Introduction
Among patients who exhibit the return of spontaneous
circulation (ROSC) upon arrival at emergency departments, and who
do not survive until hospital discharge, ~70% succumb to
post-anoxic neurological injury (1).
There is an urgent requirement for early assessment and monitoring
of brain damage following cardiac arrest. Monitoring approaches,
including neurological evaluation, cranial CT,
electroencephalography and somatosensory evoked potentials, have
been designed to assess brain damage after cardiac arrest (2). In addition, serum markers
[neuron-specific enolase (NSE) and S100B] are easily available,
observer-independent and have been indicated to reflect the
severity of brain damage accurately, as well as improve early
evaluation and the quantification of post-cardiac arrest brain
damage (3). However, these serum
markers require collection of blood samples, which is invasive and
unsustainable for continued monitoring (4). Furthermore, the results are not
available in real time.
Regional cerebral tissue oxygen saturation
(SctO2), which is monitored in a non-invasive
manner using near-infrared spectroscopy (NIRS), constitutes a
potentially feasible marker for the early assessment of brain
damage following cardiac arrest (5).
Examinations of the role of SctO2 during and
after cardiac arrest have revealed that SctO2
levels increase with high-quality cardiopulmonary resuscitation
(CPR) during cardiac arrest, and that SctO2
levels are correlated with outcomes, including ROSC and survival
(6–8). However, there is variability in
SctO2 based on the oxygen penetration of
arteries, veins, capillaries and nonvascular tissue, such that
baseline values among subjects vary by ~10% (9). Therefore, cerebral and tissue oximetry
values are more appropriate for monitoring trends than for use as
absolute indices of tissue oxygenation.
Due to the absence of baseline measurements and
uncontrolled experiments, as well as the generally limited numbers
of samples, there has been minimal discussion regarding sequential
changes in SctO2 following ROSC (10). Hypothermic therapy is the only
treatment with proven efficacy in terms of neurological outcome
after cardiac arrest (11,12). SctO2 remains
uncertain due to hypothermic reduction of the cerebral metabolic
rate of oxygen (10). Although NIRS
monitoring is growing in popularity, definitive data regarding the
benefits of its use remain sparse. Therefore, further research is
required. The aim of the present study was to elucidate variations
in SctO2 from the time of cardiac arrest
until 30 h after resuscitation in an animal model of hypothermia,
to establish the usefulness of SctO2
monitoring during and after cardiac arrest.
Materials and methods
Ethics
This was a prospective, randomized, controlled
experimental study, using a porcine model of cardiac arrest and
resuscitation. The protocol of the current study was approved by
the Animal Care and Use Committee of the Medical School of Zhejiang
University. Animal care and experiments were conducted in
accordance with the guidelines of the Institutional Animal Care and
Use Committee (13).
Animal preparation
A total of 23 healthy male domestic pigs (4–6
months; 36.5±2 kg) were supplied by Shanghai Jiagan Biological
Technology Co., Ltd. The research animals were maintained in
standard atmospheric pressure, a 12/12-h light/dark cycle, room
temperature (20–25°C), and 60–80% humidity. Animals had access to
food and water ad libitum. Animals were fasted overnight
with free access to water prior to the commencing of experiments.
Anesthesia induction was initiated by intramuscular injection of
ketamine (20 mg/kg) and completed via an ear vein injection of
sodium pentobarbital (30 mg/kg). Tracheal intubation was performed
and mechanical ventilation was applied at a rate of 25 breaths/min,
with peak inspiratory pressure of 25 cm H2O and positive
end-expiratory pressure of 5 cm H2O. After central
vascular access was achieved, hydration was maintained with 5%
dextrose and 0.9% NaCl and anesthesia was maintained with sodium
pentobarbital (8 mg/kg/h) and fentanyl (2 µg/kg/h). Ventilation
rate and airway pressure were adjusted to maintain arterial carbon
dioxide partial pressure (PaCO2) at 35–45 mmHg. Femoral
arteries and veins were cannulated for the measurement of aortic
pressure and core temperature, and for the collection of blood
samples. All catheters were flushed intermittently with saline
containing bovine heparin (5 IU/ml). Ventricular fibrillation (VF)
was induced by advancing a 5-Fr pacing catheter (EP Technologies,
Inc.) from the right external jugular vein into the right
ventricle. Catheter position was confirmed by characteristic
pressure morphology and fluoroscopy. For all animals, body
temperature was maintained at 36–38°C during the preparation.
NIRS, concept and device
Prior to the induction of VF, NIRS probes were
placed on the supraorbital region above the eyebrows, ~4 cm apart,
over the frontoparietal cortex and covered to prevent ambient light
interference. SctO2 was continuously
monitored with a Tissue Oxygenation Monitor (EGOS-600A; Suzhou
Engin Bio-medical Electronics Co., Ltd.), which measured the
difference between oxygenated and deoxygenated hemoglobin in
venous, arterial and capillary blood. This also served as an
assessment of cerebral perfusion and delivery, as well as uptake of
oxygen. SctO2 indicates adequate blood flow
and oxygen delivery in relation to oxygen consumption, rather than
directly measuring cerebral blood flow or tissue oxygenation. Since
~70% of the sampled blood is venous, normal
SctO2 is approximately 60–80%. Thus,
SctO2 decreases when oxygen supply falls
relative to oxygen uptake and requirements and increases when
oxygen supply increases relative to uptake and requirements
(5).
