Introduction
Colorectal cancer (CRC) is a common human malignancy
and the fourth leading cause of cancer-related mortality worldwide
(1). CRC is heterogeneous, and its
development has been confirmed to be associated with dietary
habits, genetic factors and epigenetic changes (2,3).
Although an increasing number of oncogenes and tumor suppressor
genes have been demonstrated to be involved in the occurrence and
development of CRC (4,5), there is still a need to discover new
prognostic markers and mechanisms for CRC progression.
Long non-coding RNAs (lncRNAs) are a group of
transcripts >200 nucleotides long that lack protein-coding
capacity (6). lncRNAs have been
reported to regulate tumorigenesis and progression in a variety of
cancer types (7). A number of
studies have confirmed that lncRNAs serve crucial roles in CRC cell
proliferation, invasion and drug resistance (8,9). Long
intergenic non-protein coding RNA 70 (LINC00707) is located on
chromosome 10p14 and has a length of 3,097 bp. LINC00707 has been
confirmed to be upregulated in lung adenocarcinoma and
hepatocellular carcinoma and act as an oncogene to promote cancer
development and progression (10–12).
LINC00707 has been demonstrated to promote the proliferation and
metastasis of gastric cancer (GC) by interacting with human antigen
R (HuR) (13), which indicates that
this molecule may also serve an important role in digestive tract
tumors. Recent studies have also revealed that LINC00707 is highly
expressed in CRC and may promote the progress of CRC by binding to
microRNA (miR)-206 (14,15).
In the present study, the expression of LINC00707 in
65 paired CRC and matched non-cancerous tissues (NCTs) was
examined. The relationship between LINC00707 and prognosis was also
analyzed. Finally, the present study explored the regulatory
mechanisms of action of LINC00707 in CRC.
Patients and methods
Sample collection
A total of 65 CRC tissues and paired adjacent NCTs
(located more than 5 cm from the cancer margin) were obtained from
patients undergoing surgery from January 2012 to December 2015 at
the First Clinical Medical College of Mudanjiang Medical University
(Mudanjiang, China). Inclusion criteria: i) Radical resection of
CRC was performed in all patients. ii) The patients were
pathologically confirmed as suffering from CRC. iii) The clinical
and pathological data of the CRC patients were complete. Exclusion
criteria: Patients who received preoperative radiotherapy or
chemotherapy. The patients were aged between 41 to 72 years (mean
age, 60 years). The CRC staging was determined based on the 7th
edition of the American Joint Committee on Cancer and the Union for
International Cancer Control staging systems. After tumor
resection, the tissue specimens were immediately snap-frozen in
liquid nitrogen and then stored at −80°C. This study was approved
by the Medical Ethics Committees of the Mudanjiang Medical
University. All patients signed a written informed consent
form.
Cell culture
Human CRC cell lines HCT116 (ATCC®
CCL-247™), HT29 (ATCC® HTB-38™), and SW480
(ATCC® CCL-228™) were purchased from the American Type
Culture Collection. The human colonic epithelial cell line NCM460
(HTX1841) was obtained from Otwo Biotech, Inc. Cells were cultured
in Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (both from Gibco; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin (both from Beyotime
Institute of Biotechnology). The cells were characterized by
Genewiz, Inc. using short tandem repeat markers. All cells were
confirmed to be mycoplasma-free and were incubated at 37°C in a
humidified atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen tissues and
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Nuclear and cytoplasmic RNA of HT29 and HCT116
cells was extracted using NE-PER nuclear and cytoplasmic extraction
reagents (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Complementary DNA (cDNA) was reverse
transcribed from 1 µg of total RNA using PrimeScript RT Master Mix
System (Takara Biotechnology Co., Ltd.). The reverse transcription
reaction was performed at 37°C for 15 min and 85°C for 5 sec. The
expression levels of LINC00707, O-GlcNAcylation transferase (OGT)
and miR-485-5p were detected by RT-qPCR using the UltraSYBR Mixture
(Low ROX) (CWbio Co., Ltd.) in a total volume of 20 µl containing
cDNA, 2X UltraSYBR Mixture, 0.2 µM of each primer and
ddH2O. The relative RNA expression was calculated using
the 2−ΔΔCq method (16)
and normalized to the internal references (β-actin or U6). The
primer sequences are listed in Table
SI. The PCR amplification procedure included a pre-denaturation
step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec,
59°C for 30 sec and 72°C for 32 sec.
Transfection
HT29 and HCT116 cells were transfected with 50 pmol
LINC00707 siRNAs (si-LINC00707), scrambled siRNA (si-blank),
miR-485-5p mimics (or NC mimics) or miR-485-5p inhibitor (or NC
inhibitor) (Shanghai GenePharma Co., Ltd.) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Following transfection for 6 h, the medium was replaced with
complete medium. The cells were cultured for 24 h prior to
subsequent experiments.
Cell Counting Kit-8 (CCK-8) and colony
formation assays
HT29 and HCT116 cells were seeded at a density of
5×103 cells/well onto 96-well plates and transfected
with si-LINC00707 and si-blank. Cell proliferation was assessed for
24, 48, 72 and 96 h after 10 µl CCK-8 reagent (Beyotime Institute
of Biotechnology) was added to each well according to the
manufacturer's instructions. The optical density values were
measured at a wavelength of 450 nm using a microplate reader
(Thermo Fisher Scientific, Inc.). For the colony formation assay, a
total of 800 HT29 and HCT116 cells were seeded into 6-well plates
and incubated at 37°C and 5% CO2 for 2 weeks. The
colonies were fixed with 4% paraformaldehyde for 15 min and
incubated with trypan blue for 15 min at room temperature. The
number of colonies (more than 50 cells) were counted using light
microscopy.
