Introduction
Osteoporosis is a systemic osteopathy. It results in
a decrease in both the density and mass of bones, ultimately
causing bone microstructure damage and increased bone fragility
(1). Osteoblasts are crucial for the
process of bone formation. Defects in osteoblast viability,
differentiation and mineralization are among the major causes of
osteoporosis (2,3). Puerarin, an isoflavone extracted from
the Chinese medicine pueraria, exhibits protective functions on the
cardiovascular system, nervous system, osteoporosis, liver injury,
and inflammation in vivo and in vitro (4). Studies have shown that puerarin can
promote the proliferation, differentiation and mineralization of
osteoblasts in vitro (5,6). In a
recent report, puerarin was found to improve bone loss in
estrogen-deficient rats, display a superior anti-osteoporosis
effect, and prevent osteoporosis in postmenopausal women (7). Although experiments have shown that
puerarin plays an important role in osteoblasts and osteoporosis
in vitro and in vivo, the exact mechanisms involved
in the anti-osteoporosis effects of puerarin remain unclear.
There are three main types of cell death: Autophagy,
apoptosis and necrosis (8). The
level of autophagy changes significantly during the process of
osteoporosis. Moreover, autophagy can regulate osteoblasts in both
directions to either promote or inhibit cell proliferation under
different conditions (9). Cells can
generate energy from the degradation pathway of autophagy. But if
the damage is exceedingly high, the cells will undergo controlled
suicide by autophagy (10). The
above findings warrant further investigation of the effect of
puerarin on autophagy in osteoblasts.
Microtubule-associated light chain 3 (LC3) exists in
the double membrane structure of autophagosomes, and is an
important marker of autophagy. There are three isoforms of LC3
proteins in mammals: LC3A, LC3B and LC3C. These three undergo
post-translational modifications during autophagy (11). The LC3 protein is immediately
synthesized by Atg4 at the carboxyl end of the protein, and LC3-I
is localized in the cytoplasm. We discovered that during autophagy,
LC3-I is modified and processed by ubiquitin-like systems,
including Atg7 and Atg3. The overall result is LC3-II with
molecular weight of 16 kDa localized in autophagic microsomes.
Thus, the presence of LC3-I and low molecular weight LC3-II in
autophagosome are both molecular markers of autophagy, and the
amount of LC3-II represents the degree of autophagy (12). To date, LC3B has been extensively
studied and identified as an autophagic membrane-associated factor
(13). Previous studies have
demonstrated that a low concentration of puerarin can promote the
viability and differentiation of osteoblastic MC3T3-E1 cells, which
may be achieved by downregulation of the expression of miR-204 and
its host gene TRPM3 (6).
However, it is not clear whether there are other targets in this
pathway. Biological information indicates that there may be a
targeting relationship between miR-204 and LC3B, but this has not
been verified in osteoblasts.
In the present study, the effects of puerarin on the
viability, differentiation and mineralization of osteoblasts were
observed. It was found that puerarin significantly upregulated the
expression of LC3B and Beclin1 leading to the formation of
autophagosomes in osteoblasts. In addition, inhibition of autophagy
in osteoblasts significantly reduced the viability and
differentiation of the cells. It is suggested that puerarin
enhances the viability and differentiation of osteoblasts by
promoting autophagy. The possible binding sites between miR-204 and
LC3B were explored based on biological information. In addition,
the overexpression of miR-204 significantly reduced the protein
level of LC3B and the formation of autophagosomes, while its
inhibition reversed this effect. To conclude, puerarin promoted the
viability and differentiation of MC3T3-E1 cells through autophagy,
a mechanism possibly associated with miR-204-mediated LC3B
upregulation.
Materials and methods
Osteoblast culture
Osteoblastic MC3T3-E1 cells were purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in a 25 cm2 flask, and
α-modified Eagle's medium (α-MEM; Wisent Inc., Canada) was added
together with 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher
Scientific, Inc.) 100 U/ml penicillin and 100 g/ml streptomycin and
maintained at 37°C in a humid 5% CO2 incubator (Sanyo
Electric Co., Ltd., Japan).
CCK-8 assay
Firstly, 5×103 cells/well were seeded in
96-well plates (Corning Inc.) at 37°C in a humid 5% CO2
incubator for 24 h, and then 200 µl of different final
concentrations (0.1, 1 and 10 µM) of puerarin were added to
experimental wells, while equivalent serum-free medium was added to
the control cells. After culturing for 24, 48, 72 h, 10 µl CCK-8
(Dojindo Molecular Technologies) was added to each well and
culturing was continued for 30 min to detected optical density (OD)
values (wavelength 450 nm) using a multi-functional enzyme labeling
instrument (BioTek Instruments, Inc.). The cell viability following
treatment with 3-MA, or puerarin + 3-MA was also detected by CCK-8
assay.
Alkaline phosphatase (ALP)
activity
Cells at a density of 5×105 cells/well
were seeded in 6-well plates. Following culture for 24 h, 2 ml of 1
µM puerarin was added to the experimental wells, while equivalent
serum-free medium was added to the control cells. Following
culturing for 72 h, the cell ALP activity was determined using an
ALP kit according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute, China). This method was also
used to detect cell ALP activity of the cells following treatment
with 3-MA, or puerarin + 3-MA.
Count of mineralized nodules
A total of 5×104 cells were added to each
well of a 24-well culture plate. After 24 h, the cells were treated
with puerarin, 3-MA, or puerarin + 3-MA. The medium was changed
every 3 days. The cells were washed 2 times with PBS and stained
with 0.2% solution of alizarin red for 30 min on day 7, 14 and 21.
