Introduction
Liver damage resulting from multiple factors
including drug misuse, alcohol abuse and pesticides represents a
major international health problem (1). To tackle this problem investigations
into the potential hepatoprotective properties of food, medicinal
plants and their bioactive constituents is currently of upmost
importance. Indeed, inflammation and reactive oxygen species
(ROS)-induced oxidative stress are major players in the
pathogenesis of many chronic diseases such as liver fibrosis
(2). The huge diversity of compounds
derived from natural products provides a rich source of potential
therapeutic agents (3). Accumulating
evidence supports the beneficial antioxidant effects of
food-derived phenolic compounds on the health of the liver, by
protecting against chemical-induced hepatotoxicity such as
ethanol-induced liver injury (4).
Carbon tetrachloride (CCl4)-intoxication is an animal
model of oxidative stress-induced liver injury that is widely used
for assessing the hepatoprotective capacity of prospective
therapeutic agents (5).
One such agent, the Gum resin of Boswellia
serrata (BS), has been used for centuries as a traditional
remedy for a variety of ailments in Ayurvedic medicine. The
anti-inflammatory, anti-atherogenic, and analgesic properties of BS
have been recognized for centuries (6). Extracts from this gum resin have
previously been demonstrated to target the humoral and adaptive
immune response (7). In vitro
studies have revealed that the boswellic acids, consisting of a
group of pentacyclic triterpenoid compounds/acids, and their
acetylated derivatives can inhibit the biosynthesis of
pro-inflammatory mediators such as leukotrienes (8), which increase cell permeability. In
particular, 3-acetyl-11-ketobeta-boswellic acid (AKBA) has been
found to be a natural inhibitor of the transcription factor NF-κB,
which is an important downstream mediator of cytokines during
inflammation (9). These
anti-inflammatory properties has been attributed to the boswellic
acids (α, β and γ-boswellic acid), acetyl-β boswellic acid,
11-keto-β-boswellic acid and acetyl-11-keto-β-boswellic acid
(10), which can also simultaneously
reduce oxidative stress (11). This
group of triterpenic acids have also been reported to exhibit
anti-cancer properties, controlling cell proliferation, metastasis,
invasion and migration by targeting cell signaling components,
including MAPK, NF-κB, TNF-α and ERK1/2 (12,13).
The aim of the present study was to elucidate the
potential hepatoprotective effects and the mechanism of action of
BS in CCl4-induced hepatocellular damage rat models.
These effects were biochemically and histologically assessed in
addition to being compared with that of silymarin, a more
well-known hepatoprotective compound (14).
Materials and methods
Chemicals and Plant Material
Chemicals used were all of analytical grade and were
purchased from Sigma-Aldrich (Merck KGaA). BS oleo-gum resin
utilized in the present study was a kind gift from Professor Dr H.
P. T. Ammon, Department of Pharmacology, Institute of
Pharmaceutical Sciences, University of Tuebingen, Germany
(Tubingen, Germany).
Animals and experimental design
Experiments on animals were performed in accordance
with the international ethical guidelines for animal care of the
United States Naval Medical Research Centre, Unit no. 3, Abbaseya,
Cairo, Egypt, accredited by the Association for Assessment and
Accreditation of Laboratory Animal Care International. The adopted
guidelines were in agreement with ‘Principles of Laboratory Animals
Care’ (NIH Publication no. 85-23, revised 1985). The study protocol
was approved by The Research Ethics Committee of the Faculty of
Pharmacy, Minya University (Minya, Egypt).
A total of 32 male Wistar rats (age, 7–8 weeks old;
average body weight, 250±25 g) were obtained from the Animal House
of Assiut University were utilized in the experimental procedures.
All animals received professional care and were kept with a 12-h
light/dark cycle at 20°C and 45% relative humidity and had free
access to water and food. Animals were randomly divided into four
test groups of eight rats each, with the experimental procedures
described as follows: i) Normal control group, which received two
intraperitoneal (i.p.) injections of olive oil per week for six
weeks; ii) CCl4-treated group, in which liver fibrosis
was induced by an i.p. injection of CCl4 (1 ml/kg 40%
CCl4, diluted in olive oil) twice weekly for 6 weeks
(15); iii) BS treatment group, in
which the rats received a daily i.p. injection of BS (150 mg/kg
body weight) for an additional two weeks directly after the end of
the six-week CCl4 treatment (16); and iv) Silymarin treatment group, in
which the rats received a daily oral dose of silymarin (100 mg/kg
body weight per oral gavage) for two weeks directly after the end
of the six week CCl4 treatment. At the end of the 8th
week, rats deeply anaesthetized by i.p. injection of 100 mg/kg
ketamine and 20 mg/kg xylazine were sacrificed by cervical
dislocation.
Sample collection
To perform the biochemical analysis, 5 ml of blood
were collected from animals that were deeply anesthetized by
intraperitoneal injections of 100 mg/kg ketamine and 20 mg/kg
xylazine by cardiac puncture. The blood samples were subsequently
centrifuged (1,000 × g, 20 min at room temperature) with the
subsequent serum isolated. Liver tissues were excised rapidly for
histological investigation and RNA isolation. A buffer with the
following composition was used for tissue homogenization for
further protein analysis: 20 mM Tris, 100 mM NaCl, 1 mM EDTA and
0.5% Triton X-100 supplemented with protease inhibitors mix. Biuret
reagent (Bio-Rad laboratories, Inc.) was used to estimate the
protein content of homogenates, using bovine serum albumin as
standard (Sigma-Aldrich; Merck KGaA). Homogenized tissue samples
were stored at −70°C until use.
Detection of nuclear NF-κB expression
by western blot analysis
Preparation of nuclear samples was performed using
Nuclear Extraction Kit (cat. no. ab113474; Abcam) according to
manufacturer's protocol. A total of 50 µg of nuclear protein from
each liver sample were used for western blot analysis according the
protocol previously described by Abouzied et al (17). Briefly, protein samples were
separated by 10% SDS-PAGE and then transferred to Hybond™ nylon
membranes. Membranes were blocked for 2 h at room temperature in 5%
non-fat milk diluted in TBS supplemented with 0.05% Tween-20 buffer
followed by an overnight incubation at 4°C with mouse monoclonal
primary antibodies against rat NF-κB p65 (1:2,000, cat. no.
TA336457; Origene Technologies, Inc.) and β-actin (1:2,000; cat.
no. TA310155; OriGene Technologies, Inc.). Alkaline
phosphatase-conjugated goat anti-mouse immunoglobulins (cat. no.
AP32278AP-N; Origene Technologies, Inc.) were diluted 1:5,000 in
the 10X diluted blocking buffer and used as a secondary antibody
that was used for incubating the membranes for 2 h at room
temperature. Visualization of protein bands was achieved by
incubating the membranes with alkaline phosphatase buffer
containing the substrate 1-step™ NBT/BCIP substrate solution (cat.
no. 34042; Thermo Fisher Scientific, Inc.). Color reactions were
stopped by rinsing the membranes with stop buffer (10 mM Tris-Cl,
pH 6.0, 5 mM EDTA). Visualized bands were analyzed using ImageJ
software (version 2.0.0; National Institutes of Health).
