); anti-cleaved caspase-9 (1:1,000; cat.
no., ab2324); and anti-Fas ligand (FasL; 1:1,000; cat. no.,
ab231011). All antibodies were purchased from Abcam, and then
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. no. sc-2357; Santa Cruz Biotechnology,
Inc.). The membranes were subjected to enhanced chemiluminescence
assay (Pierce; Thermo Fisher Scientific, Inc.) and detected using a
ChemiDoc™ XRS+ imaging system (Bio-Rad Laboratories, Inc.). The
blot densities were analyzed using the Media Cybernetics Gel-Pro
Analyzer software 6.3 (Media Cybernetics, Inc.). GAPDH was used as
the internal control.
RT-qPCR
Total RNA was extracted from colonic tissues or
RAW264.7 cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). A total of 2 µg RNA was used for the generation
of cDNA using the High Capacity cDNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.). RT-qPCR was performed by a
TaqMan™ MicroRNA Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) for miR-21-5p and a Power SYBR Green qPCR Master
Mix (Takara Biotechnology Co., Ltd.) for IL6R, STAT3, ICAM-1,
NF-κB, caspase-3, caspase-9 and FasL, ccording to the
manufacturer's protocols. The thermocycling conditions are as
follows: Initial denaturation at 95°C for 2 min; 38 of cycles of
denaturation at 95°C for 1 min, annealing at 37°C for 1 min and
elongation at 72°C for 2 min; and a final extension at 72°C for 10
min. The primer sequences for qPCR were as follows: U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
miR-21-5p forward, 5′-TAGCTTATCAGACTGATG-3′ and reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; GAPDH forward, 5′-GGAGTCCACTGGTGTCTTCA-3′
and reverse, 5′-GGGAACTGAGCAATTGGTGG-3′; IL6R forward,
5′-CTCCTGCCAGTTAGCAGTCC-3′ and reverse, 5′-TCTTGCCAGGTGACACTGAG-3′;
STAT3 forward, 5′-TGTCCGTCGTGGATCTGAC-3′ and reverse,
5′-CCTGCTTCACCACCTTCTTG-3′; ICAM-1 forward,
5′-CCCTTCCCCCCAAAACTG-3′ and reverse,
5′-GTCATTGTGAACACTGGCAGAAA-3′; NF-κB forward,
5′-CTGAACCAGGGCATACCTGT-3′ and reverse, 5′-GAGAAGTCCATGTCCGCAAT-3′;
caspase-3 forward, 5′-AAGGCAGAGCCATGGACCAC-3′ and reverse,
5′-CTGGCAGCATCATCCACACAT-3′; caspase-9 forward,
5′-ATACACCCTGGACTCGGATCC-3′ and reverse,
5′-TGCTGAAGCTTCTCACAGTCC-3′; FasL forward,
5′-GACCCAGAATACCAAGTGCAGA and reverse,
5′-CTGTTTCAGGATTTAAGGTTGGA-3′. miR-21-5p and mRNA expression data
were calculated relative to U6 and GAPDH, respectively, using the
2−∆∆Cq method (22).
Statistical analysis
All experimental data are presented as mean ±
standard deviation, and were analyzed with SPSS 19.0 (IBM Corp.).
Comparisons between two groups were evaluated using s Student's
t-test, and multiple comparisons between different groups were
evaluated using a one-way analysis of variance with Duncan's
multiple-range test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-21-5p and STAT3 in
human sera
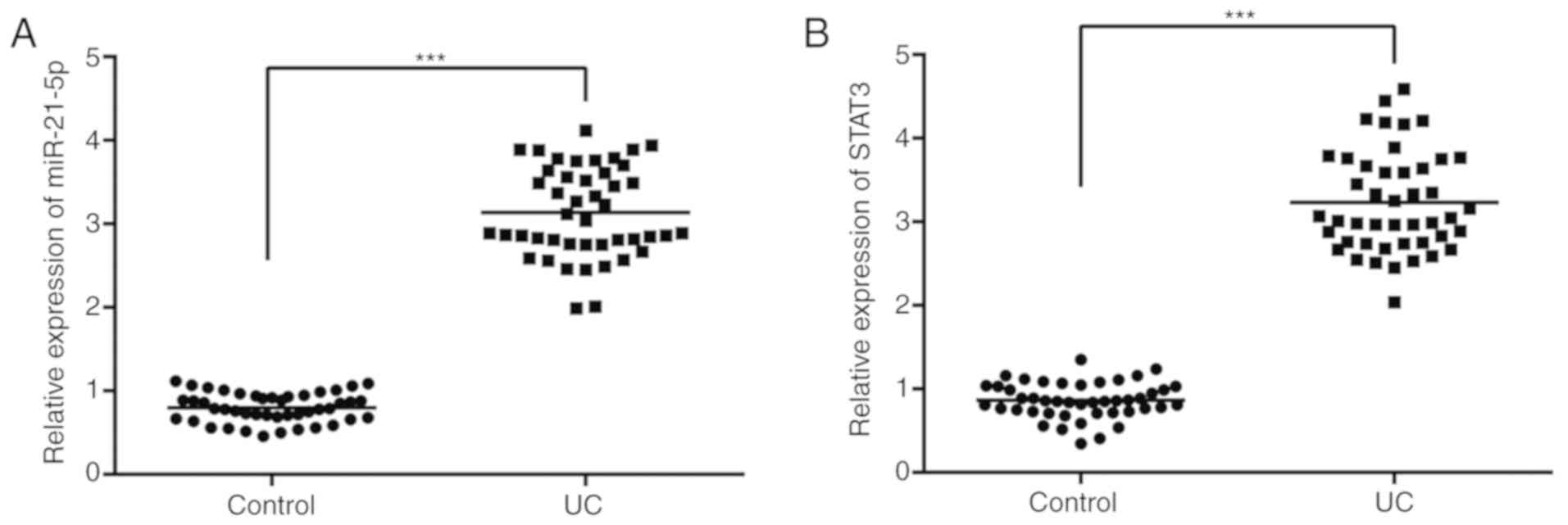
As indicated in Fig.