Three fields were randomly selected for each well under low
magnification, and the relative mineralized nodule areas were
analyzed by Image J 2× 2.1.4.7 software (Rawak Software Inc.).
Transmission electron microscopy
A total of 5×105 cells/well were seeded
in 6-well plates. After culturing for 24 h, 2 ml/well fresh culture
medium was added according to the experiment grouping (control
group, 1 µM puerarin group, 1 µM puerarin+3-MA group). Following
culturing for 72 h, 1 ml/well trypsin was added to digest the cells
in a 1.5 ml EP tube, and then the cells were centrifuged at 1,000 ×
g for 5 min and the supernatant was removed. Next glutaraldehyde
(500 ml/tube) was added and the cells were maintained at 4°C for 12
h, and then osmium acid was used to fix the cells which then
underwent uranium acetate staining, ethanol gradient dehydration
and resin embedding. Finally, the structure of the cell organelles
was observed under a transmission electron microscope (Jeol, Tokyo,
Japan; magnification, ×12,000). This method was also used to
observe cell organelles in the cells transfected with miR-204 NC,
miR-204 mimics and miR-204 inhibitor.
Western blot analysis
Cells at a density of 5×105 cells/well
were seeded in 6-well plates. After culturing for 24 h, the cells
were treated with puerarin for 0, 48 and 72 h. Cell total protein
was extracted using a total protein extraction kit (cat. no.
BC3710; Beijing Solarbio Science & Technology Co., Ltd., China)
and quantified by the BCA method. 12% SDS-PAGE was performed on a
25 g sample, and the separated proteins were electrotransfer onto a
polyvinylidene phosphatide membrane, and blocked using 5% skim milk
in TBS containing 0.1% Tween-20 (TBST) at room temperature for 1 h.
After 1 h, the membranes were washed 3 times with TBST for 15 min.
Next, primary antibodies including LC3B (cat. no. ab48394; Abcam),
Beclin1 (cat. no. ab62557; Abcam) and β-actin (cat. no. 4970; Cell
Signaling Technology, Inc.) were mixed with blocking solution
(dilution 1:1,000) separately in 4 ml and used to treat the
membranes at 4°C overnight. The membranes were then washed 3 times
with TBST for 15 min, and then incubated with horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (cat. no.
7074; Cell Signaling Technology, Inc.; dilution 1:2,000) for 1 h at
room temperature. After washing with TBST, the protein bands of
interest were displayed by enhanced chemiluminescence scenario
detection system. (Bio-Rad Laboratories, Inc.). ImageJ2× V2.1.4.7
software (National Institutes of Health, Bethesda, MD, USA) was
used to quantify the relative protein levels. The expression of
LC3B protein in the cells treated with rapamycin, 3-MA, puerarin +
3-MA, and transfected with miR-204 NC, miR-204 mimics and miR-204
inhibitor was also detected using this method.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Osteoblastic MC3T3-E1 cells were incubated with
puerarin (1 µM) for 72 h, and total RNA was extracted using TRIzol
reagent (Takara Bio, Inc.). The reverse-transcription reaction
solution (20 µl) contained 10.0 µl 5X Reverse Transcription Mix
(Takara Bio, Inc.), 1.0 µl Stem-loop RT primers (GenScript), 500 ng
miRNA, 2.0 µl HiScript Enzyme Mix (cat. no. R223; Vazyme) and
RNase-free water. The PCR amplification conditions consisted of
25°C for 5 min, 45°C for 50 min and 85°C for 50 min. Specific miRNA
stem-loop RT primers for mouse miR-204 and internal control U6 were
designed and synthesized by Shanghai Sangon Pharmaceutical Co. Ltd.
(Shanghai, China). Primers used in the stem-loop RT-qPCR were as
follows: RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGCAT-3′; PCR
upstream primer, 5′-GCGGCGGTTCCCTTTGTCATCC-3′ and downstream primer
5′-ATCCAGTGCAGGGTCCGAGG-3′ for miR-204; PCR upstream primer,
5′-CTCGCTTCGGCAGCACA-3′ and downstream primer,
5′-AACGCTTCACGAATTTGCGT-3′ for U6. The two-step PCR amplification
conditions were: pre-denaturation of 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec, 60°C for 34 sec. The fluorescence
signal detection was assessed using the Mx3000P Real-Time PCR
system (Stratagene; Agilent Technologies, Inc.).
Bioinformatics predicts miR-204 and
mouse LC3B gene binding sites
The binding sites of mouse LC3B and miR-204 were
predicted using TargetScan (http://genes.mit.edu/targetscan).
Overexpression and inhibition of
miR-204
Mouse miR-204 mimics, inhibitor and negative control
were obtained from Shanghai Gene Pharma Co., Ltd. Cells were
inoculated into 6-well plates at a density of 1×105 per
well and transfected using 4 µl Lipofectamine 2000 (Invitrogen;
Semefield Technology) according to the manufacturers protocol when
the cells reached 90% confluence. The control cells were
transfected with only 4 µl transfection reagent. Then the cells
were incubated in a humid 5% CO2 incubator at 37°C for
48 h. Cell preparation was used for RT-qPCR analysis of miR-204,
transmission electron microscopy and western blot analysis of
LC3B.
Statistical analysis
Data are expressed as mean ± standard deviation.
Data were analyzed using one-way analysis of variance with SPSS
software (version, 17.0; SPSS Inc.) or Student's t-tests.
Bonferroni post hoc test was used to compare the differences
between groups. P<0.05 is considered to indicate a statistically
significant result.