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) for TNF-α
Total RNA was isolated from freshly dissected
tissues using total RNA Kit (Hangzhou Bioer Co., Ltd.) according to
manufacturer's protocol. The amount of isolated RNA was quantified
by measuring absorbance at 260 nm. RT-PCR was performed using
RevertAid RT reverse transcription kit (Thermo Fisher Scientific,
Inc.) according to manufacturer's protocols. For the amplification
of TNF-α, the following synthetic oligonucleotides were used: TNF-α
forward, 5′-CAGCAGATGGGCTGTACCTT-3′ and reverse,
5′-AAGTAGACCTGCCCGGACTC-3′; and GAPDH forward,
5′-AGATCCACAACGGAT-3′ and reverse, 5′-TCCCTCAAGATTGTCAGCAA-3′,
where GAPDH served as the internal control (18). The reaction mixture, consisting of 2
µl cDNA, 2.5 U REDtaq® ReadymixTM PCR Reaction Mix
(Sigma-Aldrich; Merck KGaA) and 0.1 µmol/l primers (Fermentas;
Thermo Fisher Scientific, Inc.) was amplified using a Biometra
cycler® (Biometra GmbH). Thermocycling started with 4
min of pre-denaturation at 94°C followed by 30 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 1 min. Finally, the mixture was incubated at
72°C for 4 min and cooled to 4°C. Subsequent PCR products were
separated on 1.5% (v/v) agarose gels and visualized by ethidium
bromide (0.3 µg/ml final concentration) and bands were quantified
using the ImageJ software (version 2.0.0; National Institutes of
Health).
Evaluation of serum TGF-β and
interleukin-6 levels
Commercially available ELISA kits (Cusabio Biotech
Co., Ltd.) were used to measure serum levels of TGF-β (cat. no.
CSB-E04726m) and IL-6 (cat. no. CSB-E04639m) according to
manufacturer's protocol.
Biochemical analysis
Isolated sera were used for the assessment of serum
levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST) (cat. no. AT-1034; Bio-diagnostic), albumin
(cat. no. AB-1010; Bio-diagnostic), bilirubin (cat. no. BR-1110;
Bio-diagnostic), lactate dehydrogenase activity (LDH; cat. no.
278001; Spectrum Diagnostics), total cholesterol (cat. no. CH-1220;
Bio-diagnostic) and triglycerides (TG; cat. no. TR-2030;
Bio-diagnostic). Liver homogenates were used for estimating levels
of lipid peroxides (measured as malondialdehyde, MDA; cat. no.
MD-2529; Bio-diagnostic), catalase activity (cat. no. CA-2517,
Bio-diagnostic) and total anti-oxidant activity (cat. no. TA-2513,
Bio-diagnostic). Commercially available kits were used for
measuring the different biochemical parameters according to the
manufacturers' protocols.
Histopathology
Freshly isolated liver specimens were first
formalin-fixed (10% w/v in PBS, overnight at 4°C) then embedded in
paraffin following dehydration in a series of increasing ethanol
concentrations. The tissues were then cut into 5 µm-thick sections,
de-paraffinized, rehydrated and stained with hematoxylin for 7 min
and eosin for 1 min at room temperature (H&E staining) as well
as Masson's trichrome (19) or
immunostained against COX-2 using a specific COX-2 antibody
according to the method described by Wójcik et al (20). An independent pathologist blindly
estimated the degree of liver damage using an Optika B-810
microscope (Optika SRL). Knodell index was used for scoring the
degree of liver damage as follows: 0, absence of fibrosis; 1,
portal fibrosis; 2, fibrous portal expansion; 3, bridging fibrosis
(portal-portal or portal-central linkage); and 4, cirrhosis
(21).
High-performance liquid chromatography
(HPLC) and characterization of BS extract
BS oleo-gum resin used in the present study was
previously characterized by HPLC (16,22,23). It
was found to be a combination of α- and β-boswellic acids and their
derivatives, including 3-O-acetyl-α-boswellic acid,
11-keto-β-boswellic acid (AKB) and 3-O-acetyl-11-keto-β-boswellic
acid (AKBA). Based on the data obtained from the provider, the
actual concentrations of KBA and AKBA in the extract were 5.48 and
4.66%, respectively.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism (version 7; GraphPad Software, Inc.). Data were presented as
mean ± standard error of mean (SEM). Comparisons were performed
using one-way ANOVA followed by Tukey-Kramer multiple comparisons
test. Results of histopathological analyzes were performed using
non-parametric Kruskal-Wallis test followed by Bonferroni's
correction. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of BS on the activities of
serum liver enzymes
The levels of ALT, AST and LDH were evaluated in the
serum from different test groups as markers of hepatocyte
integrity. CCl4 treatment resulted in significant increases in
serum ALT and AST activities compared with healthy control animals
(P<0.001; Table I). Daily
treatment with BS (150 mg/kg) significantly reversed the elevated
serum levels of these three biomarkers induced by CCl4 (P<0.001;
Table I). The values obtained after
BS treatment were comparable to healthy control as well as
silymarin treated group (Table
I).
 | Table I.Levels of liver biomarkers in the
different test groups. |
Table I.
Levels of liver biomarkers in the
different test groups.
| Parameter | Control (n=8) | CCl4
(n=8) | CCl4 +
Boswellia extract (150 mg/kg) (n=8) | CCl4 +
Silymarin (100 mg/kg) (n=8) |
|---|
| AST (U/l) | 91±4.700 |
240±10.800c |
120.0±9.100b |
105±11.300b |
| ALT (U/l) | 40±2.300 |
108±9.700c |
60±4.800a |
45±3.700b |
| Bilirubin
(mg/dl) | 0.12±0.080 |
0.46±0.020c |
0.15±0.005b |
0.13±0.090b |
| Albumin
(mg/dl) | 5.1±0.410 |
2.9±0.170c |
4.3±0.250a |
4.8±0.180b |
| Triglycerides
(mg/dl) | 73±6.300 |
130±10.200c |
81±4.700b |
75±5.400b |
| Cholesterol
(mg/dl) | 83±3.22 |
152±5.700c | 94
±8.300b |
83.00±5.150b |
Effects of BS on serum cholesterol,
TG, albumin and bilirubin levels
CCl4 administration resulted in significant
increases in the serum levels of cholesterol, TG and bilirubin
(P<0.001) along with a significant drop in albumin levels
(P<0.001; Table I), effects that
were significantly reversed by subsequent BS administration
(P<0.001; Table I).
