Introduction
Post-herpetic neuralgia refers to pain that persists
after an acute episode of herpes zoster and resolution of the rash
(1). Post-herpetic neuralgia is a
frequent complication of herpes zoster. Approximately 12.5% of
patients with herpes zoster aged ≥50 years suffer from
post-herpetic neuralgia at 3 months after the outbreak of herpes
zoster and the risk of post-herpetic neuralgia increases sharply
with age (1).
Post-herpetic neuralgia affects nerve fibers and
skin and is characterized by a constant burning, stabbing sensation
or pain triggered by light contact with non-painful stimuli. Pain
associated with post-herpetic neuralgia is frequently refractory to
treatment and may persist for years, negatively impacting patients'
quality of life (2–4).
Numerous interventions are available for symptom
control in patients with post-herpetic neuralgia, but there is
currently no disease-modifying therapy. Oral medications are
commonly used to treat post-herpetic neuralgia, including
pregabalin, gabapentin, anti-depressants, anti-convulsants,
carbamazepine, lamotrigine and opioids. However, these treatments
may not provide effective pain relief in ~50% of patients with
post-herpetic neuralgia and long-term use of these agents is
associated with adverse effects, including dizziness, ataxia,
nausea and dependence (1,5,6).
Botulinum toxin (BTX-A) is a neurotoxic protein
produced by Clostridium botulinum. BTX-A is used in the
clinic to treat muscle spasticity through blockade of neuromuscular
transmission. BTX-A also has anti-nociceptive properties, as it
inhibits the release of sensory inflammatory mediators and
peripheral neurotransmitters and inactivates membrane sodium
channels in central neurons (7–10).
A recent study demonstrated that BTX-A has an
analgesic effect in post-herpetic neuralgia, trigeminal neuralgia
and other types of neuropathic pain (11). Several meta-analyses have reported on
the use of BTX-A in neuralgia, but these reviews investigated a
range of neuropathies and included multiple interventions
(lidocaine, saline, placebo) as comparators (11,12).
Since these meta-analyses lack a subgroup analysis, they may have
limited relevance regarding the effectiveness of BTX-A in
post-herpetic neuralgia; as the inclusion of multiple neuropathies,
each with a different underlying pathology, and several
comparators, may introduce confounding variables that produce a
biased effect.
The objective of the present systematic review and
meta-narrative was to investigate the safety and efficacy of local
administration of BTX-A vs. lidocaine in the treatment of
post-herpetic neuralgia.
Materials and methods
Literature search
The present meta-analysis and systematic review was
performed according to the recommendations of the Cochrane
Collaboration (13). Two review
authors independently searched the PubMed, Embase, Cochrane
Library, Chinese National Knowledge Infrastructure, Wanfang,
Chongqing VIP Information Co. and Chinese Biomedical Literature
Database using the following keywords: ‘Botulinum toxin type A’,
‘abobotulinumtoxinA’, ‘lidocain’, ‘botulinum toxin A’, ‘BTX-A’,
‘post-herpetic neuralgia’ and ‘placebo’. Searches were limited to
studies published between 2005 and February 2019 in the English or
Chinese languages. Additional articles were selected from manual
searches of included studies and reviews.
Inclusion criteria
The inclusion criteria were as follows: i) Study
design: Randomized controlled trials (RCTs); ii) population:
Clinically diagnosed with post-herpetic neuralgia, lesions had
crusted and healed, there was no new rash, but there was pain at
the site of the original lesion (14); iii) intervention: Subcutaneous
injection of BTX-A vs. lidocaine; patients may have been
administered treatments in addition to BTX-A or lidocaine (15); iv) treatment duration: <3
injections during <6 days of hospitalization; and v) outcome
measures, including the Visual Analogue Scale (VAS) pain scores,
McGill pain questionnaire and the rate of adverse events.
Patients had been diagnosed with post-herpetic
neuralgia according to the American Academy of Neurology 2004 or
Chinese Medical Association criteria (16). BTX-A (total dose, ≤100 units) was
administered by subcutaneous injection at the site of the herpes
lesion and subsequent pain at the proximal end of the nerve branch
in the damaged tissue. Efficacy was evaluated by pain scores on a
Visual Analogue Scale (VAS; 0, no pain; 10, most severe pain); the
effective rate, defined as the percentage of patients in which
symptoms and signs had improved and the pain was at least 25%
reduced; and the McGill pain questionnaire, which contains three
questions: ‘What Does Your Pain Feel Like?’, ‘How Does Your Pain
Change with Time?’ and ‘How Strong is Your Pain?’, with responses
that may be scored to a maximum of 78 points (higher scores
indicate stronger pain) (17).
Safety was assessed from the adverse event rate, including those
that were self-limiting.
Exclusion criteria were as follows: i) No full text
available; ii) insufficient data; iii) models of induced neuralgia;
and iv) case reports, reviews and abstracts.
Disagreements between review authors (XLL and HPH)
on study selection were resolved by discussion mediated by LLP,
until consensus was reached.
Primary and secondary outcomes
The primary outcomes were VAS pain scores at 1, 2
and 3 months after treatment and the effective rate. Secondary
outcomes were scores on the McGill pain questionnaire at one month
after treatment and the adverse event rate during follow-up.
Data extraction
Two review authors independently extracted the
following information from each of the studies included: Name of
first author, year of publication, demographic characteristics of
the study population, intervention measures and follow-up.
Disagreements among review authors on data extraction were resolved
by discussion until consensus was reached.