Results
Puerarin promotes osteoblast
viability, differentiation and mineralization
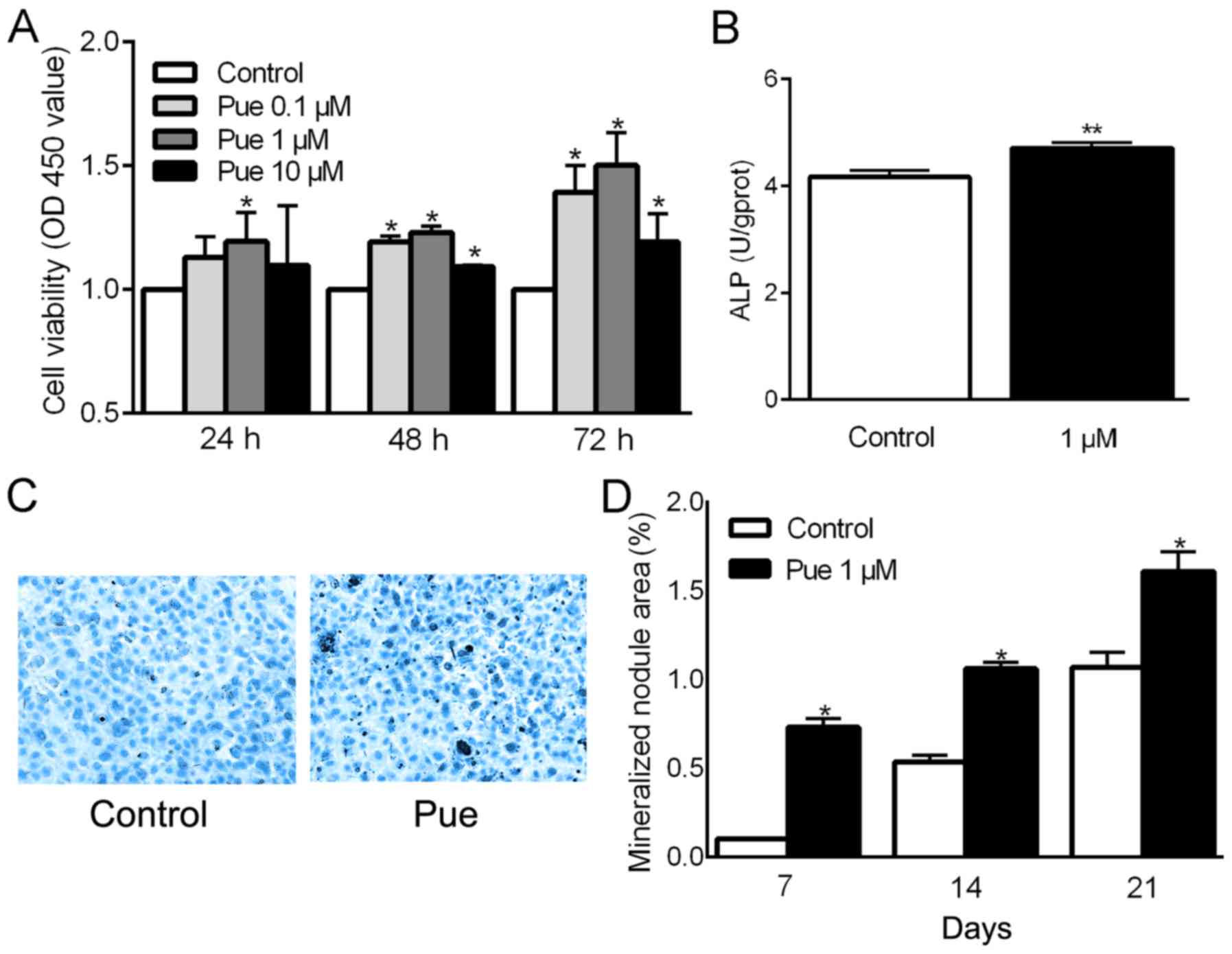
It was first ascertained whether puerarin affects
the viability, differentiation and mineralization of osteoblasts.
CCK-8 results showed that the viability of the osteoblastic
MC3T3-E1 cells treated with different concentrations of puerarin
(Pue) (0.1, 1 and 10 µM) for 24, 48 and 72 h was significantly
higher than that noted in the control cells (except for 0.1 and 10
µM puerarin at 24 h) and puerarin at the concentration of 1 µM
showed the highest effect at 72 h (P<0.05; Fig. 1A). The cells treated with 1 µM
puerarin for 72 h also had a significantly higher ALP activity
(P<0.05; Fig. 1B). In addition,
the effect of puerarin on osteoblast mineralization was further
tested by calculating the mineralized nodule area through Alizarin
Red S staining. Compared with the control group, the formation of
mineralized nodules was increased significantly in the cells
treated with 1 µM puerarin for 7, 14 and 21 days (P<0.05;
Fig. 1C and D).
