Introduction
Inflammation is closely related to inflammatory
factors such as inflammatory cytokines, which are released by the
body to initiate/stimulate the inflammatory response and
characterized by local redness, swelling, heat and pain, and may be
accompanied by fever, leucocyte overproduction, macrophage
activation, degeneration of parenchymal organs, necrosis and
functional disorders of the heart, as well as even resulting in
death (1). At present, chronic
inflammatory processes are considered to be at the core of the
pathologies of seven of the top ten leading causes of
disease-associated mortality in developed countries. Of note,
extensive evidence supports the role of inflammation in chronic
pulmonary disease (2), heart disease
(3), cancer (4), stroke (5), Alzheimer's disease (6), diabetes (7) and nephritis (8). Therefore, elucidation of the role of
inflammation in the pathogenesis of various diseases has become one
of the most urgent clinical problems to be addressed (9–12).
Pulegone is a derivative of monoterpene ketones
occurring in the leaves and flowering tops of several members of
the mint family. It is a constituent of peppermint essential oil,
which has a wide range of applications in the food, fragrance and
pharmaceutical industries (13).
Furthermore, it is known to possess anti-bacterial, anti-oxidant
and anti-inflammatory properties.
A previous study has indicated that pulegone has
potent anti-inflammatory effects to reduce the lung index in rats
with acute lung injury and alleviate inflammation-induced tissue
swelling, and that the mechanisms include reduced transcription and
expression of the inflammatory factor NLR family pyrin domain
containing 3 (NLRP3) (14). However,
the mechanisms underlying its anti-inflammatory effects have
remained to be fully elucidated.
The NLPR3 inflammasome may be activated by a variety
of endogenous or exogenous signals, which mainly include the
following: i) Pathogens and their secreted toxins (15) ii) crystal compounds (16,17),
iii) endogenous damage signals (18). Based on the above activating factors
of the NLRP3 inflammasome, ATP, the cytotoxin nigericin or certain
crystalloid substances, including calcium dihydrate pyrophosphate
(CPPD) and alum, are frequently employed to stimulate the
activation of the NLRP3 inflammasome in vitro.
The present study focused on the NLRP3 inflammasome
pathway as part of the mechanism of action or pulegone. An in
vitro THP-1 cell model of inflammation was established using
ATP and the cellular toxin nigericin to explore the
anti-inflammatory effects of pulegone, as well as the underlying
mechanisms. In general, the results indicated that treatment with
pulegone resulted in reduced secretion of interleukin (IL)-1β and
IL-18, decreased expression and co-localization of NLRP3 and
apoptosis-associated speck-like protein (ASC), decreased reactive
oxygen species (ROS) generation and inhibition of potassium
channels, which led to downstream suppression of the NLRP3
inflammasome.
Materials and methods
Cell line and culture
THP-1 cells (cat. no. 347258) were purchased from
the cell bank of the Institute of Biochemistry and Cell Biology;
Shanghai Institutes for Life Science; the Chinese Academy of
Sciences. The cells were cultured in RPMI1640 medium (Gibco; Thermo
Fisher Scientific, Inc.; cat. no. 1683014) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
1715752) at 37°C and 5% CO2 with saturated humidity.
Induced differentiation of THP-1
cells
THP-1 cells in the logarithmic growth phase were
inoculated into 96-well plates (2×105 cells per well)
and incubated with different concentrations of phorbol myristate
acetate (PMA; 5, 10, 20, 50 and 100 ng/ml) for 48 h. The
morphological changes and adherence of the THP-1 cells were then
observed under a light microscope (AE2000; Motic).
In the subsequent experiments, the cells were
stimulated with PMA (5 ng/ml) for 48 h to induce them to
differentiate into macrophages and adhere to the plates.
Activation conditions of the NLRP3
inflammasome
THP-1 cells in the logarithmic growth phase were
inoculated into 96-well plates (2×104 cells per well)
and incubated with PMA (5 ng/ml) for 48 h. The cells were washed
three times with PBS and then stimulated with lipopolysaccharide
(LPS; 10 µg/ml) for 3 h, while the Normal group was not stimulated
with LPS. The cells were washed twice with PBS and then activated
using various stimulants at different concentrations. The
concentrations of the stimulants and the stimulation times were as
follows: ATP (5 mM) and nigericin (5 µM) for 1 h; CPPD (40 µg/ml)
and CaCl2 (5 mM) for 6 h. After activation, the
supernatant was collected and the levels of IL-1β and IL-18 were
assessed via ELISA.
Cytotoxic effect of pulegone estimated
via the MTS method
THP-1 cells were seeded in 96-well plates
(2×104/well) and induced with PMA (5 ng/ml) for 48 h.
After washing three times with PBS, the THP-1 cells were treated
with different concentrations of pulegone or DMSO (six replicates
for each experimental condition). The cells were then cultured for
24 h. Subsequently, 20 µl MTS was added to each well, followed by
culture for an additional 3 h. Finally, the optical density at 490
nm was assessed using a microplate reader (Thermo Fisher
Scientific, Inc.).
Experimental grouping and
treatments
The experiment included the Normal, LPS, LPS +
ATP/nigericin, LPS + ATP/nigericin + 0.2% DMSO (solvent used to
dissolve pulegone) and pulegone (0.2, 0.1 and 0.05 mg/ml) groups.
The treatments were performed as follows: THP-1 cells were
inoculated and induced as described in the previous section. All of
the groups were then treated with LPS (10 µg/ml) for 3 h, except
for the Normal group. After washing twice with PBS, the cells were
cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc.; cat. no. 11875) containing different concentrations of
pulegone for 1 h and then activated with different stimulants, with
the concentrations of the stimulants and the stimulation times as
mentioned above. Finally, the supernatants or lysates of the cells
were collected and used for detection of the various factors.
ELISA
ELISA was used to detect IL-1β and IL-18 in the
supernatants. The ELISA kits for cytokine profiling, including
those for IL-1β and IL-18, were obtained from Boster Biological
Technology. The THP-1 supernatants were harvested for ELISA
according to the manufacturer's protocol.