Risk of bias
Two review authors independently assessed risk of
bias in the studies included using the Cochrane Risk of Bias Tool
for Randomized Controlled Trials, which evaluates the following
domains: Sequence generation, allocation concealment, blinding of
participants and personnel, blinding of outcome assessment,
incomplete outcome data, selective reporting and other bias. In
each domain, risk of bias was judged as ‘high’, ‘unclear’ or ‘low’
(18). Disagreements among review
authors on assessment of study quality were resolved by discussion
until consensus was reached.
Statistical analysis
Data analyses were performed using Review Manager
5.3 (Cochrane Collaboration). Mean differences (MDs) with 95% CIs
were calculated for continuous variables (VAS pain score and McGill
pain questionnaire) and odds ratios (ORs) with 95% CIs were
calculated for dichotomous variables (effective rate and adverse
event rate). Heterogeneity was evaluated with the
I2-test. A fixed-effects model was used for outcomes
with evidence of low heterogeneity (I2<50%) between
studies and a random-effects model was used for outcomes with
evidence of significant heterogeneity (I2>50%)
between studies. P<0.05 was considered to indicate statistical
significance (19).
Results
Study characteristics
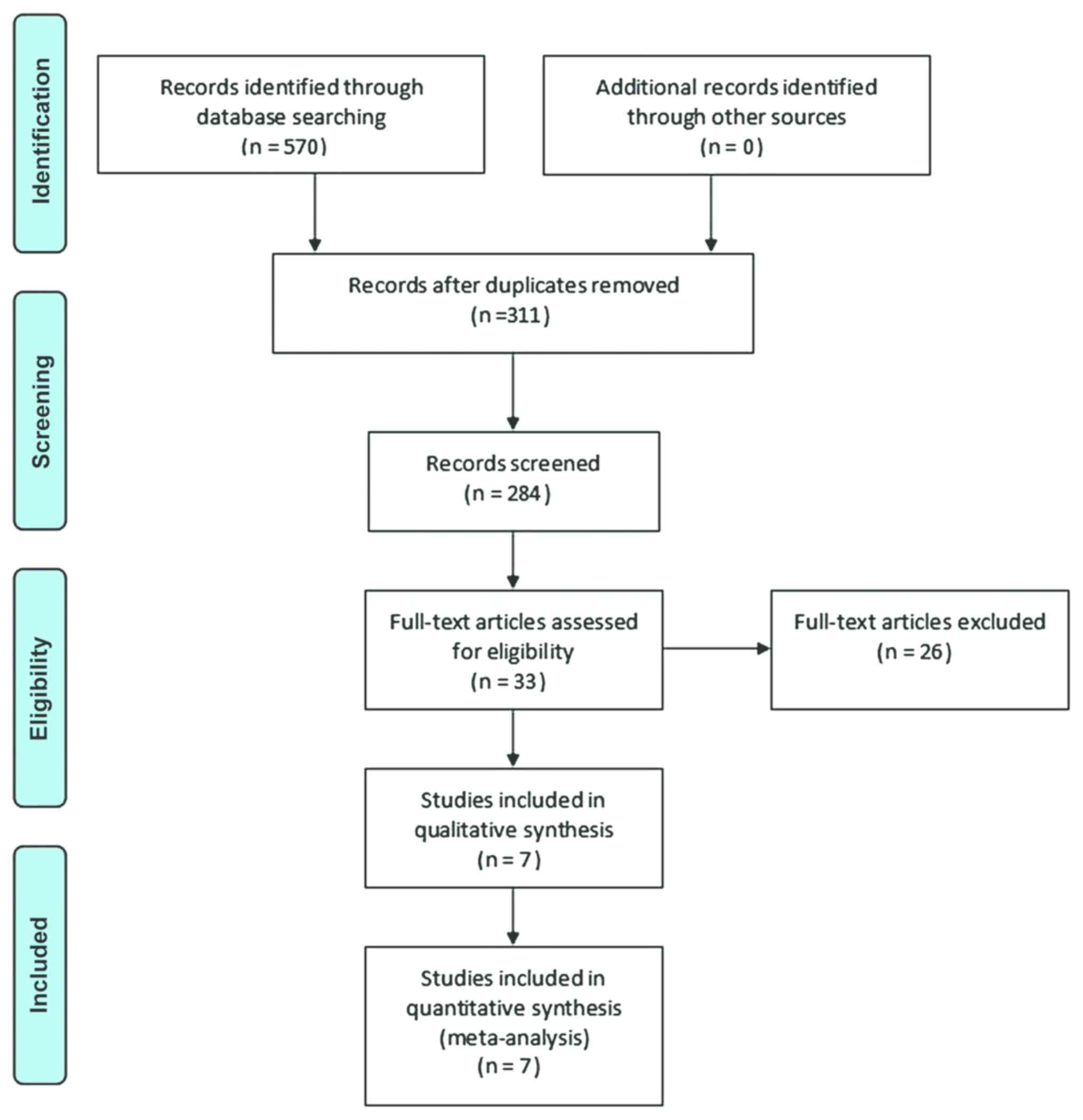
The searches identified 570 articles. Titles and
abstracts were screened, 259 duplicates were excluded and 33
articles were considered eligible for inclusion. After analyzing
the full-text articles, 26 studies were excluded. Among these, 6
articles reported incomplete data, 11 were on non-randomized
controlled trials, 8 studies did not use lidocaine as the
intervention and 1 article was a case report. Finally, 7 RCTs (8
datasets) were evaluated in the meta-analysis (Fig. 1) (20–27).
The characteristics of the studies included are
presented in Table I. The 7 eligible
RCTs included 752 patients (367 patients in the BTX-A group and 385
patients in the lidocaine group) who were followed up for 3
months.
 | Table I.Characteristics of trials
included. |
Table I.