Puerarin upregulates the expression of
Beclin1 and LC3B and promotes the formation of autophagosome
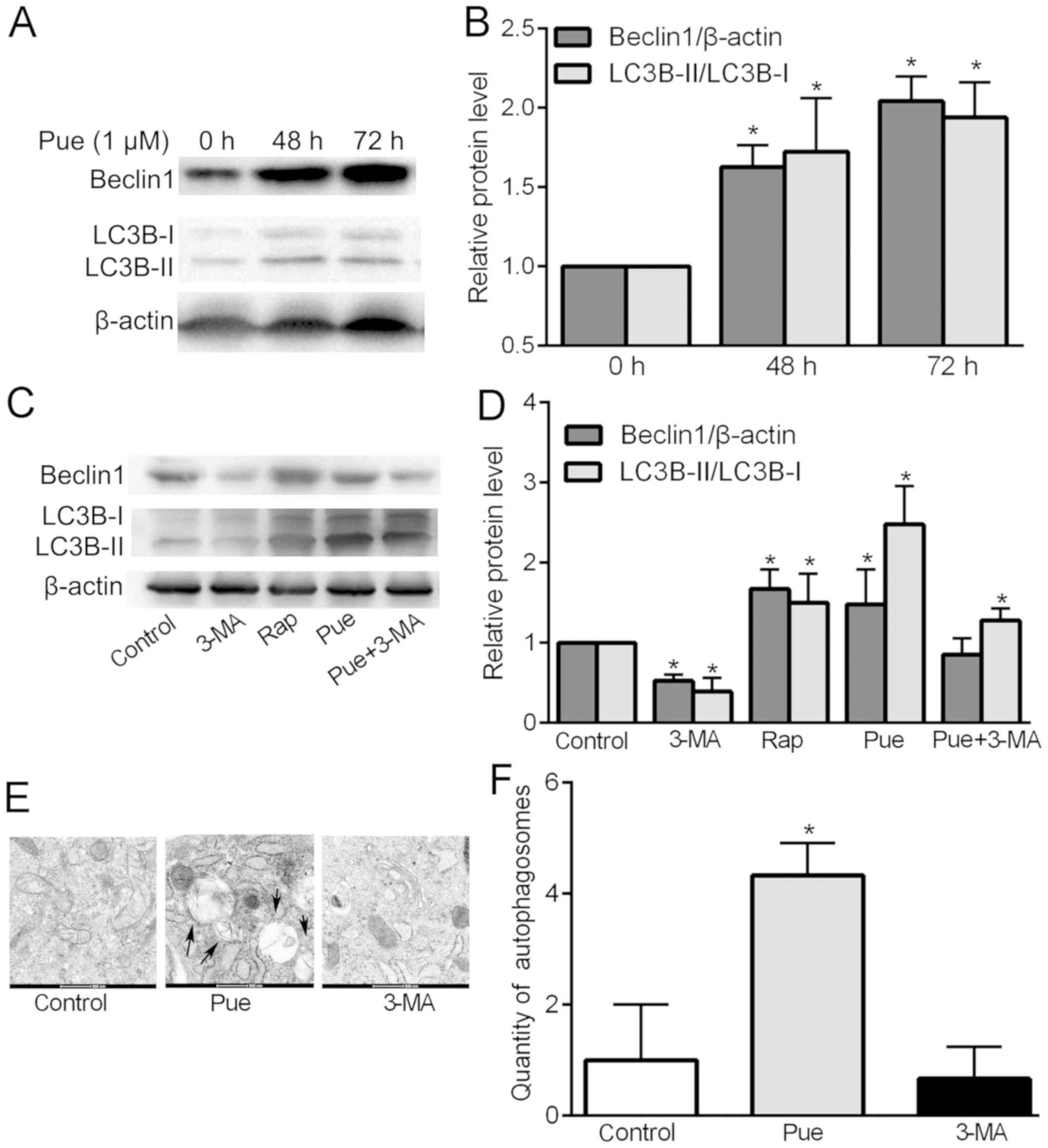
The expression levels of Beclin1 and LC3B-II/LC3B-I
were used to compare the intensity of autophagy. Puerarin at
concentrations of 0.1 and 10 µM did not effectively promote
osteoblast viability at 24 h; therefore, the expression levels of
Beclin 1 and LC3B protein were observed at 48 and 72 h compared
with levels at 0 h. The results (P<0.05; Fig. 2A and B) showed that after puerarin
treatment for 48 and 72 h, Beclin1 and LC3B expression was
significantly upregulated when compared with that at 0 h and
puerarin showed the greatest effect at 72 h. The results suggested
that the expression levels of Beclin1 and LC3B-II/LC3B-I proteins
were upregulated in the puerarin-treated cells within 72 h and the
effect was time-dependent. Therefore, in the following experiments
72 h for drug action time was selected. Next, we compared treatment
of puerarin with 3-MA, rapamycin (Rap) and puerarin (Pue)+3-MA to
confirm the intensity of puerarin in promoting autophagy. Compared
with the blank control group, puerarin showed the highest promotive
effect on LC3B expression, which exceeded that of pue+3-MA and
rapamycin, while 3-MA significantly inhibited the expression of
LC3B. For Beclin1 expression, rapamycin showed the highest
upregulation effect better than that of puerarin. 3-MA exhibited an
obvious inhibitory effect (P<0.05; Fig. 2C and D). The results showed that
puerarin treatment significantly caused upregulation of
autophagy-related proteins, especially significant promotion of the
expression of LC3B. In addition, puerarin did not significantly
upregulate Beclin1 expression after addition of 3-MA, but still
upregulated LC3B expression. This suggests that the regulation of
puerarin-induced autophagy may be related to the mediation of
LC3B.
The ultrastructure of the cells were next observed
by transmission electron microscopy, in order to confirm the
occurrence of autophagy intuitively. The morphological distribution
of organelles, nuclei and chromosomes was normal in the control and
3-MA-treated cells. There were many ring-shaped substances in the
cytoplasm of the cells treated with puerarin, which had a
double-layer membrane structure specific to autophagy (Fig. 2E). The results showed that puerarin
induced the formation of autophagosomes in the osteoblasts compared
with that noted in the control and 3-MA-treated cells (P<0.05;
Fig. 2F). This further confirmed
that puerarin promotes autophagy of osteoblasts.