Cell cycle and apoptosis
For cell cycle analysis, HT29 and HCT116 cells with
LINC00707-knockdown were stained with PI in the dark for 30 min
using a Cell Cycle and Apoptosis Analysis kit (Beyotime Institute
of Biotechnology). For cell apoptosis analysis, HT29 and HCT116
cells with LINC00707-knockdown were double stained with Annexin
V-FITC and PI at dark for 20 min using an Annexin V-FITC Apoptosis
Detection kit (Beyotime Institute of Biotechnology). The cell cycle
distribution and the cell apoptosis rates were then determined
using a FACSCanto II Flow Cytometer (BD Biosciences). The results
were analyzed using ModFit software (version 3.2, Verity Software
House, Inc.)
Bioinformatics analysis
The potential binding sites between LINC00707 and
miR-485-5p were predicted using the starBase v3.0 (http://starbase.sysu.edu.cn/) and RegRNA (http://regrna2.mbc.nctu.edu.tw/index.html)
databases.
Vector construction and luciferase
reporter assay
The fragments containing the wild-type (Wt) and
mutant (Mut) LINC00707 were synthesized and cloned into the
luciferase reporter vector pGL3 (Promega Corporation). HT29 and
HCT116 cells were co-transfected with LINC00707-Wt or -Mut
luciferase reporter vector and miR-485-5p mimics (or NC mimics)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
luciferase activities of these cells were measured using the
Dual-Luciferase® Reporter Assay System (Promega
Corporation) according to the manufacturer's instructions. All
firefly luciferase activities were normalized to Renilla luciferase
activity.
RNA immunoprecipitation (RIP)
Magna RIP kit (EMD Millipore) was used according to
the manufacturer's protocol. First, 1×107 HT29 and
HCT116 cells were washed with ice-cold PBS and lysed using RIP
lysis buffer. Then the lysates were centrifuged at 18,000 × g for
10 min at 4°C. Magnetic beads conjugated with anti-argonaute RISC
catalytic component 2 (AGO2) (cat. no. 10686-1-AP, 5 µg for RIP,
ProteinTech Group, Inc.) or anti-IgG (cat. no. PP64B, 5 µg for RIP,
EMD Millipore) antibodies were used to incubate the cell extract.
The cell extract was incubated with gentle agitation overnight at
4°C. RT-qPCR was conducted to analyze the enrichment of LINC00707
and miR-485-5p.
Western blot analysis
Total protein was extracted from HT29 and HCT116
cells transfected with miR-485-5p inhibitor or si-LINC00707 using
RIPA Lysis Buffer (Beyotime Institute of Biotechnology). The
protein concentration was measured using an Enhanced BCA Protein
Assay kit (Beyotime Institute of Biotechnology). The protein (30 µg
per lane) were separated by 10% SDS-PAGE and transferred to a PVDF
membrane. Non-fat milk (5%) was used to block the membrane at room
temperature for 1 h. The membranes were incubated with anti-human
OGT (cat. no. 11576-2-AP, 1:2,000; ProteinTech Group, Inc.) and
GAPDH (cat. no. AF0006, 1:1,000; Beyotime Institute of
Biotechnology) antibodies at 4°C overnight. The membranes were
subsequently incubated with secondary HRP-labeled goat anti-rabbit
IgG (cat. no. A0208, 1:1,000; Beyotime Institute of Biotechnology)
and HRP-labeled goat anti-mouse IgG (cat. no. A0216, 1:1,000;
Beyotime Institute of Biotechnology) antibodies. The protein levels
were detected using an enhanced chemiluminescence system (Pierce;
Thermo Fisher Scientific, Inc.). GAPDH was used as the loading
control.
Statistical analysis
The measurement data were expressed as the mean ±
standard deviation. SPSS 19.0 (IBM Corp.) was used for statistical