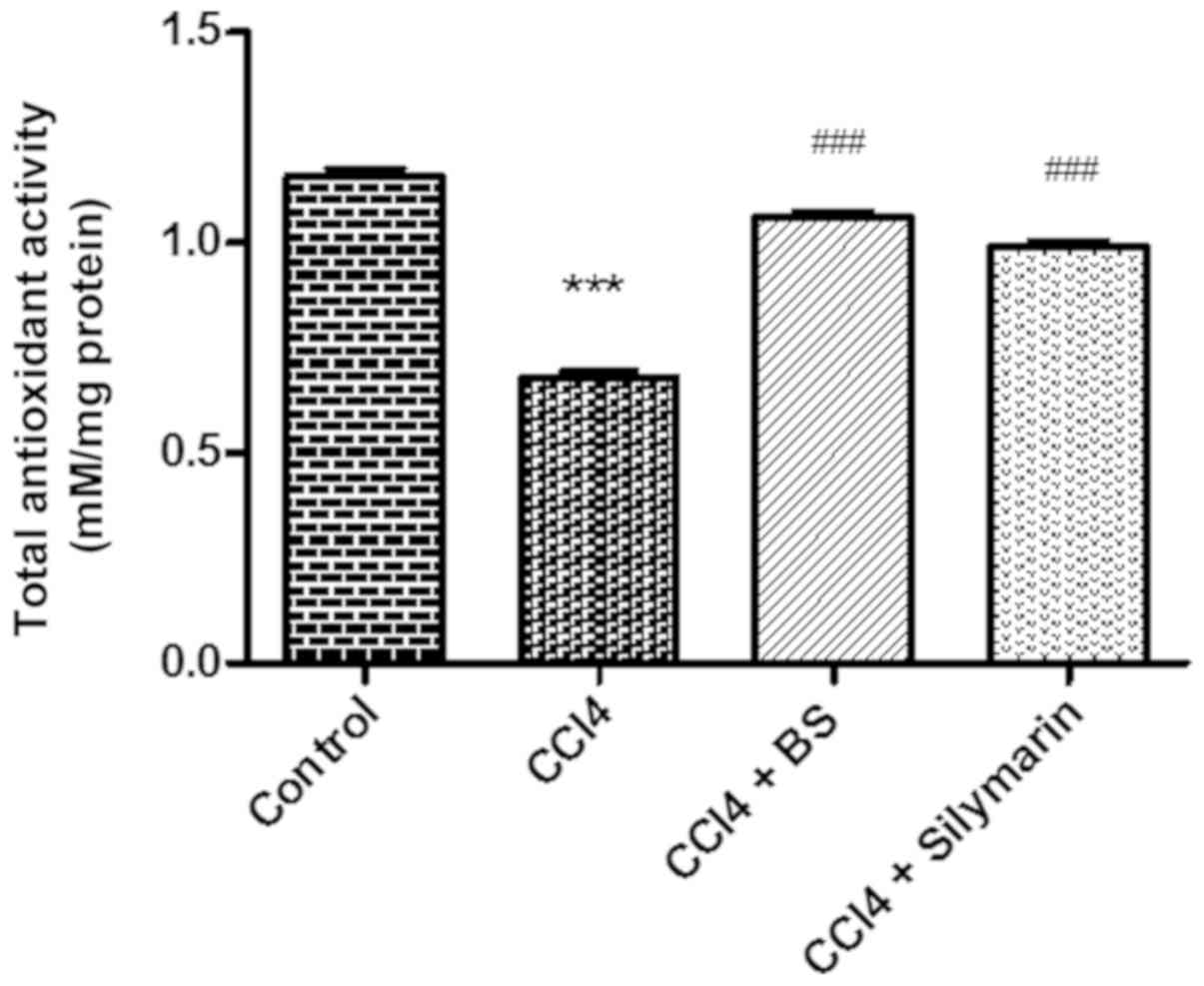
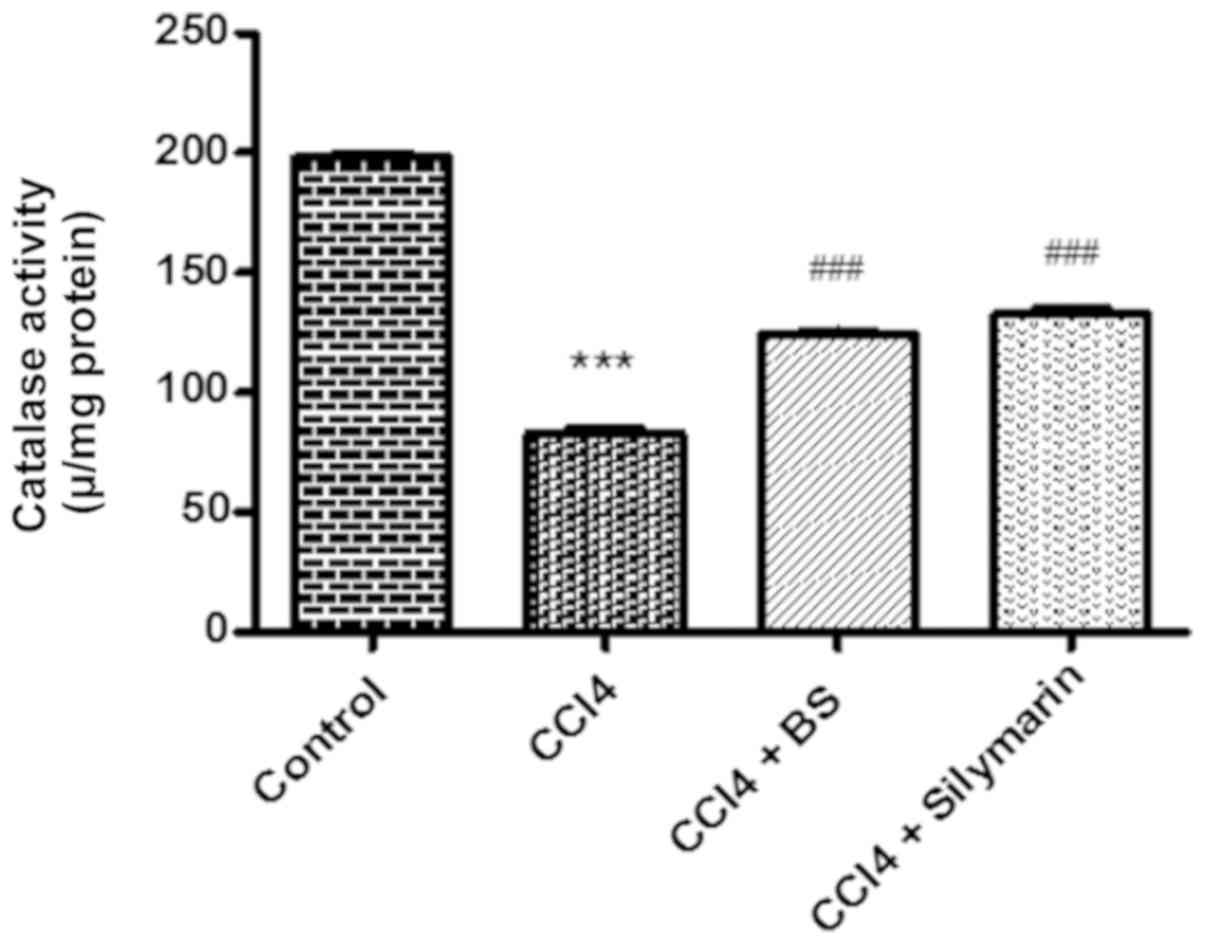
Effects of BS on total antioxidant
capacity and catalase activity
The hepatic tissue antioxidant capacity and catalase
activity were then measured in the four test groups. A significant
reduction in catalase and total antioxidant activities were
observed in the CCl4 group compared with normal control
(P<0.001, Figs. 1 and 2). Treatment with BS or silymarin resulted
in significant increases in catalase and total antioxidant activity
compared with the CCl4 group (P<0.001; Figs. 1 and 2).