Inhibition of autophagy reduces the
viability and differentiation of MC3T3-E1 osteoblasts, but has
little effect on mineralization
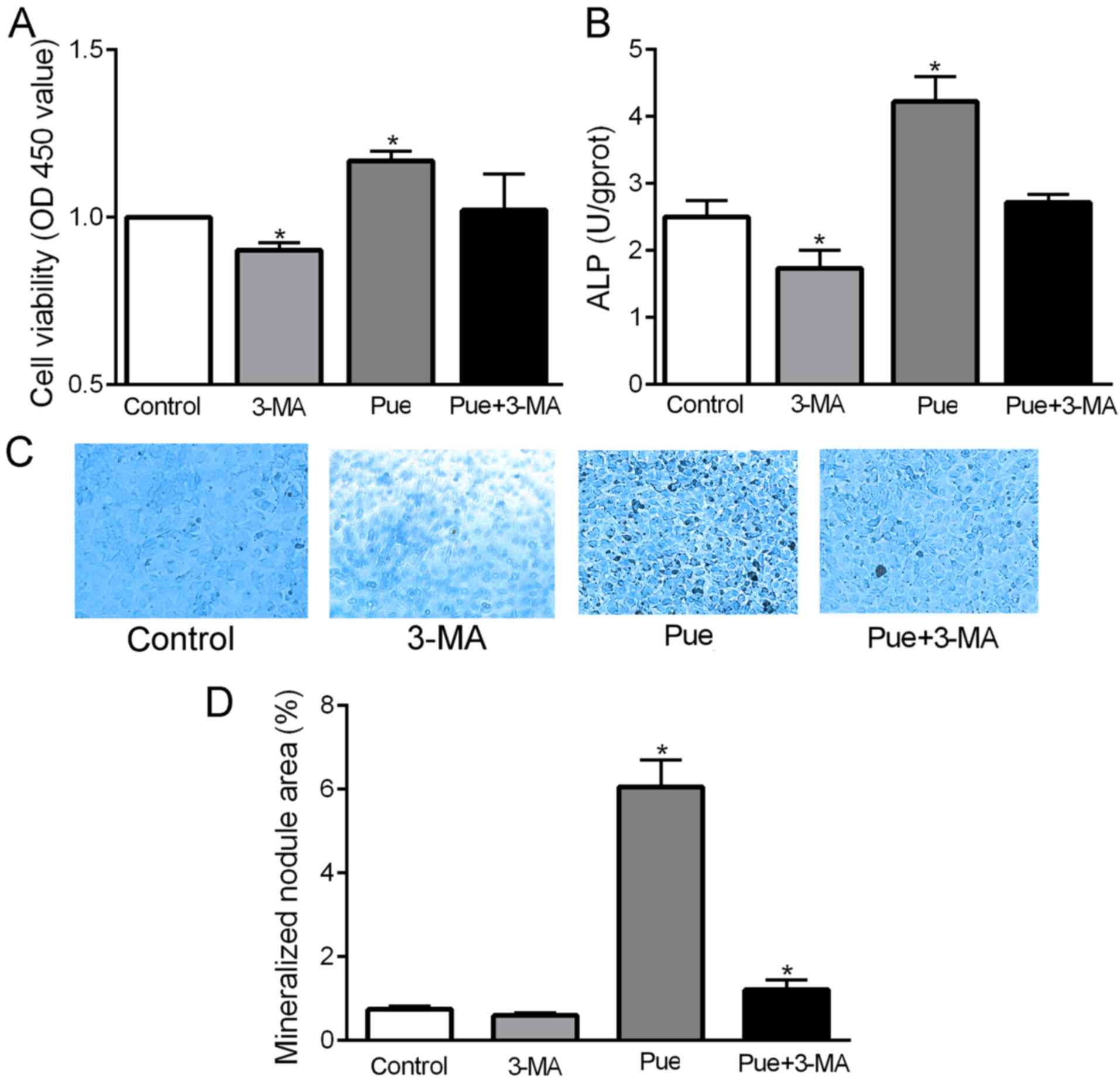
The effects of autophagy on the viability,
differentiation and mineralization of mouse MC3T3-E1 cells were
observed. Compared with the blank control, the viability and ALP
activity were significantly decreased following treatment with 3-MA
(P<0.05; Fig. 3A and B), but the
area of mineralized nodules exhibited no significant change
(P<0.05; Fig. 3C and D). These
results suggest that inhibition of autophagy can reduce the
viability and differentiation of MC3T3-E1 osteoblasts, but has
little effect on mineralization.
miR-204 regulates LC3B and affects
autophagosome formation in MC3T3-E1 cells
miR-204 is a key regulator of bone growth, which
participates in the viability and differentiation of osteoblasts.