1A, the median of miR-21-5p in the control group was 0.78 and
the median of miR-21-5p in the patients with UC was 3.12. As
demonstrated in Fig. 1B, the median
STAT3 level in the control group was 0.86 and the median STAT3
level in the patients with UC was 3.25.
General condition and histological
analysis
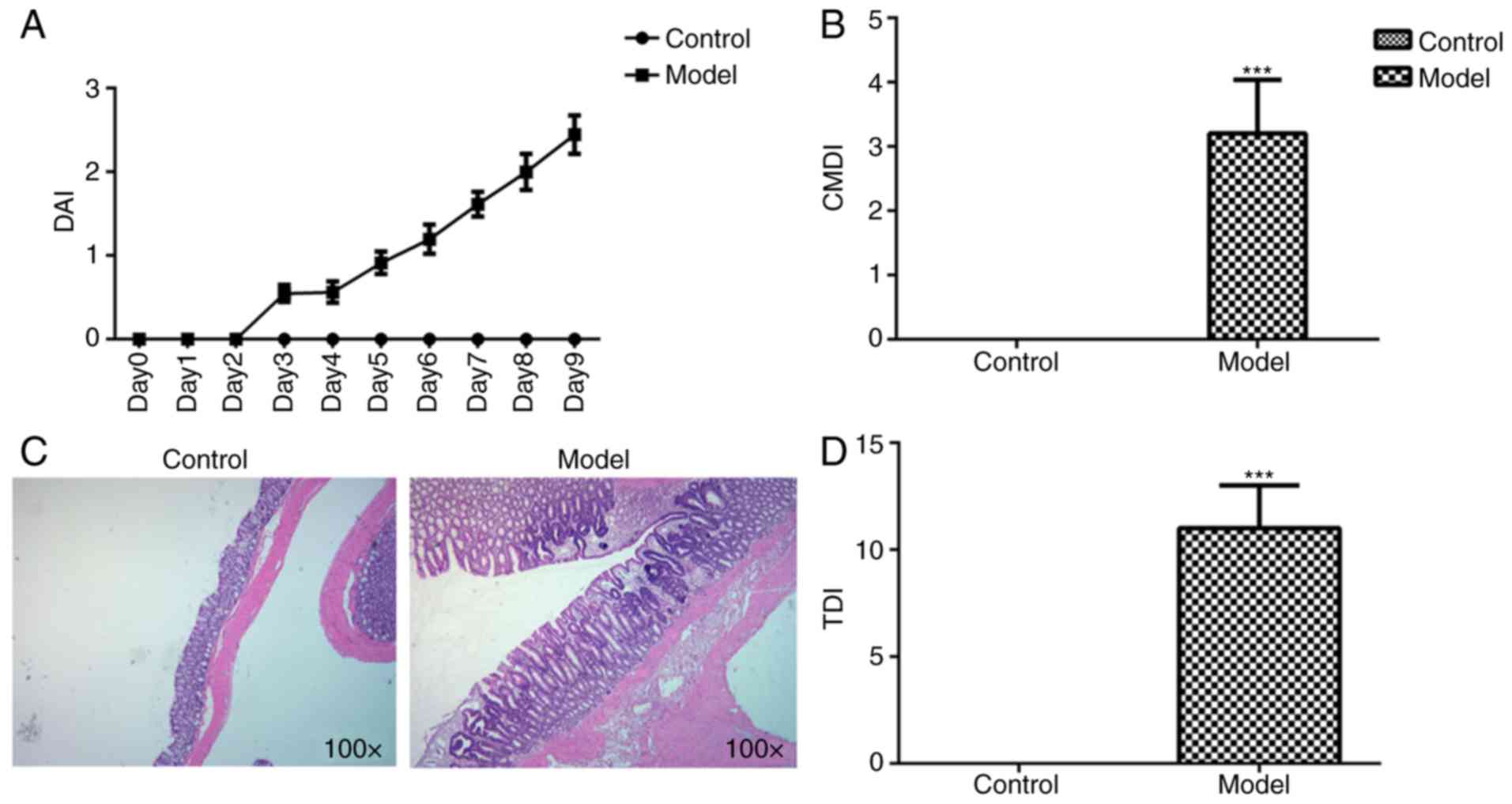
Compared with the control group, DAI increased
following prolonged DSS administration (Fig. 2A). CMDI in the model group was
markedly increased compared with that in the control group
(Fig. 2B). The colonic mucosal
morphologies in each group were observed using a microscope. Clear
layers of colonic structures, aligned mucosal epithelial cells,
regular intestinal glands and no hyperemia and edema in the stroma
were observed in the control group, while colonic mucosa edema,
congestion and inflammatory infiltration were observed in the model
group (Fig. 2C). This coincided with
an increased damage score in the model group, as compared with the
control group (Fig. 2D).
Expression of miR-21-5p in rat colon
tissues
As demonstrated in Fig.
3A, the expression of miR-21-5p in colonic tissue of model
group was increased compared with that in the control group.