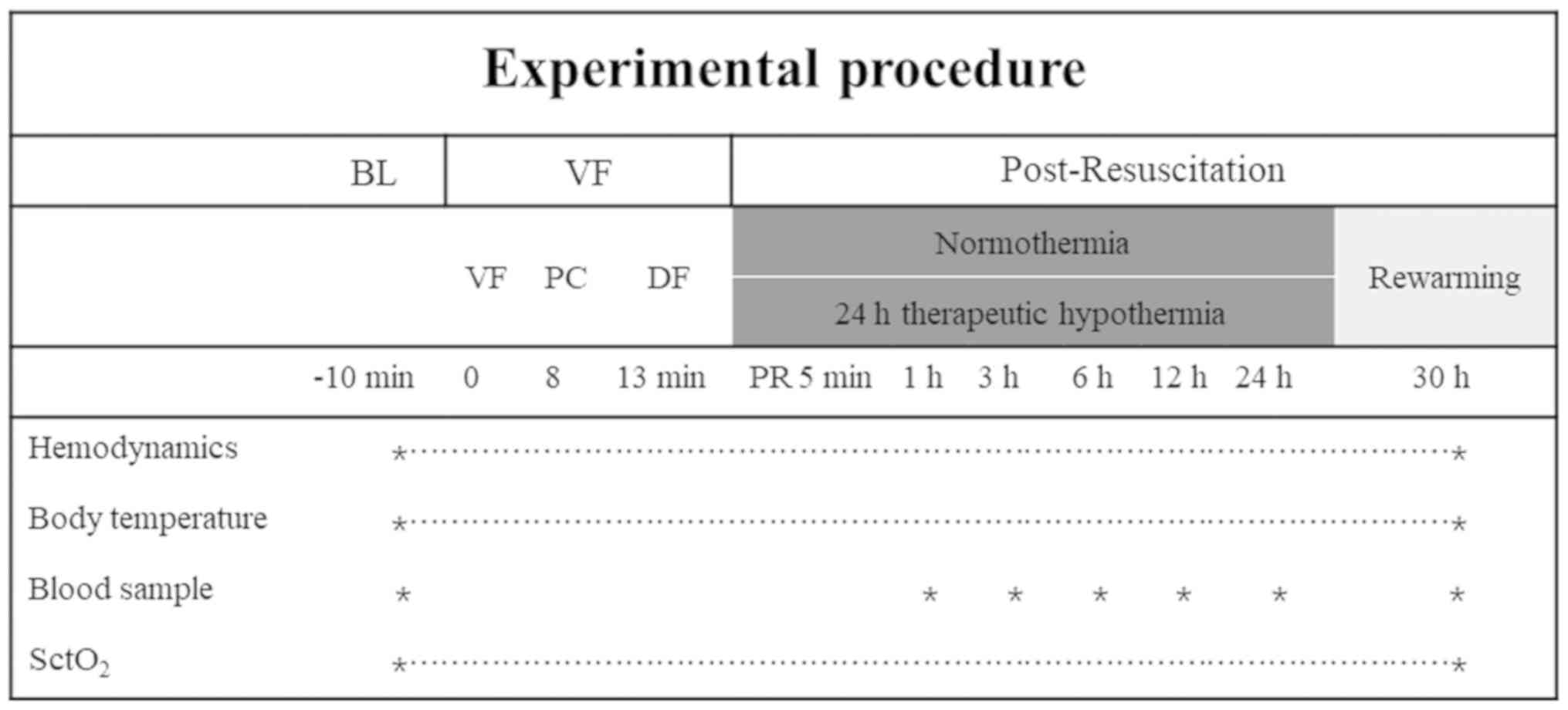
Experimental protocol
Baseline measurements were obtained 15 min prior to
the induction of VF in all groups. The animals were randomized into
three groups, using the sealed envelope method: i) Therapeutic
hypothermia (TH) group; ii) Normothermia (NT) group; and iii)
Control group. Control animals only underwent surgical preparation,
including endotracheal intubation and all venous and arterial
catheterizations, without cardiac arrest and resuscitation. In the
NT and TH groups, VF was induced via the application of a 1 mA
alternating current through the 5-Fr pacing catheter, delivered to
the right ventricular endocardium. Mechanical ventilation was
discontinued after onset of VF. After 8 min of untreated VF, CPR
was manually performed at a ratio of 30:2 (compression to
ventilation). Compression quality was continuously monitored using
the ZOLL feedback device (ZOLL Medical Corporation) to guarantee
optimal compressions (depth of 50–60 mm and rate of 100–120/min).
Ventilation was performed using a CPR simple respirator with room
air. After 2.5 min of CPR, the first bolus of epinephrine (procaine
and adrenaline injection, Fuzhou Neptunus Fuyao Pharmaceutical Co.,
Ltd.; 20 g/kg) was administered. After 5 min of CPR, defibrillation
was attempted by the delivery of a single 150-J biphasic waveform
electrical shock. ROSC was defined as an unassisted HR >100/min
demonstrated by arterial blood pressure wave forms. Following ROSC,
the SctO2, mean arterial pressure, arterial carbon
dioxide partial pressure, arterial oxygen partial pressure,
lactate, NSE and S100B were observed in all groups for 30 h after
ROSC, then all the catheters were removed, the wounds closed, the
animals taken off the ventilator when awakened and then returned to
their cages in the laboratory. For 2 weeks, one researcher took
daily measurements of several objective parameters (food/water
consumption, body weight and body surface temperature). Animals
were immediately euthanized with an intravenous injection of 150
mg/kg sodium pentobarbital upon reaching the moribund state/humane
endpoints to reduce the amount of animal suffering (Table I; 14).
 | Table I.Selected signs of the moribund
statea (14). |
Table I.
Selected signs of the moribund
statea (14).
| Impaired mobility
(unable to reach food and water) |
| Inability to
maintain upright position |
| Prolonged lack of
activity (2–3 days in duration) |
| Labored breathing
and cyanosis |
| Prolonged decreased
food and water intake |
| Extreme or
prolonged weight loss/emaciation |
| Prolonged diarrhea
or constipation |
| Biochemical or
physical evidence of organ failure |
| Bleeding from an
orifice |
|
Unconsciousness |
Following ROSC, mechanical ventilation was continued
with FiO2 of 0.21 for 30 h. In the TH group, TH was
implemented at 5 min after resuscitation via surface cooling with a
cooling blanket and ice packs, to reach a temperature of 32–34°C as
quickly as possible; this was maintained for 24 h, and was followed
by a rewarming rate of 1°C/h for 5 h. In the NT and control groups,
the temperature was maintained at approximately 36–38°C during the
30 h observation period.
Venous blood samples were collected through a
central venous catheter and the first 10 ml of blood was discarded
to ensure that the sample was not mixed with normal saline and
heparin diluent. Immediately after sampling, the blood in the tubes
was mixed by manually spinning and inverting to prevent
coagulation, carefully avoiding the formation of foam, then placed
on a mixture of water and ice to ensure a constant temperature of
≤4°C, and monitored using a thermometer. The tubes were centrifuged
within 1 h of collection at 1,500 × g for 15 min at 4°C. The plasma
was then separated and stored as aliquots in plastic tubes at −70°C
until assayed. Arterial blood samples were analyzed within 1 min of
collection. The experimental pipeline was summarized in Fig. 1.