Our previous research demonstrated that low concentrations of
puerarin promote osteoblast proliferation and differentiation by
downregulating the expression of miR-204 by targeting Runx2
(14). In the present study, the
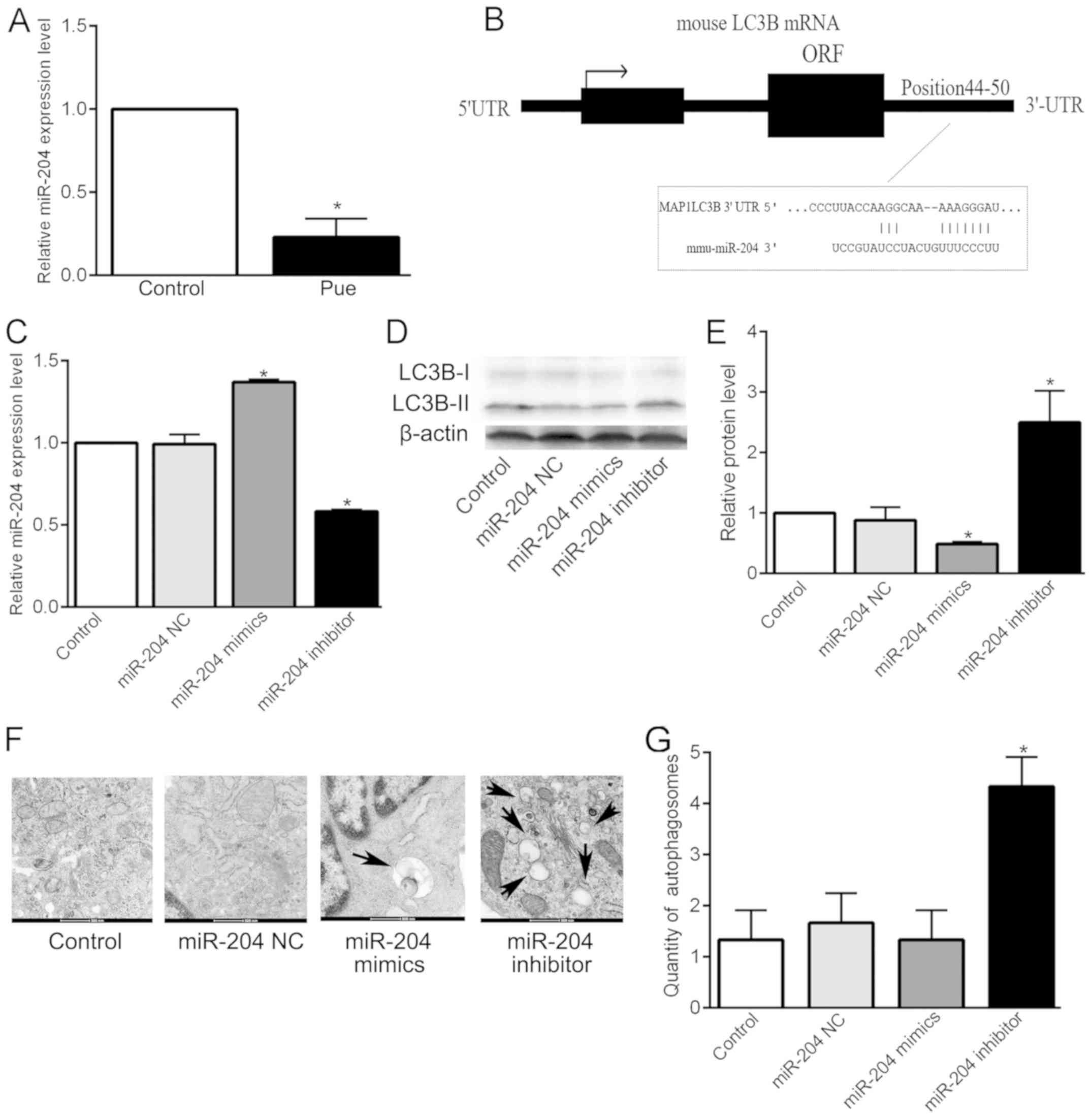
effect of puerarin on miR-204 expression was examined by stem-loop
RT-qPCR. The expression level of miR-204 was significantly
decreased following culturing of the MC3T3-E1 cells with 1 µM
puerarin for 72 h, compared with that of the cells in the absence
of puerarin (Control) (P<0.05; Fig.
4A). The results demonstrated that puerarin downregulated the
expression of miR-204. Combined with the previous validation that
puerarin promotes osteoblast viability and differentiation through
autophagy upregulation, the authors attempted to ascertain the
relationship between miR-204 and autophagy. Bioinformatic analysis
showed that the binding site of miR-204 in LC3B was a widely
conserved element between 44 and 50 bp of the LC3B 3-′UTR,
suggesting that LC3B may be a downstream target of miR-204
(Fig. 4B). Therefore, it was
speculate that miR-204 can affect the level of autophagy by
regulating LC3B. Furthermore, the effects of the overexpression and
inhibition of miR-204 on LC3B were investigated. The results of
RT-qPCR (P<0.05; Fig. 4C) showed
that transfection with miR-204 mimics or miR-204 inhibitor
significantly increased or decreased, respectively, the expression
of miR-204 mRNA. In addition, the results of the western blotting
(P<0.05; Fig. 4D and E) showed
that overexpression of miR-204 significantly decreased the
expression of LC3B protein, while inhibition of miR-204 had the
opposite effect. The results showed that the expression of LC3B
protein in osteoblasts is inversely regulated by miR-204.
Furthermore, in a previous study, double luciferase reporter gene
indicated the direct regulation of miR-204 on LC3B in renal clear
cell carcinoma cells (15). Although
this result was not validated in osteoblasts, it also supports our
hypothesis to some extent. Then, electric mirror analysis showed
that, compared with the blank control, there was no significant
difference in the number of autophagosomes in the negative control
and miR-204 mimic group, while the miR-204 inhibitor significantly
increased the number of autophagosomes in the osteoblastic MC3T3-E1
cells (P<0.05; Fig. 4F and G). In
conclusion, the results indicate that miR-204 regulates
LC3B-mediated autophagy.