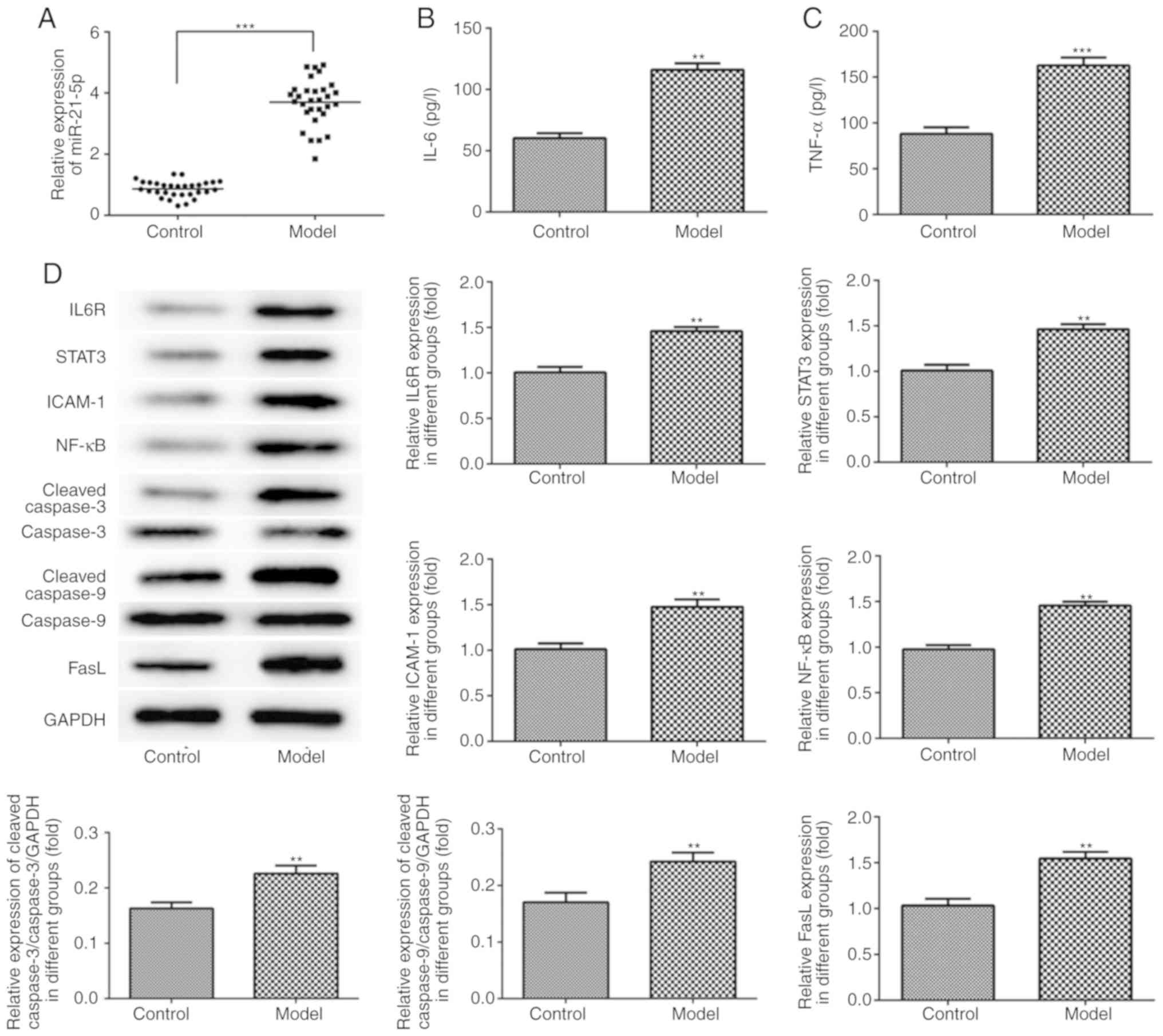 | Figure 3.miR-21-5p expression, inflammation and
apoptosis-associated protein expression in rat colon tissue. (A)
miR-21-5p expression was increased in the UC model group. (B) The
concentration of IL-6 was increased in the UC model group in rat
sera. (C) The concentration of TNF-α was increased in the UC model
group in rat sera. (D) The protein expression levels of IL6R,
STAT3, ICAM-1, NF-κB, cleaved caspase-3, cleaved caspase-9 and FasL
in rat colon tissue were examined using western blot analysis.
**P<0.01 and ***P<0.001 vs. control group. miR, microRNA; UC,
ulcerative colitis; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; IL6R, IL-6 receptor; STAT3, signal transducer and
activator of transcription; ICAM-1, intracellular adhesion
molecule-1; FasL, Fas ligand. |
Inflammation and apoptosis in rats
with DSS-induced UC
As indicated in Fig. 3B
and C, the concentrations of IL-6 and TNF-α in the rat sera
were increased in the model group compared with those in the