Measurement
Hemodynamics, electrocardiogram data and blood
temperature were continuously recorded using a patient monitoring
system (BeneView T6; Shenzhen Mindray Bio-Medical Electronics Co.,
Ltd.). Coronary perfusion was calculated as the difference between
decompression diastolic aortic and time-coincident right atrial
pressure, which was measured at the end of each minute of
precordial compression. SctO2 was
continuously monitored at baseline and at 1, 3, 6, 12, 24 and 30 h
after ROSC. Venous and arterial blood samples were collected at the
same time points. Serum concentrations of NSE and S100B were
measured using ELISA assay kits (MEXN-R0832, Shanghai Meixuan
Biotechnology Co., Ltd.). Arterial blood gas and lactate were
measured using a Blood Gas/Electrolyte Analyzer (Model 5700;
Instrumentation Laboratory). All assays were performed in a blinded
manner by an independent member of the laboratory staff.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0, IBM Corp.). Changes in
SctO2 hemodynamic measurements over time were
compared using the one-sample Wilcoxon signed rank test. The
results are presented as the median (interquartile range), mean
(standard deviation), or percent (%), as indicated. Comparisons
between time-based measurements within each group were performed
using a repeated-measurement ANOVA. If there was a significant
difference in the overall comparison of groups, comparisons between
any other 2 groups were performed using a Bonferroni test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
During the study period, 20/23 pigs were
successfully resuscitated after cardiac arrest and were observed
for 30 h (8/9 pigs in the TH group, 8/9 pigs in the NT group and
4/5 pigs in the control group). One pig in NT group was
successfully resuscitated, then succumbed 12 h later. There were no
significant differences in baseline characteristics, including
hemodynamics and body temperature, among the three groups (Table II). Throughout CPR, no differences
were observed in the duration of CPR, epinephrine dosage,
hemodynamics, number of shocks required to establish ROSC, or
subsequent incidence of recurrent VF between the TH and NT groups
(Table III). Following ROSC, mean
arterial pressure (MAP) was initially 117.9±11.3 mmHg, then
remained >100 mmHg throughout the 30 h observation period.
 | Table II.Baseline characteristics. |
Table II.
Baseline characteristics.
| Variables | TH group (n=9) | NT group (n=9) | Control group
(n=5) | P-value |
|---|
| Body weight,
kg | 36.3 (3.1) | 36.9 (2.7) | 36.2 (3.0) | 0.901 |
| Heart rate,
beats/min | 108.6 (11.0) | 105.4 (13.4) | 104.4 (5.0) | 0.762 |
| Mean aortic
pressure, mmHg | 113.6 (12.0) | 121.4 (12.4) | 119.8 (7.8) | 0.395 |
| End-tidal
CO2, mmHg | 39.4 (3.3) | 40.1 (2.9) | 39.6
(1.7) | 0.872 |
| Core temperature,
°C | 37.9 (0.3) | 38.0 (0.3) | 37.9
(0.4) | 0.891 |
| ROSC | 8/9 | 7/9 | 5/5 | 0.172 |
 | Table III.Characteristics during
cardiopulmonary resuscitation. |
Table III.
Characteristics during
cardiopulmonary resuscitation.
| Variables | TH group (n=8) | NT group (n=7) | P-value |
|---|
| Duration of CPR,
min | 5 (0) | 5.6 (1) | 0.158 |
| Number of shocks to
ROSC | 1.7 (1.4) | 3.29 (2.5) | 0.084 |
| Epinephrine dosage,
mg | 71.5 (71.5) | 1025.7 (395.6) | 0.345 |
| Prevalence of
recurrent VF | 0.8 (1.4) | 1.7 (2.4) | 0.762 |
| CPP in
PC1, mmHg | 17.8 (2.6) | 17.4 (3.2) | 0.379 |
| CPP in
PC2, mmHg | 22.8 (3.4) | 21.9 (2.4) | 0.591 |
| CPP in
PC3, mmHg | 28.6 (5.1) | 28.7 (3.2) | 0.115 |
| CPP in
PC4, mmHg | 37.8 (4.4) | 37.1 (3.9) | 0.892 |
| CPP in
PC5, mmHg | 27.9 (5.2) | 27.1 (4.7) | 0.691 |
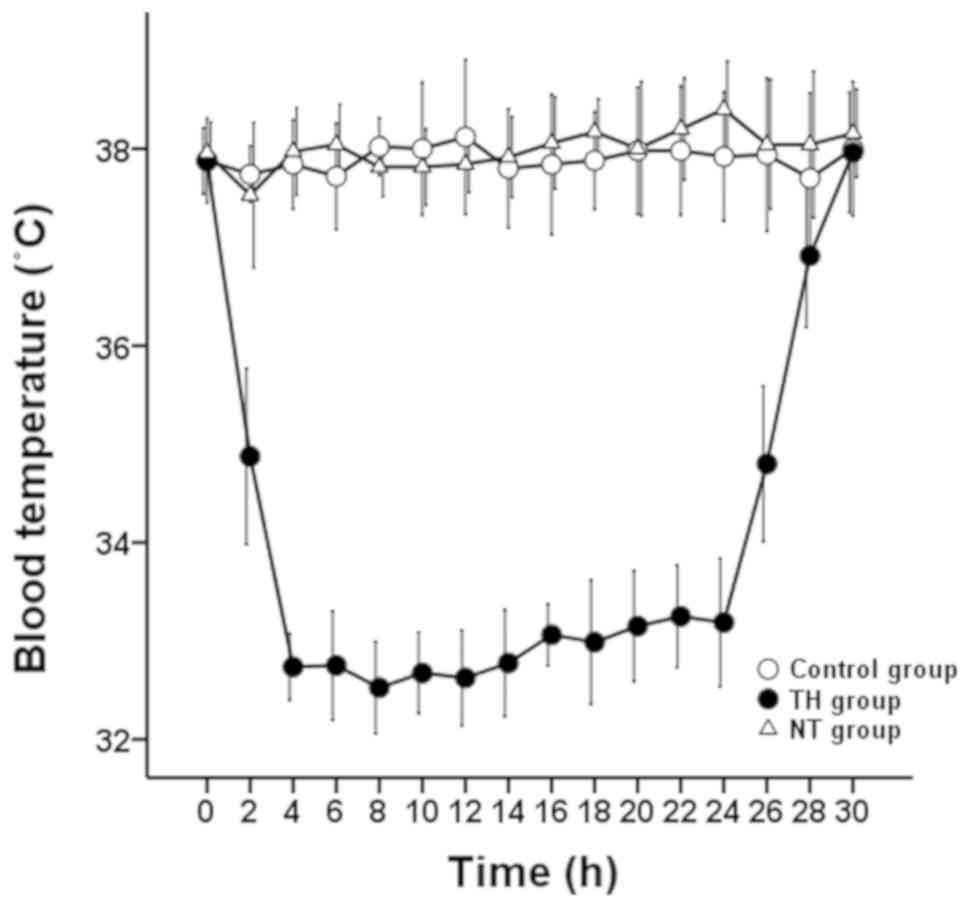
In the TH group, body temperature was rapidly
reduced from 37.9±0.3°C to 34.9±0.9°C within 2 h after ROSC.