Discussion
The development of osteoblasts generally occurs in
three stages: Proliferation, maturation of the extracellular
matrix, and mineralization (16). At
the maturation stage of the extracellular matrix, an increase in
the synthesis and secretion of alkaline phosphatase (ALP), a
homodimer glycoprotein secreted by osteoblasts, is considered the
early stage of osteoblast differentiation and a prerequisite for
the beginning of mineralization (17). Mineralization is the last stage of
osteoblast differentiation and the primary marker of bone
formation. Shortages or the inactivity of osteoblasts are two of
the main pathological bases of osteoporosis. Promoting the
proliferation, differentiation, and mineralization of osteoblasts
and improving their function are of great significance to
preventing and treating osteoporosis.
During autophagy, cytoplasmic materials surrounded
by bilateral membrane structures are called autophagosomes, the
direct markers of autophagy. Autophagosome can bind with lysosomes
to degrade related cytoplasmic structures. In addition, autophagy
also occurs during starvation, quality control of intracellular
protein organelles, inhibition of tumorigenesis, and antigen
presentation. Ideally, evidence consistently shows that autophagy
also plays an important role in cell differentiation and
development. Beclin-1 and microtubule-associated light chain 3
(LC3) are the major autophagic proteins involved in the formation
of autophagosomes (18,19). Early changes in these proteins may
affect our further study of bone development. Over the past few
years, more and more attention has been paid to the effect of
autophagy on osteoporosis. The effects of autophagy on osteoblasts
are also controversial. Some studies have found that autophagy
promotes osteoblast proliferation and differentiation but does not
promote mineralization. This effect may be related to the
upregulation of BMP2, phosphorylation of SMAD1/5/8 proteins,
transcription of RUNX-2, OSX and SMAD-7 expression and activation
of the MEK/ERK pathway (20–22). However research has also shown that
autophagy can promote mineralization (23). Our studies have found that puerarin,
at a concentration of 1 µM, promotes the viability, differentiation
and mineralization of osteoblasts. It also increased the expression
of autophagy-related factors LC3B and Beclin 1, as well as the
formation of autophagosomes. Then inhibition of autophagy
significantly reduced the viability and differentiation of
osteoblasts, but had no significant effect on mineralization.
Therefore, we speculated that the change in the level of autophagy
may not be the main reason why puerarin promotes the mineralization
of osteoblasts. We then investigated the mechanism through which
autophagy promotes osteoblast proliferation and differentiation.
The results showed that miR-204/LC3B participated in the regulatory
mechanism. miRNAs are a large family of small non-coding RNAs that
regulate gene expression. miR-204 is a negative regulator involved
in the regulation of a variety of biological activities (24). Research indicates that it is
downregulated during puerarin-promoted osteoblast viability and
differentiation (14). In the
present study, puerarin downregulated the expression of miR-204,
and bioinformatics analysis revealed that the binding site for
miR-204 in LC3B mRNA is a widely conserved element located between
44 and 50 bp of the 3′-UTR of LC3B. Furthermore, overexpression of
miR-204 significantly reduced the expression of LC3B protein and
the number of autophagosomes, whereas inhibition of miR-204 was
reversed. The above results confirmed that puerarin promotes the
viability and differentiation of MC3T3-E1 cells by enhancing
LC3B-mediated autophagy by downregulating miR-204, but there is
still a lack of evidence confirming the direct effect of miR-204 on
LC3B at the gene level. Thus, the specific mechanism requires
further study. In the present study, changes in LC3B regulated by
miR-204 were observed and assessed. However, autophagy-related
regulator Beclin1 was also upregulated after puerarin treatment.
Based on software prediction and bioinformatic analysis, miR-204
cannot directly target Beclin1. Nevertheless, it has been found
that microRNAs usually play a role in clusters (25). The effect of other microRNAs in the
expression profile of Beclin1 after puerarin treatment still
remains unclear. In fact, studies have shown that autophagy-related
factors, such as Beclin1, can directly or indirectly regulate the
expression of microRNAs (26,27).
This suggests that regulatory factors and microRNAs are not
independent, and they regulate each other in a bidirectional
manner. In addition, the downstream molecule of autophagy has not
been clearly studied, although it may be related to the mTOR
pathway or osteogenesis-related markers such as Runx2 and related
animal experiments can further explain the issue. In summary, the
results from the present study demonstrated that puerarin
significantly promoted the viability, differentiation and
mineralization of osteoblasts. It also increased the expression of
autophagy-related factors such as LC3B and Beclin1, as well as the
formation of autophagosomes. However, the cell viability and
differentiation decreased after inhibition of autophagy.
Furthermore, we found that downregulation of miR-204 promoted
protein expression of autophagy-associated factor LC3B and
formation of autophagosomes. The above results suggest that
puerarin promotes the viability and differentiation of MC3T3-E1
cells by enhancing LC3B-mediated autophagy by downregulating
miR-204. These findings provide a better understanding of the role
of puerarin in bone biology.
Acknowledgements
Not applicable.
Funding
The present study was supported by A Project Funded
by the Priority Academic Program Development of Jiangsu Higher
Education Institutions (Integration of Chinese and Western
Medicine).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QF, SYC, RY, XWZ and XQZ contributed to the study
design, statistical analyses, data interpretation, manuscript
preparation and the literature search. FMZ contributed to the study
design, data collection and statistical analyses. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suvarna V, Sarkar M, Chaubey P, Khan T,
Sherje A, Patel K and Dravyakar B: Bone health and natural
products- an insight. Front Pharmacol. 9:9812018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saint-Pastou Terrier C and Gasque P: Bone
responses in health and infectious diseases: A focus on
osteoblasts. J Infect. 75:281–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei SY, Chen Y and Xu XY: Progress on the
pharmacological research of puerarin: A review. Chin J Nat Med.