PCR analysis
PCR was used to detect NLRP3, caspase-1, IL-1β and
IL-1α in the cell lysates. Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and the
RNA quality and concentration were measured using a Nanodrop 1000
Spectrophotometer (Thermo Fisher Scientific, Inc.). Approximately 1
µg of total RNA was reverse-transcribed into complementary DNA
using a FastQuant cDNA Kit (Tiangen Biotech Co. Ltd.) following the
manufacturer's protocol. Quantitative PCR (95°C for 15 min followed
by 39 cycles of 95°C for 10 sec and 63.8°C for 30 sec, with a final
extension step of 65°C for 5 sec.) was performed using a CFX96TM
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.). The
change in gene expression was calculated by 2−ΔΔC
(19). The primer sequences used
were as follows: NLRP3 forward, 5′-TGGCTGTAACATTCGGAGATTG-3′ and
reverse, 5′-GAAGTCACCGAGGGCGTTGT-3′; caspase-1 forward,
5′-GGAAACAAAAGTCGGCAGAG-3′ and reverse, 5′-ACGCTGTACCCCAGATTTTG-3′;
IL-1β forward, 5′-GGCCCTAAACAGATGAAGTGCT-3′ and reverse,
5′-TGCCGCCATCCAGAGG-3′; IL-1α forward, 5′-CAGTGAAATTTGACATGGGTG-3′
and reverse, 5′-CAGGCATCTCCTTCAGCAG-3′; GADPH forward,
5′-CCACATCGCTCAGACACCAT-3′ and reverse,
5′-GGCAACAATATCCACTTTACCAGAGT-3′.
Immunofluorescence
THP-1 cells were stimulated to differentiate into
macrophages induced with PMA on coverslips at a density of
5×105. After 48 h, the cells were stimulated with LPS
for 3 h, followed by treatment with ATP (5 mM) and nigericin (5 µM)
for 1 h. Samples were washed twice with PBS and then fixed in 4%
paraformaldehyde at room temperature for 30 min. This was followed
by washing three times with PBS and subsequent treatment with 0.2%
Triton X-100 for 15 min. The cells were then blocked with blocking
buffer (5% bovine serum albumin; Gibco; Thermo Fisher Scientific,
Inc. cat. no. 1715752) for 1 h and incubated overnight with primary
antibodies against NLRP3 (dilution ratio 1:100; AdipoGen; cat. no.
AG-20B-0014) and ASC (dilution ratio 1:100; Novus Biologicals Ltd.;
cat. no. NBP1-78978). This was added in error) at 4°C, followed by
incubation with FITC-tagged secondary antibody IgG (H+L; dilution
ratio 1:100 Proteintech Group, Inc.; cat. no. SA00003-1) and Alexa
Fluor 594-tagged secondary antibody IgG (H+L; dilution ratio 1:100;
Proteintech Group, Inc.; cat. no. SA00006-4) for 1 h at room
temperature. After washing with PBS, the cells were mounted and
images were captured using a BA200 digital microscopic imaging
system (Motic Electric Group Co., Ltd.). The Image-Pro Plus 6.0
image analysis software (Media Cybernetics, Inc.) was used to
measure the integrated optical density of all images collected and
the average optical density of each image was determined.
ROS
THP-1 cells were seeded in 48-well plates
(5×105/well) and stimulated with 5 ng/ml PMA and 10
µg/ml LPS, and then treated with pulegone (0.2 or 0.1 mg/ml) for 1
h. ATP or nigericin was then added for 1 h. After this stimulation,
a 2′,7′-dichlorodihydrofluorescein diacetate ROS probe (0.5 µM) was
added to the cells, followed by incubation for 30 min at 37°C in
the dark. A fluorescence microscope (DMI3000; Leica) was employed
to observe the cells after incubation.
Detection of potassium ions in the
supernatant
THP-1 cells were seeded in 24-well plates
(5×105/well) and stimulated with 5 ng/ml PMA and 10
µg/ml LPS, followed by treatment with pulegone (0.2 mg/ml) for 1 h.
ATP or nigericin was then added for 1 h. Subsequently, the THP-1
cells were incubated with KCl (100 mM) or RPMI 1640 medium only,
without serum. The IL-1β levels in the supernatant were assayed via
ELISA at 1 h post-incubation.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS 20.0
software (IBM Corp.). Comparisons between groups were performed
using ANOVAs with Fisher's protected least significant difference
(LSD) tests used to determine the significance between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Induced differentiation of THP-1
cells
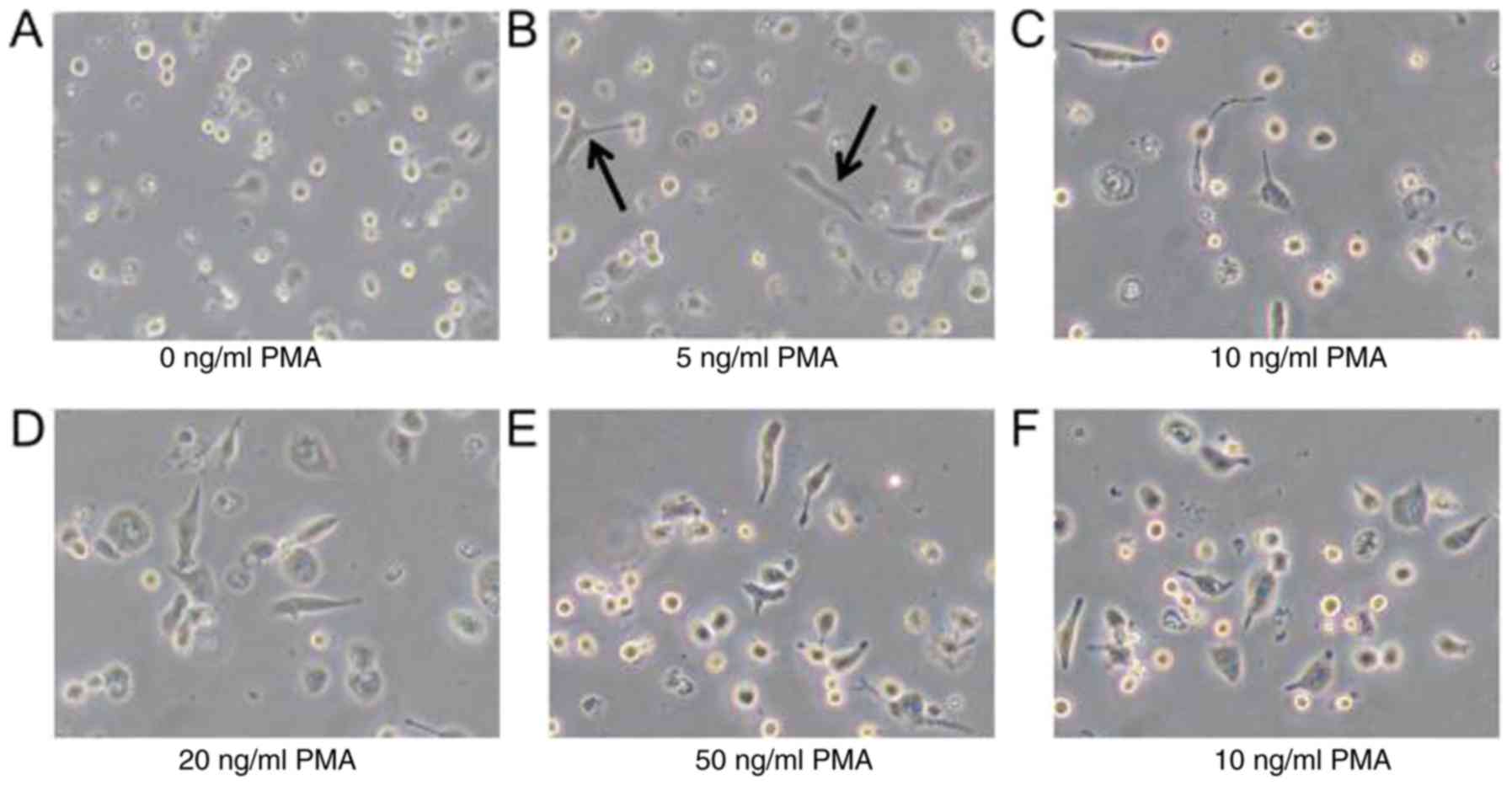
Following incubation with different concentrations
of PMA, the THP-1 cells became attached to the dish, while they did
not adhere in the absence of PMA treatment. However, there was no
significant difference in the level of adherence between the groups
treated with different concentrations of PMA (Fig. 1).