Characteristics of trials
included.
|
|
| Age (years) | Sex
(male/female) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| First author
(year) | Patients, total
(study completion) | BTX-A group | Lidocain group | BTX-A group | Lidocain group | Interventions | Outcome measures | (Refs.) |
|---|
| Dai (2018) | 71 (71) | 64.5±8.9 | 66.2±8.4 | 18/14 | 21/18 | BTX-A+gabapentin
vs. Lidocain+gabapentin | VAS score, adverse
events | (20) |
| Yang (2014) | 400 (400) | 56.32±5.69 | 56.34±4.88 | 120/80 | 115/85 | BTX-A vs.
Lidocain+carbamazepine | VAS score,
effective rate | (21) |
| Xiao (2009)
(2010) | 40 (38) | 70±15.41 | 65±14.20 | 11/9 | 8/12 | BTX-A+gabapentin
vs. Lidocain+gabapentin | VAS score,
effective rate, adverse events | (22,23) |
| Zhu (2018) | 65 (65) | / | / | / | / |
BTX-A+Pregabalin+vitamin B1 vs.
Lidocain+Pregabalin+vitamin B1 | VAS score,
effective rate | (24) |
| Xue (2017) | 60 (60) | 53.37±6.28 | 53.78±6.34 | 16/14 | 15/15 | BTX-A+Pregabalin
vs. Lidocain+Pregabalin | VAS score,
effective rate | (25) |
| Yuan (2015) | 56 (56) | 58±3.5 | 57±4.3 | 16/12 | 11/17 | BTX-A vs.
Lidocain | Mcgill pain
questionaire | (26) |
| Liu (2009) | 60 (60) | 56.36±0.9 | 56.8±0.9 | 13/17 | 14/16 | BTX-A vs.
Lidocain+carbamazepine | VAS score,
effective rate, Mcgill pain questionaire | (27) |
Risk of bias in the studies
included
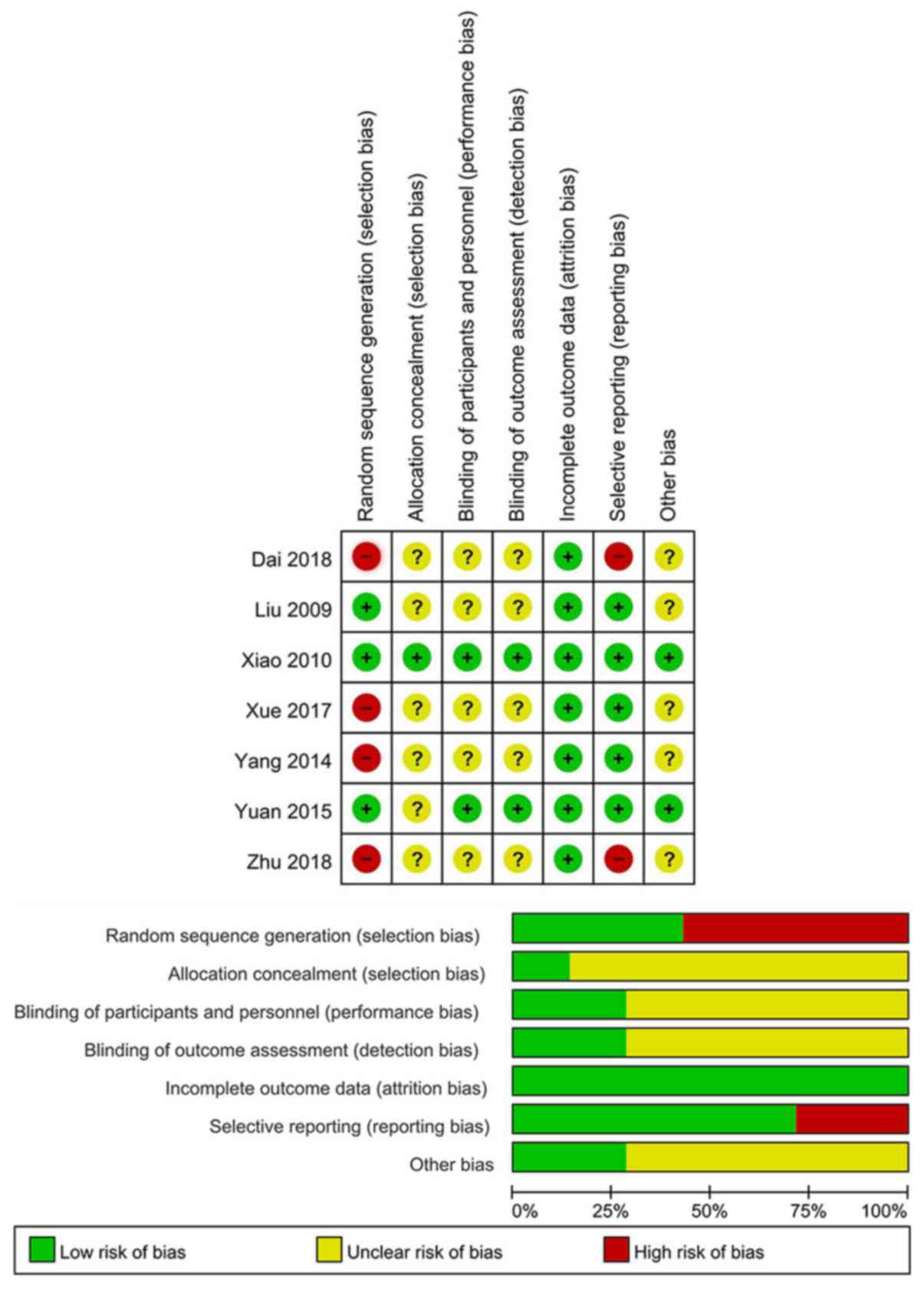
A total of 3 trials described random sequence
generation (22,23,26,27). In
addition, 1 trial adequately reported allocation concealment
(22,23). Furthermore, 2 trials detailed the
methods of blinding (22,23,25). All
trials reported information on incomplete outcome data (20–27).
