Introduction
Acute pancreatitis (AP) is one of the most common
causes of hospital admissions worldwide and the incidence continues
to rise annually (1). Although the
majority of patients present with mild AP, which can be promptly
diagnosed and cured, a notable number of patients go on to develop
severe AP, causing systemic inflammatory responses, multiple-organ
failure and even death (2). Despite
improvements in the diagnosis and treatment of AP, the mortality
rate of the disease remains ~5% (3).
Gallstones and alcohol abuse represent the first and second most
frequent causes of AP, respectively. However, hyperlipidemia is
also a rare but important etiology of AP (4–6). The
typical presentation of hyperlipidemic pancreatitis (HP) is a
patient with a pre-existing abnormal lipid profile (7). A serum triglyceride (TG) level ≥1,000
mg/dl has been reported to precipitate an episode of AP (7). Inflammation and abnormal lipid
metabolism are primary contributors to the pathogenesis of HP
(8). A rapid reduction in serum TG
levels can effectively prevent further worsening of a patient's
condition and the routine management of HP is often similar to that
of AP caused by other factors (9).
Multiple treatment strategies have been used for the management of
patients with HP (10). The
therapeutic mechanisms are largely based on a combination of the
inflammatory response, abnormal lipid metabolism, extensive
accumulation of free fatty acids and microcirculatory disturbance
(2,11,12).
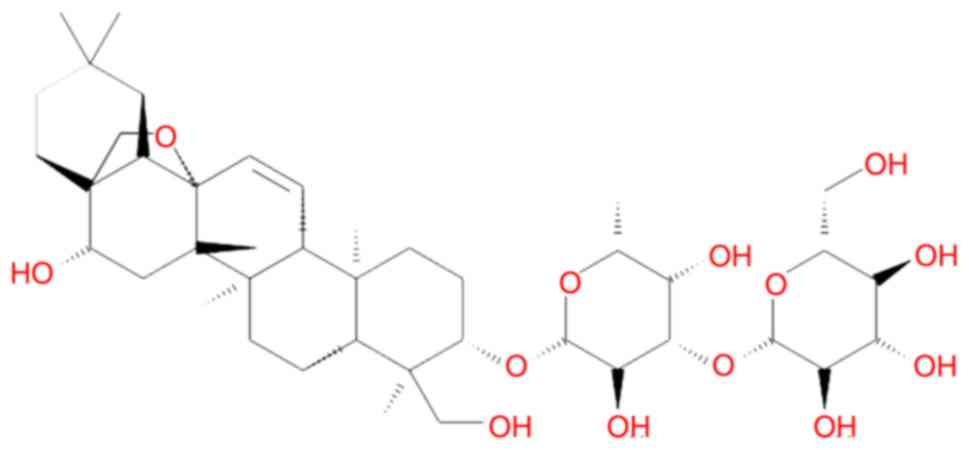
The saikosaponins (SSs), including saikosaponin a
(SSa; Fig. 1), are the most active
ingredients isolated from Radix Bupleuri, which is used in
traditional Chinese medicine (13).
This herb has long been utilized for inflammatory diseases, chronic
hepatitis, influenza and digestive ulcers (14). Emerging evidence has suggested that
SSs display anti-inflammatory, antiviral, antioxidant, antitumor,
immune regulatory and central nervous system protective effects
(13,15). Among the SSs, SSa has attracted
considerable attention due to its significant anti-inflammatory
activity (16). Direct inhibition of
proinflammatory cytokine expression and regulation of the
inflammatory response via specific signaling pathways, such as the
NF-κB signaling pathway, have been identified as critical
mechanisms underlying the anti-inflammatory activity of SSa
(17). Additionally, previous
studies have confirmed that SSa can regulate lipid metabolism and
promote cholesterol efflux in early atherosclerosis, SSa may also
serve as a potential peroxisome proliferator-activated receptor
(PPAR)-γ agonist, significantly boosting the expression of PPAR-γ
(18). PPARs usually interact
closely with NF-κB, suppressing its signaling pathway and
subsequently inhibiting the release of proinflammatory cytokines
(19). Furthermore, PPAR agonists
have been demonstrated to serve a pivotal role in controlling lipid
metabolism and are capable of inhibiting AP-associated inflammatory
responses (20,21). However, the effect of SSa on HP and
the underlying mechanisms remain unclear.
The current study aimed to investigate the
therapeutic effect and underlying mechanisms of SSa in rats with
HP. A rat model of hyperlipidemia-induced pancreatitis was employed
to assess lipid and proinflammatory cytokine profiles, as well as
the expression of PPAR-γ and NF-κB.
Materials and methods
Reagents
SSa was purchased from Beijing Baiaolaibo Technology
Co., Ltd. The assay kits for total cholesterol (TC, cat. no.
A111-1-1), TG (cat. no. A110-1-1), low-density
lipoprotein-cholesterol (LDL-C; cat. no. A113-1-1), high-density
lipoprotein cholesterol (HDL-C; cat. no. A112-1-1), myeloperoxidase
(MPO; cat. no. A044-1-1), amylase (AMY; cat. no. C016-1-1) and
lipase (cat. no. A054-1-1) were purchased from Nanjing Jiancheng
Bioengineering Institute. ELISA kits for rat tumor necrosis factor
(TNF)-α (cat. no. MM-0180R1), interleukin (IL)-1β (cat. no.
MM-0047R1), IL-6 (cat. no MM-91067O2) and IL-10 (cat. no.
MM-0195R1) were obtained from Zhongsheng Beikong Bio-Technology.
Additionally, rabbit anti-rat PPAR-γ antibody (cat. no. ab59256),
rabbit anti-rat NF-κB antibody (cat. no. ab32536), rabbit anti-rat
phosphorylated-(p)-NF-κB antibody (cat. no. ab86299), rabbit
anti-rat inhibitor of κ-Bα (cat. no. Iκ-Bα) antibody (cat. no.
ab32518), rabbit anti-rat p-Iκ-Bα antibody (cat. no. ab92700) and
rabbit anti-rat myeloid differentiation primary response protein
(Myd88) antibody (cat. no. ab2064) were purchased from Abcam.
Animals
50 Male Sprague-Dawley (SD) rats (8 weeks of age,
200–230 g) were purchased from the Centre of Experimental Animals
at the Nanjing Medical University. All the animal experiments were
performed in collaboration with Zhejiang Traditional Chinese
Medicine University and were approved by the Ethics Committee of
Zhejiang Traditional Chinese Medicine University. Additionally, all
animals received humane care according to the criteria outlined in
the ‘Guide for the Care and Use of Laboratory Animals’ prepared by
the National Academy of Sciences and published by the National
Institutes of Health (22). The
animals were housed at 20±2°C, 55±10% humidity, with 12-h
light/dark cycles and free access to water and food and were
allowed to acclimatize for one week prior to experimentation.
Model preparation and animal
grouping
SD rats were randomly divided into two groups. A
group of 10 rats receiving a normal diet were regarded as the
control group (n=10). 40 hyperlipidemic animal models were
established according to previous publications (23,24) and
received a high-fat diet for two weeks to induce hyperlipidemia.
Serum lipid levels, including TC and TG, were also measured and met
the hyperlipidemic criteria (23).