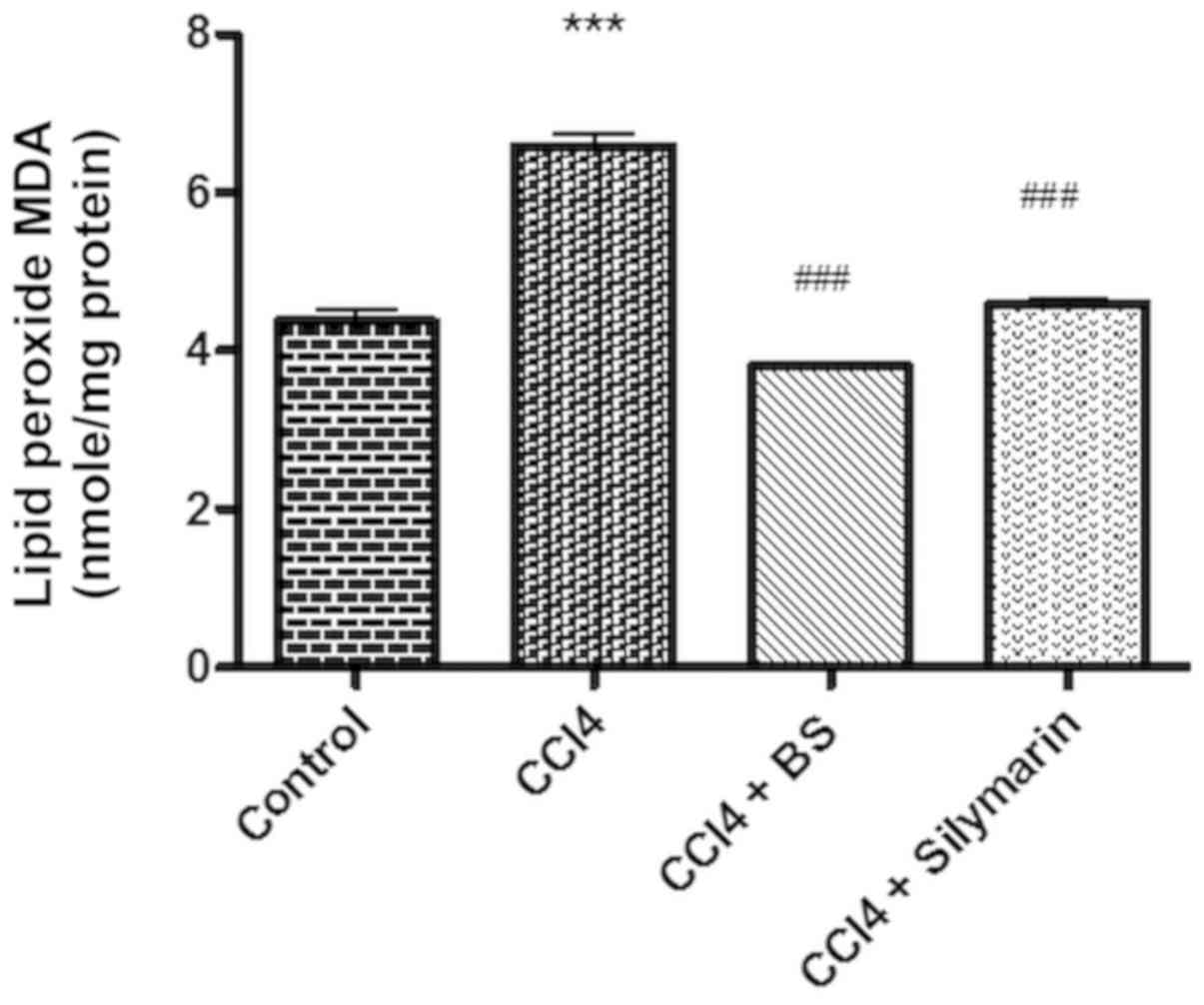
Effects of BS on lipid peroxidation in
hepatic tissues
For the present study, malondialdehyde (MDA) levels
in hepatic tissues were used as a marker for lipid peroxidation.
CCl4 treatment induced significant increases in tissue MDA levels
compared with normal control (P<0.001; Fig. 3). BS treatment resulted in a
significant reduction in the elevated MDA levels compared with the
CCl4-treated group (P<0.001; Fig.
3), to levels that were comparable to those of healthy control
and the silymarin-CCl4-treated group.
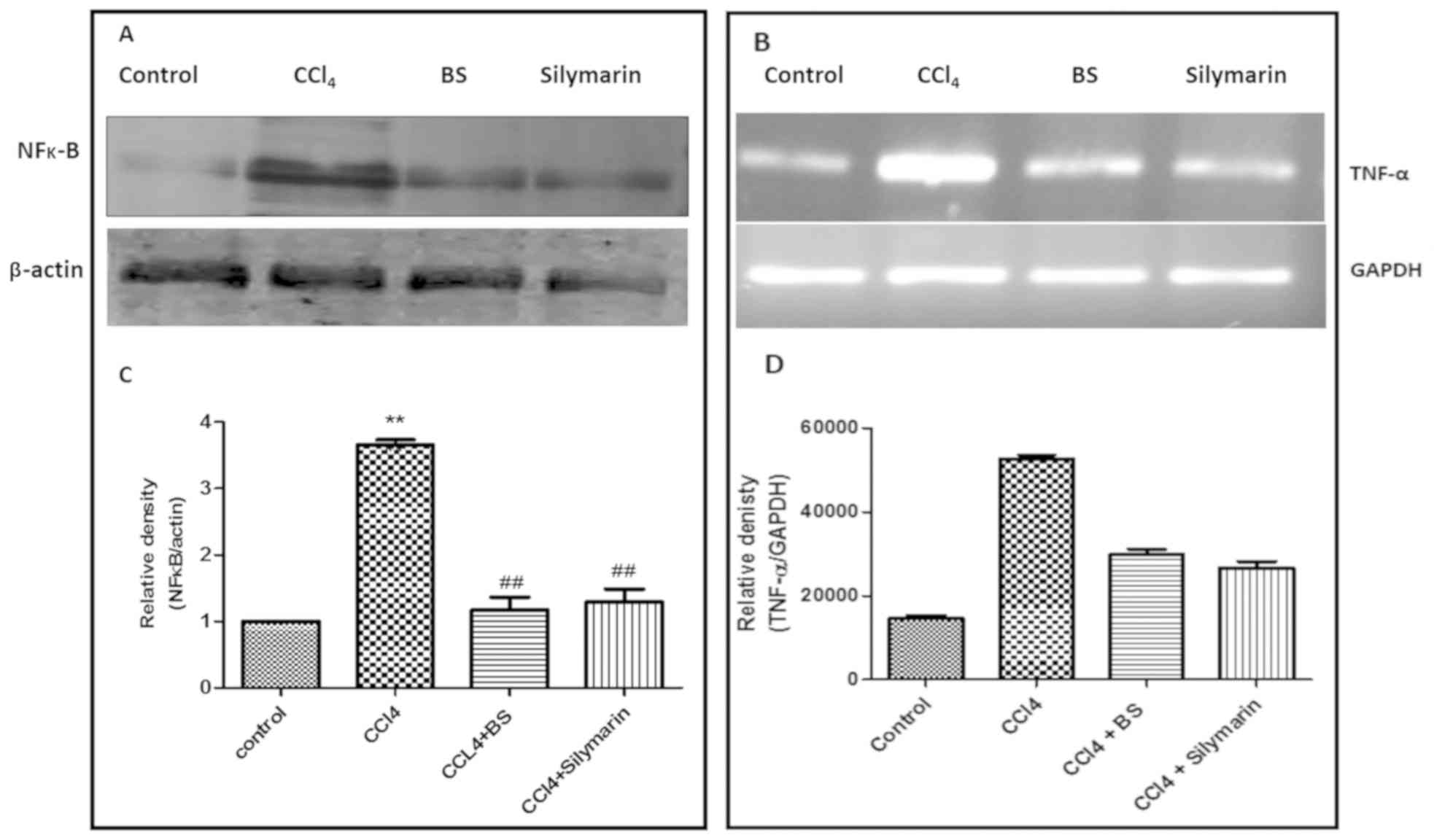
Effects of BS on the expression and
transcription levels of nuclear NF-κB and TNF-α
Changes in nuclear NF-κB p65 protein expression and
TNF-α transcription in liver tissues from the four treatment groups
were next measured to assess the anti-inflammatory activity of BS
(Fig. 4A and B). CCl4 treatment
resulted in an increase in the expression of the pro-inflammatory
markers, TNF-α mRNA and NF-κB proteins, compared with healthy
controls. BS administration reversed this CCl4-induced effect to
levels comparable with control and similar to that induced by
silymarin treatment (Fig. 4A-D).