Reporting bias was described by 5 trials (Fig. 2) (21–23,25–27).
Funnel plots for publication bias were not evaluated due to the
small number of trials included in the present meta-analysis.
Primary outcomes
VAS pain score
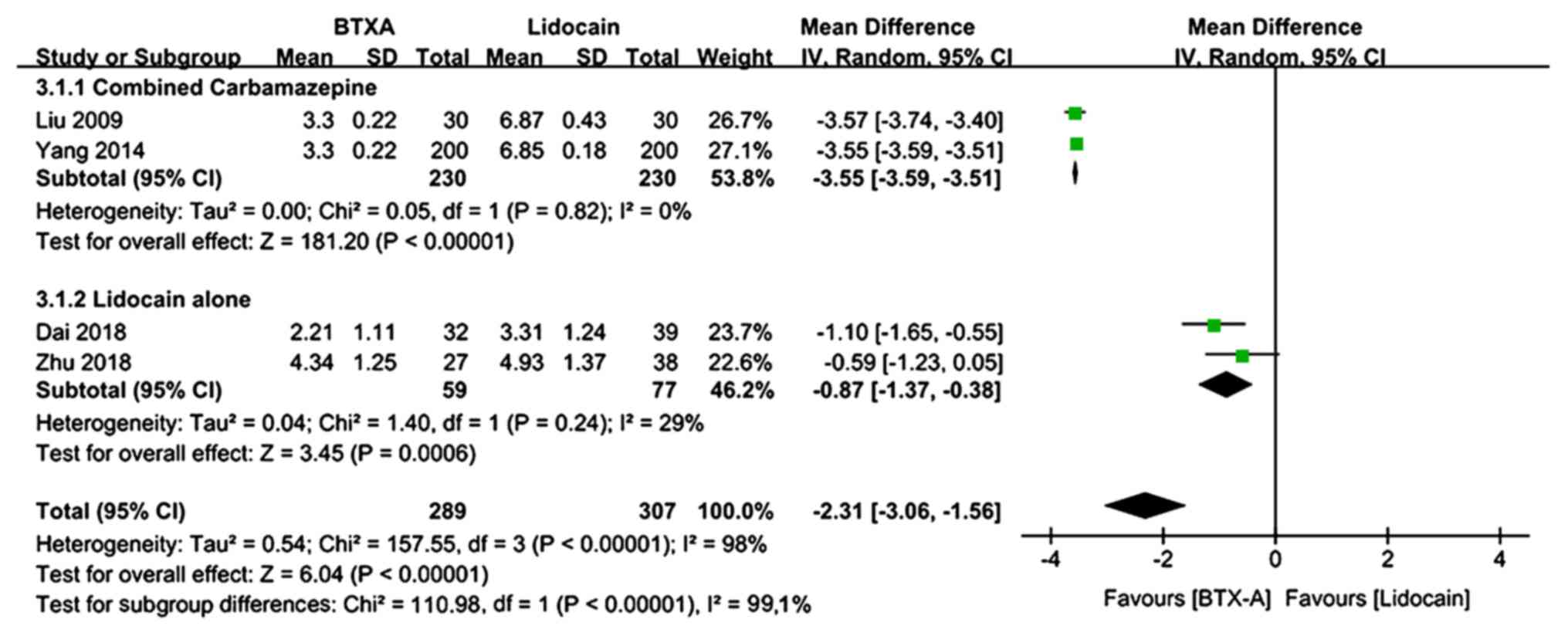
The VAS pain scores at 1 month of follow-up were
provided by 4 trials (569 patients, including 289 patients in the
BTX-A group and 307 patients in the lidocaine group) (20,21,24,27).
There was evidence of heterogeneity between studies. The
meta-analysis revealed a significantly lower VAS pain score in
patients who received BTX-A for post-herpetic neuralgia compared to
those who received lidocaine (MD=−2.31; 95% CI: −3.06, −1.56;
Z=6.04; P<0.00001; Fig. 3). In a
subgroup analysis, patients who received lidocaine were stratified
by use of oral carbamazepine. At 1 month of follow-up, the VAS pain
score was significantly lower in patients who received BTX-A for
post-herpetic neuralgia compared to those who received lidocaine
alone or lidocaine plus oral carbamazepine (lidocaine alone:
MD=−0.87; 95% CI: −1.37, −0.38; P=0.0006; lidocaine plus oral
carbamazepine: MD=−3.55; 95% CI: −3.59, −3.51; P<0.00001;
Fig. 3).