The acute pancreatitis model was then further induced according to
the methods described by Niyaz et al (25) and Shi et al (26). After a 12-h fast, the hyperlipidemic
rats were anesthetized by the intraperitoneal injection of
pentobarbital sodium (30 mg/kg). An abdominal midline incision was
made and the rat's abdominal cavity was exposed. Subsequently, rats
received a retrograde infusion of 5% sodium taurocholate (0.1
ml/100 g; Sigma-Adlrich; Merck KGaA) into the bile-pancreatic duct
to create the AP model. The biliopancreatic duct that enters the
duodenum was clipped using a vascular clip for 5 min to prevent the
solution from entering the bile duct, allowing the induction of the
HP model. The control group (sham operation) was subjected to the
same procedure; however, the sodium taurocholate was replaced with
an equal volume of saline (0.1 ml/100 g). During the operation and
the following 12 h, there was a mortality rate of ~23% in acute
pancreatitis animals. The remaining HP rats were then randomly
assigned into three groups (n=10 in each group): Model group, low
SSa group (LSSa, 10 mg/kg) and high SSa (HSSa, 20 mg/kg). The
animal grouping strategy used in the current study was similar to a
previous study (26). The dose of
SSa administrated in the current study was determined according to
a previous publication (16). SSa
was administrated by intraperitoneal injection 1 h before inducing
HP. The same volume of normal saline was injected into the control
and model group. All rats were anesthetized with 30 mg/kg
pentobarbital sodium, 12 h after surgery. Blood samples were
collected via cardiac puncture and centrifuged at 900 × g for 10
min to obtain the serum sample which was stored at −80°C for
subsequent analysis. The pancreatic tissues were immediately
removed, snap frozen in liquid nitrogen and stored at −80°C for
subsequent analysis.
Histopathological examination
Pancreatic tissues from each group were fixed in 4%
paraformaldehyde for 12 h at room temperature and embedded in
paraffin. The tissues sections (4 µm thick) were stained with
hematoxylin and eosin (H&E) for 5 min and 1 min, respectively,
at room temperature and then observed under a light microscope
(×400 magnification) for histopathological examination. The degree
of pancreatic injury was histologically scored according to the
standard scale described by Schmidt et al (27), including the graded assessment of
pancreatic edema, inflammatory cell infiltration and acinar
necrosis in pancreatic tissues.
Pancreas wet/dry (W/D) ratio
After the rats were sacrificed, freshly collected
pancreatic tissues were weighed in the wet state. The pancreatic
tissues were then dried at 80°C for 48 h and the final dry weight
was obtained. The W/D ratio was used to assess the edema of the
pancreas.
MPO assay
The activity of MPO, an inflammatory marker
associated with neutrophil infiltration (28), was measured in the pancreatic tissues
with three replicates using the aforementioned test kit according
to the manufacturer's instructions.
Measurement of lipid profile, AMY and
lipase activity in the serum
Blood biochemical parameters of the lipid profile
were assessed. The concentrations of TC, TG, LDL-C and HDL-C in rat
serum were measured using the aforementioned assay kits according
to manufacturer's instructions with an automatic biochemistry
analyzer (Uni Cel Dx C 800Synchron; Beckman Coulter, Inc.) in
triplicate. The AMY and lipase concentration in serum were also
measured according to manufacturer's instructions in
triplicates.
ELISA
Serum levels of inflammatory cytokines TNF-α, IL-1β,
IL-6 and IL-10 were determined using an ELISA with three
replicates. The assays were performed in triplicate using the
corresponding ELISA kits and a microplate reader according to the
manufacturer's instructions.
Western blot assay
Pancreatic tissues from each group were obtained
immediately after the rats were sacrificed. Total protein was
extracted using RIPA lysis buffer (Beyotime Institute of
Biotechnology) from the pancreatic tissues and the protein
concentrations were measured using a bicinchoninic acid assay kit.
Subsequently, equal amounts of each protein sample (100 µg) were
separated via SDS-PAGE on 10% gels and transferred onto
nitrocellulose filter membranes. After blocking with 5% non-fat
milk at room temperature for 1 h, the membranes were incubated at
4°C overnight with primary antibodies against PPAR-γ (1:1,000),
p-NF-κB p65 (1:5,000), NF-κB p65 (1:2,000), IκBα (1:5,000), p-IκBα
(1:5,000) and MyD88 (1:1,000). Subsequently, the membranes were
further incubated with fluorescently-labeled secondary antibodies
(cat. no. ab205718; 1:5,000; Abcam) for 1 h at room temperature and
exposed to X-ray film. The bands were scanned and quantified using
ImageJ 1.48 software (National Institutes of Health). β-actin (cat.
no. ab179467, 1:5,000; Abcam) was used as an internal control to
determine protein levels and each experiment were performed in
triplicate.
Statistical analysis
All data are expressed as mean ± SD. Statistical
analysis was performed using SPSS software (version 19.0; IBM
Corp.). Statistical differences between the groups were determined
using one-way ANOVA followed by the Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
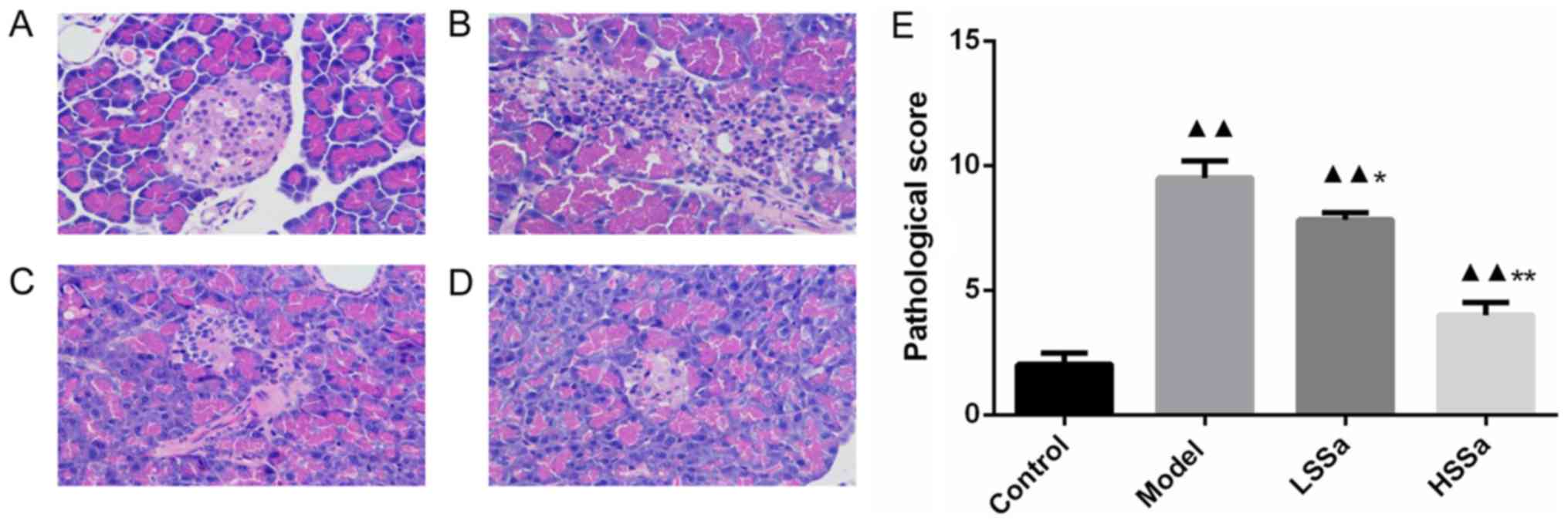
Histopathological analysis of
pancreatic tissue
The pancreatic tissues from all the groups were
collected and stained with H&E (Fig.
2A-D). Pancreatic tissue of control rats displayed a normal
pathological score and showed clear tissue structure with no
obvious abnormality in the pancreatic ducts and acini. Pancreatic
tissue obtained from rats in the model group displayed interstitial
edema, interstitial hyperemia, necrosis and neutrophil granulocyte
infiltration. The pathological score was also significantly
increased in the model group compared with the control group
(P<0.01; Fig. 2E). However, an
improvement in pathological changes was observed following SSa
treatment at both doses and pathological scores were reduced
compared with those of the model group (P<0.05; Fig. 2E).