analysis. The differences between groups were evaluated by
two-tailed Student's t-test, χ2 test or one-way ANOVA.
One-way ANOVA, followed by Tukey's multiple comparison test, was
used to analyze the differences among multiple groups. Survival
analysis was performed using the Kaplan-Meier method and the log
rank test. Univariate and multivariate analyses were performed on
the basis of a Cox proportional hazard model. Correlations between
the LINC00707 and miR-485-5p expression levels, the LINC00707 and
OGT expression levels, and between OGT and miR-485-5p expression
levels were analyzed using Pearson's correlation coefficient test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
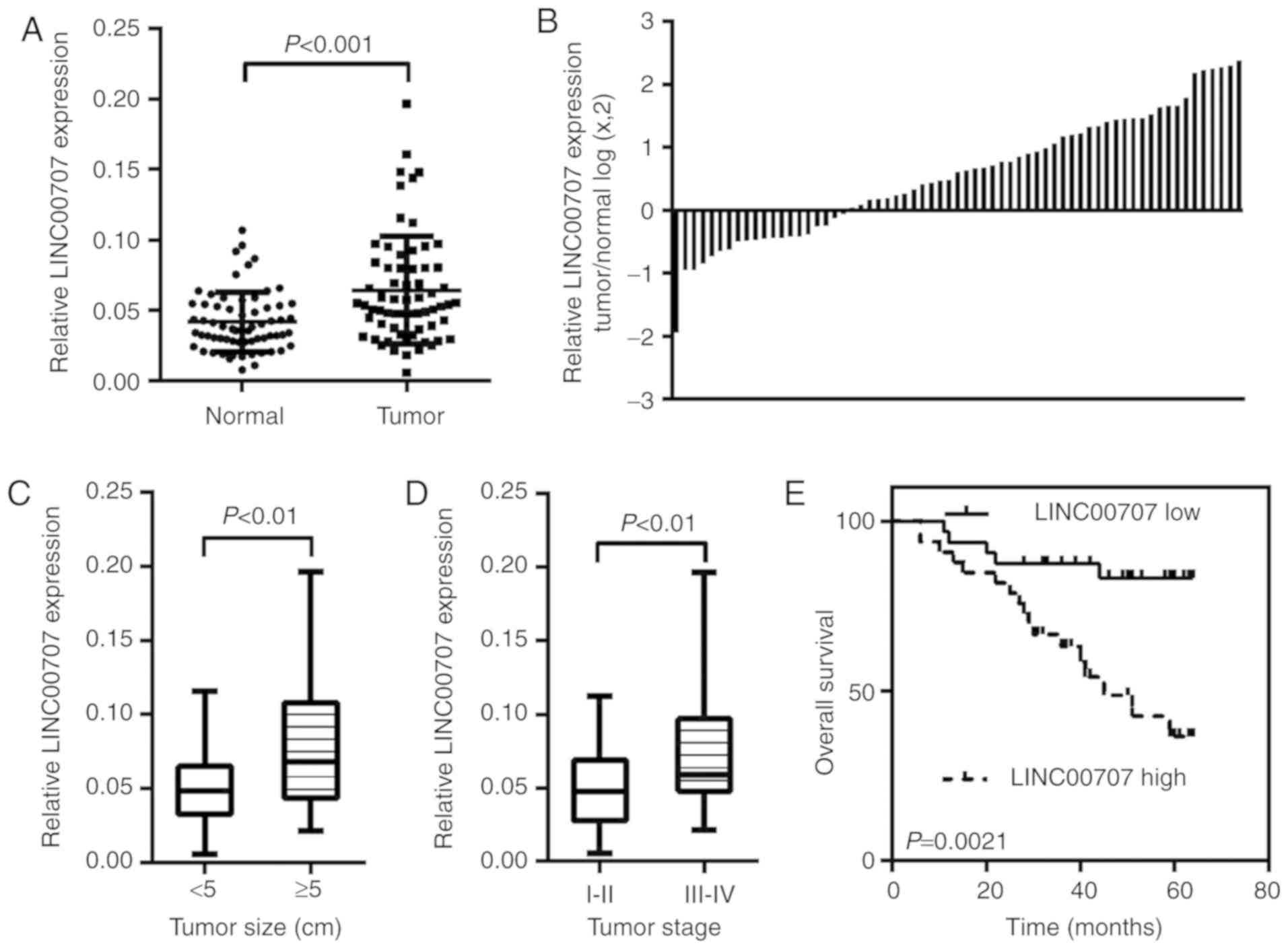
LINC00707 is upregulated in CRC
tissues
The expression level of LINC00707 was confirmed by
RT-qPCR in 65 paired CRC tissues and matched NCTs. The results
revealed that LINC00707 expression was significantly increased in
tumor tissues compared with that in the corresponding NCTs
(P<0.001; Fig. 1A and B).
Additionally, the expression of LINC00707 was notably upregulated
in patients with large tumor size (>5 cm; P=0.0014; Fig. 1C) and advanced tumor stage (stage
III–IV; P=0.0028; Fig. 1D).
Collectively, these data suggested that the upregulation of
LINC00707 was present in CRC and may serve a role in CRC
progression.
Upregulation of LINC00707 is
positively associated with tumor progression and poor prognosis in
patients with CRC
To assess the potential association between
LINC00707 expression and the clinicopathological characteristics of
patients with CRC, the CRC samples were assigned to a high
LINC00707-expression group (n=33; relative expression level of
LINC00707 ≥ median value) and a low LINC00707-expression group
(n=32; relative expression level of LINC00707 < median value).
As presented in Table I, high
expression of LINC00707 was associated with large tumor size
(P=0.017) and advanced tumor stage (P=0.013), whereas it was not
associated with age, sex or differentiation degree (Table I).
 | Table I.Clinicopathological characteristics
of 65 patients with CRC. |
Table I.
Clinicopathological characteristics
of 65 patients with CRC.
|
| LINC00707 |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low | High | P-value |
|---|
| Age, years |
|
| 0.257 |
|
<60 | 12 | 17 |
|
|
≥60 | 20 | 16 |
|
| Sex |
|
| 0.919 |
|
Male | 13 | 13 |
|
|
Female | 19 | 20 |
|
| Tumor size, cm |
|
| 0.017a |
|
<5 | 23 | 14 |
|
| ≥5 | 9 | 19 |
|
| Degree of
differentiation |
|
| 0.283 |
| Well
and moderate | 28 | 25 |
|
|
Poor | 4 | 8 |
|
| Tumor stage |
|
| 0.013a |
|
I+II | 17 | 13 |
|
|
III+IV | 15 | 20 |
|
To assess the prognostic value of LINC00707, the
effects of LINC00707 expression on the overall survival (OS) of
patients with CRC were analyzed. Kaplan-Meier analysis demonstrated
that patients with high LINC00707 expression presented a poorer OS
compared with those with low LINC00707 expression (log rank=9.453,
P=0.0021; Fig. 1E). Univariate
analysis demonstrated that the degree of differentiation, tumor
stage and LINC00707 expression level were significantly associated
with the OS of patients with CRC. Furthermore, multivariate
analysis indicated that tumor stage (HR, 10.967; 95% CI,
2.471–48.671; P=0.002) and LINC00707 expression (HR, 3.129; 95% CI,
1.091–8.968; P=0.034) were independent prognostic factors for CRC
(Table II).
 | Table II.Univariate and multivariate
regression analyses of parameters associated with the prognosis of
CRC patients. |
Table II.