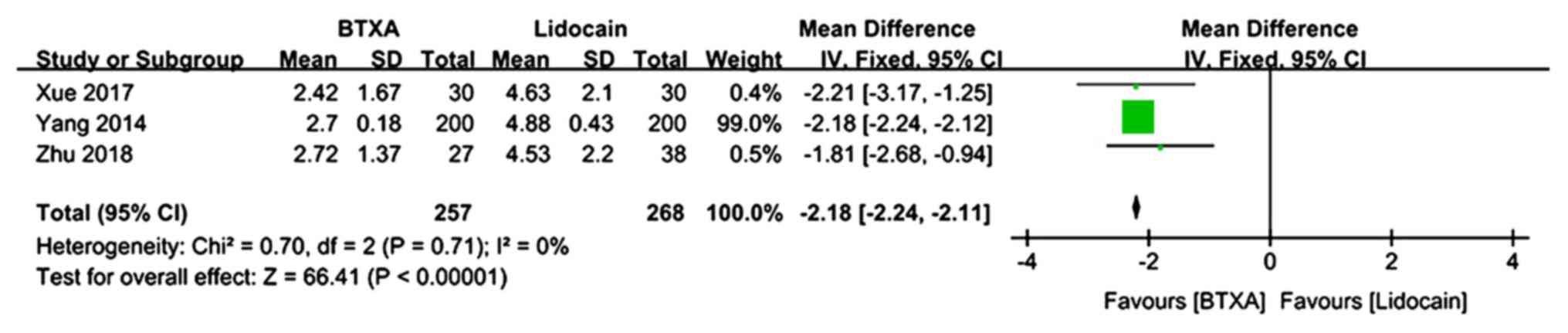
The VAS pain scores at 2 months of follow-up were
described in 3 trials (n=525 patients; 257 patients in the BTX-A
group and 268 patients in the lidocaine group) (21,24,25).
There was no evidence of heterogeneity between studies. The
meta-analysis demonstrated a significantly lower VAS pain score in
patients who received BTX-A for post-herpetic neuralgia compared to
those who received lidocaine (MD=−2.18; 95% CI: −2.24, −2.11;
P<0.00001; Fig. 4).
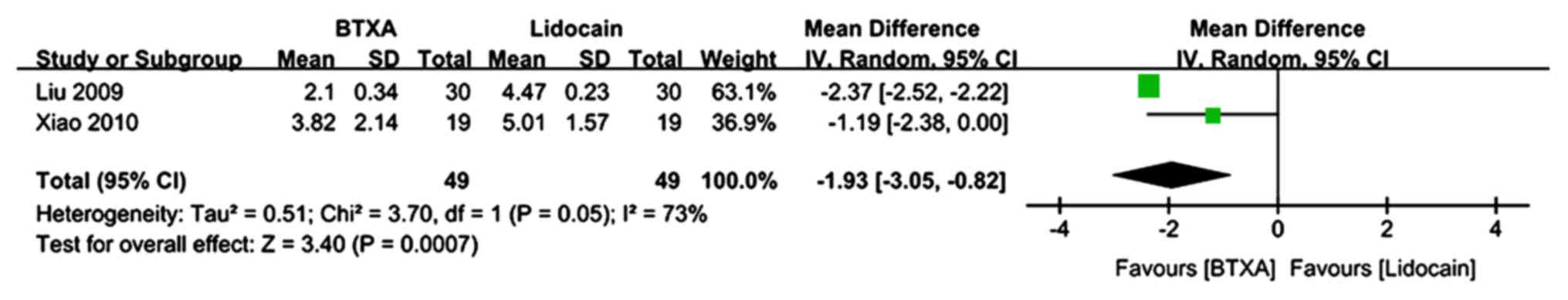
The VAS pain scores at 3 months of follow-up were
described in 2 trials (n=98 patients; 49 patients in the BTX-A
group and 49 patients in the lidocaine group) (22,23,27).
There was evidence of heterogeneity between studies. The
meta-analysis demonstrated a significantly lower VAS pain score in
patients who received BTX-A for post-herpetic neuralgia compared to
those who received lidocaine (MD=−1.93; 95% CI: −3.05, −0.82;
Z=3.4; P=0.0007; Fig. 5).
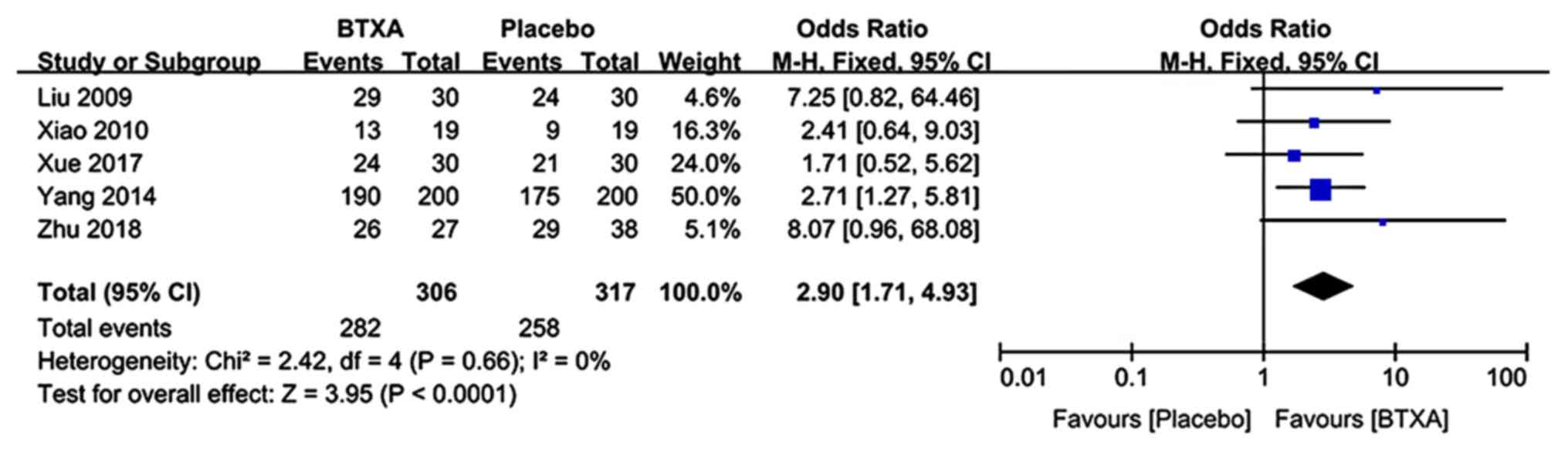
Effective rate
Data reporting on the effective rate were included
in 5 trials (n=623 patients; 306 patients in the BTX-A group and
317 patients in the lidocaine group) (21–23,25,27).