Thereafter, a temperature of 32–34°C was maintained until 24 h
after ROSC, followed by rewarming at a rate of 1°C/h for 5 h
(Fig. 2). The temperature of animals
in the control and NT groups were maintained at 37–38°C throughout
the experiment.
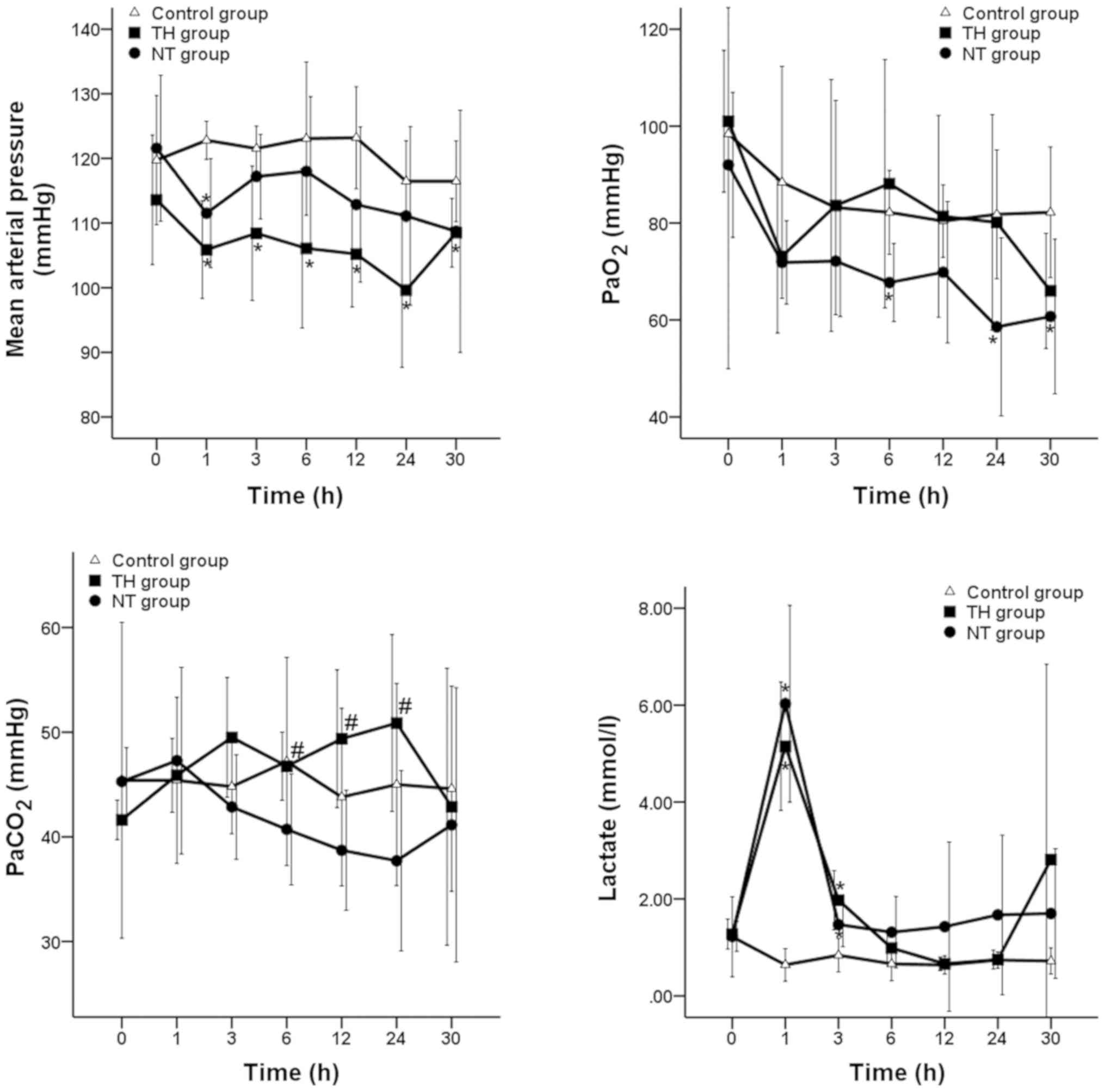
Fig. 3 indicated the
course of MAP, arterial oxygen partial pressure (PaO2),
PaCO2 and lactate throughout the 30 h following ROSC in
the three groups. After ROSC, MAP decreased but remained at a
normal physiological level of >98 mmHg in all animals. In the TH
group, post-resuscitation PaCO2 gradually increased and
was significantly greater compared with the NT group at 6, 12 and
24 h (P<0.05), then decreased during the rewarming period. There
no significant differences were observed in arterial
PaO2 or lactate between the NT and TH groups.