Effects of BS on the expression levels
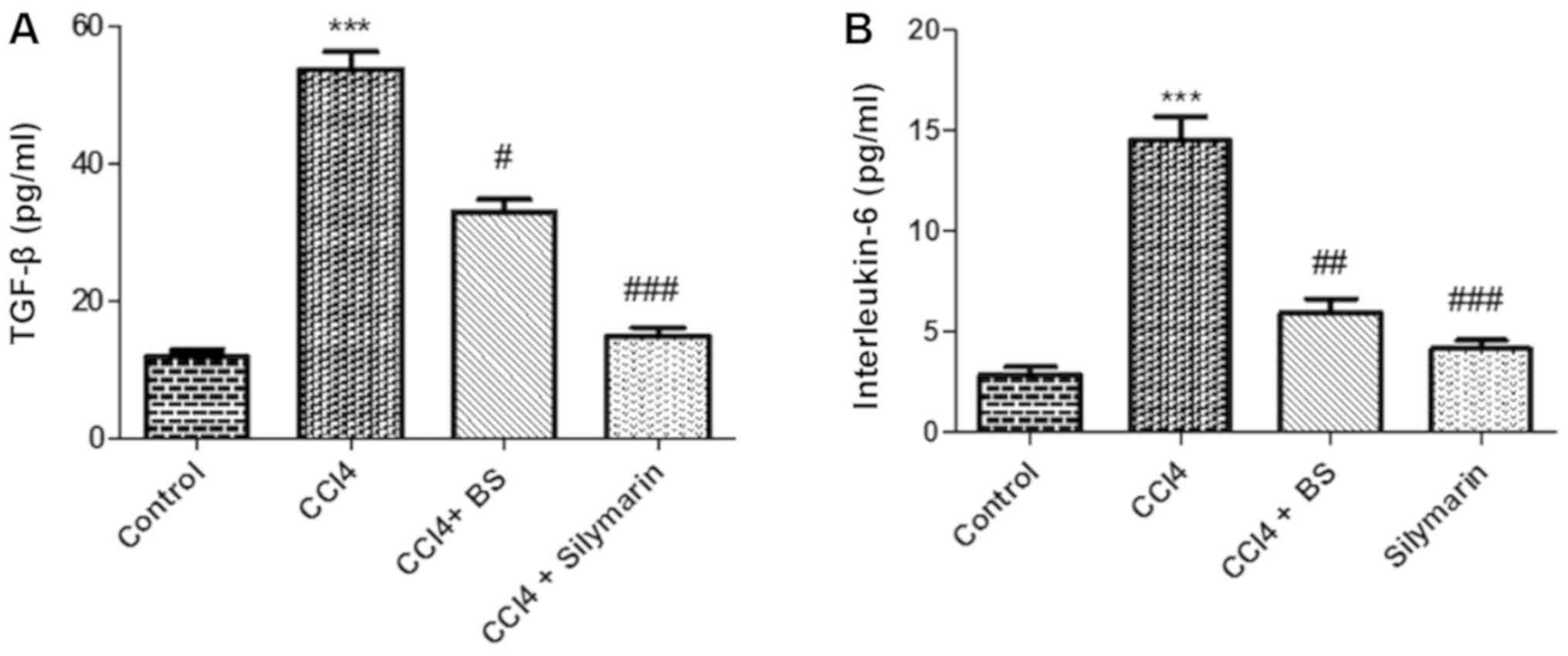
of TGF-β and interleukin-6
To evaluate the effect of different treatments on
the expression of inflammatory markers, the levels of the
pro-inflammatory markers TGF-β and IL-6 were measured in the serum
from the four treatment groups. CCl4 treatment significantly
increased the serum levels of both markers as a result of the
induced inflammatory response (Fig. 5A
and B). Subsequent treatment with BS partially but
significantly reversed the increased expression of TGF-β and IL-6
to values comparable to those obtained after silymarin treatment
(Fig. 5A and B).
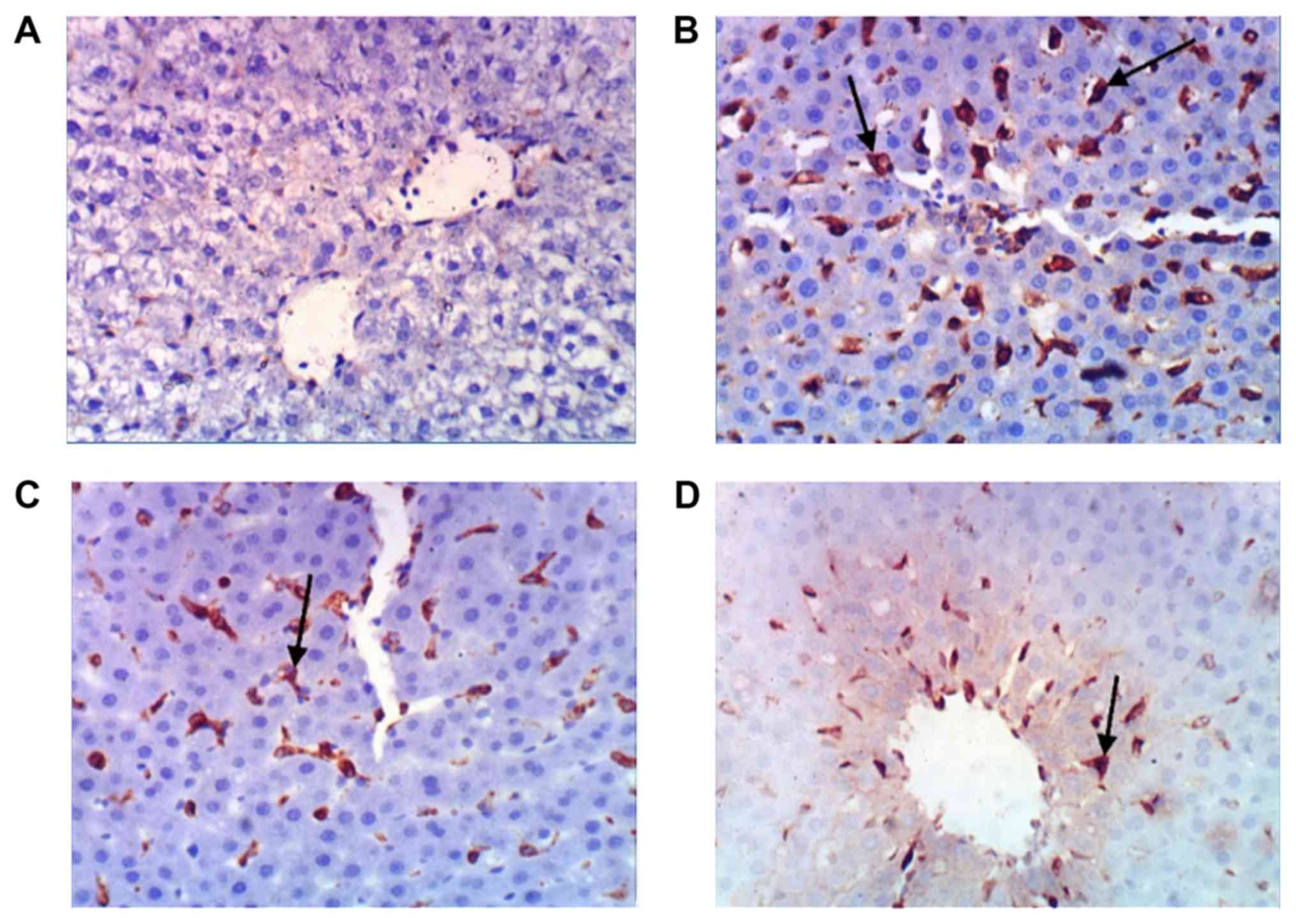
Histopathological analysis
Liver sections from the four treatment groups were
subsequently stained against COX-2 to investigate the extent of
inflammation. Upon CCl4 treatment, strong COX-2 expression was
detected in infiltrated mononuclear phagocytes compared with
control sections (Fig. 6A and B).
This expression was ameliorated following BS or silymarin treatment
(Fig. 6C and D), suggestive of their
curative effects.