Activation of the NLRP3
inflammasome
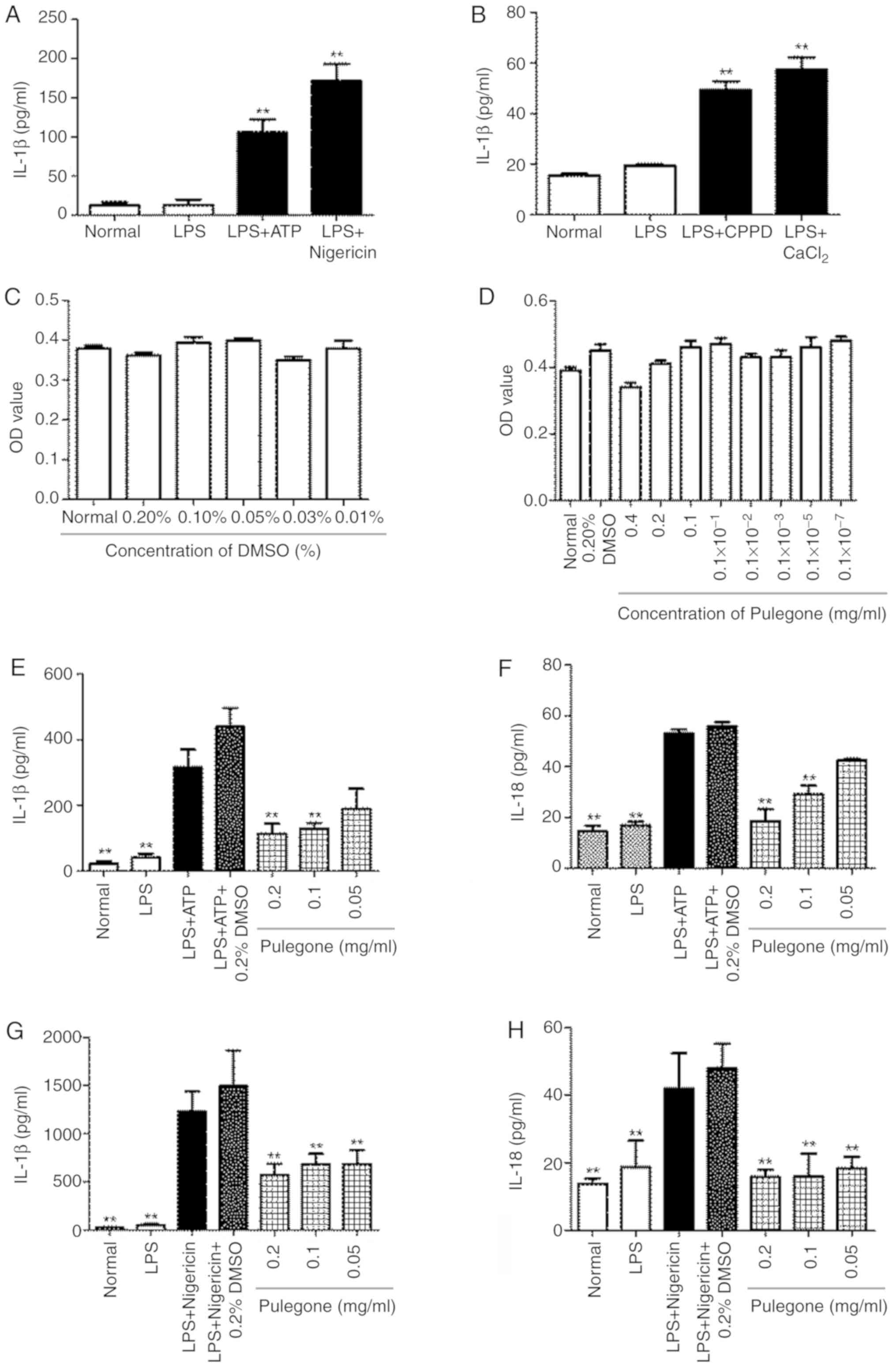
The results indicated that the IL-1β and IL-18
content in the supernatants of the cells was significantly
increased in the LPS + ATP/nigericin group and the LPS +
CPPD/CaCl2 group (P<0.01). However, the LPS +
ATP/nigericin treatment had a greater impact on IL-1β and IL-18
levels. Therefore, LPS + ATP/nigericin was selected for
establishing the in vitro model for use in the subsequent
experiments (Fig. 2A and B).
Cytotoxic effect of pulegone on THP-1
cells
The in vitro cytotoxicity experiments
indicated that the vehicle DMSO (0.01–0.2%) was not toxic to THP-1
cells (Fig. 2C). Furthermore,
pulegone (0–0.2 mg/ml) had no toxic effect on THP-1 cells (Fig. 2D). Therefore, pulegone was used at
concentrations of ≤0.2 mg/ml in all subsequent experiments.
Effect of pulegone on IL-1β and IL-18
secretion
As compared with those in the Normal group, the
contents of IL-1β and IL-18 were significantly increased in the LPS
+ ATP and LPS + nigericin groups (P<0.05). The pulegone groups
(0.2 and 0.1 mg/ml) demonstrated significantly reduced
hypersecretion of IL-1β and IL-18 (P<0.05) compared with that in
the model group. Furthermore, there was a dose-dependent effect
between the amount of pulegone used and the levels of the
inflammatory cytokines, and these inhibitory effects of pulegone
were most prominent at 0.2 mg/ml. Furthermore, addition of DMSO
(0.2%) was indicated to have no significant influence on the
hypersecretion of IL-1β and IL-18 (Fig.
2E-H).