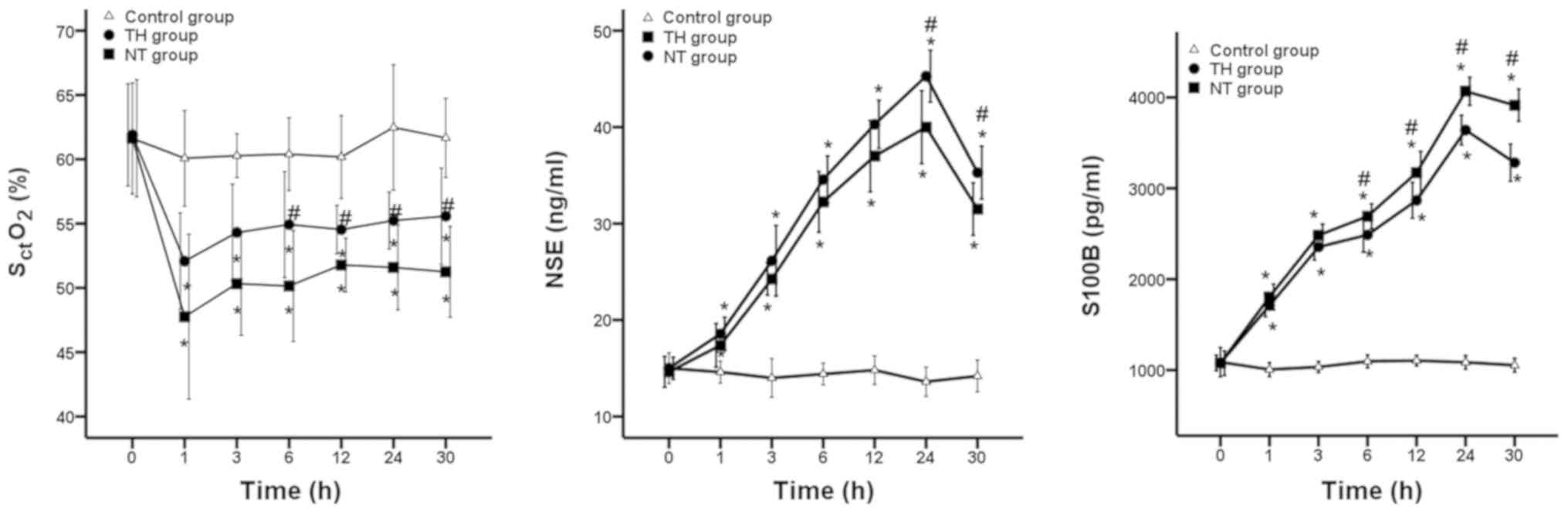
Biomarkers of brain damage (NSE,
S100B) by blood samples
Following ROSC, serum levels of NSE were
significantly increased to 40.00±3.78 ng/ml and 45.29±2.69 ng/ml in
all resuscitated animals in the TH and NT groups during the
hypothermic period, then decreased to 31.50±2.73 ng/ml and
35.29±2.75 ng/ml at rewarming time (all P<0.05; Fig. 4). Serum levels of S100B were
significantly increased to 3640.75±162.93 pg/ml and 4067.86±154.07
pg/ml in all resuscitated animals in the TH and NT groups during
the hypothermic period, then decreased to 3282.75±205.42 pg/ml and
3914.86±177.64 pg/ml at rewarming time (all P<0.05; Fig. 4). Throughout the experiment, serum
levels of NSE and S100B increased after VF and resuscitation, then
declined at rewarming time in both TH and NT groups. However, NSE
and S100B were lower in the TH group compared with the NT group
after ROSC; these differences were significant at 12 and 6 h after
ROSC (all P<0.05; Fig. 4).
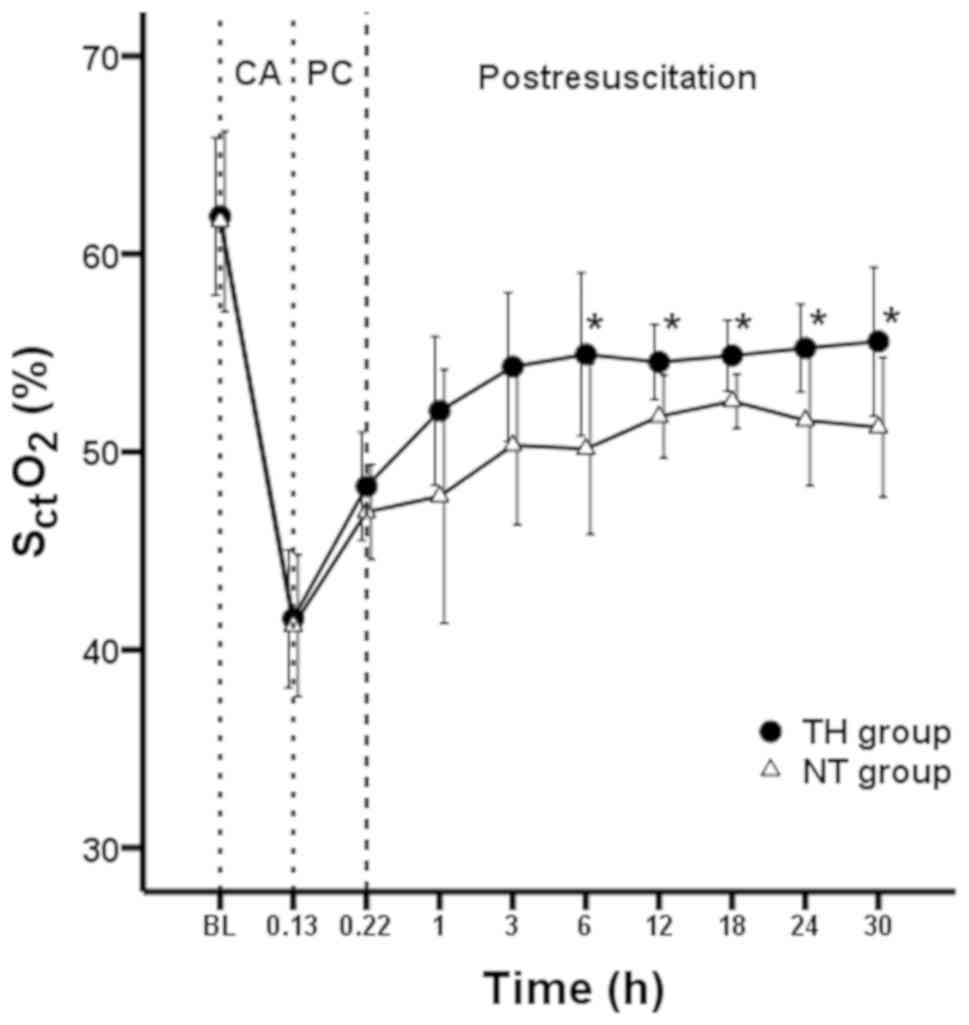
SctO2 obtained
by NIRS
Mean SctO2 was monitored from
the initiation of VF (baseline) until 30 h following ROSC, and the
mean SctO2 values at baseline were 61.9%
(4.0%) in the TH group and 61.6% (4.5%) in the NT group (P=0.155).
The mean SctO2 values after 8 min of VF were
41.6% (3.5%) and 41.2% (3.6%) (P=0.664) in the TH and NT groups,
respectively (Fig. 5). The mean
SctO2 significantly decreased after 8 min of
VF in the two groups, compared with SctO2 at
baseline (P<0.001 and P<0.001). During 5 min of CPR, the mean
SctO2 values gradually increased to 48.3%
(2.74%) and 47.0% (2.40%), but there were no significant
differences between the TH and NT groups (P=0.524). Following ROSC,
a progressive increase in mean SctO2 was
observed in both groups, such that stable values were reached at
approximately 12 h after ROSC in the two groups.
SctO2 values increased to 55.2% (2.2%) in the
TH group and 51.2% (3.5%) in NT group at 24 h after ROSC (P=0.024).
Finally, SctO2 values further increased to
55.6% (3.8%) in the TH group and 51.2% (3.5%) in the NT group
during the rewarming period (P=0.039; Fig. 5). Throughout the experiment,
SctO2 declined after VF, then increased
following ROSC in the TH and NT groups. However,
SctO2 values in the TH group were greater
compared with the NT group at 6 h after ROSC (54.9% vs. 50.1%;
P=0.047).