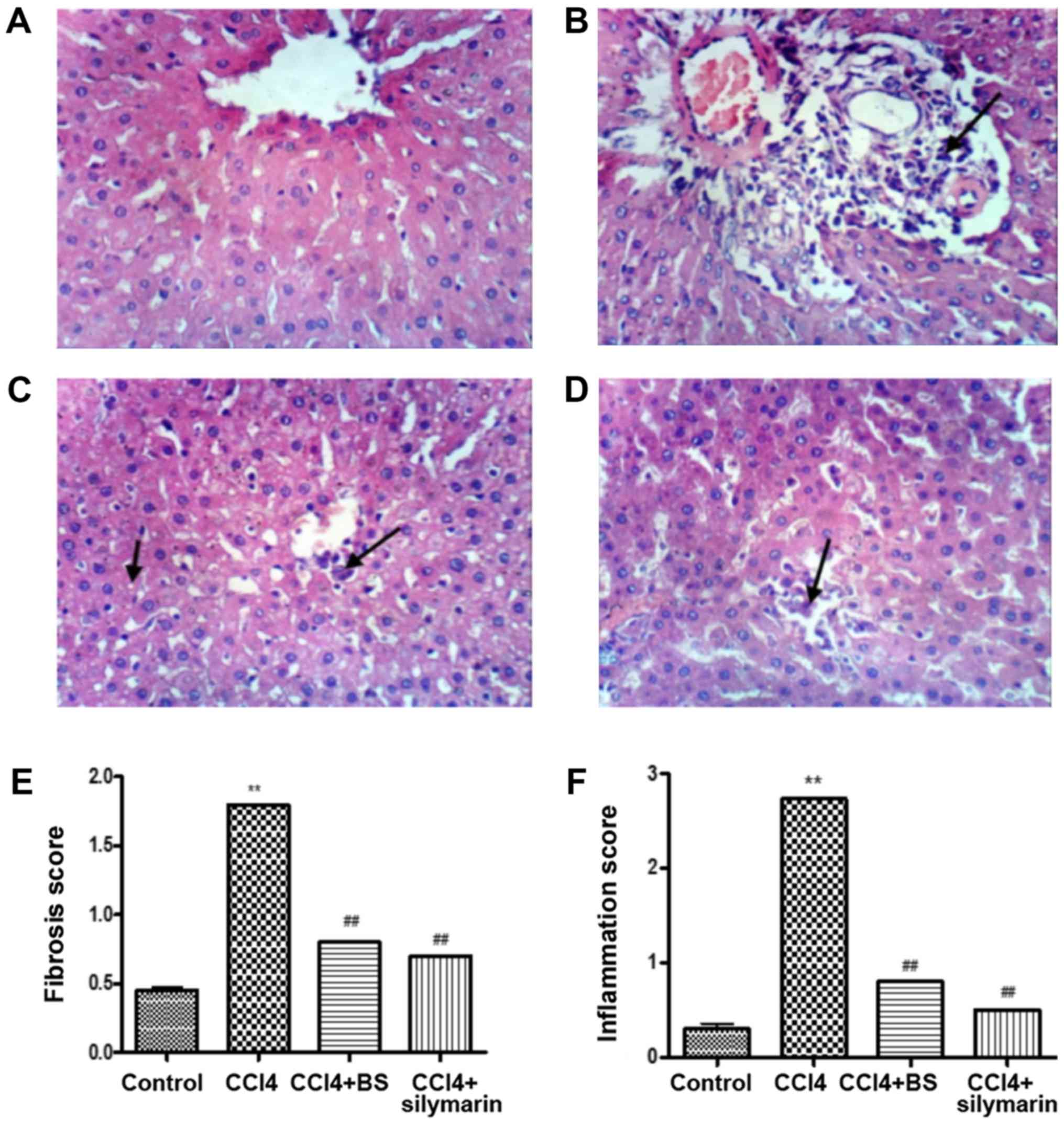
Examination of liver sections stained using H&E
reflected normal morphology and architecture in the control group
(Fig. 7A). In contrast, extensive
hepatocellular damage including portal inflammation, venous
congestion, and fatty changes in the form of hepatocyte
vacuolization and fatty droplets were observed in liver tissue
sections from animals treated with CCl4 (Fig. 7B). BS treatment ameliorated
CCl4-induced liver damage, an effect that was similar to
that achieved by silymarin treatment (Fig. 7C and D). BS and silymarin treatment
significantly reversed CCl4-induced histopathological
damage in terms of fibrosis and inflammation scores (P<0.05;
Fig. 7E and F).
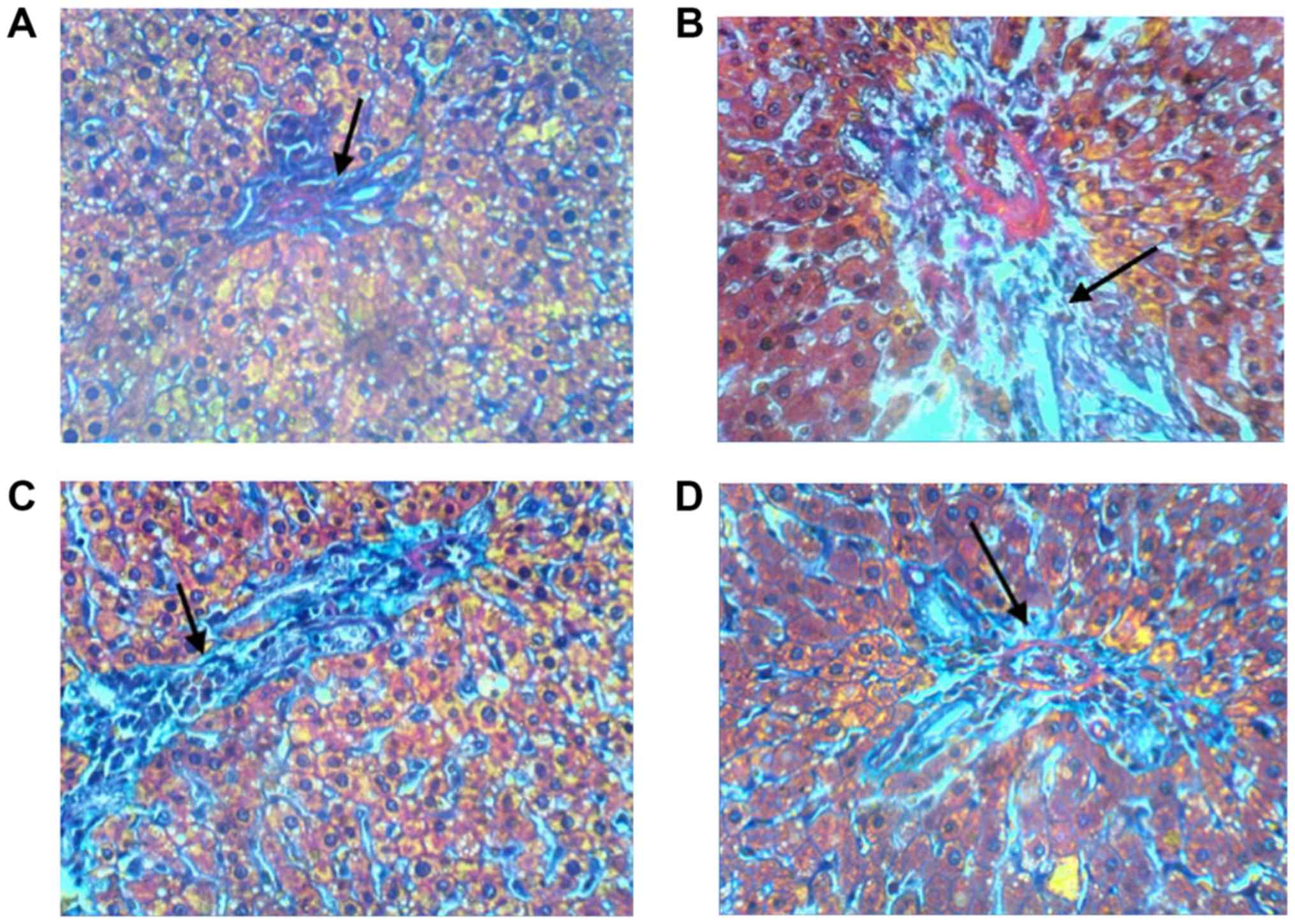
Masson's trichrome staining was performed to
evaluate the extent of liver fibrosis. Liver sections prepared from
normal control rats exhibited normal architecture with minimal
accumulation of collagen fibers (Fig.