Univariate and multivariate
regression analyses of parameters associated with the prognosis of
CRC patients.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | Subset | P-value | HR (95% CI) | P-value | HR (95% CI) |
|---|
| Age, years | ≥60 vs. <60 | 0.519 | 0.759
(0.329–1.753) | 0.998 | 1.001
(0.401–2.502) |
| Sex | Male vs.
female | 0.724 | 1.170
(0.491–2.790) | 0.383 | 1.516
(0.595–3.862) |
| Tumor size, cm | ≥5 vs. <5 | 0.197 | 1.739
(0.750–4.033) | 0.441 | 1.530
(0.519–4.510) |
| Degree of
differentiation | Poor vs. well and
moderate | 0.007a | 3.303
(1.381–7.898) | 0.149 | 2.301
(0.741–7.143) |
| Tumor stage | III+IV/I+II | 0.001a | 12.042
(2.805–51.684) | 0.002a | 10.967
(2.471–48.671) |
| LINC00707 | High/low | 0.005a | 4.255
(1.560–11.610) | 0.034a | 3.129
(1.091–8.968) |
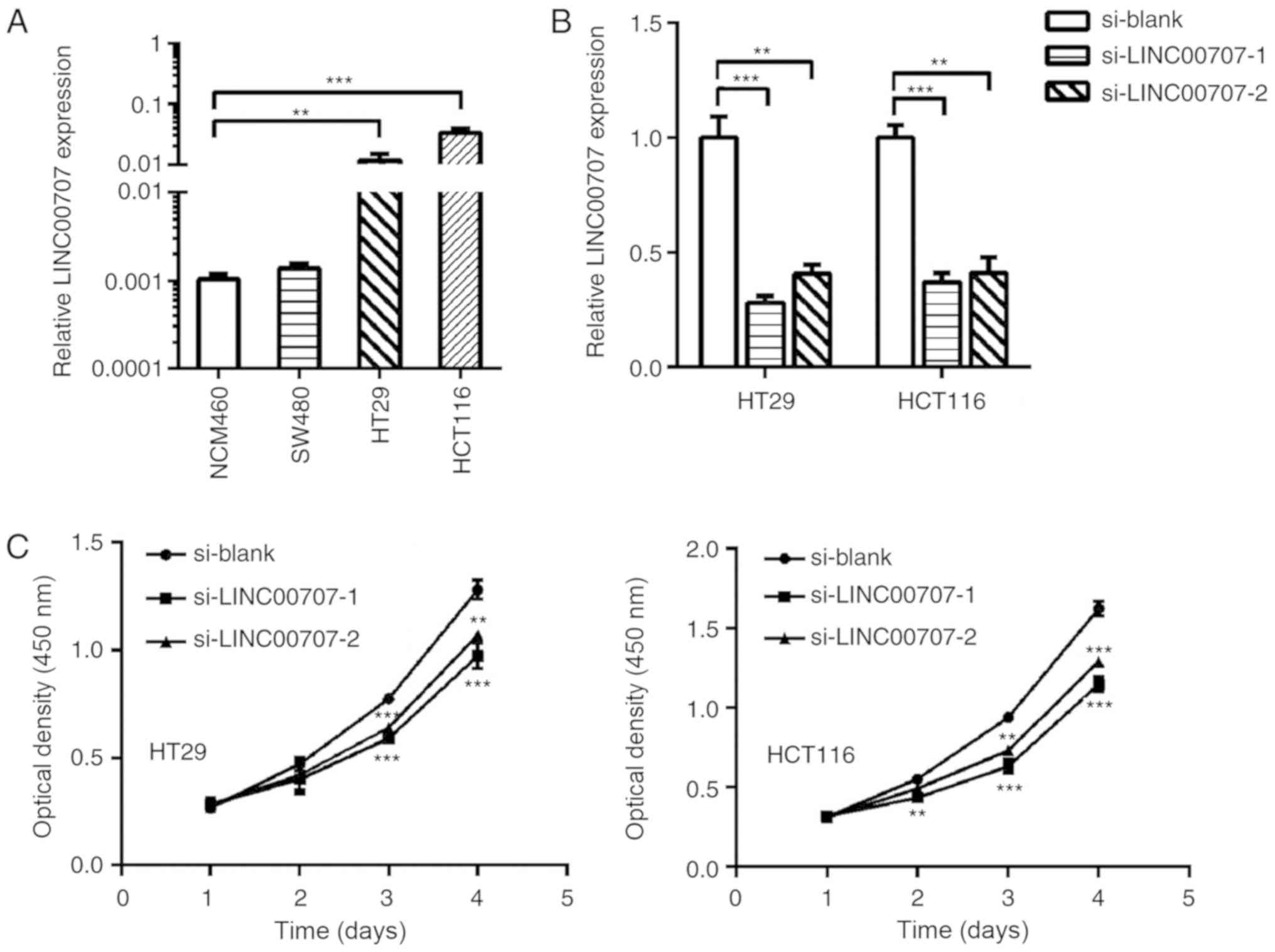
LINC00707 promotes CRC cell
proliferation
To further investigate the detailed functional role
of LINC00707 in CRC, three CRC cell lines and a normal human
intestinal epithelial cell line, NCM460, were subjected to RT-qPCR
to examine LINC00707 expression (Fig.
2A). Since high expression of LINC00707 was identified to be
associated with large tumor size, the role of LINC00707 in
promoting CRC cell proliferation was investigated. LINC00707
expression in HT29 and HCT116 cells was observed to be decreased by
siRNAs (Fig. 2B). The results also
revealed that the downregulation of LINC00707 significantly
decreased cell proliferation and colony formation abilities of CRC
cells (Fig. 2C and D). Furthermore,
the effect of LINC00707 on the cell cycle of CRC cells was
determined by flow cytometry. The results demonstrated that
LINC00707 silencing induced cell cycle arrest at the G1 phase
compared with the control cells (Figs.
2E and S1). No effect was
observed on the apoptotic rates (data not shown). Thus, it was
concluded that LINC00707 may promote cell proliferation in CRC.