Discussion
The present study monitored
SctO2 and biomarkers of brain injury in the
overall course from the time of cardiac arrest until 30 h after
ROSC in the TH and NT groups. Hemodynamics and PaO2 were
similar in both treatment groups. SctO2
declined after VF, then increased in both treatment groups. NSE and
S100B began to show differences from 6 h onwards, when values were
compared between the TH and NT groups. SctO2
was significantly greater in the TH group compared with the NT
group at 6 h after ROSC. Therefore, the results of the present
study indicated that therapeutic hypothermia could increase
SctO2.
The current study used a model of out-of-hospital
cardiac arrest (OHCA), which comprised 8 min of untreated VF,
followed by CPR at a ratio of 30:2. Earlier studies (15,16) have
demonstrated that this model is feasible to explore monitoring of
and protection against multiple organ injuries after resuscitation
(for example in brain injuries). Furthermore, OHCA is associated
with increased levels of morbidity and mortality worldwide and the
majority of patients who attain ROSC often exhibit poorer
neurologic outcomes, compared with those who experience in-hospital
cardiac arrest (17). Therefore, it
was hypothesized that it might be useful to evaluate the values and
dynamics of continuous SctO2 measurements
during OHCA, particularly around the time of ROSC (18).
The biomarkers NSE and S100B are associated with the
neurological outcomes and serve important roles in prognostication
because they are nearly universally accessible and are inexpensive
(3). In the present study, a
consistent trend between S100B and NSE in NT and TH groups was
observed throughout the 30 h observation period. Furthermore, the
levels of S100B and NSE were higher in NT and TH groups compared
with the control group, which indicated the presence of brain
damage after cardiac arrest in the two groups. The level of S100B
in TH group was lower compared with the NT group from 6 h after
ROSC, while the NSE level was lower in TH group beginning at 24 h
after ROSC compared with NT group. These finding were consistent
with those of previous studies (19,20). NSE
levels have indicated promising results only at later stages
(>24 h after cardiac arrest) (21). S100B serum levels can assess overall
cerebral outcome and survival following cardiac arrest at earlier
points than other methods (22).
However, these markers require the collection of blood samples,
which is invasive and unsustainable for continued monitoring.
Furthermore, the results are not available in real time.
To maximize the effectiveness of CPR and increase
the likelihood that victims will survive without major neurological
deficits, continuous monitoring is required to determine the
balance between cerebral oxygen demand and delivery (11,23).
NIRS is a noninvasive optical technique that uses near-infrared
spectrum photons (700–1,300 nm) to calculate hemoglobin saturation
(24,25). At these wavelengths, oxyhemoglobin
and deoxyhemoglobin exhibit different absorption properties. A
tissue oximeter could calculate the total concentrations of
oxyhemoglobin and deoxyhemoglobin, thus enabling assessments of
mixed oxygen saturation in tissues. As NIRS measures the oxygen
saturation in all vessels <1 mm diameter (including arteries,
capillaries and venules) (26), it
can provide readings in instances of low blood flow, independently
of pulsatile flow, including the flow exhibited during cardiac
arrest. Its prognostic value has been validated in recent studies
(5,27). NIRS could offer a large clinical
benefit in that it alerts clinicians promptly, enabling
implementation of corrective interventions. A meta-analysis
indicated clinicians should consider NIRS saturation trends during
resuscitative efforts (5,28).
The present study investigated sequential changes
and the physiological significance of SctO2
during therapeutic hypothermia and normal temperature immediately
following ROSC (29–31). After cardiac arrest,
SctO2 decreased, then progressively increased
in the TH and NT groups. This decrease could be explained by the
onset of different pathophysiological mechanisms after cardiac
arrest. During 5 min of CPR, a steep increase in
SctO2 was observed, which is likely to be
related the initiation of CPR, as described in a previous study
(32).
Within the first few hours after ROSC, the NT group
showed a significant decrease in cerebral oxygenation, compared
with baseline levels. Low SctO2 in this
context might be related to an inadequate oxygen supply to meet
cerebral oxygen demand, and may indicate cerebral ischemia caused
by unstable hemodynamics, hypoxia or reduced PaCO2,
rather than a cerebral metabolic suppression. Another cause of the
reduction in SctO2 values is the continuation
of a no-reflow phenomenon exhibited by the brain, which can be
caused by post-ischemic hypoperfusion, increased blood viscosity,
reduced small-vessel caliber, or impaired microvascular perfusion.
As a result, cerebral blood flow might be reduced, regardless of
normal blood pressure. Some studies have shown that severe brain
damage might cause metabolic depression and hyperemia and that
increased SctO2 may occur in the very early
stage of post-cardiac arrest syndrome (8,27).
Therefore, the significance of higher or lower
SctO2 in the early post-resuscitation phase
remains to be elucidated. In the present study, the overall trend
following ROSC demonstrated a progressive increase, which reached
stable values after ~6 h in both groups.
Significant increases in SctO2
were observed at 6 h after ROSC in the TH group, compared with the
NT group, which is consistent with the findings of a previous study
(7). TH reduces cerebral oxygen
consumption (33,34) and suppresses cerebral reperfusion
injury, which is characterized by increased intracellular levels of
glutamate and oxygen free-radical reactions; both of these
phenomena occurring when cerebral blood flow is restored after
resuscitation (35,36). Experimental studies and previous
clinical trials have demonstrated that mild hypothermia may be
superior to NT for the maintenance of cerebral oxygenation and
neurological function (37,38). However, no significate differences
were observed in SctO2 between NT and TH groups at the
early stages (1, 3 h) of post-resuscitation. In the TH group, the
body temperature decreased and only reached a stable level at 3 h
after ROSC. Perhaps the unstable temperature affected the
protective outcome of hypothermia and caused the no significant
P-values, similar to a previous study (15).
The present study possessed several limitations. One
potential limitation was that the sample size of animals was small,
but all the tests suggested that there was a difference between
groups. Focusing on the SctO2 in a larger
sample clinical study should be performed in the future.
