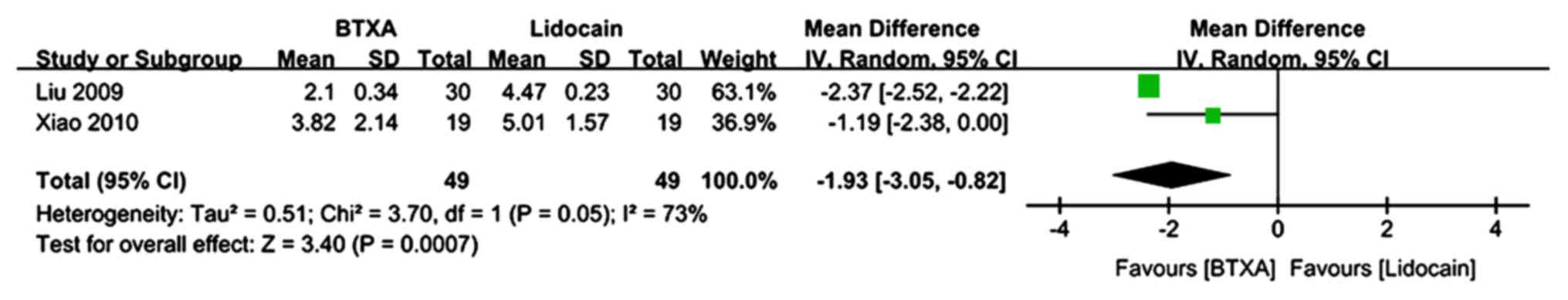
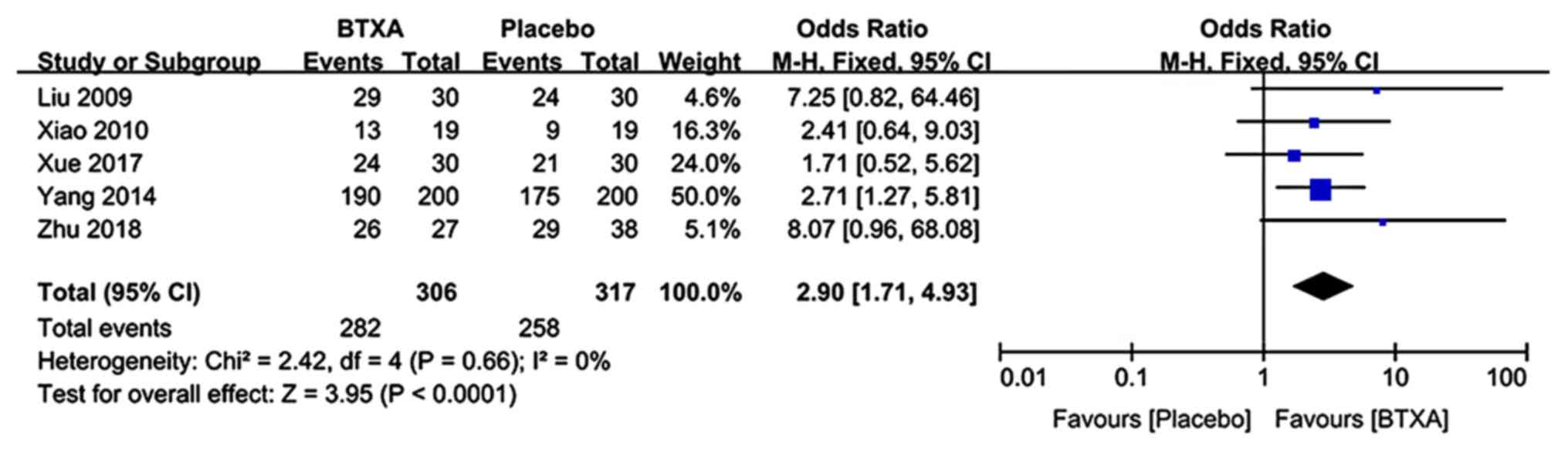
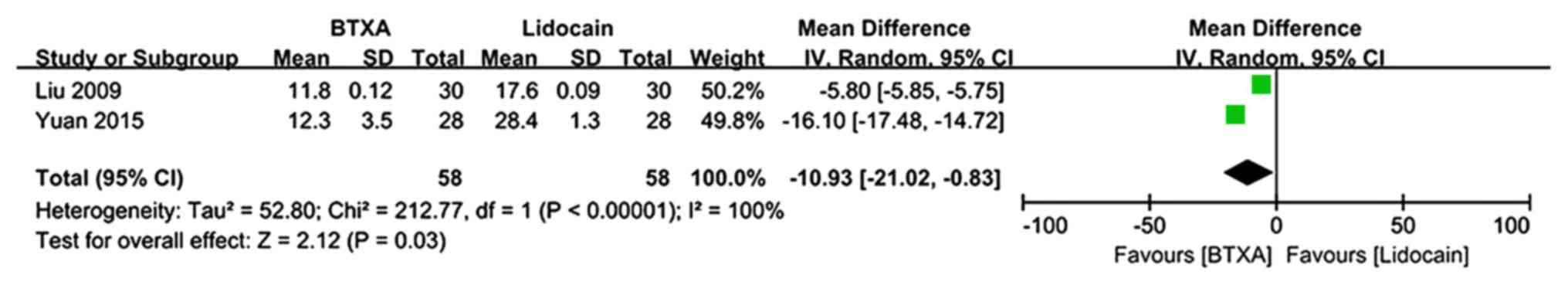
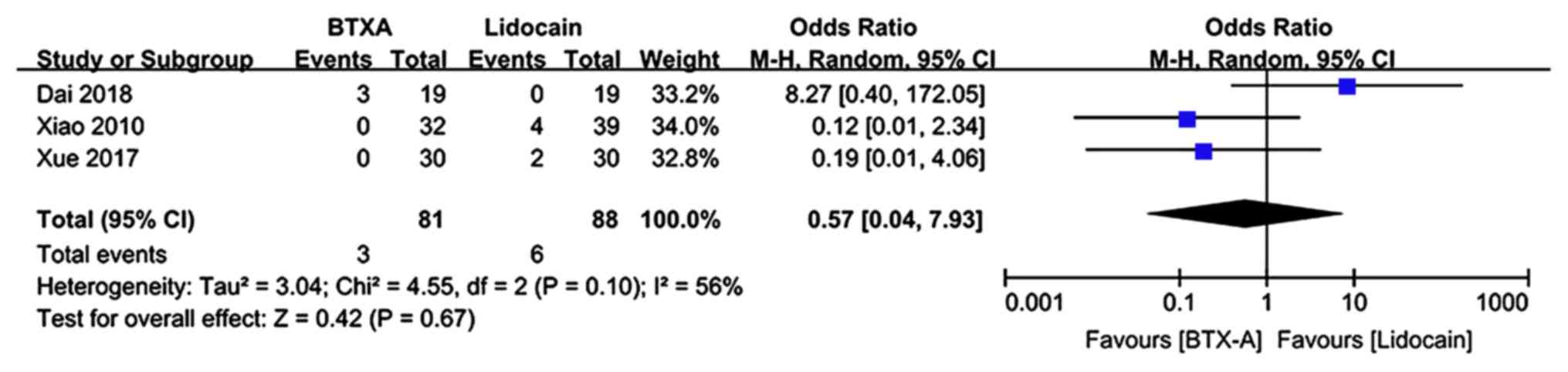
|
1
|
Forbes HJ, Thomas SL, Smeeth L, Clayton T,
Farmer R, Bhaskaran K and Langan SM: A systematic review and
meta-analysis of risk fact-ors for postherpetic neuralgia. Pain.
157:30–54. 2015. View Article : Google Scholar
|
|
2
|
Weaver BA: The burden of herpes zoster and
postherpetic neuralgia in the United States. J Am Osteopath Assoc.
107 (Suppl 1):S2–S7. 2007.PubMed/NCBI
|
|
3
|
Drolet M, Brisson M, Schmader KE, Levin
MJ, Johnson R, Oxman MN, Patrick D, Blanchette C and Mansi JA: The
impact of herpes zoster and postherpetic neuralgia on
health-related quality of life: A prospective study. CMAJ.
182:1731–1736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feller L, Khammissa RAG, Fourie J,
Bouckaert M and Lemmer J: Postherpetic neuralgia and trigeminal
neuralgia. Pain Res Treat. 1–6. 2017. View Article : Google Scholar
|
|
5
|
Dosenovic S, Jelicic Kadic A, Miljanovic
M, Biocic M, Boric K, Cavar M, Markovina N, Vucic K and Puljak L:
Interventions for neuropathic pain: An overview of systematic
reviews. Anesth Analg. 125:643–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shackleton T, Ram S, Black M, Ryder J,
Clark GT and Enciso R: The efficacy of botulinum toxin for the
treatment of trigeminal and post-herpetic neuralgia: A systematic
review with meta-analyses. Oral Surg Oral Med Oral Pathol Oral
Radiol. 122:61–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durham PL and Cady R: Insights into the
mechanism of onabotulinumtoxinA in chronic migraine. Headache.