12:407–414. 2014.PubMed/NCBI
|
|
5
|
Shan Z, Cheng N, Huang R, Zhao B and Zhou
Y: Puerarin promotes the proliferation and differentiation of
MC3T3-E1 cells via microRNA-106b by targeting receptor activator of
nuclear factor-κB ligand. Exp Ther Med. 15:55–60. 2018.PubMed/NCBI
|
|
6
|
Zeng X, Feng Q, Zhao F, Sun C, Zhou T,
Yang J and Zhan X: Puerarin inhibits TRPM3/miR-204 to promote
MC3T3-E1 cells proliferation, differentiation and mineralization.
Phytother Res. 32:996–1003. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suthon S, Jaroenporn S, Charoenphandhu N,
Suntornsaratoon P and Malaivijitnond S: Anti-osteoporotic effects
of Pueraria candollei var. mirifica on bone mineral density and
histomorphometry in estrogen-deficient rats. J Nat Med. 70:225–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang GM, Tan Y, Wang H, Peng L, Chen HT,
Meng XJ, Li LL, Liu Y, Li WF and Shan H: The relationship between
autophagy and the immune system and its applications for tumor
immunotherapy. Mol Cancer. 18:172019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen G, Ren H, Shang Q, Qiu T, Yu X, Zhang
Z, Huang J, Zhao W, Zhang Y, Lian g and Jiang X: Autophagy as a
target for glucocorticoid-induced osteoporosis therapy. Cell Mol
Life Sci. 75:2683–2693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurley JH and Young LN: Mechanisms of
autophagy initiation. Annu Rev Biochem. 86:225–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YK and Lee JA: Role of the mammalian
ATG8/LC3 family in autophagy: Differential and compensatory roles
in the spatiotemporal regulation of autophagy. BMB Rep. 49:424–430.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moulis M and Vindis C: Autophagy in
metabolic age-related human diseases. Cells. 7:E1492018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan XQ, Zeng XW, Zhang YY, Feng Q, Zhao
FM, Jiang ZQ and Sun C: Puerarin promotes the viability and
differentiation of MC3T3-E1 cells by miR-204-regulated Runx2
upregulation. Mol Med Rep. 16:6262–6268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mikhaylova O, Stratton Y, Hall D, Kellner
E, Ehmer B, Drew AF, Gallo CA, Plas DR, Biesiada J, Meller J and
Czyzyk-Krzeska MF: VHL-regulated MiR-204 suppresses tumor growth
through inhibition of LC3B-mediated autophagy in renal clear cell
carcinoma. Cancer Cell. 21:532–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Lan Y, He Y, He C, Xu F, Zhang Y,
Zhao Y and Liu Y: 99Tc-MDP-induced human osteoblast proliferation,
differentiation and expression of osteoprotegerin. Mol Med Rep.
16:1801–1809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma U, Pal D and Prasad R: Alkaline
phosphatase: An overview. Indian J Clin Biochem. 29:269–278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reggiori F and Ungermann C: Autophagosome
maturation and fusion. J Mol Biol. 429:486–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbosa MC, Grosso RA and Fader CM:
Hallmarks of aging: An autophagic perspective. Front Endocrinol
(Lausanne). 9:7902019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang C, Wei L, Song B, Chen L, Liu J, Deng
B, Pan X and Shao L: Involvement of autophagy in tantalum
nanoparticle-induced osteoblast proliferation. Int J Nanomedicine.
12:4323–4333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darcy A, Meltzer M, Miller J, Lee S,
Chappell S, Ver Donck K and Montano M: A novel library screen
identifies immunosuppressors that promote osteoblast
differentiation. Bone. 50:1294–1303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng Y, Zhang W, Fan H and Xu P:
Water-soluble Nano-pearl powder promotes MC3T3-E1 cell
differentiation by enhancing autophagy via the MEK/ERK signaling
pathway. Mol Med Rep. 18:993–1000. 2018.PubMed/NCBI
|
|
23
|
Kim IR, Kim SE, Baek HS, Kim BJ, Kim CH,
Chung IK, Park BS and Shin SH: The role of kaempferol-induced
autophagy on differentiation and mineralization of osteoblastic
MC3T3-E1 cells. BMC Complement Altern Med. 16:3332016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun M, Zhou X, Chen L, Huang S, Leung V,
Wu N, Pan H, Zhen W, Lu W and Peng S: The regulatory roles of
microRNAs in bone remodeling and perspectives as biomarkers in
osteoporosis. BioMed Res Int. 2016:16524172016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang FE, Zhang C, Maminishkis A, Dong L,
Zhi C, Li R, Zhao J, Majerciak V, Gaur AB, Chen S and Miller SS:
MicroRNA-204/211 alters epithelial physiology. FASEB J.
24:1552–1571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Liang J, Li Y and Li J, Yang X,
Zhang X, Han S, Li S and Li J: Down-regulation of miRNA-30a
alleviates cerebral ischemic injury through enhancing beclin
1-mediated autophagy. Neurochem Res. 39:1279–1291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng B, Zhu H, Gu D, Pan X, Qian L, Xue
B, Yang D, Zhou J and Shan Y: MiRNA-30a-mediated autophagy
inhibition sensitizes renal cell carcinoma cells to sorafenib.
Biochem Biophys Res Commun. 459:234–239. 2015. View Article : Google Scholar : PubMed/NCBI
|