control group. Compared with the control group, the expression
levels of IL6R, STAT3, ICAM-1, NF-κB, cleaved caspase-3, cleaved
caspase-9 and FasL in the rat colon tissue were increased in the
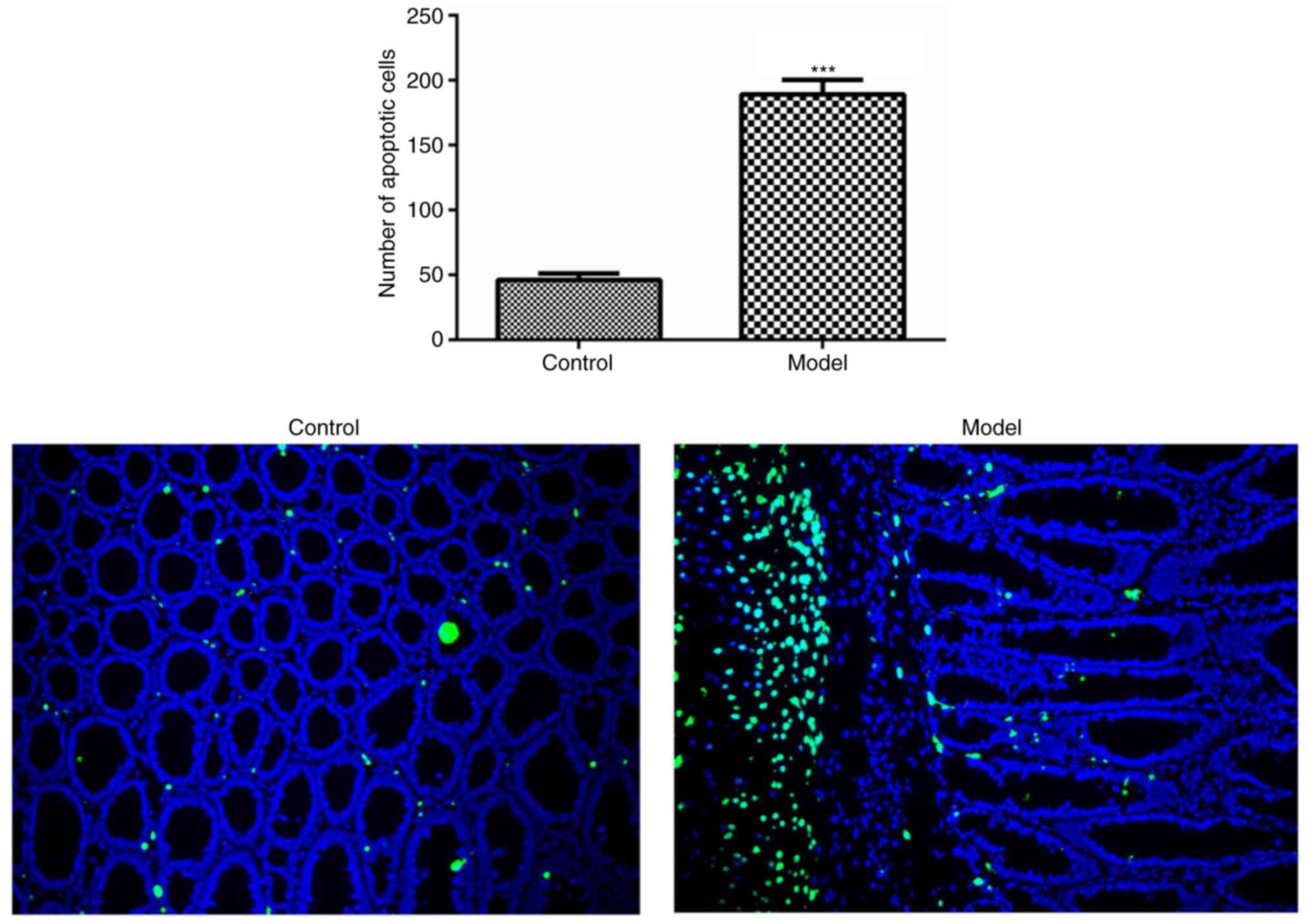
model group, as detected by western blot analysis (Fig. 3D). In addition, the level of
apoptosis in the colonic epithelial cells was increased in the
model group, as compared with that in the control group (Fig. 4).
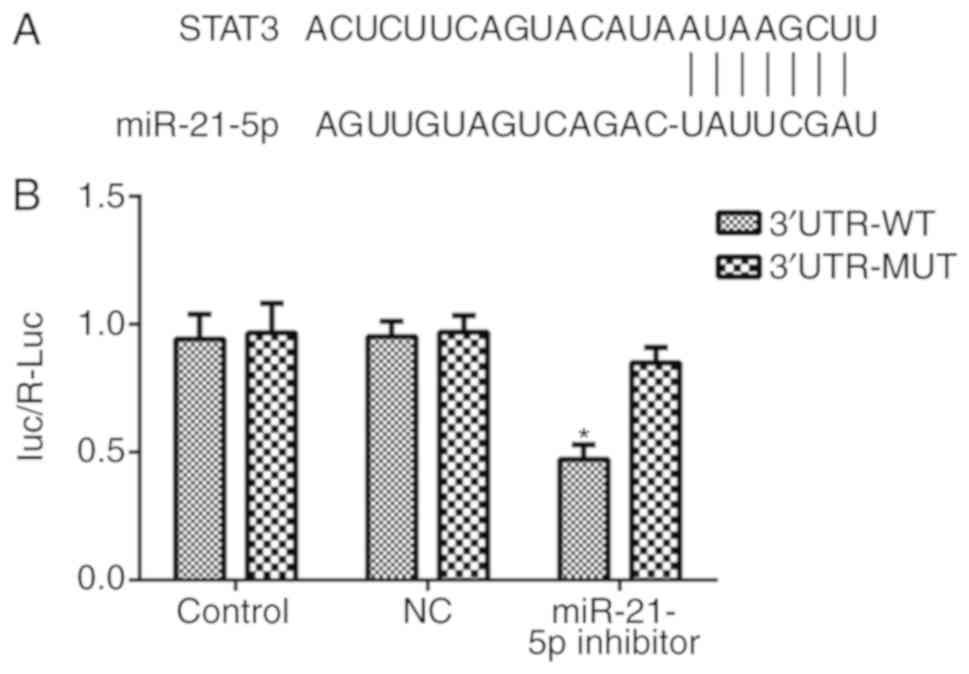
miR-21-5p directly targets STAT3
Bioinformatics analysis was performed using the
TargetScan tool to predict the potential targets of miR-21-5p, and
the results indicated that STAT3 is one of the target genes of
miR-21-5p (Fig. 5A). A
dual-luciferase reporter assay was used to confirm this prediction.
As indicated in Fig. 5B, the
overexpression of miR-21-5p clearly decreased the expression of
STAT3 in the 3′UTR-WT group, while no difference was observed in
the 3′UTR-MUT group, suggesting that miR-21-5p directly targets
STAT3.