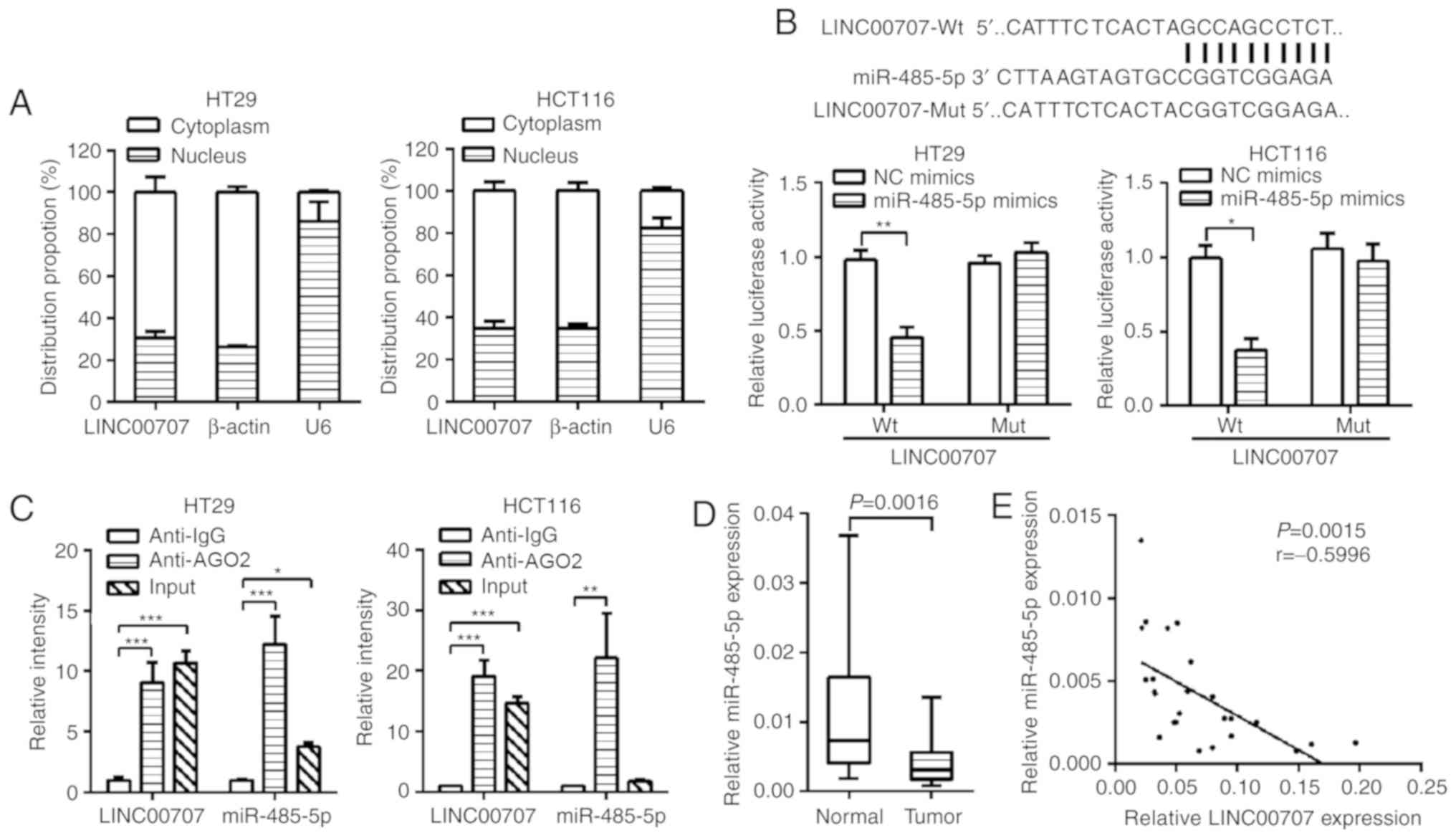
LINC00707 acts as a competing
endogenous RNA (ceRNA) to bind miR-485-5p
To further investigate the underlying mechanism of
LINC00707 in CRC, RT-qPCR was performed to detect the localization
of LINC00707 in HT29 and HCT116 cells. As presented in Fig. 3A, LINC00707 was mostly distributed in
the cytoplasm. Therefore, LINC00707 was hypothesized to participate
in post-transcriptional regulation by acting as a ceRNA. The
starBase database analysis revealed that LINC00707 could combine
with 26 potential microRNAs (miRNAs), whereas only three potential
miRNAs were identified to bind to LINC00707 according to the RegRNA
database. The intersection of the predicted data from these two
databases showed that miR-485-5p was most likely to combine with
LINC00707 (Fig. S2). In addition, a
luciferase reporter vector containing LINC00707 was constructed to
demonstrate the binding between LINC00707 and miR-485-5p. The
results revealed that miR-485-5p mimics could decrease the
luciferase activity of LINC00707-Wt, but could not affect the
luciferase activity of LINC00707-mut, suggesting that the binding
between LINC00707 and miR-485-5p was sequence-dependent (Figs. 3B and S3A). In addition, RIP assay was performed
using anti-AGO2 (the key component that associates with miRNAs) in
the HT29 and HCT116 extract, and LINC00707 and miR-485-5p were
demonstrated to be enriched in the AGO2 compared with anti-IgG
immunoprecipitates (Fig. 3C),
suggesting that LINC00707 may bind to miR-485-5p through AGO2.
Additionally, the expression levels of miR-485-5p in CRC tissues
were detected, and the results revealed that miR-485-5p was
downregulated in CRC tissues (Fig.
3D). The expression of LINC00707 was also revealed to be
inversely correlated with that of miR-485-5p (P=0.0015, r=−0.5996;
Fig. 3E).