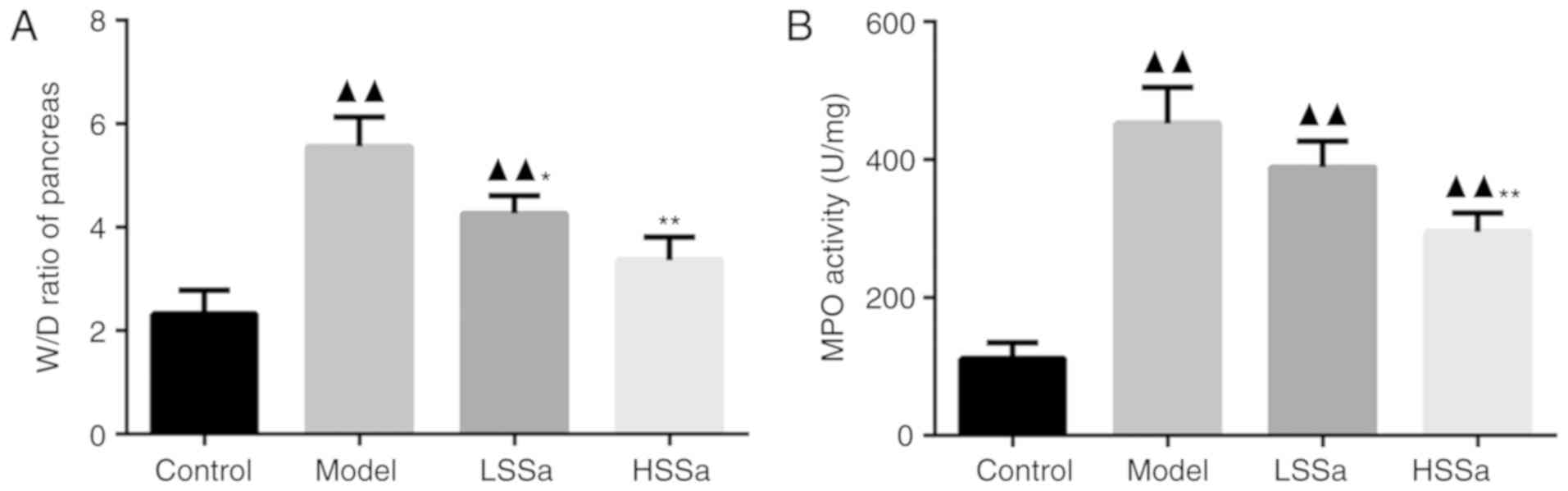
Effects of SSa on the pancreas W/D
ratio and MPO activity
The effect of SSa on pancreatic edema was
represented by the W/D ratio. Compared with that of the control,
the W/D ratio was significantly increased in the model group and
was reduced following the administration of SSa in dose-dependent
manner (P<0.05; Fig. 3A). For MPO
activity, a significant increase in pancreatic MPO activity was
observed in the model group compared with the control group
(P<0.01; Fig. 3B) and SSa
inhibited this HP-induced MPO activity, especially in the high
dosage group (P<0.05).
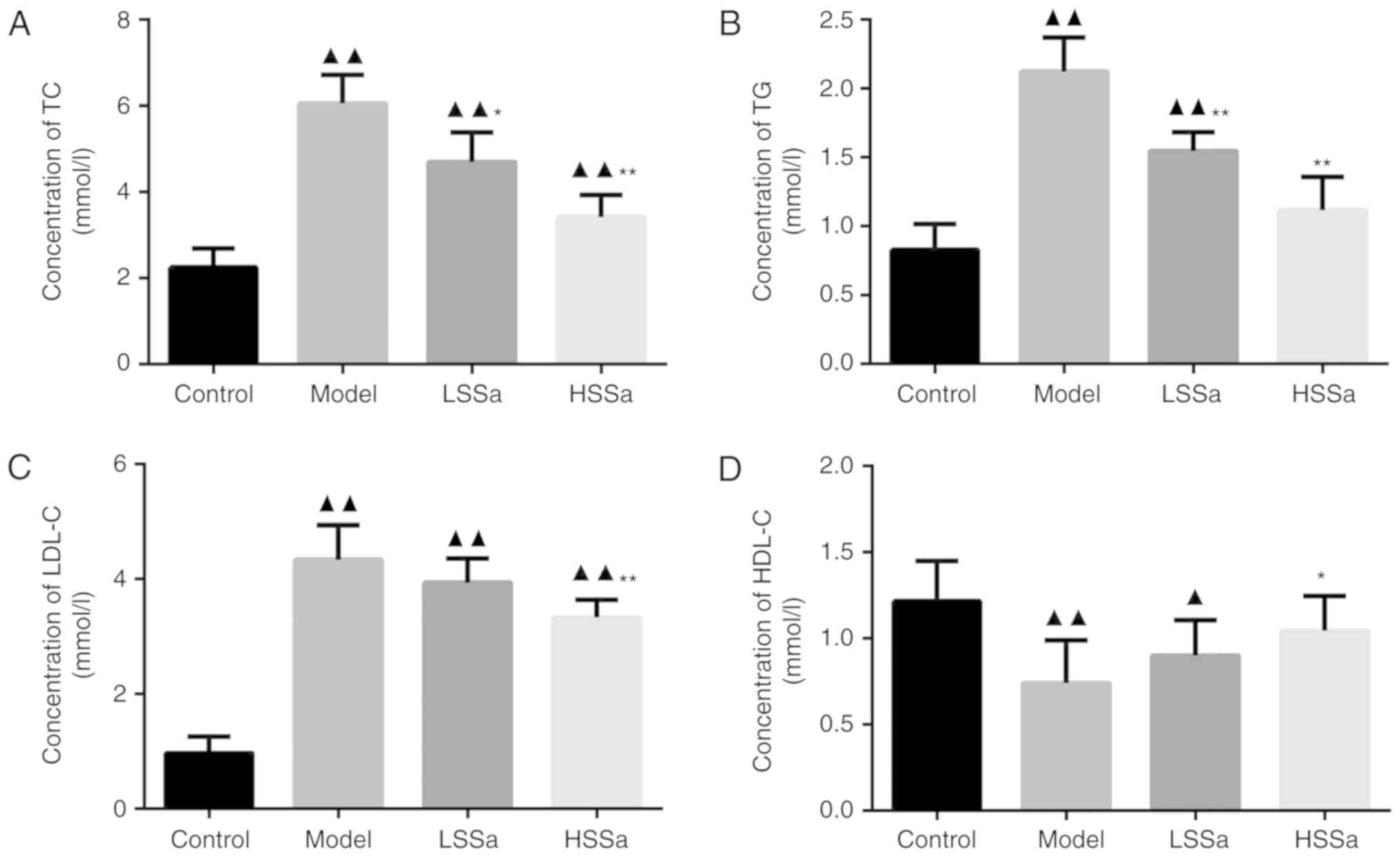
Determination of serum TC, TG, LDL-C
and HDL-C levels
The levels of serum TC, TG, LDL-C and HDL-C were
assessed to evaluate the alterations in lipid profiles in different
groups. As shown in Fig. 4, the
serum concentration of TC, TG, LDL-C and HDL-C varied among the
groups. In the model group, the TC, TG and LDL-C levels were
significantly higher than those in the control group (P<0.01),
which indicated that high lipid levels were associated with HP. The
level of TC in the model group and SSa groups was significantly
higher than that in the control group (P<0.01). The
administration of SSa (LSSa and HSSa group) significantly decreased
the level of TC compared with the model group, particularly in the
HSSa group (P<0.01; Fig. 4A).
Regarding TG levels in serum, a similar pattern was also observed.