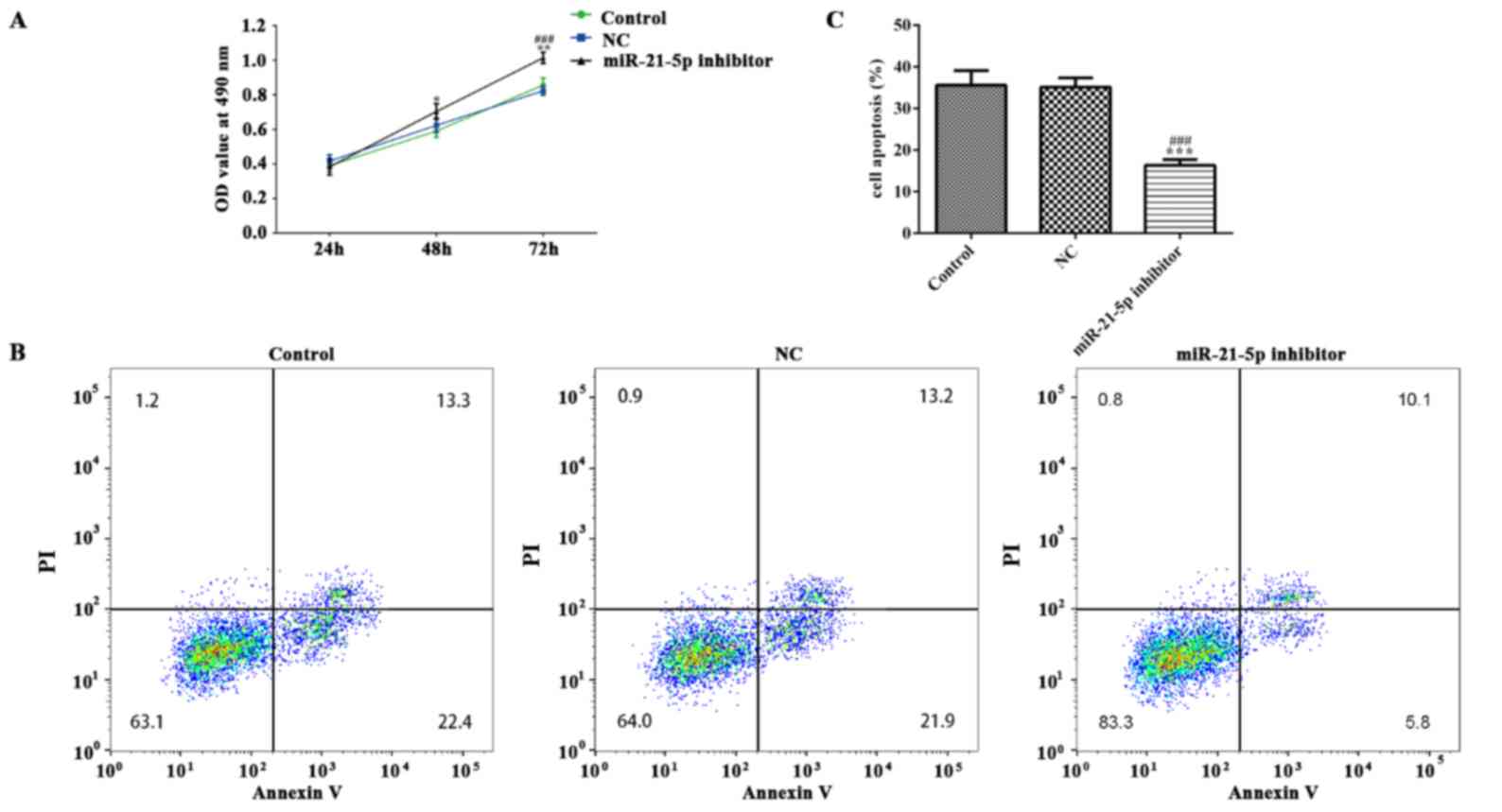
Effect of miR-21-5p on LPS-induced
RAW264.7 cell inflammation, viability and apoptosis
The RAW264.7 cell line was used to investigate the
role of miR-21-5p. As demonstrated in Fig. 6A, miR-21-5p was downregulated in
RAW264.7 cells following transfection with miR-21-5p inhibitor,
while no difference was observed between the NC and control groups.
As indicated in Fig. 6B and C, the
concentration levels of IL-6 and TNF-α in RAW264.7 cells were
markedly decreased in the miR-21-5p inhibitor group, as compared
with the NC and control groups. miR-21-5p inhibition suppressed the
mRNA expression levels of IL6R, STAT3, ICAM-1, NF-κB, caspase-3,
caspase-9 and FasL in RAW264.7 cells, as compared with that in the
NC and control groups (Fig. 6D). As
shown in Fig. 7, the expression of
IL6R, STAT3, ICAM-1, NF-κB, cleaved caspase-3, cleaved caspase-9
and FasL in RAW264.7 cells was decreased in RAW264.7 cells
transfected with miR-21-5p inhibitor. MTT assay and flow cytometry
analyses were conducted to investigate the effects of miR-21-5p on
RAW264.7 cell viability and apoptosis. As demonstrated in Fig. 8, the viability of RAW264.7 cells was
markedly increased while the apoptosis of RAW264.7 cells was
obviously decreased in the miR-21-5p inhibitor group, as compared
with the NC and control groups.
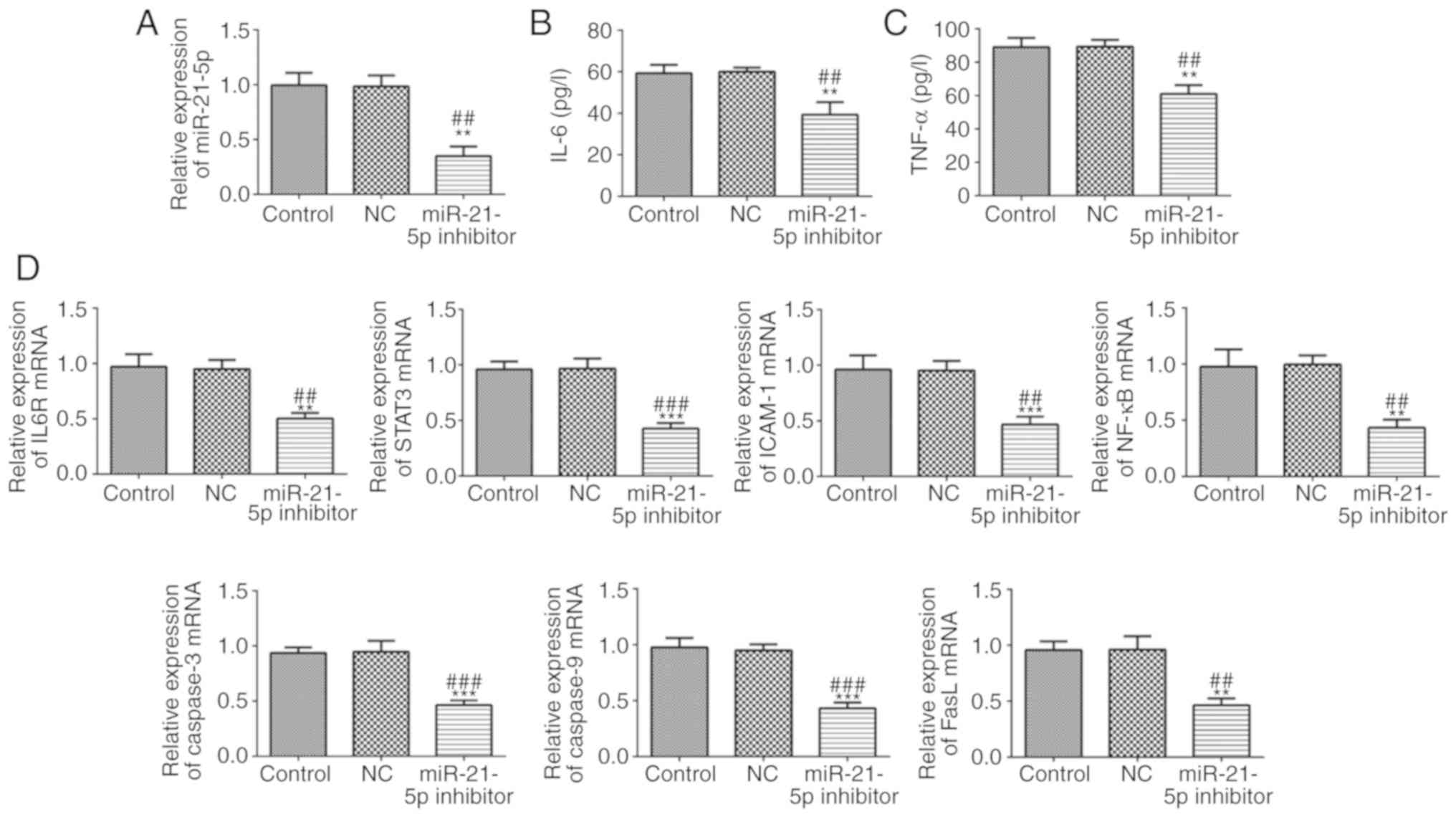 | Figure 6.miR-21-5p inhibition improves the
expression of inflammation and apoptosis-associated proteins. (A)
miR-21-5p expression was decreased following cell transfection with
miR-21-5p inhibitor. (B) The concentration of IL-6 was decreased in