8A). Tissues from rats following the administration of
CCl4 showed disrupted tissue architecture, extended
collagen fibers, formation of large fibrous septa, separation of
the pseudolobe and accumulation of collagen as observed by intense
signals of collagen staining (Fig.
8B). BS and silymarin treatment reversed these pathological
changes and decreased collagen deposition (Fig. 8C and D).
Discussion
In the present study, CCl4-treated rats
exhibited severe inflammatory reactions in the form of increased
levels of NF-κB, TNF-α, TGF-β, IL-6, and COX-2 expression. In
addition, lipid peroxidation was increased following
CCl4 treatment, which was accompanied with depleted
endogenous antioxidant capacity. As a consequence of
CCl4-induced liver damage, hepatocytes lost their
membrane integrity and the ability to conjugate or excrete
bilirubin, as reflected by the observed elevations in serum
bilirubin and liver enzymes levels and activities (ALT, AST and
LDH) and disrupted lipid profiles, concordant with a previous
report (24).
BS treatment effectively alleviated
CCl4-induced liver injury as it normalized the
expression levels of TNF-α and NF-κB. It also reversed signs of the
inflammation as reported by the observed normalization of tissue
COX-2 expression and serum levels of TGF-β and IL-6. BS treatment
reduced lipid peroxidation, recovered antioxidant capacity and
increased catalase activity following preceding CCl4
treatment. BS also normalized lipid profiles, levels and activities
of hepatic enzymes and serum bilirubin levels in addition to
improving cell integrity.
These effects of BS were comparable to those
observed following silymarin treatment, a well-documented
hepatoprotective agent. Silymarin consists of a complex of
flavonolignans derived from the milk thistle and is famous for its
antioxidant, anti-inflammatory, antifibrotic and properties in the
liver (25).
Metabolism of xenobiotics such as CCl4, among other
molecules, usually result in the generation of free reactive
radicals, a main inducer of hepatocellular injury (26). The hepatotoxic effects of
CCl4 is caused by the intensive oxidative stress
generated from its metabolism into highly reactive free radicals
(27,28). These free radicals attack several
molecules causing lipid peroxidation, loss of membrane integrity,
leakage of intracellular enzymes, inflammation and liver injury
(17). Prolonged liver injury leads
to the development of fibrosis, cirrhosis and finally
hepatocellular carcinoma. However, fibrosis can be reversed by
removing the causative agents, including relieving oxidative
stress, modifying the inflammatory response, preventing activation
of hepatic stellate cells (HSCs) or reducing the production of
extracellular matrix (ECM) components, to permit hepatocyte
regeneration (29).
However, chronic hepatic injury impairs the
regenerative capacity of hepatocytes by activating apoptosis. In
response to injury, Kupffer cells (KCs) release cytokines and
inflammatory factors, including interleukins, COX-2, TNF-α and
TGF-β as reported in the present and a previous study (28). This activates and transforms HSCs
into hepatic myofibroblasts, which in turn deposit components of
the ECM to initiate fibrosis (30).
TNF-α along with interleukins serve a role in the
overexpression of cyclooxygenase-2 and activating the
transcriptional factor NF-κB (31).
Activated NF-κB amplifies the inflammatory response by activating
the transcription of genes involved in the inflammatory response
and induces fibrosis by stimulating TGF-β signaling and HSCs
activation (32). Cyclooxygnase-2
catalyzes the synthesis of prostanoids that are involved in
inflammation, fibrosis and oncogenesis (33).
Membrane integrity of intact liver cells prevents
excessive leakage of liver enzymes, including ALT, AST and LDH from
the cytosol into the circulation. As membrane integrity becomes
lost in response to CCl4-induced hepatotoxicity, liver enzymes
escape into the circulation reflecting the severity of the damage
(34), and the hepatocytes lose
their ability to conjugate or excrete bilirubin, resulting in
elevated serum bilirubin levels (24).
Based on the observed antioxidant effects of BS, its
administration alleviated oxidative stress leading to diminished
cellular damage and improved hepatocyte integrity and
functionality. This was evident by the reductions in serum
bilirubin and liver enzymes levels, in addition to the
downregulation of cytokines and profibrogenic factors, including
TNF-α, NF-κB, IL-6, TGF-β and COX-2. This was confirmed by the
positive effects of BS on regenerating liver cells by histological
analysis of liver tissue sections.
The antioxidant capacity of natural products is
generally associated with the content of polyphenolic compounds in
their composition (35). Among other
constituents, BS oleo-gum resin is rich in pentacyclic terpenoids
that exhibited anti-inflammatory effects by targeting multiple
pathways in a range of disorders, including rheumatoid arthritis,
ulcerative colitis, bronchial asthma, osteoarthritis and cancer
(12). AKBA and boswellic acids have
been previously reported to inhibit the activation of NF-κB and
production of TNF-α, IL-1, −2, −4 and −6 (7).
Histopathological evaluation of H&E and Masson's
trichrome staining showed that CCl4-treatment induced
histopathological damage and increased collagen deposition.
Treatment with BS resulted in the reduction in fibrosis scoring and
achieved marked improvements in reversing these pathological
changes. This could be due to the observed ability of BS to recover
endogenous antioxidant mechanisms, resulting in the free radicals
being scavenged to allow hepatocyte regeneration. This antioxidant
effect breaks the vicious cycle of continuous inflammation-fibrosis
resulting from the continuous activation of KCs, causing a release
of proinflammatory factors, activation of HSCs and the deposition
of ECM, aiding fibrosis.
In addition to the low costs on the long-term use,
no adverse effects were reported following the use of BS in
treating whole rats (36) or colon
cancer cell lines (37).
Collectively, these findings lead to the conclusion
that BS oleo-gum resin is effective in alleviating
CCl4-induced inflammation and fibrosis in the rat liver.
This effect may be due to its antioxidant properties and its
ability to recover endogenous antioxidant capacity whilst
downregulating the inflammatory response. These effects reported in
the present study in addition to the lack of reported adverse
effects, suggest BS to be a potential therapeutic hepatoprotective
agent.
Acknowledgements
The authors would like to thank Professor Kawkab A.
Ahmed, Professor of Pathology, Faculty of Veterinary Medicine,
Cairo University (Cairo, Egypt), for her assistance in the analysis
of histopathological data.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HME performed the western blot analysis and
histochemistry, edited and revised the manuscript; MAF performed
the biochemical analysis and took part in writing the manuscript;
EMM performed animal maintenance, drug administration, interpreted
the data and took part in writing the manuscript; MAA took part in
writing and revising the manuscript and the statistical analysis of
data; AMS provided Boswellia and took part in data analysis and
verification; MMA designed the study, and revised and edited the
manuscript.
Ethics approval and consent to
participate
Experiments on animals were performed in accordance
with the international ethical guidelines for animal care of the
United States Naval Medical Research Centre, Unit No. 3, Abbaseya,
Cairo, Egypt, accredited by the Association for Assessment and
Accreditation of Laboratory Animal Care International. The present
study was approved by The Research Ethics Committee of the Faculty
of Pharmacy, Minya University (Minya, Egypt).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jia C: Advances in the regulation of liver
regeneration. Expert Rev Gastroenterol Hepatol. 5:105–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biswas SK: Does the Interdependence
between oxidative stress and inflammation explain the antioxidant
paradox? Oxid Med Cell Longev. 2016:56989312016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rates SM: Plants as source of drugs.