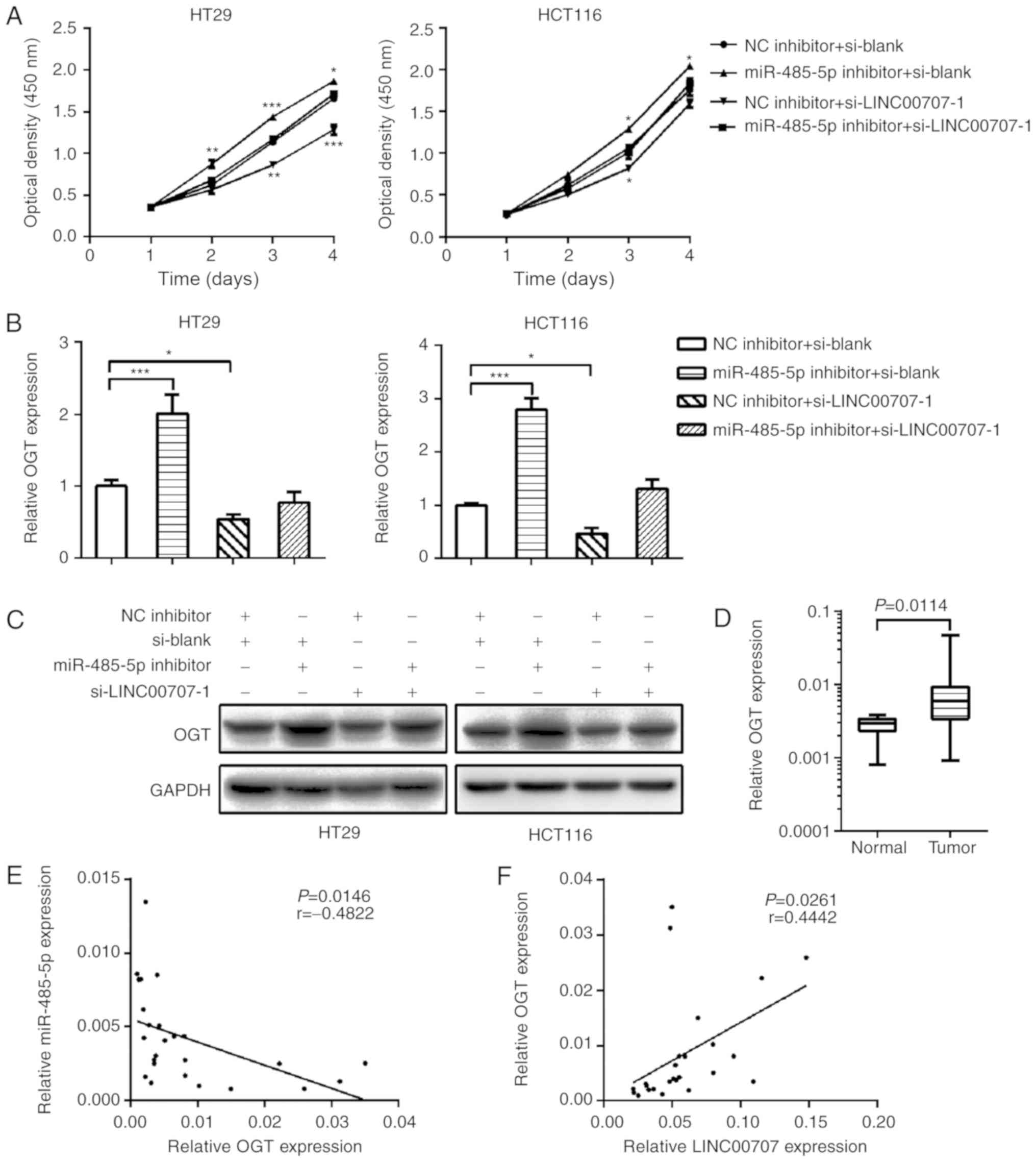
LINC00707 exerts a tumor-promoting
function in CRC by regulating miR-485-5p
To investigate whether LINC00707 promotes cell
proliferation in CRC by sponging miR-485-5p, the effect of
miR-485-5p inhibition on the proliferation of LINC00707-knockdown
HT29 and HCT116 cells was investigated. miR-485-5p inhibition was
observed to reverse the effect of LINC00707-knockdown on CRC cell
proliferation (Figs. 4A and S3B). A Previous study demonstrated that
miR-485-5p inhibits CRC cell proliferation by regulating OGT
(17). In the present study,
LINC00707 knockdown significantly reduced the endogenous mRNA and
protein expression levels of OGT in CRC cells compared with the
negative control group (Fig. 4B and
C). Silencing of miR-485-5p in LINC00707-knockdown cells
restored the expression level of OGT, indicating that LINC00707 may
bind miR-485-5p to regulate the expression of OGT. The expression
of OGT in 25 of the 65 pairs CRC tissues was also examined, and the
results revealed that OGT was notably upregulated in CRC tissues
compared with that in NCTs (Fig.
4D). Correlation analysis demonstrated that the expression of
OGT was negatively correlated with miR-485-5p levels (P=0.0146,
r=−0.4822; Fig. 4E) and positively
correlated with LINC00707 levels (P=0.0261, r=0.4442; Fig. 4F) in CRC tissues.