SSa treatment significantly reduced TG levels in the LSSa group
compared with the control group (P<0.01). Furthermore, no
statistically significant difference was observed between the
control and HSSa groups, suggesting a pronounced effect of SSa in
lowering TG levels (Fig. 4B). There
was also no significant difference in serum LDL-C levels between
the model group and LSSa group, but LDL-C levels in the HSSa group
were significantly decreased compared with those in the model group
(P<0.01; Fig. 4C). By contrast,
the model group displayed lower HDL-C levels than the control group
and SSa treatment increased the levels of HDL-C compared with those
in the model group (Fig. 4D).
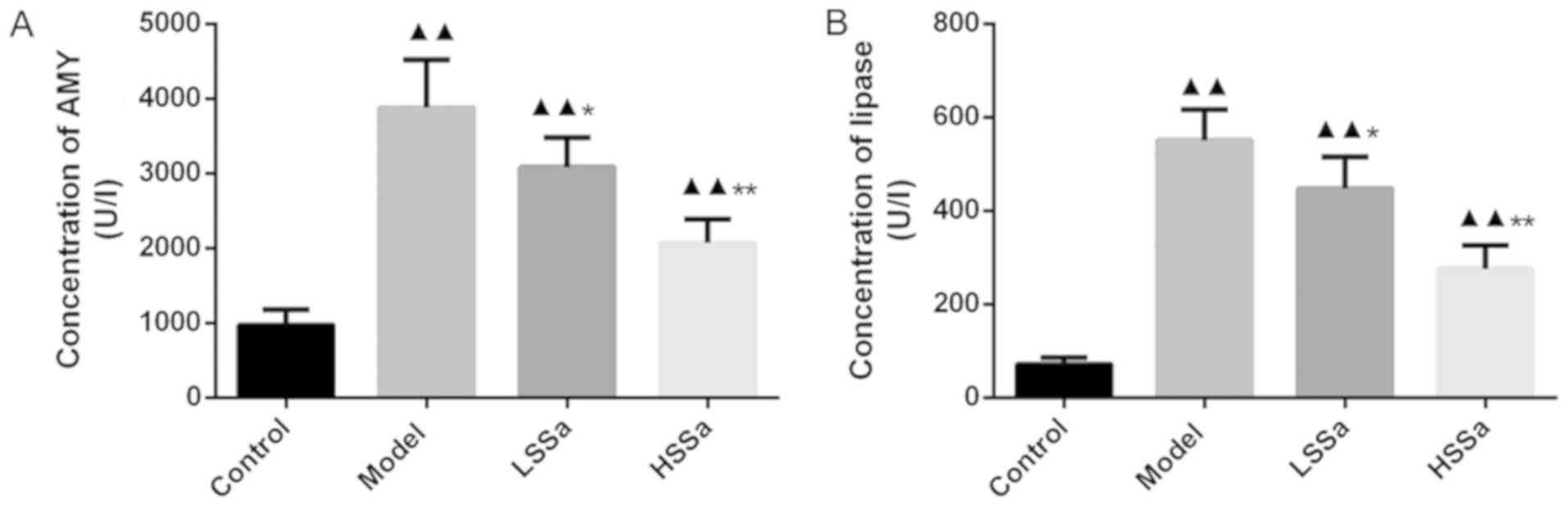
Serum levels of AMY and lipase
activity
Serum levels of AMY and lipase activity were
subsequently determined (Fig. 5A and
B). Compared with the control group, the levels of AMY and
lipase were significantly increased in the model group (both
P<0.01). Following SSa treatment, significant differences were
observed between the model group and SSa groups (P<0.05).
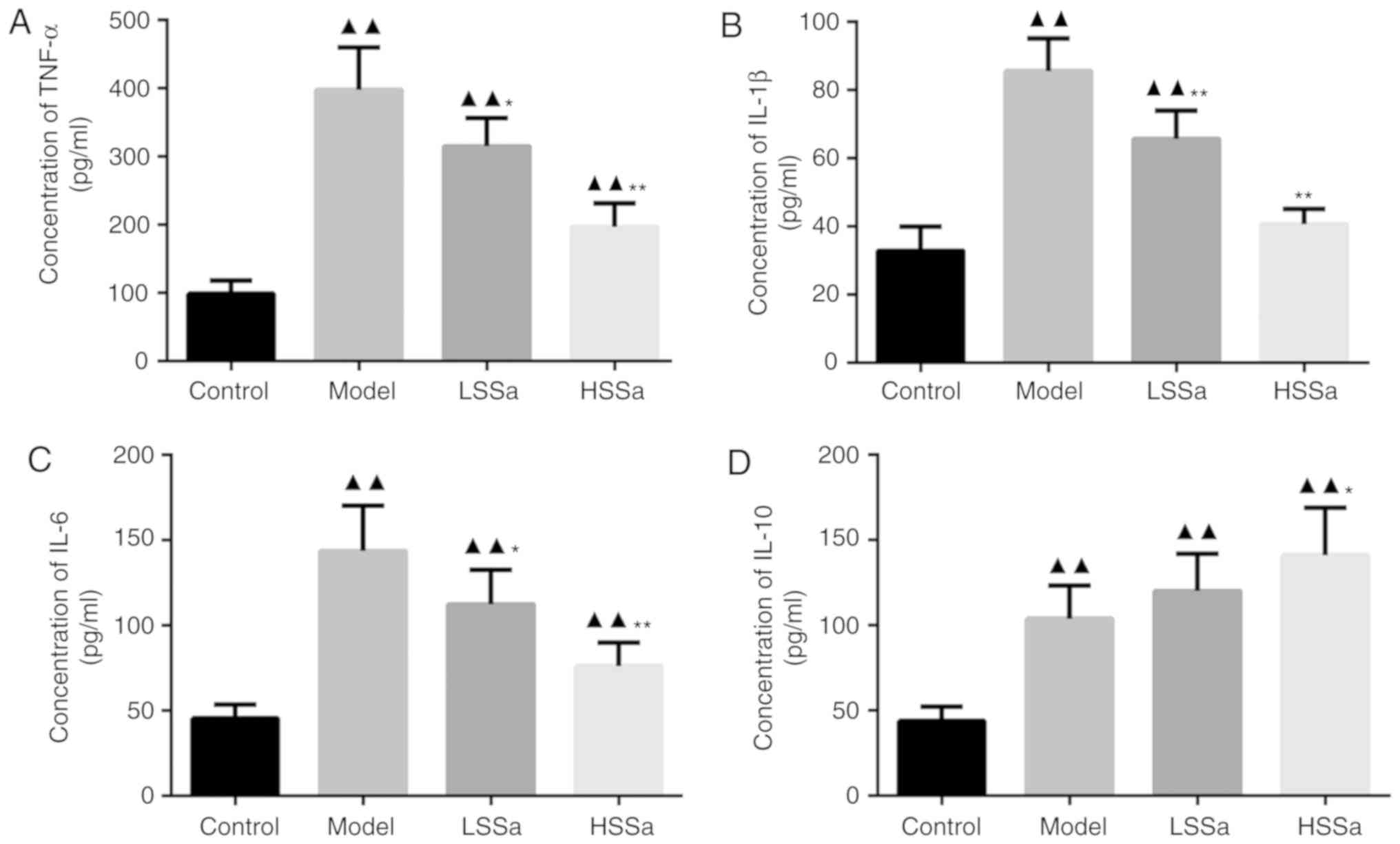
Serum levels of TNF-α, IL-1β, IL-6 and
IL-10
The expression levels of inflammatory cytokines in
the serum were measured by ELISA. The significantly higher levels
of TNF-α, IL-1β and IL-6 in the model group compared with those in
the control group (P<0.01) suggested that HP may be associated
with the overproduction of inflammatory cytokines and the
inflammatory response (Fig. 6).