Toxicon. 39:603–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Wang X, Liu R, Liu Y, Zhang T, Fu H
and Hai C: Oleanolic acid co-administration alleviates
ethanol-induced hepatic injury via Nrf-2 and ethanol-metabolizing
modulating in rats. Chem Biol Interact. 221:88–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui Y, Yang X, Lu X, Chen J and Zhao Y:
Protective effects of polyphenols-enriched extract from Huangshan
Maofeng green tea against CCl4-induced liver injury in mice. Chem
Biol Interact. 220:75–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimmatkar N, Thawani V, Hingorani L and
Khiyani R: Efficacy and tolerability of Boswellia serrata extract
in treatment of osteoarthritis of knee-a randomized double blind
placebo controlled trial. Phytomedicine. 10:3–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ammon HP: Modulation of the immune system
by Boswellia serrata extracts and boswellic acids. Phytomedicine.
17:862–867. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Ning Z, Lu C, Zhao S, Wang J, Liu
B, Xu X and Liu Y: Triterpenoid resinous metabolites from the genus
Boswellia: Pharmacological activities and potential
species-identifying properties. Chem Cent J. 7:1532013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cuaz-Pérolin C, Billiet L, Baugé E, Copin
C, Scott-Algara D, Genze F, Büchele B, Syrovets T, Simmet T and
Rouis M: Antiinflammatory and antiatherogenic effects of the
NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in
LPS-challenged ApoE-/- mice. Arterioscler Thromb Vasc Biol.
28:272–277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchele B, Zugmaier W and Simmet T:
Analysis of pentacyclic triterpenic acids from frankincense gum
resins and related phytopharmaceuticals by high-performance liquid
chromatography. Identification of lupeolic acid, a novel
pentacyclic triterpene. J Chromatogr B Analyt Technol Biomed Life
Sci. 791:21–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Umar S, Umar K, Sarwar AH, Khan A, Ahmad
N, Ahmad S, Katiyar CK, Husain SA and Khan HA: Boswellia serrata
extract attenuates inflammatory mediators and oxidative stress in
collagen induced arthritis. Phytomedicine. 21:847–856. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu JJ, Toy WC, Liu S, Cheng A, Lim BK,
Subramaniam T, Sum CF and Lim SC: Acetyl-keto-β-boswellic acid
induces lipolysis in mature adipocytes. Biochem Biophys Res Commun.
431:192–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Altmann A, Poeckel D, Fischer L,
Schubert-Zsilavecz M, Steinhilber D and Werz O: Coupling of
boswellic acid-induced Ca2+ mobilisation and MAPK activation to
lipid metabolism and peroxide formation in human leucocytes. Br J
Pharmacol. 141:223–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vargas-Mendoza N, Madrigal-Santillán E,
Morales-González A, Esquivel-Soto J, Esquivel-Chirino C,
García-Luna Y, González-Rubio M, Gayosso-de-Lucio JA and
Morales-González JA: Hepatoprotective effect of silymarin. World J
Hepatol. 6:144–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Li Z, Li H, Li J and Yu C:
Metabolic profile changes of CCl4-liver fibrosis and inhibitory
effects of jiaqi ganxian granule. Molecules. 21:6982016. View Article : Google Scholar
|
|
16
|
Shehata AM, Quintanilla-Fend L, Bettio S,
Singh CB and Ammon HP: Prevention of multiple low-dose
streptozotocin (MLD-STZ) diabetes in mice by an extract from gum
resin of Boswellia serrata (BE). Phytomedicine. 18:1037–1044. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abouzied MM, Eltahir HM, Abdel Aziz MA,
Ahmed NS, Abd El-Ghany AA, Abd El-Aziz EA and Abd El-Aziz HO:
Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT and
caspase-3 expression in experimental rat model. Tumour Biol.
36:1763–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Zheng H, Liu Y, Lin J, Zhong X, Xu
W, Hong Z and Peng J: Anti-inflammatory effects of total alkaloids
from Rubus alceifolius Poir [corrected]. on non-alcoholic fatty
liver disease through regulation of the NF-κB pathway. Int J Mol
Med. 31:931–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suvik A and Effendy AWM: The use of
modified Masson's trichrome staining in collagen enaluation in
wound healing study. Malays J Vet Res. 3:39–47. 2012.
|
|
20
|
Wójcik M, Ramadori P, Blaschke M, Sultan
S, Khan S, Malik IA, Naz N, Martius G, Ramadori G and Schultze FC:
Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue
macrophages in normal rat liver and to recruited mononuclear
phagocytes in liver injury and cholangiocarcinoma. Histochem Cell
Biol. 137:217–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunt EM: Grading and staging the
histopathological lesions of chronic hepatitis: The Knodell
histology activity index and beyond. Hepatology. 31:241–246. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ammon HP: Boswellic acids in chronic
inflammatory diseases. Planta Med. 72:1100–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tawab MA, Kaunzinger A, Bahr U, Karas M,
Wurglics M and Schubert-Zsilavecz M: Development of a
high-performance liquid chromatographic method for the
determination of 11-keto-beta-boswellic acid in human plasma. J
Chromatogr B Biomed Sci Appl. 761:221–227. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tabor E: Hepatocellular carcinoma: Global
epidemiology. Dig Liver Dis. 33:115–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rasool M, Iqbal J, Malik A, Ramzan HS,
Qureshi MS, Asif M, Qazi MH, Kamal MA, Chaudhary AG, Al-Qahtani MH,
et al: Hepatoprotective effects of Silybum marianum (Silymarin) and
Glycyrrhiza glabra (Glycyrrhizin) in combination: A possible
synergy. Evid Based Complement Alternat Med. 2014:6415972014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaeschke H, Gores GJ, Cederbaum AI, Hinson
JA, Pessayre D and Lemasters JJ: Mechanisms of hepatotoxicity.
Toxicol Sci. 65:166–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Recknagel RO, Glende EA Jr, Dolak JA and
Waller RL: Mechanisms of carbon tetrachloride toxicity. Pharmacol
Ther. 43:139–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cohen-Naftaly M and Friedman SL: Current
status of novel antifibrotic therapies in patients with chronic
liver disease. Therap Adv Gastroenterol. 4:391–417. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer DH, Bachem MG and Gressner AM:
Modulation of hepatic lipocyte proteoglycan synthesis and
proliferation by Kupffer cell-derived transforming growth factors
type beta 1 and type alpha. Biochem Biophys Res Commun.
171:1122–1129. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gambhir S, Vyas D, Hollis M, Aekka A and
Vyas A: Nuclear factor kappa B role in inflammation associated
gastrointestinal malignancies. World J Gastroenterol. 21:3174–3183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng J and Hada T: The significance of
COX-2 and COX-2 inhibitors in liver fibrosis and liver cancer. Cur
Med Che Anti Infl Anti Allergy Agents. 4:199–206. 2005. View Article : Google Scholar
|
|
34
|
Brattin WJ, Glende EA Jr and Recknagel RO:
Pathological mechanisms in carbon tetrachloride hepatotoxicity. J
Free Radic Biol Med. 1:27–38. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Younis T, Khan MR and Sajid M: Protective
effects of Fraxinus xanthoxyloides (Wall.) leaves against
CCl4 induced hepatic toxicity in rat. BMC Complement
Altern Med. 16:4072016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh P, Chacko KM, Aggarwal ML, Bhat B,
Khandal RK, Sultana S and Kuruvilla BT: A-90 day gavage safety
assessment of Boswellia serrata in rats. Toxicol Int. 19:273–278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi M, Sung B, Shen Y, Hur K, Link
A, Boland CR, Aggarwal BB and Goel A: Boswellic acid exerts
antitumor effects in colorectal cancer cells by modulating
expression of the let-7 and miR-200 microRNA family.
Carcinogenesis. 33:2441–2449. 2012. View Article : Google Scholar : PubMed/NCBI
|