There was no evidence of heterogeneity between studies. The
meta-analysis demonstrated a significantly higher effective rate in
patients who received BTX-A for post-herpetic neuralgia compared to
those who received lidocaine (OR: 2.9; 95% CI: 1.71, 4.13;
P<0.0001; Fig. 6).
Secondary outcomes
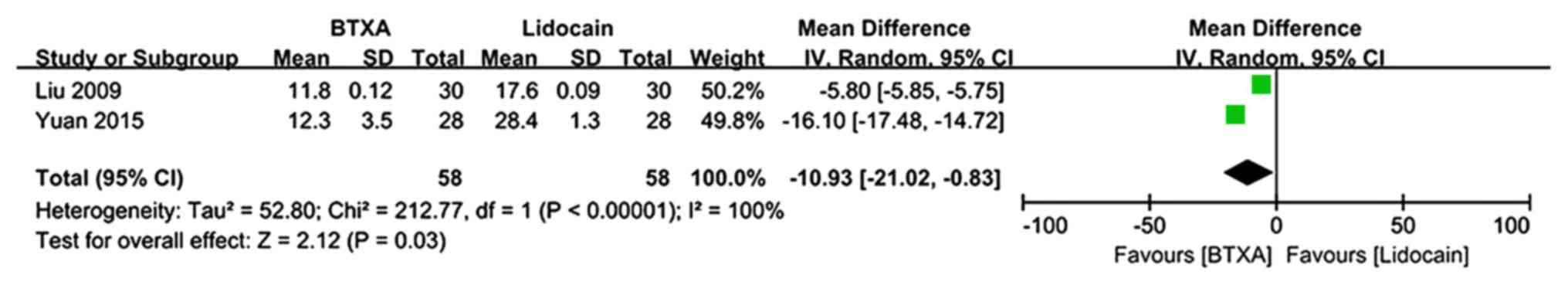
McGill pain questionnaire
Data from the McGill pain questionnaire at 1 month
of follow-up were included in 2 trials (n=116 patients; 58 patients
in the BTX-A group and 58 patients in the lidocaine group)
(26,27). There was evidence of heterogeneity
between studies. The meta-analysis demonstrated a significantly
lower score on the McGill pain questionnaire in patients who
received BTX-A for post-herpetic neuralgia compared to those who
received lidocaine (MD=−10.93; 95% CI: −21.02, −0.83; Z=2.12;
P=0.03; Fig. 7).
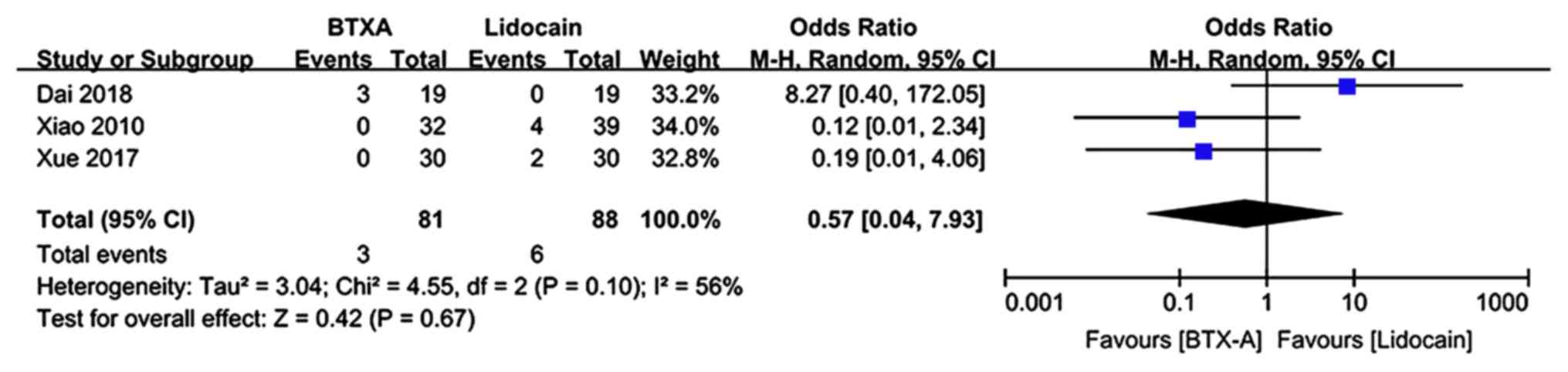
Adverse event rate
Adverse events during the follow-up period were
described in 3 trials (n=169 patients; 81 patients in the BTX-A
group and 88 patients in the lidocaine group). There was evidence
of significant heterogeneity between studies. The meta-analysis
revealed no significant difference in the adverse event rate in
patients who received BTX-A for post-herpetic neuralgia compared to
those who received lidocaine (OR: 0.57; 95% CI: 0.04, 7.93; P=0.67;
Fig. 8).