However, the levels of TNF-α, IL-1β and IL-6 were reduced following
the administration of SSa, especially in the HSSa group, although
not to the levels of the untreated controls. The levels of TNF-α in
the model group increased significantly, by ~3-fold, compared with
those in the control group (P<0.01) and were significantly
decreased in the LSSa and HSSa groups compared with those in the
model group (P<0.05; Fig. 6A).
Similar outcomes were also observed for the expression levels of
IL-1β and IL-6, with slight differences among the experimental
groups (Fig. 6B and C). The model
group had the highest levels of these three cytokines, followed by
the LSSa and HSSa groups. The control group displayed the lowest
expression levels of these three cytokines. The expression levels
of IL-10, an anti-inflammatory cytokine (29), in the model group were higher than
those in the control group (P<0.01) and were increased following
SSa treatment (Fig. 6D). When HP
model rats were treated with SSa, the expression levels of IL-10
significantly increased in the HSSa group (P<0.05; Fig. 6D). The aforementioned results
suggested that SSa might have a therapeutic effect against
inflammation in HP.
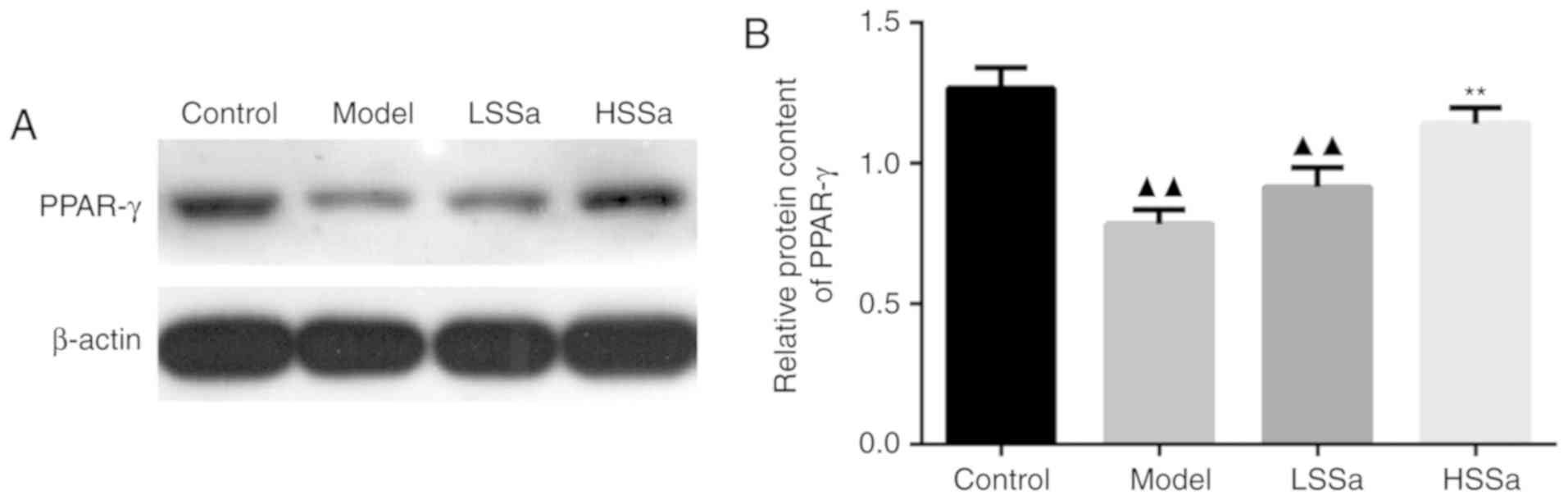
SSa promoted the expression of PPAR-γ
in pancreatic tissues
The expression of PPAR-γ in pancreatic tissue was
determined by western blotting to investigate the role of PPAR-γ in
HP, as well as the effect of SSa on PPAR-γ expression. The protein
expression levels of PPAR-γ were significantly decreased in the
model group compared with those in the control group (P<0.01;
Fig. 7A and B), but enhanced by SSa
at both the low and high dosage compared with those in the model
group, especially in HSSa group with significant difference
compared to model group (P<0.01). This indicated that the
expression levels of PPAR-γ in pancreatic tissue were reduced by HP
and furthermore, the administration of SSa effectively increased
PPAR-γ expression, inferring that SSa may be regarded as an ideal
PPAR-γ agonist.
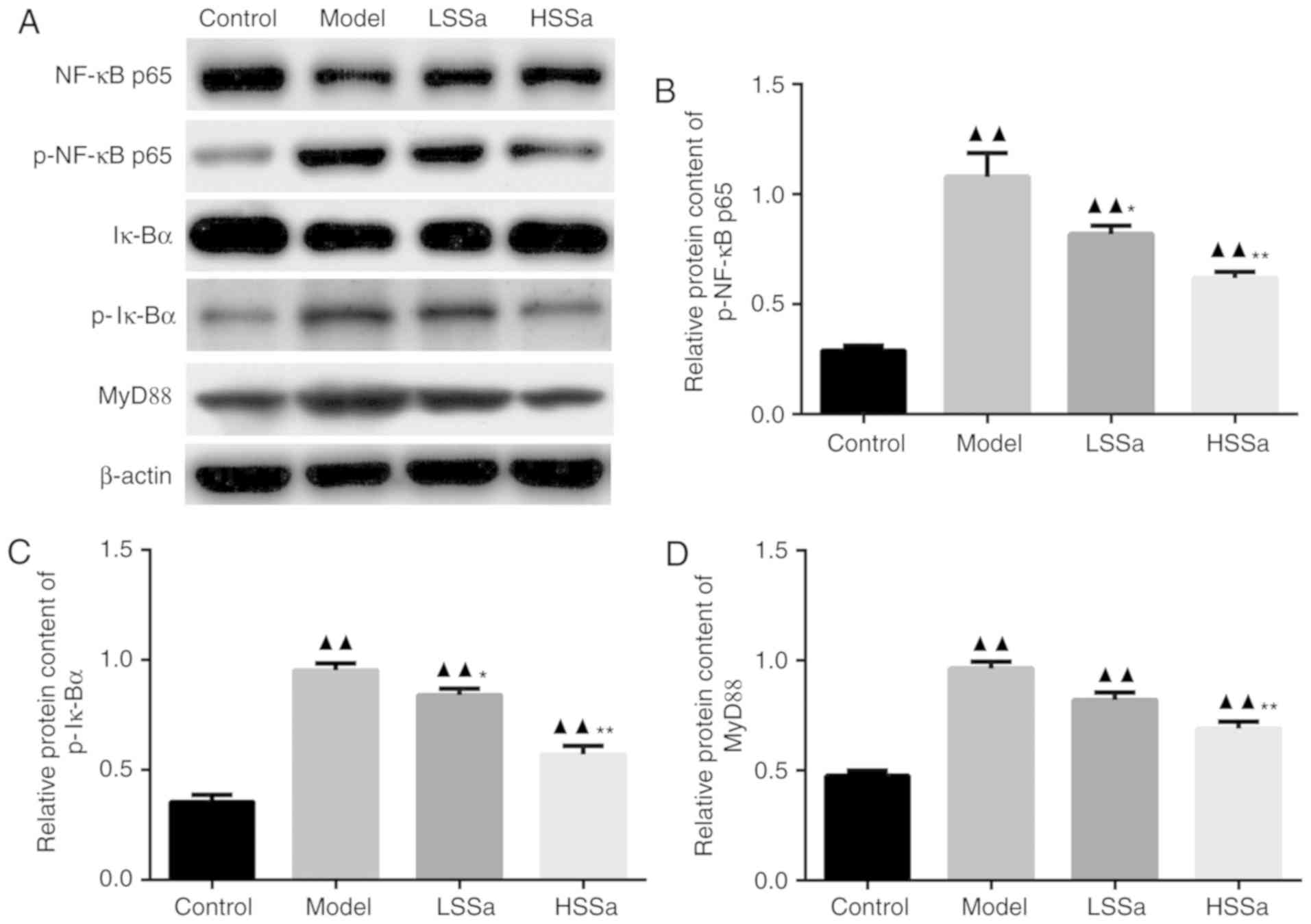
SSa suppressed NF-κB signaling in
pancreatic tissues
Following the determination of the therapeutic role
of SSa in the inflammatory response, it was speculated that this
may be regulated by transcription factors involved in inflammatory
pathways. To investigate whether SSa served an anti-inflammatory
role in HP via the NF-κB signaling pathway, the protein expression
of NF-κB, Iκ-Bα and MyD88 were detected by western blot analysis
(Fig. 8A). The results showed that
in the model group, the levels of p-NF-κB p65, p-Iκ-Bα and MyD88
were elevated compared with those in the control group (P<0.01)
(Fig. 8B-D). Whereas, treatment with
SSa limited this HP-enhanced expression of p-NF-κB p65, p-Iκ-Bα and
MyD88 in both the LSSa and the HSSa group. The expression of
p-NF-κB p65, p-Iκ-Bα were significantly inhibited in LSSa group
(P<0.05), and the expression of p-NF-κB p65, p-Iκ-Bα and MyD88
in HSSa group were significant decreased compared to the model
group (P<0.01) (Fig. 8B-D). These
results suggested that SSa inhibited the activation of the NF-κB
signaling pathway in pancreatic tissues, thus suppressing the
inflammatory response and regulating the release of proinflammatory
cytokines.