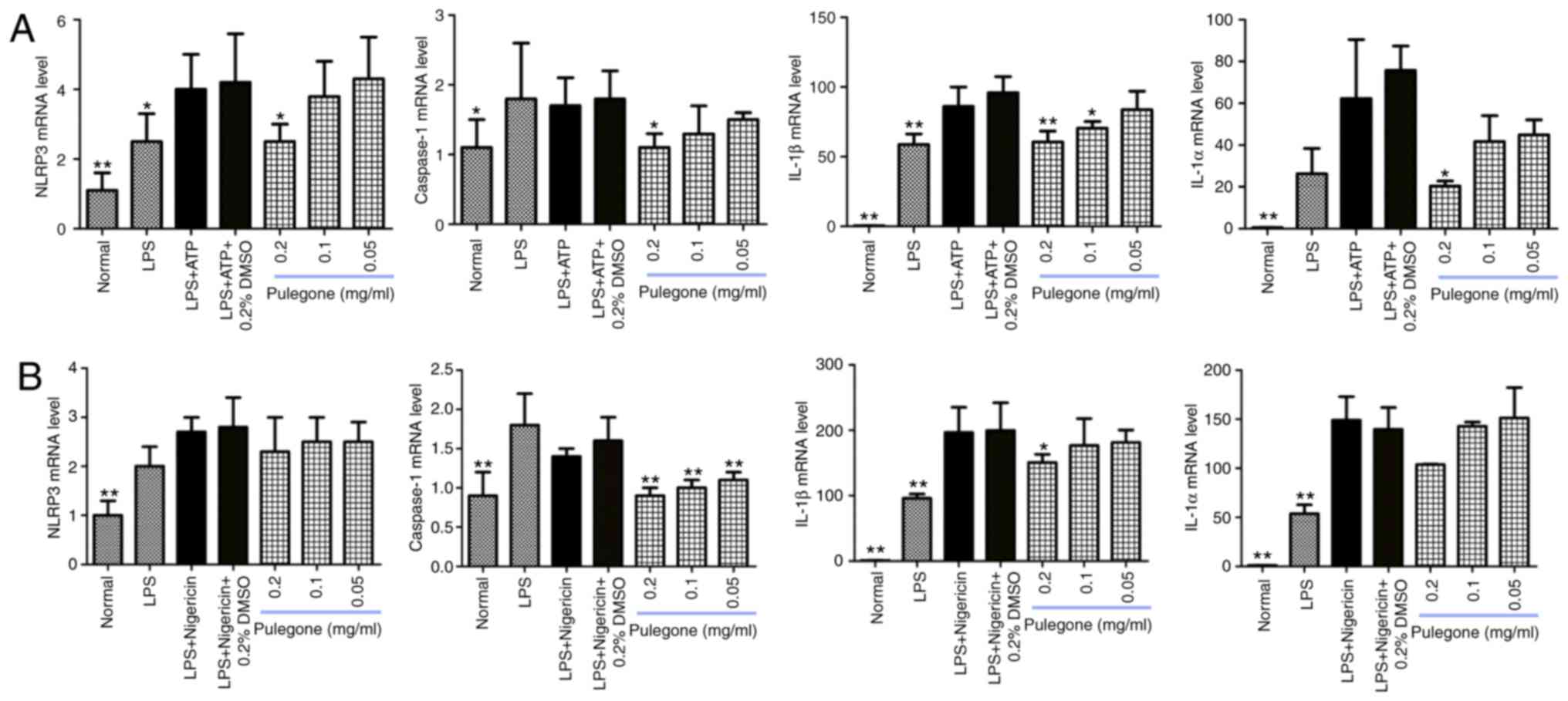
Inhibitory effect of pulegone on mRNA
levels in THP-1 cells
Following the addition of pulegone, the mRNA levels
of NLRP3, caspase-1, IL-1β and IL-1α in the LPS + ATP (Fig. 3A) and LPS + nigericin groups
(Fig. 3B) exhibited a decreasing
trend. The inhibitory effects of pulegone were most prominent at
0.2 mg/ml.
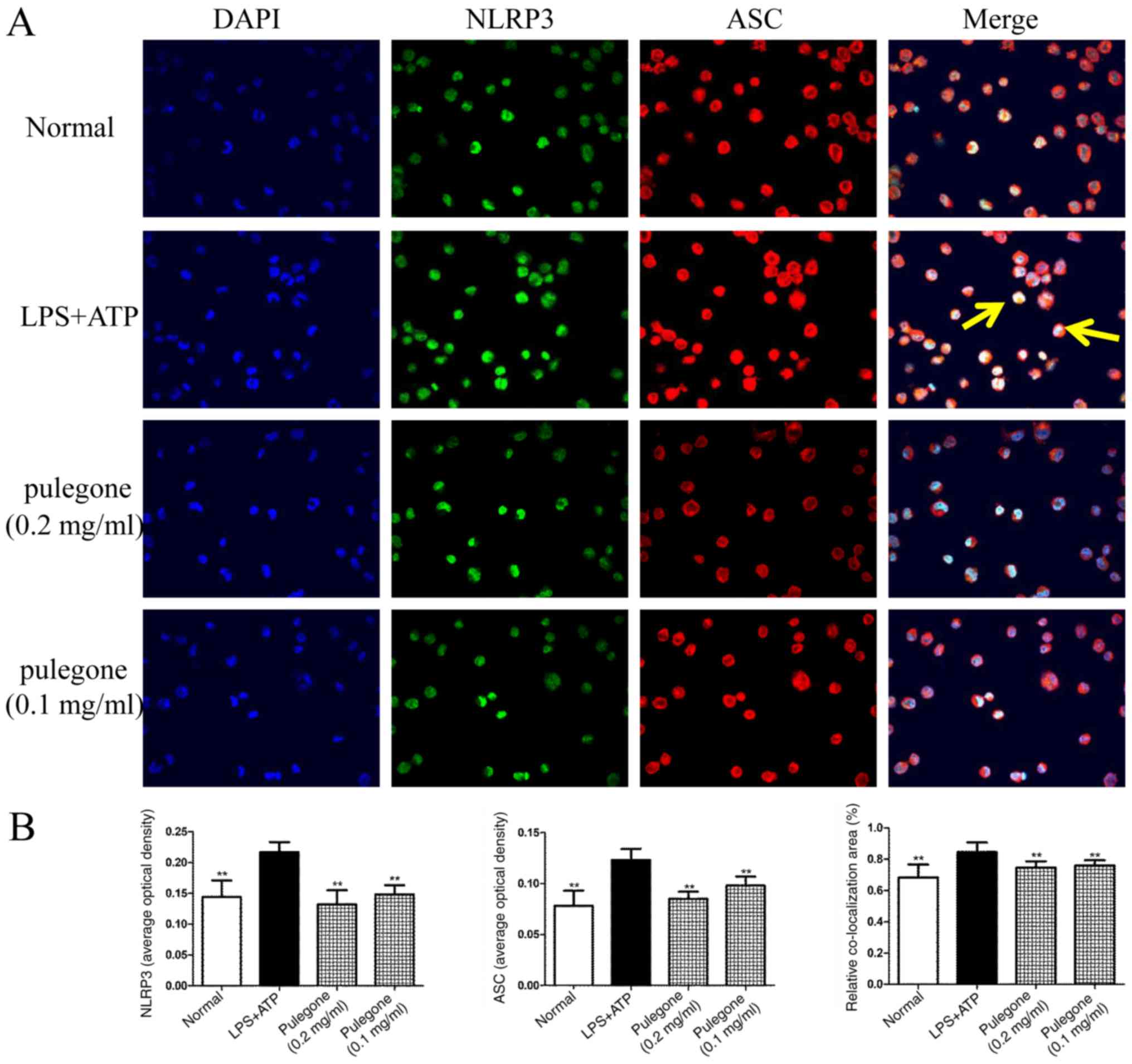
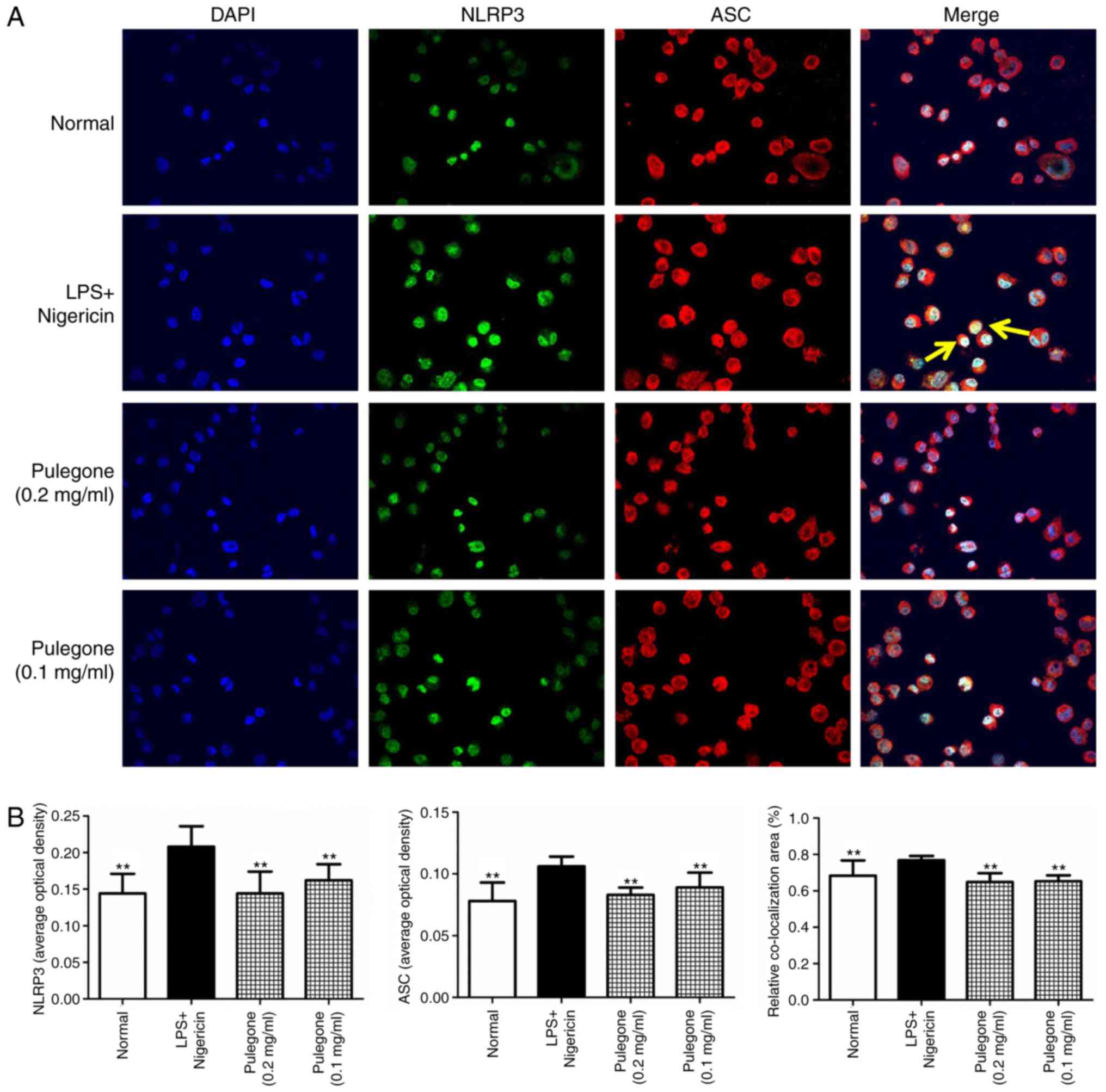
Co-localization of NLRP3 and ASC
After challenge with LPS + ATP or LPS + nigericin,
the protein levels of NLRP3 and ASC in the model groups were
significantly increased compared with those in the Normal group
(P<0.01). Double immunofluorescent labeling revealed
co-localization of the NLRP3 and ASC proteins in these THP-1 cells.
Following treatment with pulegone (0.2 or 0.1 mg/ml), the protein
expression of NLRP3 and ASC was significantly inhibited (P<0.01)
and the co-localization of the NLRP3 and ASC proteins was also
significantly reduced (P<0.01). The inhibitory effects of
pulegone were most prominent at the concentration of 0.2 mg/ml
(Figs. 4 and 5).