Additionally, cerebral hemodynamic parameters (transcranial doppler
or jugular bulb oxygenation) were not assessed in conjunction with
changes in SctO2, although a combination of
assessment with these parameters could improve understanding of
cerebral hemodynamic disturbances. However, jugular bulb
oxygenation is an invasive technique that is difficult to perform
in post-cardiac arrest animals. In addition, its use in such
animals is difficult to justify. NIRS is feasible for use in
monitoring the oxygen saturation in all vessels <1 mm diameter
within the human brain, but anatomical differences in the thickness
of the forehead between pigs and humans might limit detection
capabilities. A higher rate of rewarming of 1°C/h was also applied,
based on the finding in a previous study (39). However, current guidelines indicate
that patients should be rewarmed at a rate of 0.25–0.5°C/h
(11). Additionally, although all
animals were continuously monitored under anesthesia throughout the
experiment, post-resuscitation neurologic function was not
evaluated. Lastly, one approach alone is unlikely to reflect the
brain injury accurately after cardiac arrest. A multimodal approach
for neurologic assessment has been demonstrated to be effective and
the optimal sequential combination of tests requires further
investigation (40).
The finding of the present study indicated that,
following cardiac arrest, therapeutic hypothermia could increase
SctO2 following ROSC and it was demonstrated
that it could improve overall neurological outcome. Additionally,
SctO2 was feasible for use as an early marker
of brain damage during and after cardiac arrest.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the 2015
Welfare Scientific Research Project from the Chinese Ministry of
Health (grant no. 2015SQ00050), Key joint research project of
Chinese Ministry of Health & Zhejiang Province (grant no.
2018271879), Welfare scientific research project of Zhejiang
Province (grant nos. LGF18H150003 and LGD19H150003), Zhejiang
Provincial Medical Science Foundation (grant no. 2017KY389) and
Yuyao Medical Science and Technology Project (grant no.
2017YZD01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ and ZL designed the study, CW and JX analyzed and
summarized the literature and were responsible for writing the
manuscript. XJ and QC analyzed the data. XL, AQ, MW performed the
experiments. MZ, ZL, AQ, CW and JX assisted in providing
constructive analysis and interpreted the data. All authors read
and approve the final manuscript.
Ethics approval and consent to
participate
The protocol of the current study was approved by
the Animal Care and Use Committee of the Medical School of Zhejiang
University. Animal care and experiments were conducted according to
Institutional Animal Care and Use Committee guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CPR
|
cardiopulmonary resuscitation
|
|
MAP
|
mean arterial pressure
|
|
NIRS
|
near-infrared spectroscopy
|
|
NSE
|
neuron-specific enolase
|
|
NT
|
normothermia
|
|
PaO2
|
arterial oxygen partial pressure
|
|
PaCO2
|
arterial carbon dioxide partial
pressure
|
|
ROSC
|
return of spontaneous circulation
|
|
SctO2
|
cerebral tissue oxygen saturation
|
|
TH
|
therapeutic hypothermia
|
References
|
1
|
Nolan JP, Neumar RW, Adrie C, Aibiki M,
Berg RA, Bbttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac arrest syndrome: epidemiology, pathophysiology,
treatment, and prognostication: A scientific statement from the
International Liaison Committee on Resuscitation; the American
Heart Association Emergency Cardiovascular Care Committee; the
Council on Cardiovascular Surgery and Anesthesia; the Council on
Cardiopulmonary, Perioperative, and Critical Care; the Council on
Clinical Cardiology; the Council on Stroke (Part II). Int Emerg
Nurs. 18:8–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moseby-Knappe M, Pellis T, Dragancea I,
Friberg H, Nielsen N, Horn J, Kuiper M, Roncarati A, Siemund R,
Undén J, et al: Head computed tomography for prognostication of
poor outcome in comatose patients after cardiac arrest and targeted
temperature management. Resuscitation. 119:89–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duez CHV, Grejs AM, Jeppesen AN, Schroder
AD, Søreide E, Nielsen JF and Kirkegaard H: Neuron-specific enolase
and S-100b in prolonged targeted temperature management after
cardiac arrest: A randomised study. Resuscitation. 122:79–86. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawkes MA and Rabinstein AA: Neurological
prognostication after cardiac arrest in the era of target
temperature management. Curr Neurol Neurosci Rep. 19:102019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheeren TWL, Kuizenga MH, Maurer H,
Struys MMRF and Heringlake M: Electroencephalography and brain
oxygenation monitoring in the perioperative period. Anesth Analg.
128:265–277. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singer AJ, Ahn A, Inigo-Santiago LA, Thode
HC Jr, Henry MC and Parnia S: Cerebral oximetry levels during CPR
are associated with return of spontaneous circulation following
cardiac arrest: An observational study. Emerg Med J. 32:353–356.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cournoyer A, Iseppon M, Chauny JM, Denault
A, Cossette S and Notebaert E: Near-infrared spectroscopy
monitoring during cardiac arrest: A systematic review and
meta-analysis. Acad Emerg Med. 23:851–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ibrahim AW, Trammell AR, Austin H, Barbour
K, Onuorah E, House D, Miller HL, Tutt C, Combs D, Phillips R, et
al: Cerebral oximetry as a real-time monitoring tool to assess
quality of in-hospital cardiopulmonary resuscitation and post
cardiac arrest care. J Am Heart Assoc. 4:e0018592015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bougle A, Daviaud F, Bougouin W, Rodrigues
A, Geri G, Morichau-Beauchant T, Lamhaut L, Dumas F and Cariou A:
Determinants and significance of cerebral oximetry after cardiac
arrest: A prospective cohort study. Resuscitation. 99:1–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinoshita K, Sakurai A and Ihara S: The
pitfalls of bedside regional cerebral oxygen saturation in the
early stage of post cardiac arrest. Scand J Trauma Resusc Emerg
Med. 23:952015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nolan JP, Soar J, Cariou A, Cronberg T,
Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K and
Sandroni C: European resuscitation council and european society of
intensive care medicine guidelines for post-resuscitation care
2015: Section 5 of the european resuscitation council guidelines
for resuscitation 2015. Resuscitation. 95:202–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nielsen N, Wetterslev J, Cronberg T,
Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J,
Kuiper M, et al: Targeted temperature management at 33°C versus
36°C after cardiac arrest. N Engl J Med. 369:2197–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wolfle TL: 50 years of the Institute for
Laboratory Animal Research (ILAR): 1953–2003. ILAR J. 44:324–337.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nemzek JA, Xiao HY, Minard AE, Bolgos GL
and Remick DG: Humane endpoints in shock research. Shock. 21:17–25.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Jin X, Chen Q, Wu C, Li Z, Zhou G,
Xu Y, Qian A, Li Y and Zhang M: Faster hypothermia induced by
esophageal cooling improves early markers of cardiac and
neurological injury after cardiac arrest in swine. J Am Heart
Assoc. 7:e0102832018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Chen Q, Jin X, Wu C, Li Z, Zhou G,
Xu Y, Qian A, Li Y and Zhang M: Early initiation of continuous
renal replacement therapy induces fast hypothermia and improves
post-cardiac arrest syndrome in a porcine model. Shock. 52:456–467.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Myat A, Song KJ and Rea T: Out-of-hospital
cardiac arrest: Current concepts. Lancet. 391:970–979. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prosen G, Strnad M, Doniger SJ, Markota A,
Stožer A, Borovnik-Lesjak V and Mekiš D: Cerebral tissue oximetry
levels during prehospital management of cardiac arrest-A
prospective observational study. Resuscitation. 129:141–145. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuzhuget R, Starodubtsev V, Ignatenko P,
Starodubtseva A, Voroshilina O, Ruzankin P and Karpenko A: The role
of stump pressure and cerebral oximetry in predicting ischaemic
brain damage during carotid endarterectomy. Brain Inj.