Discussion
The present meta-analysis revealed that BTX-A has
greater efficacy than lidocaine for post-herpetic neuralgia based
on the VAS pain scores at 1, 2 and 3 months after treatment, the
effective rate and the McGill pain questionnaire. There was no
difference in the adverse event rate between treatments and BTX-A
administration was not associated with any serious adverse events.
Although the present meta-analysis was based on articles identified
during a comprehensive search of seven databases, the results
should be interpreted with caution. Of note, the results are based
on a small sample size and risk of bias was unclear in five of the
seven RCTs included (20,21,24,25,27).
Previously, several meta-analyses have reported on
the use of BTX-A in neuralgia. Meng et al (11) analyzed 12 RCTs and, consistent with
the present results, their analysis suggested that BTX-A is a safe
and more effective option than saline for alleviating neuropathic
pain. They included studies investigating BTX-A for a range of
neuropathies, including post-herpetic neuralgia, peripheral
neuropathic pain, thoracic outlet syndrome, piriformis syndrome,
spinal cord injury, trigeminal neuralgia and diabetic neuropathic
pain, but did not perform a subgroup analysis investigating
post-herpetic neuralgia alone. Shackleton et al (6) analyzed 6 RCTs and revealed that BTX-A
was more effective than placebo for managing trigeminal neuralgia
and post-herpetic neuralgia but did not perform any subgroup
analysis investigating post-herpetic neuralgia alone. Yang et
al (12) analyzed 4 RCTs and
demonstrated the efficacy of BTX-A for post-herpetic neuralgia;
however, their results may have been confounded by the inclusion of
multiple interventions (lidocaine, saline, placebo) as comparators.
The present meta-analysis did not include any RCTs comparing BTX-A
to placebo or saline. Although such RCTs are considered rigorous
high-quality studies (28), the
clinical relevance of including saline or placebo as comparators is
questionable.
Postherpetic neuralgia has a negative impact on
patients' quality of life and represents an economic burden on
individuals and the healthcare system (29). One study investigated the impact of
post-herpetic neuralgia on quality of life in individuals aged ≥50
years in 6 European countries (Spain, Portugal, The Netherlands,
Belgium, Sweden and Switzerland). In 37% of individuals,
post-herpetic neuralgia had a high impact on quality of life,
affecting enjoyment of life, general activity, mood, sleep and
walking ability (30). A systematic
review of published data investigating healthcare resource use and
costs associated with herpes zoster in Europe revealed that
post-herpetic neuralgia incurred outpatient costs (medical visits,
diagnostic tests and medications), hospitalization and inpatient
costs, as well as costs associated with sick leave (absenteeism)
(31). Future research should
include a comparison of the psychosocial and economic burden of
post-herpetic neuralgia in patients treated with BTX-A vs.
lidocaine.
The present meta-analysis had certain limitations.
First, the numbers of clinical trials and patients included were
small. Furthermore, certain studies were judged as having ‘high
risk’ of bias and it was not possible to assess publication bias,
as an insufficient number of studies was included. In addition,
there were significant differences in the baseline characteristics
of the patients treated with BTX-A or lidocaine in several of the
included trials, representing a potential source of heterogeneity
in the present analyses. As another limitation, certain patients
were administered therapies in addition to BTX-A or lidocaine,
which may make it difficult to draw conclusions about several
outcomes. Furthermore, there is currently no gold standard measure
of treatment success for neuralgia; therefore, the present study
reports on a variety of outcomes. Finally, there was evidence of
heterogeneity between trials. This will likely be reduced in the
future as data from more RCTs become available and a gold standard
measure of treatment success is established.
In conclusion, the present meta-analysis indicates
that BTX-A has potential as an effective treatment for
post-herpetic neuralgia. Large well-designed RCTs are required to
substantiate this conclusion.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Department of Sichuan Province (grant nos. 2013SZZ002
and 2018JY0404), the Health Commission of Sichuan Province (grant
no. 16PJ557), the government of Luzhou (grant nos. 14ZC0071-LH09
and 2016LZXNYD-G03), Southwest Medical University (grant no.
2013ZRQN068) and the Project Program of Neurosurgical Clinical
Research Center of Sichuan Province (grant no. 17082).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XLL made substantial contributions to research
concept, screening process, identification of eligible studies and
manuscript preparation. SZ and XZ performed data analysis and
prepared the manuscript. HPH screened, identified eligible studies
and analyzed the data. ZZ and LLP performed the data extraction and
quality evaluation. LGC conceived the research study and supervised
the other authors to ensure integrity of the analysis. All authors
reviewed, read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BTX-A
|
botulinum toxin A
|
|
RCT
|
randomized controlled trial
|
References
|
1
|
Forbes HJ, Thomas SL, Smeeth L, Clayton T,
Farmer R, Bhaskaran K and Langan SM: A systematic review and
meta-analysis of risk fact-ors for postherpetic neuralgia. Pain.
157:30–54. 2015. View Article : Google Scholar
|
|
2
|
Weaver BA: The burden of herpes zoster and
postherpetic neuralgia in the United States. J Am Osteopath Assoc.
107 (Suppl 1):S2–S7. 2007.PubMed/NCBI
|
|
3
|
Drolet M, Brisson M, Schmader KE, Levin
MJ, Johnson R, Oxman MN, Patrick D, Blanchette C and Mansi JA: The
impact of herpes zoster and postherpetic neuralgia on
health-related quality of life: A prospective study. CMAJ.