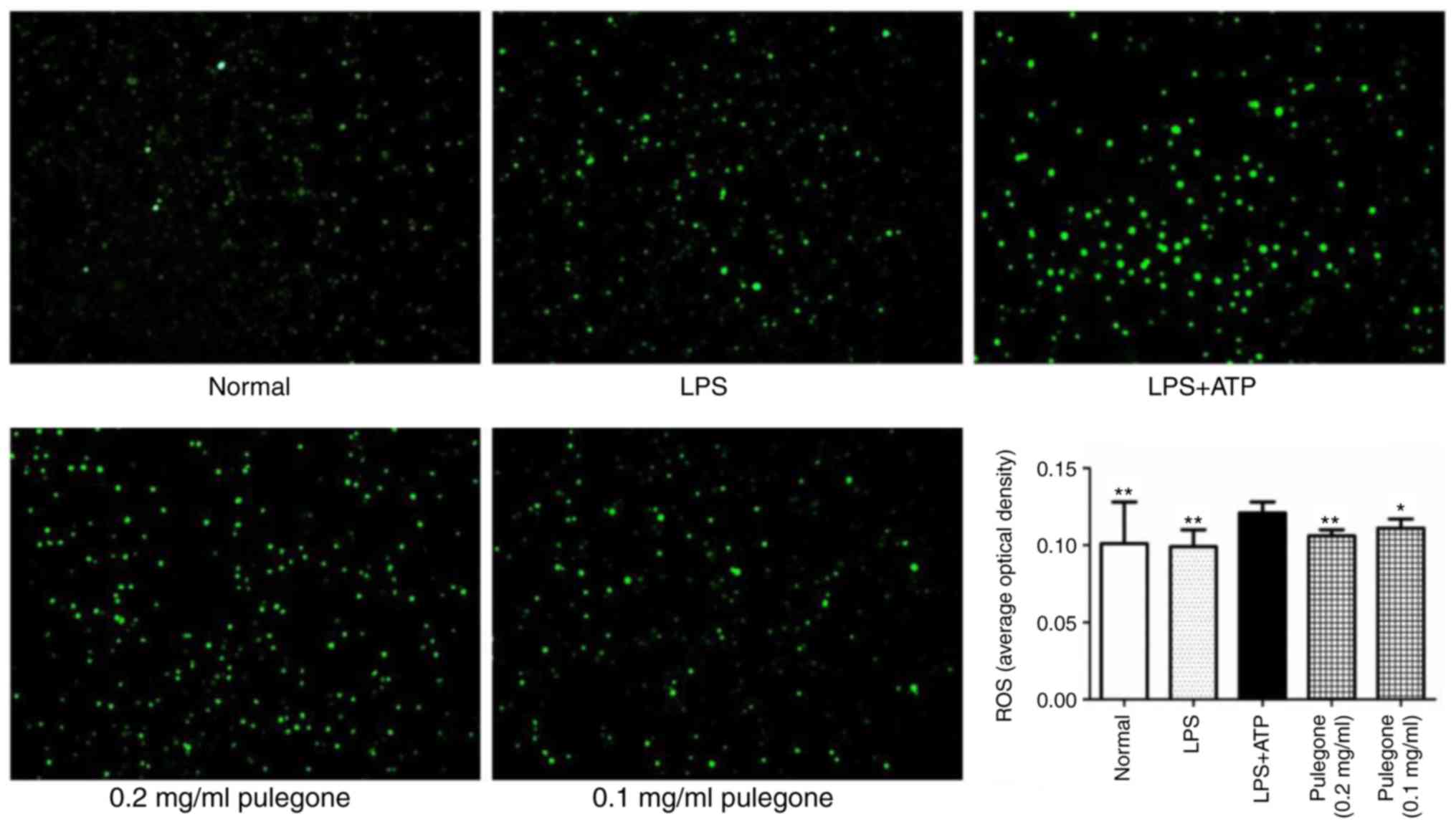
Reduction of ROS levels by
pulegone
After challenge with LPS + ATP, the ROS levels in
the THP-1 cells of the model group were significantly increased
compared with those in the Normal group (P<0.01). Of note, the
pulegone groups (0.2 and 0.1 mg/ml) demonstrated significantly
reduced ROS levels (P<0.01). The inhibitory effects of pulegone
were most prominent at the concentration of 0.2 mg/ml (Fig. 6).
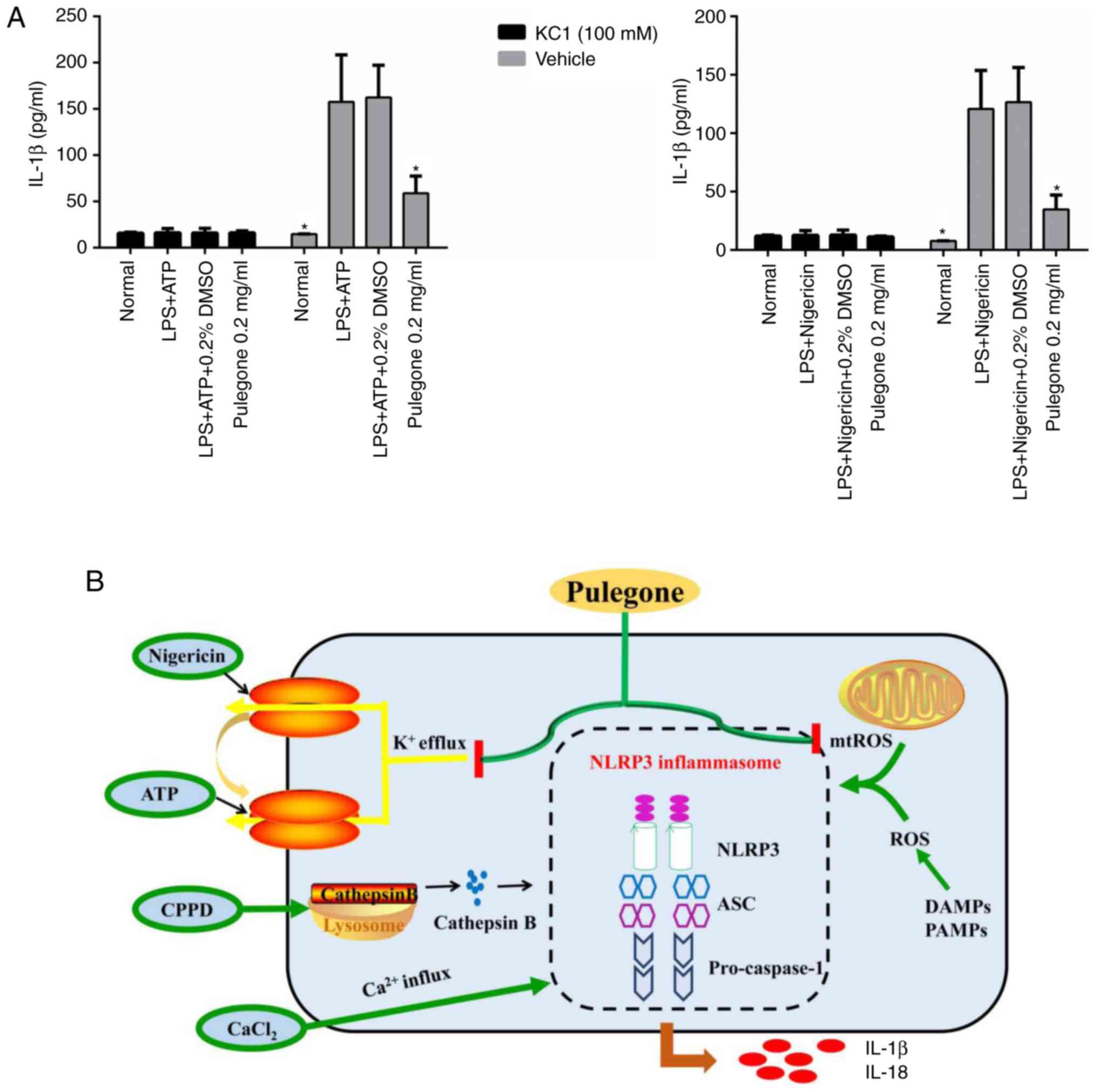
Effect of potassium ions on the
secretion of IL-1β
Compared with that in the Normal group, the IL-1β
content in the supernatant was significantly increased in the LPS +
ATP and LPS + nigericin groups in the absence of potassium ions
(P<0.05). In the pulegone (0.2 mg/ml) group, the hypersecretion
of IL-1β (P<0.05) was significantly reduced compared with that
in the model group. However, after pre-treatment with potassium
ions, there was no significant difference among the four groups
(Normal, LPS + ATP, LPS + nigericin and 0.2 mg/ml pulegone)
(Fig. 7A).