Discussion
Hyperlipidemia is often a triggering factor for AP
and the incidence of AP may also be accompanied by lipid metabolic
disorders (30). Except for the
commonly associated indicators, such as high levels of AMY and
lipase, patients diagnosed with typical HP predominantly present
with a pre-existing abnormal lipid profile, with a serum TG level
of >1,000 mg/dl (7). Furthermore,
a previous study reported an earlier age of onset, severe
pathological grade and higher mortality rates in patients with HP,
compared with those with AP from other causes (31). In the present study, high levels of
TC, TG and LDL-C were observed in HP rat models and the
administration of SSa effectively attenuated the abnormal lipid
profile.
Despite the complicated pathogenesis of HP, HP
ultimately results in the activation of local and systemic
inflammatory responses, as well as overproduction of inflammatory
mediators (32). Inflammatory
cytokines such as TNF-α, IL-1, IL-6 and IL-8, as well as a number
of other cytokines, have a vital role in the occurrence and
development of HP (33).
Furthermore, activation of TNF-α induces the uncontrolled release
of inflammatory mediators, including IL-6 and IL-8 (34). In a study carried out by Pérez et
al (35), the upregulation of
TNF-α, IL-6 and IL-1β in abdominal tissue was observed following
the induction of pancreatitis in Wistar rats.
It is well known that the NF-κB signaling pathway is
implicated in the pathogenesis of numerous inflammatory diseases
such as asthma, arthritis (36,37). It
is also hypothesized that the pathway exerts detrimental effects by
inducing the expression of proinflammatory cytokines and activating
the transcription of downstream inflammatory genes like Iκ-Bα,
causing tissue damage and even organ failure (36,38). The
activation of the NF-κB signaling pathway is frequently observed in
pancreatitis (39). Upon activation,
this pathway controls the expression of proinflammatory cytokines
including TNF-α, IL-1β, IL-6 and monocyte chemoattractant
protein-1, and the regulation of the NF-κB signaling pathway has
been confirmed as a treatment for pancreatitis in various studies
(40,41). In vitro studies indicate that
SSa strongly inhibits the expression of proinflammatory cytokines
including TNF-α, IL-1β and IL-6 and increases IL-10 expression
levels via the NF-κB pathway in LPS-treated macrophages and 3T3-L1
adipocytes (42,43). In mice with
lipopolysaccharide-induced acute lung injury, SSa exerted a
critical anti-inflammatory effect by inhibiting the expression of
TNF-α and IL-1β, suppressing the NF-κB and NLR family pyrin domain
containing 3 (NLRP3) signaling pathways (27). The NLRP3 inflammasome serves an
important role in the maturation of IL-1β and the initiation of
inflammatory cascades (44). Another
study also found that NLRP3 mitigated injury to the pancreas and
lungs, the inflammatory response and neutrophil infiltration in
NLRP3−/− SAP mice (45).
Similar results were observed in a rat model of chronic
constriction injury, where SSa inhibited the levels of TNF-α, IL-1β
and IL-2 and reduced the elevated expression of MAPK and NF-κB in
the spinal cord, thus attenuating neuropathic pain (46).
In the current study, following the administration
of a hyperlipidemic diet and sodium taurocholate to SD rats, the
expression levels of inflammatory and chemotactic cytokines TNF-α,
IL-1β and IL-6 were significantly increased (P<0.01) and NF-κB
signaling was activated. Also, SSa treatment significantly
decreased the expression levels of TNF-α, IL-1β and IL-6 and
increased that of IL-10 (P<0.05). The results indicated that SSa
has the ability to inhibit the secretion of pro-inflammatory
cytokines and promote the production of anti-inflammatory cytokines
to attenuate inflammation. Furthermore, the NF-κB signaling pathway
was also regulated by administration of SSa. These results
suggested that SSa served a protective role in the inflammatory
response by regulating the NF-κB signaling pathway and reducing the
expression of proinflammatory cytokines. SSa may also promote the
expression of anti-inflammatory cytokines, thus modulating the
inflammatory response and attenuating organ injury.
Our study showed that the expression of PPAR-γ was
inhibited in HP rats and following treatment with SSa, the
expression of PPAR-γ was effectively restored. PPARs are
lipid-sensing nuclear receptors that are involved in metabolic
diseases, including obesity, type 2 diabetes and various
cardiovascular diseases (47). A
series of studies have demonstrated the importance of PPARs in
regulating inflammatory pathways in AP, by closely interacting with
transcription factors such as NF-κB (48,49).
Moreover, the preservation of PPAR expression by its agonists
usually modulates inflammation by suppressing the activity of
NF-κB, increasing the expression of Iκ-Bα and thereby inhibiting
their nuclear transcriptional activity and signaling pathways
(25). Previous studies investigated
PPARs in inflammatory diseases and also found that PPAR activation
mediates the NF-κB pathway and associated factors such as Iκ-Bα,
MyD88 and toll-like receptor (TLR) 4 (50,51). It
was also revealed that TLR4 contributes to the inflammation and
tissue damage in AP (52). The NF-κB
signaling pathway can be activated or inhibited by a number of
factors, such as PPARs and TNF-α (53). Meanwhile, the upregulation of NF-κB
by TNF-α can induce the release of proinflammatory cytokines
(54).
In present study, during the operation and the
following 12 h, there was a mortality rate of ~23% in acute
pancreatitis animals. Previous studies reported similar mortality
rates. In studies by Turkyilmaz et al (55,56), the
mortality rate at 24 h after the operation was 43.75% in the acute
pancreatitis rats. In a study by Hughes et al (57), the overall survival rate of the acute
pancreatitis rats was 40% at 72 h and survival curves showed that
the mortality rate at 12 h was ~15%. In a study by Chen et
al (58), the mortality rate was
55% at 48 h in the acute hemorrhagic pancreatitis rat group. The
cause of mortality in rats may be due to multiple-organ injury and
failure after the induction of the acute pancreatitis model.
Furthermore, a limitation of the present study is that the effect
of SSa in hyperlipidemia rats without AP was not included,
therefore it will be considered in future research.