Discussion
Currently, emerging evidence has demonstrated that
lncRNAs serve increasingly important roles in cancer development
and progression. For example, lncRNA-OOC1 has been identified to
serve a tumor suppressive role in CRC by destabilizing HuR
(18). LINC00659 is a novel
oncogenic lncRNA involved in CRC cell growth by regulating the cell
cycle (19). LINC00707 is a recently
verified lncRNA that has been reported to promote lung
adenocarcinoma cell proliferation and migration by regulating Cdc42
(10). LINC00707 can promote
hepatocellular carcinoma progression by activating the
extracellular-signal-regulated kinase pathway (11). These studies suggest that LINC00707
is a crucial lncRNA with carcinogenic effects in various types of
cancer. In the present study, the significant upregulation of
LINC00707 in CRC tissues was observed, which was consistent with
the high expression of LINC00707 observed in CRC reported recently
by Zhu et al (14) and Shao
et al (15). Since the
majority of the clinical specimens used in these studies were
collected recently and the prognostic information could not be
provided, no analysis of the association between LINC00707 and
prognosis was presented. In the present study, the association
between the expression of LINC00707 and the prognosis of patients
with CRC was investigated, and the results revealed that high
expression of LINC00707 is indicative of poor prognosis in patients
with CRC. In addition, the results of the present study
demonstrated that LINC00707 could promote CRC cell proliferation,
which is consistent with the function of LINC00707 as reported by
other studies (14,15).
lncRNAs serve various functions closely related to
their cellular localization (20).
Cytosolic lncRNAs are frequently involved in post-transcriptional,
translational and post-translational regulatory processes by
interactions with various proteins or RNA molecules (21). The majority of cytoplasmic lncRNAs
serve key roles in the development of human cancers by acting as
ceRNAs of miRNAs (22,23). LINC00707 has been demonstrated to
induce hepatocellular carcinoma progression through sponging
miR-206 and modulating the expression of CDK14 (12). LINC00707 was recently reported to
promote osteogenesis by sponging miR-370-3p (24). LINC00707 can act as a molecular
sponge for miR-876 to promote the malignant progression of breast
cancer (25). LINC00707 mitigates
LPS-induced inflammation and apoptosis in PC-12 cells by targeting
miR-30a-5p/Neurod 1 (26). It has
also been reported that LINC00707 promotes CRC cell proliferation
and invasion through sponging miR-206 and regulating the expression
of NOTCH3, transmembrane 4 L6 family member 1 and formin-like 2
(14,15). These studies indicate that LINC00707,
as a ceRNA, serves an important role in numerous physiological and
pathophysiological processes, especially in the occurrence and
development of human tumors. The subcellular localization of
LINC00707 was also examined in the present study, and the
cytoplasmic location of LINC00707 indicated the potential ceRNA
role of LINC00707 in CRC. To date, LINC00707 has been confirmed to
bind miR-206, miR-370-3p, miR-876 and miR-30a-5p (12,24–26). As
all lncRNAs, LINC00707 can bind multiple miRNAs, as long as they
share a miRNA response element. ChIPBase, LncRNAab and starBase
were used by Tu et al (12)
to identify the interaction between miR-206 and LINC00707 at
binding sites 2926–2933. Jia et al (24) demonstrated that miR-370-3p may bind
to nucleotides 744–749 of LINC00707 using the LncRNABase and
NONCODE databases. Li et al (25) used starBase and LncBase databases to
identify two putative binding sites of miR-876 to LINC00707,
593–599 and 604–610. Zhu et al (26) used starBase to identify one putative
binding site of miR-30a-5p to LINC00707 at nucleotides 1379–1385.
Based on these studies, it may be concluded that there are numerous
miRNAs that can bind to different locations of LINC00707. Although
it has been reported that LINC00707 can sponge miR-206 in CRC, no
explanation has been provided on the selection of miR-206 from a
large number of miRNAs that LINC00707 could bind (14,15).
Since LINC00707 may bind more miRNAs in CRC, other than miR-206,
the intersection of the starBase and RegRNA databases was analyzed
in the present study to further predict miRNAs that may be sponged
by LINC00707 in CRC. The results revealed that miR-485-5p, as an
intersection of these two databases, is likely to bind to
LINC00707. Subsequent experiments also confirmed that LINC00707
could bind miR-485-5p and prevent its normal function. miR-485-5p
is a tumor suppressor gene in a number of human cancers, such as
GC, lung adenocarcinoma, melanoma and breast cancer (27–30).
Previous studies have demonstrated the anticarcinogenic role of
miR-485-5p in CRC (17,31). The present study demonstrated that
LINC00707 could sponge miR-485-5p and inhibit the tumor suppressor
effect of miR-485-5p in CRC. The effects of LINC00707 on OGT, a
target gene of miR-485-5p, were further examined. The results
revealed that the expression of LINC00707 was positively correlated
with OGT levels in CRC tissues, indicating that LINC00707 may exert
a tumor-promoting function in CRC by regulating miR-485-5p.
A limitation of this study should be noted. The
effect of LINC00707 in vivo was not detected. A recent study
has reported that LINC00707 promotes CRC growth in vivo
(15). The authors used HCT116
cells, which is a type of cells also used in the present study, in
a xenograft tumor experiment on nude mice, to knock down the
expression of LINC00707. According to the results of Shao et
al (15), LINC00707 could
promote the proliferation of CRC in vivo.
In conclusion, the results of the present study
revealed that LINC00707 was upregulated in CRC tissues and the
overexpression of LINC00707 was positively associated with tumor
progression and poor prognosis in patients with CRC. In addition,
LINC00707 promoted CRC cell proliferation. Further investigation of
the underlying mechanism revealed that LINC00707 may function as a
ceRNA to sponge miR-485-5p and regulate the expression of the
target genes of miR-485-5p. These results indicated that
LINC00707/miR-485-5p may promote CRC cell proliferation, providing
new insights into CRC mechanisms and identifying potential
therapeutic targets for CRC.
Supplementary Material
Supporting Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the Fundamental
Research Project for the Colleges and Universities of Heilongjiang
Province (grant no. 2018-KYYWFMY-0071).
Availability of data and materials
All data generated during the present study are
included in this published article.