RAW264.7 cells transfected with miR-21-5p inhibitor. (C) The
concentration of TNF-α was decreased in RAW264.7 cells transfected
with miR-21-5p inhibitor. (D) The mRNA expression levels of IL6R,
STAT3, ICAM-1, NF-κB, caspase-3, caspase-9 and FasL were analyzed
by reverse transcription-quantitative polymerase chain reaction.
**P<0.01 and ***P<0.001 vs. control group.
##P<0.01 and ###P<0.001 vs. NC group.
miR, microRNA; NC, negative control; IL-6, interleukin-6; tumor
necrosis factor-α; IL6R, IL-6 receptor; STAT3, signal transducer
and activator of transcription; ICAM-1, intracellular adhesion
molecule-1; FasL, Fas ligand. |
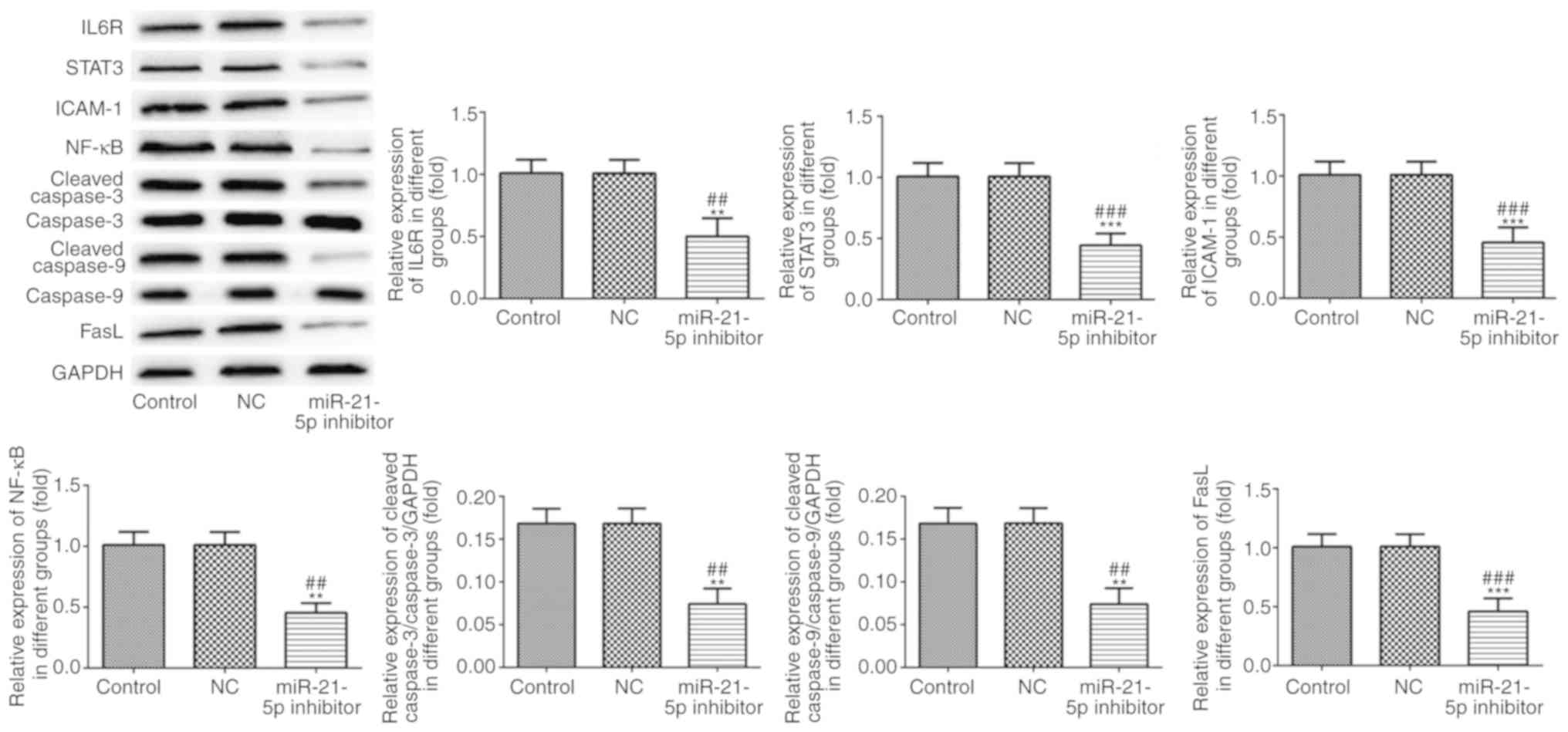 | Figure 7.Protein expression levels of IL6R,
STAT3, ICAM-1, NF-κB, cleaved caspase-3, cleaved caspase-9 and FasL
were analyzed by western blot analysis. **P<0.01 and
***P<0.001 vs. control group. ##P<0.01 and
###P<0.001 vs. NC group. IL6R, IL-6 receptor; STAT3,
signal transducer and activator of transcription; ICAM-1,
intracellular adhesion molecule-1; FasL, Fas ligand; NC, negative
control. |
Discussion
In the present study, the expression levels of
miR-21-5p and STAT3 was detected in the sera of patients with UC
and tissues of UC rats. The general condition, histological
changes, levels of inflammation-associated factors and apoptosis,
and the mechanism and function of miR-21-5p in RAW264.7 cells, were
also analyzed. It was identified that miR-21-5p inhibition mediated
the IL6R/STAT3 pathway in UC rats to decrease the levels of
inflammation and apoptosis, suggesting that miR-21-5p may be an
important therapeutic target in human ulcerative colitis.