In conclusion, the effect and therapeutic mechanism
of SSa on rats with HP were investigated in present study. The
results illustrated that SSa effectively improved lipid metabolism
and significantly decreased the levels of MPO, AMY and lipase,
especially at a high dosage (P<0.01). Following the
administration of SSa, the levels of proinflammatory cytokines
TNF-α, IL-1β and IL-6 were reduced, particularly at the high
dosage, and the level of IL-10 expression was increased.
Furthermore, as a potential agonist of PPAR-γ, SSa activated the
expression of PPAR-γ and also suppressed the NF-κB signaling
pathway in pancreatic tissues. These results indicated that the
administration of SSa attenuated HP in rats by ameliorating lipid
metabolism and inhibiting the release of proinflammatory cytokines,
via suppressing the NF-κB signaling pathway and promoting the
expression of PPAR-γ. Collectively, these results suggested that
SSa may be a promising agent for the treatment of HP.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and
Technology of Traditional Chinese Medicine of Zhejiang (grant no.
2017ZA120); Science and Technology Development Plan of Lin'an
(grant no. 201604) and the Guided Science and Technology Project of
Quzhou (grant no. 2016091).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PF and YX conceived the study. BT and XT analyzed
and interpreted the data. PF, YB, SZ and HS collected the data,
searched the literature, drafted the manuscript and plotted the
figures and tables. PF was a major contributor in writing the
manuscript and YX critically revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Dijk SM, Hallensleben NDL, van
Santvoort HC, Fockens P, van Goor H, Bruno MJ and Besselink MG;
Dutch Pancreatitis Study Group, : Acute pancreatitis: Recent
advances through randomised trials. Gut. 66:2024–2032. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quinlan JD: Acute pancreatitis. Am Fam
Physician. 90:632–639. 2014.PubMed/NCBI
|
|
4
|
Sekimoto M, Takada T, Kawarada Y, Hirata
K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S, et
al: JPN Guidelines for the management of acute pancreatitis:
Epidemiology, etiology, natural history, and outcome predictors in
acute pancreatitis. J Hepatobiliary Pancreat Surg. 13:2–6. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lippi G, Valentino M and Cervellin G:
Laboratory diagnosis of acute pancreatitis: In search of the Holy
Grail. Crit Rev Clin Lab Sci. 49:18–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Machicado JD and Yadav D: Epidemiology of
recurrent acute and chronic pancreatitis: Similarities and
differences. Dig Dis Sci. 62:1683–1691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toskes PP: Hyperlipidemic pancreatitis.
Gastroenterol Clin North Am. 19:783–791. 1990.PubMed/NCBI
|
|
8
|
Wang R, Yan Z, Wu X, Ji K, Wang H and Zang
B: Rosiglitazone attenuates renal injury caused by hyperlipidemic
pancreatitis. Int J Clin Exp Pathol. 8:4332–4343. 2015.PubMed/NCBI
|
|
9
|
Mao EQ, Tang YQ and Zhang SD: Formalized
therapeutic guideline for hyperlipidemic severe acute pancreatitis.
World J Gastroenterol. 9:2622–2626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramírez-Bueno A, Salazar-Ramírez C,
Cota-Delgado F, de la Torre-Prados MV and Valdivielso P:
Plasmapheresis as treatment for hyperlipidemic pancreatitis. Eur J
Intern Med. 25:160–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yadav D and Pitchumoni CS: Issues in
hyperlipidemic pancreatitis. J Clin Gastroenterol. 36:54–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grauvogel J, Daemmrich TD, Ryschich E,
Gebhard MM and Werner J: Chronic alcohol intake increases the
severity of pancreatitis induced by acute alcohol administration,
hyperlipidemia and and pancreatic duct obstruction in rats.
Pancreatology. 10:603–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Li X, Huang N, Liu R and Sun R: A
comprehensive review and perspectives on pharmacology and
toxicology of saikosaponins. Phytomedicine. 50:73–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang F, Dong X, Yin X, Wang W, You L and
Ni J: Radix Bupleuri: A review of traditional uses, botany,
phytochemistry, pharmacology, and toxicology. Biomed Res Int.
2017:75975962017.PubMed/NCBI
|
|
15
|
Yuan B, Yang R, Ma Y, Zhou S, Zhang X and
Liu Y: A systematic review of the active saikosaponins and extracts
isolated from Radix Bupleuri and their applications. Pharm Biol.
55:620–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du ZA, Sun MN and Hu ZS: Saikosaponin a
ameliorates LPS-induced acute lung injury in mice. Inflammation.
41:193–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu CN, Yuan ZG, Zhang XL, Yan R, Zhao YQ,
Liao M and Chen JX: Saikosaponin a and its epimer saikosaponin d
exhibit anti-inflammatory activity by suppressing activation of
NF-κB signaling pathway. Int Immunopharmacol. 14:121–126. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He D, Wang H, Xu L, Wang X, Peng K, Wang
L, Liu P and Qu P: Saikosaponin-a attenuates oxidized LDL uptake
and prompts cholesterol efflux in THP-1 cells. J Cardiovasc
Pharmacol. 67:510–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang D, Zhao Q, Liu H, Guo Y and Xu H:
PPAR-α Agonist WY-14643 inhibits LPS-induced inflammation in
dynovial fibroblasts via NF-kB pathway. J Mol Neurosci. 59:544–553.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Griesbacher T, Pommer V, Schuligoi R,
Tiran B and Peskar BA: Anti-inflammatory actions of
perfluorooctanoic acid and peroxisome proliferator-activated
receptors (PPAR) alpha and gamma in experimental acute
pancreatitis. Int Immunopharmacol. 8:325–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding JL, Zhou ZG, Zhou XY, Zhou B, Wang L,
Wang R, Zhan L, Sun XF and Li Y: Attenuation of acute pancreatitis
by peroxisome proliferator-activated receptor-α in rats: The effect
on Toll-like receptor signaling pathways. Pancreas. 42:114–122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals.
Publication No. 85-23(rev.). 327:963–965. 2011.
|
|
23
|
Cao Y, Bei W, Hu Y, Cao L, Huang L, Wang
L, Luo D, Chen Y, Yao X, He W, et al: Hypocholesterolemia of
Rhizoma Coptidis alkaloids is related to the bile acid by
up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine.
19:686–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Zhao X, Gu L, Lv C, He B, Liu Z,
Hou P, Bi K and Chen X: Simultaneous determination of five free and
total flavonoids in rat plasma by ultra HPLC-MS/MS and its
application to a comparative pharmacokinetic study in normal and
hyperlipidemic rats. J Chromatogr B Analyt Technol Biomed Life Sci.
3953-3954:1–10. 2014. View Article : Google Scholar
|
|
25
|
Niyaz B, Zhao KL, Liu LM, Chen C, Deng WH,
Zuo T, Shi Q and Wang WX: Rosiglitazone attenuates the severity of
hyperlipidemic severe acute pancreatitis in rats. Exp Ther Med.
6:989–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi C, Hou C, Zhu X, Huang D, Peng Y, Tu
M, Li Q and Miao Y: SRT1720 ameliorates sodium taurocholate-induced
severe acute pancreatitis in rats by suppressing NF-κB signalling.
Biomed Pharmacother. 108:50–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmidt J, Rattner DW, Lewandrowski K,
Compton CC, Mandavilli U, Knoefel WT and Warshaw AL: A better model
of acute pancreatitis for evaluating therapy. Ann Surg. 215:44–56.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Badiei A, Chambers ST, Gaddam RR, Fraser R
and Bhatia M: Cystathionine-gamma-lyase gene silencing with siRNA
in monocytes/macrophages protects mice against acute pancreatitis.