Authors' contributions
HW, HL and HD designed the study, analyzed the data
and wrote the manuscript. HW, TZ, XL and JS performed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The protocol for this study was approved by the
Medical Ethics Committee of the Mudanjiang Medical University
(Mudanjiang, China). All patients signed a written informed consent
form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fearon ER: Molecular genetics of
colorectal cancer. Annu Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sameer AS: Colorectal cancer: Molecular
mutations and polymorphisms. Front Oncol. 3:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi L and Ding Y: Screening of tumor
suppressor genes in metastatic colorectal cancer. Biomed Res Int.
2017:27691402017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Necsulea A, Soumillon M, Warnefors M,
Liechti A, Daish T, Zeller U, Baker JC, Grutzner F and Kaessmann H:
The evolution of lncRNA repertoires and expression patterns in
tetrapods. Nature. 505:635–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Z, Liu J, Chen C, Zhou Q, Yang S, Wang
G, Song J, Li Z, Zhang Z, Xu J, et al: The biological effect and
clinical application of long noncoding RNAs in colorectal cancer.
Cell Physiol Biochem. 46:431–441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang S, Sun Z, Zhou Q, Wang W, Wang G,
Song J, Li Z, Zhang Z, Chang Y, Xia K, et al: MicroRNAs, long
noncoding RNAs, and circular RNAs: Potential tumor biomarkers and
targets for colorectal cancer. Cancer Manag Res. 10:2249–2257.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma T, Ma H, Zou Z, He X, Liu Y, Shuai Y,
Xie M and Zhang Z: The long intergenic noncoding RNA 00707 promotes
lung adenocarcinoma cell proliferation and migration by regulating
Cdc42. Cell Physiol Biochem. 45:1566–1580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Luo Z, Yao T, Li W and Pu J:
LINC00707 promotes hepatocellular carcinoma progression through
activating ERK/JNK/AKT pathway signaling pathway. J Cell Physiol.
234:6908–6916. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tu J, Zhao Z, Xu M, Chen M, Weng Q, Wang J
and Ji J: LINC00707 contributes to hepatocellular carcinoma
progression via sponging miR-206 to increase CDK14. J Cell Physiol.
234:10615–10624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie M, Ma T, Xue J, Ma H, Sun M, Zhang Z,
Liu M, Liu Y, Ju S, Wang Z and De W: The long intergenic
non-protein coding RNA 707 promotes proliferation and metastasis of
gastric cancer by interacting with mRNA stabilizing protein HuR.
Cancer Lett. 443:67–79. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, He G, Wang Y, Hu Y, Zhang Z and
Qian X: Long intergenic noncoding RNA 00707 promotes colorectal
cancer cell proliferation and metastasis by sponging miR-206. Onco
Targets Ther. 12:4331–4340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao HJ, Li Q, Shi T, Zhang GZ and Shao F:
LINC00707 promotes cell proliferation and invasion of colorectal
cancer via miR-206/FMNL2 axis. Eur Rev Med Pharmacol Sci.
23:3749–3759. 2019.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chai Y, Du Y, Zhang S, Xiao J, Luo Z, He F
and Huang K: MicroRNA-485-5p reduces O-GlcNAcylation of Bmi-1 and
inhibits colorectal cancer proliferation. Exp Cell Res.
368:111–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L and
Song X: Long noncoding RNA OCC-1 suppresses cell growth through
destabilizing HuR protein in colorectal cancer. Nucleic Acids Res.
46:5809–5821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai KW, Lo YH, Liu H, Yeh CY, Chen YZ,
Hsu CW, Chen WS and Wang JH: Linc00659, a long noncoding RNA, acts
as novel oncogene in regulating cancer cell growth in colorectal
cancer. Mol Cancer. 17:722018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genomics Proteomics Bioinformatics. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan JJ and Tay Y: Noncoding RNA: RNA
regulatory networks in cancer. Int J Mol Sci. 19:E13102018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia B, Wang Z, Sun X, Chen J, Zhao J and
Qiu X: Long noncoding RNA LINC00707 sponges miR-370-3p to promote
osteogenesis of human bone marrow-derived mesenchymal stem cells
through upregulating WNT2B. Stem Cell Res Ther. 10:672019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Li Y and Sun H: MicroRNA-876 is
sponged by long noncoding RNA LINC00707 and directly targets
metadherin to inhibit breast cancer malignancy. Cancer Manag Res.
11:5255–5269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu S, Zhou Z, Li Z, Shao J, Jiao G, Huang
YE and Lin Y: Suppression of LINC00707 alleviates
lipopolysaccharide-induced inflammation and apoptosis in PC-12
cells by regulated miR-30a-5p/Neurod 1. Biosci Biotechnol Biochem.
83:2049–2056. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jing LL and Mo XM: Reduced miR-485-5p
expression predicts poor prognosis in patients with gastric cancer.
Eur Rev Med Pharmacol Sci. 20:1516–1520. 2016.PubMed/NCBI
|
|
28
|
Mou X and Liu S: MiR-485 inhibits
metastasis and EMT of lung adenocarcinoma by targeting Flot2.
Biochem Biophys Res Commun. 477:521–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Li J, Ren J and Zhang D:
MicroRNA-485-5p represses melanoma cell invasion and proliferation
by suppressing Frizzled7. Biomed Pharmacother. 90:303–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lou C, Xiao M, Cheng S, Lu X, Jia S, Ren Y
and Li Z: MiR-485-3p and miR-485-5p suppress breast cancer cell
metastasis by inhibiting PGC-1α expression. Cell Death Dis.
7:e21592016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu XX, Xu XN, He BS, Sun HL, Xu T, Liu XX,
Chen XX, Zeng KX, Wang SK and Pan YQ: microRNA-485-5p functions as
a tumor suppressor in colorectal cancer cells by targeting CD147. J
Cancer. 9:2603–2611. 2018. View Article : Google Scholar : PubMed/NCBI
|