182:1731–1736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feller L, Khammissa RAG, Fourie J,
Bouckaert M and Lemmer J: Postherpetic neuralgia and trigeminal
neuralgia. Pain Res Treat. 1–6. 2017. View Article : Google Scholar
|
|
5
|
Dosenovic S, Jelicic Kadic A, Miljanovic
M, Biocic M, Boric K, Cavar M, Markovina N, Vucic K and Puljak L:
Interventions for neuropathic pain: An overview of systematic
reviews. Anesth Analg. 125:643–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shackleton T, Ram S, Black M, Ryder J,
Clark GT and Enciso R: The efficacy of botulinum toxin for the
treatment of trigeminal and post-herpetic neuralgia: A systematic
review with meta-analyses. Oral Surg Oral Med Oral Pathol Oral
Radiol. 122:61–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durham PL and Cady R: Insights into the
mechanism of onabotulinumtoxinA in chronic migraine. Headache.
51:1573–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucioni A, Bales GT, Lotan TL, McGehee DS,
Cook SP and Rapp DE: Botulinum toxin type A inhibits sensory
neuropeptide release in rat bladder models of acute injury and
chronic inflammation. BJU Int. 101:366–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin MC, Wakita M, Xie DJ, Yamaga T, Iwata
S, Torii Y, Harakawa T, Ginnaga A, Kozaki S and Akaike N:
Inhibition of membrane Na+ channels by A Type botulinum
toxin at femtomolar concentrations in central and peripheral
neurons. J Pharmacol Sci. 118:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolly JO and O'Connell MA:
Neurotherapeutics to inhibit exocytosis from sensory neurons for
the control of chronic pain. Curr Opin Pharmacol. 12:100–108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng F, Peng K, Yang JP, Ji FH, Xia F and
Meng XW: Botulinum toxin-A for the treatment of neuralgia: A
systematic review and meta-analysis. J Pain Res. 11:2343–2351.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Hu XH and Liu LH: Systematic
evaluation of botulinum toxin A injection in the treatment of
post-herpetic neuralgia. J Yangtze Univ. 13:6–10. 2016.
|
|
13
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions. Cochrane Collaboration.
2011:March 19–2018
|
|
14
|
Paisley P and Serpell M: Diagnosis and
management of postherpetic neuralgia. Practitioner. 259:2–3.
2015.
|
|
15
|
Nalamachu S and Morley-Forster P:
Diagnosing and managing postherpetic neuralgia. Drugs Aging.
29:863–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luu M: Practice parameter: Treatment of
postherpetic neuralgia: An evidence-based reportof the quality
standards subcommittee of the American Academy of Neurology.
Douleurs Evaluation-Diagnostic-Traitement. 6:1792005. View Article : Google Scholar
|
|
17
|
Melzack R: The McGill pain questionnaire:
Major properties and scoring methods. Pain. 1:277–299. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions. Cochrane Collaboration
Version 5.1.0. 2010.
|
|
19
|
Peng K, Chen WR, Meng XW, Zhang J and Ji
FH: Intra-articular dexmedetomidine in knee arthroscopy: A
systematic review and meta-analysis. Sci Rep. 8:40892018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai YE and Liu L: Effect of botulinum
toxin A on postherpetic neuralgia after herpes zoster. Contemporary
medicine forum. 16:103–104. 2018.
|
|
21
|
Yang F, Li Y and Liu XB: The effectiveness
observation and the plasma β-endorphin effects of botulinum toxin
for treating postherpetic euralgia. Hebei Med. 937–940. 2014.
|
|
22
|
Xiao L, Jiang J, Zhang Q, Sha T, Luo Y,
Zheng H, Zhuang X and Zhang D: Therapeutic effect of Botulinum
toxin a in the treatment of postherpetic neuralgia by subcutaneous
injection. Chin J Pain Med. 15:140–143. 2009.(In Chinese).
|
|
23
|
Xiao L, Mackey S, Hui H, Xong D, Zhang Q
and Zhang D: Subcutaneous injection of botulinum toxin a is
beneficial in postherpetic neuralgia. Pain Med. 11:1827–1833. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu MM and Liu KF: Efficacy of botulinum
toxin type A in the treatment posthe-rpetic neuralgia. China J
Leprosy Skin Dis. 34:473–474. 2018.
|
|
25
|
Xue R and Yan F: Clinical efficacy and
safety of BTXA in Post herpetic neuralgia. Med Sci J Central South
China. 619–621. 2017.
|
|
26
|
Yuan YK, Wang HB, Jia B, Qiao b and Wang
J: Efficacy of local injection of botulinum toxin a in the
treatment of postherpetic neuralgia. Chin J Phys Med Rehabil.
37:694–695. 2015.(In Chinese).
|
|
27
|
Liu HP: A clinical study on the
therapeutic effects of botulinum toxin type A in t-he treatment of
postherpetic. Lan Zhou Univ; 2009
|
|
28
|
Apalla Z, Sotiriou E, Lallas A, Lazaridou
E and Ioannides D: Botulinum toxin A in postherpetic neuralgia: A
parallel, randomized, double-blind, single-dose, placebo-controlled
trial. Clin J Pain. 29:857–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matthews S, De Maria A, Passamonti M,
Ristori G, Loiacono I, Puggina A and Curran D: The economic burden
and impact on quality of life of herpes zoster and postherpetic
neuralgia in individuals aged 50 years or older in Italy. Open
Forum Infect Dis. 6:ofz0072019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lukas K, Edte A and Bertrand I: The impact
of herpes zoster and post-herpetic neuralgia on quality of life:
Patient-reported outcomes in six European countries. Z Gesundh
Wiss. 20:441–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gater A, Uhart M, McCool R and Préaud E:
The humanistic, economic and societal burden of herpes zoster in
Europe: A critical review. BMC Public Health. 15:1932015.
View Article : Google Scholar : PubMed/NCBI
|