Appl Microbiol Biotechnol. 100:337–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bazzoni F, Tamassia N, Rossato M and
Cassatella MA: Understanding the molecular mechanisms of the
multifaceted IL-10-mediated anti-inflammatory response: Lessons
from neutrophils. Eur J Immunol. 40:2360–2368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radojkovic M, Stojanovic M, Radojkovic D,
Jeremić L, Stanojević G, Damnjanovic Z and Stevanović G:
Hyperlipidemia in acute pancreatitis: Concomitant disorder or a
cause? Facta Universitatis. 12:57–60. 2014.
|
|
31
|
Nagayama D and Shirai K:
Hypertriglyceridemia-induced pancreatitis. Nihon Rinsho Jap J Clin
Med. 71:16022013.
|
|
32
|
Habtezion A: Inflammation in acute and
chronic pancreatitis. Curr Opin Gastroenterol. 31:395–399. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samanta J, Singh S, Arora S, Muktesh G,
Aggarwal A, Dhaka N, Kant Sinha S, Gupta V, Sharma V and Kochhar R:
Cytokine profile in prediction of acute lung injury in patients
with acute pancreatitis. Pancreatology. 18:878–884. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Malleo G, Mazzon E, Siriwardena AK and
Cuzzocrea S: Role of tumor necrosis factor-alpha in acute
pancreatitis: From biological basis to clinical evidence. Shock.
28:130–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pérez S, Pereda J, Sabater L and Sastre J:
Pancreatic ascites hemoglobin contributes to the systemic response
in acute pancreatitis. Free Radic Biol Med. 81:145–155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao Z, Zhang P, Yao Y, Lu G, Tong Z, Yan
B, Tu L, Yang G and Zhou J: Deguelin Attenuates allergic airway
inflammation via inhibition of NF-κb pathway in mice. Int J Biol
Sci. 13:492–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Spiga R, Marini MA, Mancuso E, Di Fatta C,
Fuoco A, Perticone F, Andreozzi F, Mannino GC and Sesti G: Uric
acid is associated with inflammatory biomarkers and induces
inflammation via activating the NF-κB signaling pathway in HepG2
cells. Arterioscler Thromb Vasc Biol. 37:1241–1249. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pan LF, Yu L, Wang LM, He JT, Sun JL, Wang
XB, Wang H, Bai ZH, Feng H and Pei HH: Augmenter of liver
regeneration (ALR) regulates acute pancreatitis via inhibiting
HMGB1/TLR4/NF-κB signaling pathway. Am J Transl Res. 10:402–410.
2018.PubMed/NCBI
|
|
40
|
Li G, Wu X, Yang L, He Y, Liu Y, Jin X and
Yuan H: TLR4-mediated NF-κB signaling pathway mediates
HMGB1-induced pancreatic injury in mice with severe acute
pancreatitis. Int J Mol Med. 37:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shi Q, Liao KS, Zhao KL, Wang WX, Zuo T,
Deng WH, Chen C, Yu J, Guo WY, He XB, et al: Hydrogen-rich saline
attenuates acute renal injury in sodium Taurocholate-induced severe
acute pancreatitis by inhibiting ROS and NF-κB pathway. Mediators
Inflamm. 2015:6850432015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu J, Luo C, Wang P, He Q, Zhou J and
Peng H: Saikosaponin A mediates the inflammatory response by
inhibiting the MAPK and NF-kappaB pathways in LPS-stimulated RAW
264.7 cells. Exp Ther Med. 5:1345–1350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SO, Park JY, Jeon SY, Yang CH and Kim
MR: Saikosaponin a, an active compound of Radix Bupleuri,
attenuates inflammation in hypertrophied 3T3-L1 adipocytes via
ERK/NF-κB signaling pathways. Int J Mol Med. 35:1126–1132. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ren JD, Ma J, Hou J, Xiao WJ, Jin WH, Wu J
and Fan KH: Hydrogen-rich saline inhibits NLRP3 inflammasome
activation and attenuates experimental acute pancreatitis in mice.
Mediators Inflamm. 2014:9308942014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu Q, Zhai Z, Wang Y, Xu L, Jia P, Xia P,
Liu C, Zhang X, Qin T and Zhang H: NLRP3 deficiency alleviates
severe acute pancreatitis and pancreatitis-associated lung injury
in a mouse model. Biomed Res Int. 2018:12949512018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou X, Cheng H, Xu D, Yin Q, Cheng L,
Wang L, Song S and Zhang M: Attenuation of neuropathic pain by
saikosaponin a in a rat model of chronic constriction injury.
Neurochem Res. 39:2136–2142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Laganà AS, Vitale SG, Nigro A, Sofo V,
Salmeri FM, Rossetti P, Rapisarda AM, La Vignera S, Condorelli RA,
Rizzo G and Buscema M: Pleiotropic actions of peroxisome
proliferator-activated receptors (PPARs) in Dysregulated metabolic
homeostasis, inflammation and cancer: Current evidence and future
perspectives. Int J Mol Sci. 17(pii): E9992016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang W, Szatmary P, Wan M, Bharucha S,
Awais M, Tang W, Criddle DN, Xia Q and Sutton R: Translational
insights into peroxisome Proliferator-activated receptors in
experimental acute pancreatitis. Pancreas. 45:167–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jakkampudi A, Jangala R, Reddy BR, Mitnala
S, Nageshwar Reddy D and Talukdar R: NF-κB in acute pancreatitis:
Mechanisms and therapeutic potential. Pancreatology. 16:477–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park MH, Park JY, Lee HJ, Kim DH, Chung
KW, Park D, Jeong HO, Kim HR, Park CH, Kim SR, et al: The novel
PPAR α/γ dual agonist MHY 966 modulates UVB-induced skin
inflammation by inhibiting NF-κB activity. PLoS One. 8:e768202013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin MH, Chen MC, Chen TH, Chang HY and
Chou TC: Magnolol ameliorates lipopolysaccharide-induced acute lung
injury in rats through PPAR-γ-dependent inhibition of NF-kB
activation. Int Immunopharmacol. 28:270–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Awla D, Abdulla A, Regnér S and Thorlacius
H: TLR4 but not TLR2 regulates inflammation and tissue damage in
acute pancreatitis induced by retrograde infusion of taurocholate.
Inflamm Res. 60:1093–1098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jeon Y, Jung Y, Kim MC, Kwon HC, Kang KS,
Kim YK and Kim SN: Sargahydroquinoic acid inhibits TNFα-induced
AP-1 and NF-κB signaling in HaCaT cells through PPARα activation.
Biochem Biophys Res Commun. 8(450): 1553–1559. 2014. View Article : Google Scholar
|
|
54
|
Luo G, Li F, Li X, Wang ZG and Zhang B:
TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via
the NF-κB pathway. Mol Med Rep. 17:6605–6611. 2018.PubMed/NCBI
|
|
55
|
Turkyilmaz S, Cekic AB, Usta A, Alhan E,
Kural BV, Ercin C and Sağlam K: Ethyl pyruvate treatment
ameliorates pancreatic damage: Evidence from a rat model of acute
necrotizing pancreatitis. Arch Med Sci. 15:232–239. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Turkyilmaz S, Usta A, Cekic AB, Alhan E,
Kural BV and Ercin C: N-acetylcysteine amid reduces pancreatic
damage in a rat model of acute necrotizing pancreatitis. J Surg
Res. 203:383–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hughes CB, el-Din AB, Kotb M, Gaber LW and
Gaber AO: Calcium channel blockade inhibits release of TNF alpha
and improves survival in a rat model of acute pancreatitis.
Pancreas. 13:22–28. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen X, Valente JF and Alexander JW: The
effect of Sennosides on bacterial translocation and survival in a
model of acute hemorrhagic pancreatitis. Pancreas. 18:39–46. 1999.
View Article : Google Scholar : PubMed/NCBI
|