31:1944–1950. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanchez-de-Toledo J, Chrysostomou C, Munoz
R, Lichtenstein S, Sao-Avilés CA, Wearden PD, Morell VO, Clark RS,
Toney N and Bell MJ: Cerebral regional oxygen saturation and serum
neuromarkers for the prediction of adverse neurologic outcome in
pediatric cardiac surgery. Neurocrit Care. 21:133–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calderon LM, Guyette FX, Doshi AA,
Callaway CW and Rittenberger JC; Post Cardiac Arrest Service, :
Combining NSE and S100B with clinical examination findings to
predict survival after resuscitation from cardiac arrest.
Resuscitation. 85:1025–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Böttiger BW, Möbes S, Glätzer R, Bauer H,
Gries A, Bärtsch P, Motsch J and Martin E: Astroglial protein S-100
is an early and sensitive marker of hypoxic brain damage and
outcome after cardiac arrest in humans. Circulation. 103:2694–2698.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stocchetti N, Le Roux P, Vespa P, Oddo M,
Citerio G, Andrews PJ, Stevens RD, Sharshar T, Taccone FS and
Vincent JL: Clinical review: Neuromonitoring-an update. Crit Care.
17:2012013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollard V, Prough DS, DeMelo AE, Deyo DJ,
Uchida T and Stoddart HF: Validation in volunteers of a
near-infrared spectroscope for monitoring brain oxygenation in
vivo. Anesth Analg. 82:269–277. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McCormick PW, Stewart M, Ray P, Lewis G,
Dujovny M and Ausman JI: Measurement of regional cerebrovascular
haemoglobin oxygen saturation in cats using optical spectroscopy.
Neurol Res. 13:65–70. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Backer D, Ospina-Tascon G, Salgado D,
Favory R, Creteur J and Vincent JL: Monitoring the microcirculation
in the critically ill patient: Current methods and future
approaches. Intensive Care Med. 36:1813–1825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wik L: Near-infrared spectroscopy during
cardiopulmonary resuscitation and after restoration of spontaneous
circulation: A valid technology? Curr Opin Crit Care. 22:191–198.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Genbrugge C, Eertmans W, Meex I, Van
Kerrebroeck M, Daems N, Creemers A, Jans F, Boer W, Dens J and De
Deyne C: What is the value of regional cerebral saturation in
post-cardiac arrest patients? A prospective observational study.
Crit Care. 20:3272016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meex I, Dens J, Jans F, Boer W, Vanhengel
K, Vundelinckx G, Heylen R and De Deyne C: Cerebral tissue oxygen
saturation during therapeutic hypothermia in post-cardiac arrest
patients. Resuscitation. 84:788–793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abdul-Khaliq H, Schubert S, Troitzsch D,
Huebler M, Boettcher W, Baur MO and Lange PE: Dynamic changes in
cerebral oxygenation related to deep hypothermia and circulatory
arrest evaluated by near-infrared spectroscopy. Acta Anaesthesiol
Scand. 45:696–701. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JK, Brady KM, Mytar JO, Kibler KK,
Carter EL, Hirsch KG, Hogue CW, Easley RB, Jordan LC, Smielewski P,
et al: Cerebral blood flow and cerebrovascular autoregulation in a
swine model of pediatric cardiac arrest and hypothermia. Crit Care
Med. 39:2337–2345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Genbrugge C, De Deyne C, Eertmans W,
Anseeuw K, Voet D, Mertens I, Sabbe M, Stroobants J, Bruckers L,
Mesotten D, et al: Cerebral saturation in cardiac arrest patients
measured with near-infrared technology during pre-hospital advanced
life support. Results from Copernicus I cohort study.
Resuscitation. 129:107–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hegnauer AH and D'Amato HE: Oxygen
consumption and cardiac output in the hypothermic dog. Am J
Physiol. 178:138–142. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mezrow CK, Sadeghi AM, Gandsas A, Shiang
HH, Levy D, Green R, Holzman IR and Griepp RB: Cerebral blood flow
and metabolism in hypothermic circulatory arrest. Ann Thorac Surg.
54:609–615. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Busto R, Globus MY, Dietrich WD, Martinez
E, Valdes I and Ginsberg MD: Effect of mild hypothermia on
ischemia-induced release of neurotransmitters and free fatty acids
in rat brain. Stroke. 20:904–910. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chopp M, Knight R, Tidwell CD, Helpern JA,
Brown E and Welch KM: The metabolic effects of mild hypothermia on
global cerebral ischemia and recirculation in the cat: Comparison
to normothermia and hyperthermia. J Cereb Blood Flow Metab.
9:141–148. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakatani Y, Nakayama T, Nishiyama K and
Takahashi Y: Effect of target temperature management at 32–34°C in
cardiac arrest patients considering assessment by regional cerebral
oxygen saturation: A multicenter retrospective cohort study.
Resuscitation. 126:185–190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ostadal P, Mlcek M, Kruger A, Horakova S,
Skabradova M, Holy F, Svoboda T, Belohlavek J, Hrachovina V,
Taborsky L, et al: Mild therapeutic hypothermia is superior to
controlled normothermia for the maintenance of blood pressure and
cerebral oxygenation, prevention of organ damage and suppression of
oxidative stress after cardiac arrest in a porcine model. J Transl
Med. 11:1242013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu X, Ma L, Sun S, Xu J, Zhu C and Tang W:
The effects of the rate of postresuscitation rewarming following
hypothermia on outcomes of cardiopulmonary resuscitation in a rat
model. Crit Care Med. 42:e106–e113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim JH, Kim MJ, You JS, Lee HS, Park YS,
Park I and Chung SP: Multimodal approach for neurologic
prognostication of out-of-hospital cardiac arrest patients
undergoing targeted temperature management. Resuscitation.
134:33–40. 2019. View Article : Google Scholar : PubMed/NCBI
|