51:1573–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucioni A, Bales GT, Lotan TL, McGehee DS,
Cook SP and Rapp DE: Botulinum toxin type A inhibits sensory
neuropeptide release in rat bladder models of acute injury and
chronic inflammation. BJU Int. 101:366–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin MC, Wakita M, Xie DJ, Yamaga T, Iwata
S, Torii Y, Harakawa T, Ginnaga A, Kozaki S and Akaike N:
Inhibition of membrane Na+ channels by A Type botulinum
toxin at femtomolar concentrations in central and peripheral
neurons. J Pharmacol Sci. 118:33–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolly JO and O'Connell MA:
Neurotherapeutics to inhibit exocytosis from sensory neurons for
the control of chronic pain. Curr Opin Pharmacol. 12:100–108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng F, Peng K, Yang JP, Ji FH, Xia F and
Meng XW: Botulinum toxin-A for the treatment of neuralgia: A
systematic review and meta-analysis. J Pain Res. 11:2343–2351.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Hu XH and Liu LH: Systematic
evaluation of botulinum toxin A injection in the treatment of
post-herpetic neuralgia. J Yangtze Univ. 13:6–10. 2016.
|
|
13
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions. Cochrane Collaboration.
2011:March 19–2018
|
|
14
|
Paisley P and Serpell M: Diagnosis and
management of postherpetic neuralgia. Practitioner. 259:2–3.
2015.
|
|
15
|
Nalamachu S and Morley-Forster P:
Diagnosing and managing postherpetic neuralgia. Drugs Aging.
29:863–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luu M: Practice parameter: Treatment of
postherpetic neuralgia: An evidence-based reportof the quality
standards subcommittee of the American Academy of Neurology.
Douleurs Evaluation-Diagnostic-Traitement. 6:1792005. View Article : Google Scholar
|
|
17
|
Melzack R: The McGill pain questionnaire:
Major properties and scoring methods. Pain. 1:277–299. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins J and Green S: Cochrane handbook
for systematic reviews of interventions. Cochrane Collaboration
Version 5.1.0. 2010.
|
|
19
|
Peng K, Chen WR, Meng XW, Zhang J and Ji
FH: Intra-articular dexmedetomidine in knee arthroscopy: A
systematic review and meta-analysis. Sci Rep. 8:40892018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai YE and Liu L: Effect of botulinum
toxin A on postherpetic neuralgia after herpes zoster. Contemporary
medicine forum. 16:103–104. 2018.
|
|
21
|
Yang F, Li Y and Liu XB: The effectiveness
observation and the plasma β-endorphin effects of botulinum toxin
for treating postherpetic euralgia. Hebei Med. 937–940. 2014.
|
|
22
|
Xiao L, Jiang J, Zhang Q, Sha T, Luo Y,
Zheng H, Zhuang X and Zhang D: Therapeutic effect of Botulinum
toxin a in the treatment of postherpetic neuralgia by subcutaneous
injection. Chin J Pain Med. 15:140–143. 2009.(In Chinese).
|
|
23
|
Xiao L, Mackey S, Hui H, Xong D, Zhang Q
and Zhang D: Subcutaneous injection of botulinum toxin a is
beneficial in postherpetic neuralgia. Pain Med. 11:1827–1833. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu MM and Liu KF: Efficacy of botulinum
toxin type A in the treatment posthe-rpetic neuralgia. China J
Leprosy Skin Dis. 34:473–474. 2018.
|
|
25
|
Xue R and Yan F: Clinical efficacy and
safety of BTXA in Post herpetic neuralgia. Med Sci J Central South
China. 619–621. 2017.
|
|
26
|
Yuan YK, Wang HB, Jia B, Qiao b and Wang
J: Efficacy of local injection of botulinum toxin a in the
treatment of postherpetic neuralgia. Chin J Phys Med Rehabil.
37:694–695. 2015.(In Chinese).
|
|
27
|
Liu HP: A clinical study on the
therapeutic effects of botulinum toxin type A in t-he treatment of
postherpetic. Lan Zhou Univ; 2009
|
|
28
|
Apalla Z, Sotiriou E, Lallas A, Lazaridou
E and Ioannides D: Botulinum toxin A in postherpetic neuralgia: A
parallel, randomized, double-blind, single-dose, placebo-controlled
trial. Clin J Pain. 29:857–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matthews S, De Maria A, Passamonti M,
Ristori G, Loiacono I, Puggina A and Curran D: The economic burden
and impact on quality of life of herpes zoster and postherpetic
neuralgia in individuals aged 50 years or older in Italy. Open
Forum Infect Dis. 6:ofz0072019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lukas K, Edte A and Bertrand I: The impact
of herpes zoster and post-herpetic neuralgia on quality of life:
Patient-reported outcomes in six European countries. Z Gesundh
Wiss. 20:441–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gater A, Uhart M, McCool R and Préaud E:
The humanistic, economic and societal burden of herpes zoster in
Europe: A critical review. BMC Public Health. 15:1932015.
View Article : Google Scholar : PubMed/NCBI
|