UC is a disease of the mucosal lining limited to the
innermost layers of colon with cryptitis and crypt abscesses.
Previous studies have demonstrated that miRNAs are associated with
the progress of UCL: For example, miR-146 rs2910164 was identified
to be associated with a decreased risk of UC in an Asian population
(23). The miRNA-200b mediated
inflammatory responses in an RAC-beta serine/threonine-protein
kinase-dependent manner to suppress tumor development (24). miR-184 and miR-490-5p may regulate
inflammatory signaling pathways, and attenuate inflammation and
tissue injury in the colons of rats with DSS-induced UC (25). However, the understanding of the
effect of miR-21-5p in DSS-induced UC remains limited.
In recent years, the role of miR-21-5p in a number
of diseases has been studied due to its important functions
(26–28). miR-21-5p downregulation may inhibit
proliferation and apoptosis in esophageal squamous cell carcinoma
(ESCC) cells and may represent a novel therapeutic target for the
treatment of ESCC (29). miR-21-5p
was overexpressed in response to the inflammatory cytokines in beta
cell lines, and miR-21-5p inhibition could alleviate inflammation
in beta cell lines (30). In
traumatic brain injury, miR-21-5p suppressed inflammation by
regulating the expression of inflammatory cytokines and NF-kB
signaling and inhibited cellular apoptosis by regulating the
expression of apoptosis factors and Akt signaling (31). We therefore hypothesized that
miR-21-5p may be associated with inflammation and apoptosis in
DSS-induced UC. In the present study, it was observed that
miR-21-5p expression was clearly increased in the sera of patients
with UC and rat colon tissue with DSS-induced UC. STAT3 is an
inflammation-responsive transcription factor with a role in several
types of cancer (32). STAT3
signaling is activated by inflammatory cytokines including IL-6,
IL-10 and IL-22 (33). STAT3 and the
STAT3 signaling pathway have been identified to serve a prominent
role in colitis (34). Whether
miR-21-5p regulates inflammation and apoptosis with STAT3 remains
unknown. The results of the present study identified STAT3 to be a
target of miR-21-5p, and demonstrated that miR-21-5p inhibition may
regulate the expression of the STAT3-associatied pathway and
apoptosis-associated proteins to alleviate inflammation and
decrease the level apoptosis in RAW264.7 cells.
In conclusion, the present study indicated that
miR-21-5p was downregulated in the sera and colon tissue of UC
compared with healthy people and the control group. In addition,
miR-21-5p inhibition may downregulate the expression of IL-6,
TNF-α, IL6R, STAT3, ICAM-1, NF-κB, cleaved caspase-3, cleaved
caspase-9 and FasL to alleviate the inflammation and apoptosis. In
addition, miR-21-5p may exert its role partly by targeting STAT3,
and miR-21-5p may be a therapeutic target for the treatment of
UC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL conceptualized and developed the study design and
performed the majority of the experiments. XL and YY acquired the
data which analyzed by XL, YY and ST. XL and ST wrote the
manuscript and XL and YY suggested appropriate modifications which
were corrected by ST.
Ethics approval and consent to
participate
The study was approved by the Human Ethics Committee
Review Board of Renmin Hospital of Wuhan University, and informed
consent was obtained from each patient.
Patient consent for publication
Informed consent was obtained from each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gillen CD, Walmsley RS, Prior P, Andrews
HA and Allan RN: Ulcerative colitis and Crohn's disease: A
comparison of the colorectal cancer risk in extensive colitis. Gut.
35:1590–1592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z: MicroRNA: A matter of life or
death. World J Biol Chem. 1:41–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kinose Y, Sawada K, Nakamura K and Kimura
T: The role of MicroRNAs in ovarian cancer. Biomed Res Int.