Discussion
It has been reported that pulegone possesses
anti-inflammatory (20),
anti-secretory, anti-pyretic (21),
anti-oxidant (22),
anti-cholinesterase (23,24), free radical scavenging and
lipoxygenase inhibitory effects (25). However, the anti-inflammatory effects
of pulegone have only been reported to lead to the release of
inflammatory mediators, while the mechanisms underlying its
anti-inflammatory effects have remained to be fully elucidated. The
aim of the present study was to assess the inflammatory mediators
influenced by pulegone and to further investigate the mechanisms of
action with regard to the NLRP3 inflammasome.
The inflammatory cytokines IL-1β and IL-18 are
initially produced as inactive precursor proteins and require the
generation of a signaling platform termed the inflammasome, which
processes IL-1β and IL-18 into their mature bioactive forms prior
to being released from the cells (26–28).
Thus, the secretion of mature IL-1β requires two signals. Regarding
the first, innate receptors (such as Toll-like receptor 4) are
engaged by an LPS ligand from Gram-negative bacteria to activate
NF-κB and induce transcription of the gene encoding the inactive
pro-IL-1β precursor (29). In terms
of the second signal, inflammasome activation is triggered via
mediators, including ATP or nigericin. Therefore, LPS + ATP and LPS
+ nigericin were employed in the present study to generate the
in vitro cell models.
Inflammasomes consist of an NLR protein, e.g. NLRP3,
that recruits the adapter protein ASC and caspase-1 into an
oligomeric complex, which leads to the auto-proteolytic processing
of pro-caspase-1 to its active form that is able to cleave the
pro-IL-1β and pro-IL-18 precursors into their mature secreted forms
(30,31). The activation of the inflammasome may
also lead to a necrotic type of cell death termed pyroptosis
(32). The NLRP3 inflammasome is
able to recognize danger signals from pathogen-associated molecular
patterns and damage-associated molecular patterns, and this
recognition activates four different responses within the cells
(33): i) Changes in potassium
concentration, ii) induction of ROS production, iii) changes in
calcium ion concentration and iv) induction of lysosomal damage.
Pulegone treatment is able to suppress these effects of the NLRP3
inflammasome with regard to potassium channel activity and
generation of ROS, which may serve to induce the pro-inflammatory
cytokine-mediated immune responses, as illustrated in Fig. 7B.
Subsequent to NLRP3 activation, the recruitment of
the ASC adapter protein, cleavage of pro-caspase-1 into activated
caspase-1, and the maturation and secretion of IL-1β and IL-18
occur, inducing pyroptosis and inflammatory tissue injury (33).
The efflux of potassium ions from the THP-1 cells
was reported to be mediated by stimulation of purinoceptor 7 by the
activator ATP, but the mechanism of activation via nigericin occurs
via the formation of a transmembrane pore in the plasma membrane
(34–38). The results of the present study
indicated that the hypersecretion of IL-1β and IL-18 into the
supernatant of THP-1 macrophages induced by ATP and nigericin was
significantly inhibited by treatment with 0.2 or 0.1 mg/ml
pulegone, with the inhibitory effects of pulegone being most
prominent at the concentration of 0.2 mg/ml. Pulegone at 0.2 or 0.1
mg/ml also significantly reduced the mRNA expression of NLRP3,
caspase-1, IL-1β and IL-1α, and the expression of the NLRP3 and ASC
proteins was also significantly downregulated, as assessed by
immunofluorescence methods, suggesting that pulegone had a potent
anti-inflammatory effect in vitro and that its mechanism of
action involved suppression of the activation of the NLRP3
inflammasome.
In addition, the anti-inflammatory properties of
pulegone in THP-1 macrophages induced by CPPD and CaCl2
were investigated in the present study. The endocytosis of CPPD
crystals may cause lysosomal damage or rupture, leading to release
of cathepsin into the cytosol, which is able to induce the
activation of the NLRP3 inflammasome. By contrast, CaCl2
activates the NLRP3 inflammasome by increasing the intracellular
concentration of calcium ions. The results of preliminary
experiments (39) revealed that 0.2
and 0.1 mg/ml pulegone had a significant inhibitory effect on the
secretion of IL-1β by THP-1 macrophages induced by CPPD or
CaCl2, suggesting that the anti-inflammatory effect of
pulegone may affect the function of calcium ion channels and induce
lysosomal damage; however, the underlying mechanisms remained to be
determined.
The results of the present study suggested that
pulegone significantly inhibited the hypersecretion of IL-1β and
IL-18, as well as the high expression of NLRP3, pro-caspase-1 and
pro-IL-1, and the generation of ROS in THP-1 macrophages induced by
ATP and nigericin. In addition, potassium ions were indicated to
affect the secretion of IL-1β, as high concentrations of potassium
ions from the extracellular environment significantly reduced the
hypersecretion of IL-1β induced by LPS + ATP/nigericin in THP-1
cells. As such, it is apparent that pulegone is able to suppress
the NLRP3 inflammasome by means of inhibition of potassium channels
and decreased ROS generation. It appears that auto-immune responses
are situated downstream of NLRP3, which may therefore act as a link
between inflammation and immunity. However, only the downstream
products/targets of the NLRP3 pathway were investigated in the
present study and whether pulegone also inhibits upstream
stimulatory factors of the NLRP3 pathway remains to be
investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Nature
Science Foundation of China (grant nos. 81473399, J1310034-09);
Department Pharmacology, Sichuan Provincial Science and Technology,
Sichuan Province Youth Science and Technology Innovation Team
(grant no. 2014TD0007).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY designed and performed experiments, analyzed data
and wrote the paper; QL, HL, FW, RL performed experiments and
analyzed data; NZ designed experiments and analyzed data; RL
designed experiments, analyzed data, and provided editorial input.
All authors approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He L, Wang T, Chen BW, Lu FM and Xu J:
Puerarin inhibits apoptosis and inflammation in myocardial cells
via PPARα expression in rats with chronic heart failure. Exp Ther
Med. 18:3347–3356. 2019.PubMed/NCBI
|
|
2
|
Gan WQ, Man SF, Senthilselvan A and Sin
DD: Association between chronic obstructive pulmonary disease and
systemic inflammation: A systematic review and a meta-analysis.
Thorax. 59:574–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Awan Z and Genest J: Inflammation
modulation and cardiovascular disease prevention. Eur J Prev
Cardiol. 22:719–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawabori M and Yenari MA: Inflammatory
responses in brain ischemia. Curr Med Chem. 22:1258–1277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amor S, Puentes F, Baker D and van der
Valk P: Inflammation in neurodegenerative diseases. Immunology.
129:154–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biondi-Zoccai GG, Abbate A, Liuzzo G and
Biasucci LM: Atherothrombosis, inflammation, and diabetes. J Am
Coll Cardiol. 41:1071–1077. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Z and Zheng F: Immune cells and
inflammation in diabetic nephropathy. J Diabetes Res.
2016:18416902016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clevers H: At the crossroads of
inflammation and cancer. Cell. 118:671–674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta RA and Dubois RN: Colorectal cancer
prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev
Cancer. 1:11–21. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill F and Coussens LM: Cancer: An
inflammatory link. Nature. 431:405–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adlard ER: Handbook of Essential Oils.
Science, Technology and Applications. Chromatographia. 72:10212010.
View Article : Google Scholar
|
|
14
|
Chen F, Wei G, Xu J, Ma H and Wang Q:
Naringin ameliorates the high glucose-induced rat mesangial cell
inflammatory reaction by modulating the NLRP3 Inflammasome. BMC
Complementary Alternative Med. 18:1922018. View Article : Google Scholar
|
|
15
|
Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE,
Lee Y, Seo YJ, Lee YH, Shi G, Zouboulis CC, et al:
Propionibacterium acnes activates the NLRP3 inflammasome in human
sebocytes. J Invest Dermatol. 134:2747–2756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guarda G, Dostert C, Staehli F, Cabalzar
K, Castillo R, Tardivel A, Schneider P and Tschopp J: T cells
dampen innate immune responses through inhibition of NLRP1 and
NLRP3 inflammasomes. Nature. 460:269–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Darisipudi MN, Thomasova D, Mulay SR,
Brech D, Noessner E, Liapis H and Anders HJ: Uromodulin triggers
IL-1β-dependent innateimmunity via the NLRP3 inflammasome. J Am Soc
Nephrol. 23:1783–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu F, Wen T, Wang F, Sang W and Zeng N:
Protective effect of cinnamicaldehyde in endotoxin poisoning mice.
Immunopharmacol Immunotoxicol. 38:455–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawata JK, Kameda M and Miyazawa M:
Cyclooxygenase-2 inhibitory effects of monoterpenoids with a
p-menthane skeleton. Int J Essent Oil Ther. 2:145–148. 2008.
|
|
21
|
Ortiz de Urbina AV, Martín ML, Montero MJ,
Morán A and San Román L: Sedating and antipyretic activity of
essential oil of Calamintha sylvatica subsp. Ascendens J
Ethnopharmacol. 25:165–171. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruberto G and Baratta MT: Antioxidant
activity of selected essential oil components in two lipid model
systems. Food Chem. 69:167–174. 2000. View Article : Google Scholar
|
|
23
|
Ryan MF and Byrne O: Plant-insect
coevolution and inhibition of acetylcholinesterase. J Chem Ecol.
14:1965–1975. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyazawa M, Watanabe H and Kameoka H:
Inhibition of acetylcholinesterase activity by monoterpenoids with
a p-menthane skeleton. J Agric Food Chem. 45:677–679. 1997.
View Article : Google Scholar
|
|
25
|
Demirci B, Temel HE, Portakal T,
Kırmızıbekmez H, Demirci F and Başer KHC: Inhibitory effect of
Calamintha nepeta subsp. glandulosa essential oil on lipoxygenase.
Turk J Biochem. 36:290–295. 2011.
|
|
26
|
Cerretti DP, Kozlosky CJ, Mosley B, Nelson
N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T,
Cannizzaro LA, et al: Molecular cloning of the interleukin-1 beta
converting enzyme. Science. 256:97–100. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thornberry NA, Bull HG, Calaycay JR,
Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner
JR, Aunins J, et al: A novel heterodimeric cysteine protease is
required for interleukin-1 beta processing in monocytes. Nature.
356:768–774. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghayur T, Banerjee S, Hugunin M, Butler D,
Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et
al: Caspase-1 processes IFN-gamma-inducing factor and regulates
LPS-induced IFN-gamma production. Nature. 386:619–623. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skeldon AM, Faraj M and Saleh M: Caspases
and inflammasomes in metabolic inflammation. Immunol Cell Biol.
92:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tack CJ, Stienstra R, Joosten LA and Netea
MG: Inflammation links excess fat to insulin resistance: The role
of the interleukin-1 family. Immunol Rev. 249:239–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinon F, Burns K and Tschopp J: The
inflammasome: A molecular platform triggering activation of
inflammatory caspases and processing of proIL-beta. Mol Cell.
10:417–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanaja SK, Rathinam VA and Fitzgerald KA:
Mechanisms of inflammasome activation: Recent advances and novel
insights. Trends Cell Biol. 25:308–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jo EK, Kim JK, Shin DM and Sasakawa C:
Molecular mechanisms regulating NLRP3 inflammasome activation. Cell
Mol Immunol. 13:148–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He Y, Zeng MY, Yang D, Motro B and Núñez
G: NEK7 is an essential mediator of NLRP3 activation downstream of
potassium efflux. Nature. 530:354–357. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rivers-Auty J and Brough D: Potassium
efflux fires the canon: Potassium efflux as a common trigger for
canonical and noncanonical NLRP3 pathways. Eur J Immunol.
45:2758–2761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rühl S and Broz P: Caspase-11 activates a
canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J
Immunol. 45:2927–2936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Segovia J, Sabbah A, Mgbemena V, Tsai SY,
Chang TH, Berton MT, Morris IR, Allen IC, Ting JP and Bose S:
TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux
activates NLRP3/ASC inflammasome during respiratory syncytial virus
infection. PLoS One. 7:e296952012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muñoz-Planillo R, Kuffa P, Martníez-Colón
G, Smith BL, Rajendiran TM and Núñez G: K+ efflux is the
common trigger of NLRP3 inflammasome activation by bacterial toxins
and particulate matter. Immunity. 38:1142–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Xu F, Wen T, et al: Effect of
essential oil from Schizonepeta tenuifolia on the mechanism of
NLRP3 inflammasome activation in THP-1 cells. J Chin Med Materials.
40:689–694. 2017.(In Chinese).
|