2014:2493932014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen N, Huang X and Li J: Upregulation of
miR-129-5p affects laryngeal cancer cell proliferation,
invasiveness, and migration by affecting STAT3 expression. Tumour
Biol. 37:1789–1796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen
N, Zhang P, Wang F, Yang J, Yang J, et al: Overexpression of miR-21
in patients with ulcerative colitis impairs intestinal epithelial
barrier function through targeting the Rho GTPase RhoB. Biochem
Biophys Res Commun. 434:746–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polytarchou C, Hommes DW, Palumbo T,
Hatziapostolou M, Koutsioumpa M, Koukos G, van der Meulen-de Jong
AE, Oikonomopoulos A, van Deen WK, Vorvis C, et al: MicroRNA214 is
associated with progression of ulcerative colitis, and inhibition
reduces development of colitis and colitis-associated cancer in
mice. Gastroenterology. 149:981–992.e11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ranjha R, Meena NK, Singh A, Ahuja V and
Paul J: Association of miR-196a-2 and miR-499 variants with
ulcerative colitis and their correlation with expression of
respective miRNAs. PLoS One. 12:e01734472017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis A, Felice C, Kumagai T, Lai C, Singh
K, Jeffery RR, Feakins R, Giannoulatou E, Armuzzi A, Jawad N, et
al: The miR-200 family is increased in dysplastic lesions in
ulcerative colitis patients. PLoS One. 12:e01736642017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi CC: Study on the mechanism of miRNA-21
in regulating intestinal barrier function and inflammation related
colon cancer. Shanghai Jiaotong University; 2014
|
|
11
|
Thorlacius-Ussing G, Nielsen BS, Andersen
V, Holmstrøm K and Pedersen AE: Expression and localization of
miR-21 and miR-126 in mucosal tissue from patients with
inflammatory bowel disease. Inflamm Bowel Dis. 23:739–752. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gryshkova V, Fleming A, McGhan P, De Ron
P, Fleurance R, Valentin JP and Nogueira da Costa A: miR-21-5p as a
potential biomarker of inflammatory infiltration in the heart upon
acute drug-induced cardiac injury in rats. Toxicol Lett. 286:31–38.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buoli Comani G, Panceri R, Dinelli M,
Biondi A, Mancuso C, Meneveri R and Barisani D: miRNA-regulated
gene expression differs in celiac disease patients according to the
age of presentation. Genes Nutr. 10:4822015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T: Study on the relationship between
intestinal barrier and STAT3 signaling pathway in ulcerative
colitis. Tianjin Medical University; 2013
|
|
15
|
Li T, Zhu XD, Yang Y and Zhai YH: Effect
of tongxie yaofang on the expression of IL-6 and IL6R in the
hypothalamus of rats with ulcerative colitis. Chin J Exp Trad Med
Formulae. 23:103–108. 2017.
|
|
16
|
Wang Q, Bie YL, Wang D and Fan WT: Effect
of dandelion polysaccharide on IL-6 /STAT3 signaling pathway in
ulcerative colitis rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
33:422–425. 2017.(In Chinese). PubMed/NCBI
|
|
17
|
Murthy S, Cooper HS, Yoshitake H, Meyer C,
Meyer CJ and Murthy NS: Combination therapy of pentoxifylline and
TNFalpha monoclonal antibody in dextran sulphate-induced mouse
colitis. Aliment Pharmacol Ther. 13:251–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
19
|
Paiotti AP, Ribeiro DA, Silva RM, Marchi
P, Oshima CT, Neto RA, Miszputen SJ and Franco M: Effect of COX-2
inhibitor lumiracoxib, the anti-TNF-α etanercept and your
association on TNBS-induced colitis in Wistar rats. J Mol Histol.
43:307–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dieleman LA, Palmen MJ, Akol H, Bloemena
E, Peña AS, Meuwissen SG and Van Rees EP: Chronic experimental
colitis induced by dextran sulphate sodium (DSS) is characterized
by Th1 and Th2 cytokines. Clin Exp Immunol. 114:385–391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agarwal V, Bell GW, Nam J and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Wang Y and Zhu Y: Association of
miRNA-146a rs2910164 and miRNA-196 rs11614913 polymorphisms in
patients with ulcerative colitis: A meta-analysis and review.
Medicine (Baltimore). 97:e122942018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng S, Wang H, Fan H, Zhang L, Hu J, Tang
Q, Shou Z, Liu X, Zuo D, Yang J, et al: Over-expressed miRNA-200b
ameliorates ulcerative colitis-related colorectal cancer in mice
through orchestrating epithelial-mesenchymal transition and
inflammatory responses by channel of AKT2. Int Immunopharmacol.
61:346–354. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Ma Z, Cui YH, Dong HS, Zhao JM,
Dou CZ, Liu HR, Li J and Wu HG: Effects of herb-partitioned
moxibustion on the miRNA expression profiles in colon from rats
with DSS-induced ulcerative colitis. Evid Based Complement Alternat
Med. 2017:7673012017. View Article : Google Scholar
|
|
26
|
Liu Z, Yu M, Fei B, Fang X, Ma T and Wang
D: miR-21-5p targets PDHA1 to regulate glycolysis and cancer
progression in gastric cancer. Oncol Rep. 40:2955–2963.
2018.PubMed/NCBI
|
|
27
|
Fromm B, Tosar JP, Lu Y, Halushka MK and
Witwer KW: Human and cow have identical miR-21-5p and miR-30a-5p
sequences, which are likely unsuited to study dietary uptake from
cow milk. J Nutr. 148:1506–1507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Li B, Li Q, Wei S, He Z, Huang X,
Wang L, Xia Y, Xu Z, Li Z, et al: Exosomal miR-21-5p derived from
gastric cancer promotes peritoneal metastasis via
mesothelial-to-mesenchymal transition. Cell Death Dis. 9:8542018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li XH, Chen D, Li M, Gao X, Shi G and Zhao
H: The CADM2/Akt pathway is involved in the inhibitory effect of
miR-21-5p downregulation on proliferation and apoptosis in
esophageal squamous cell carcinoma cells. Chem Biol Interact.
288:76–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lakhter AJ, Pratt RE, Moore RE, Doucette
KK, Maier BF, DiMeglio LA and Sims EK: Beta cell extracellular
vesicle miR-21-5p cargo is increased in response to inflammatory
cytokines and serves as a biomarker of type 1 diabetes.
Diabetologia. 61:1124–1134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ge XT, Huang S, Gao H, Han Z, Chen F,
Zhang S, Wang Z, Kang C, Jiang R, Yue S, et al: miR-21-5p
alleviates leakage of injured brain microvascular endothelial
barrier in vitro through suppressing inflammation and apoptosis.
Brain Res. 1650:31–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hua Y, Drew P and Richard J: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dang V, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al: Control of
T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell.
146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JZ, Suzanne VS, Hailiang H, Ng SC,
Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, et al:
Association analyses identify 38 susceptibility loci for
inflammatory bowel disease and highlight shared genetic risk across
populations. Nat Genet. 47:979–986. 2015. View Article : Google Scholar : PubMed/NCBI
|