Introduction
Cystic echinococcosis (CE) is a zoonotic parasitic
disease with worldwide prevalence, which is caused by infection
with Echinococcus granulosus(Eg) (1). For example, infection is more common in
countries and regions with animal husbandry (1,2).
Xinjiang Uygur Autonomous Region of China is one of the areas with
the highest prevalence of CE. According to a survey, the Eg
infection rate is 3.1–31.5% and the prevalence rate of CE disease
is 0.5–5.0% (1). In ~65% of affected
individuals, the disease has no specific clinical signs and is not
distinguishable from any other diseases with similar signs.
Clinical symptoms depend on the organs involved (e.g. lung, liver,
brain and bone), the number, size and location of cysts, the
invasion of cysts into adjacent organs and the induction of
immunological reactions, including asthma and anaphylaxis (3).
Liver fibrosis is a pathological process in chronic
Eg infection. Infection of Eg stimulates and activates hepatic
stellate cells, resulting in secretion of a large number of
collagen fibers and thereby causing excessive deposition of
extracellular matrix (ECM). This process is regulated by a variety
of cytokines (4–6). Studies have indicated that the cytokine
transforming growth factor-β1 (TGF-β1) has a crucial role in wound
healing and fibrosis of tissues and organs (7–12). It is
also an important factor mediating cellular processes, including
cell growth, differentiation, apoptosis and cellular homeostasis
(13). It has been indicated that
TGF-β1 cytokines are involved in chronic Eg infection (14,15). At
the same time, TGF-β1 is an important cytokine in the development
and progression of liver fibrosis. Overexpression of TGF-β1 in the
liver may lead to severe liver fibrosis. However, how TGF-β1
influences liver fibrosis in hepatic CE remains a topic requiring
further investigation. In the present study, the expression of
TGF-β1 in liver tissues and serum of patients with hepatic CE was
detected to investigate the association between TGF-β1 and hepatic
fibrosis and its significance in early diagnosis.
Materials and methods
Case source and grouping
A total of 30 patients with hepatic CE admitted to
the First Affiliated Hospital of Xinjiang Medical University
(Urumqi, China) between July 1, 2013 and June 1, 2017 were enrolled
in the present study. The age of the patients ranged from 9 to 74
years (median age, 28 years) and the male-to-female ratio was
12/18. Paired liver lesion tissue and normal tissue were obtained
from each patient. Healthy control subjects (matched with the
hepatic CE patients according to age and sex) visited the First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China)
for medical examination. Name, age, sex and hospitalization date
were checked to confirm that there were no duplicate cases.
Inclusion criteria
The diagnosis of patients with hepatic CE (patients
confirmed by B-mode ultrasonography first, underwent liver cystic
hydatidosis partial hepatectomy and their biopsy specimen could be
collected) was in accordance with the classification diagnostic
criteria formulated by the World Health Organization (WHO)
echinococcosis unofficial working group (16); the classification was intended to
follow the natural history of CE and started with undifferentiated
simple cysts, as presumably, hydatid cysts evolve from these
structures. This was confirmed by intra-operative and
post-operative pathological diagnosis (16). The patients were grouped based on
their WHO classification as follows: CL, as group 1-Active group:
Cysts developing and are usually fertile (contained numerous viable
protoscoleces); Type CE1-CE3, as group
2-Transition group: Cysts starting to degenerate, but usually still
contain viable protoscoleces; Type CE4-CE5,
as group 3-Inactive group: Degenerated or partially or totally
calcified cysts-unlikely to be fertile. The 30 hepatic CE patients
were classified into stages of CL (0 cases), CE1 (13
cases), CE2 (11 cases), CE3a/b (0 cases),
CE4 (5 cases) and CE5 (1 case). All study
subjects had provided written informed consent and the study
protocols were approved by the ethics committee of the First
Affiliated Hospital of Xinjiang Medical University (Urumqi, China;
approval nos. ZACUS-201304255002 and 20160218-14) and received
informed consent from all subjects. For the minors (<18 years of
age) that participated in the study, written informed consent was
provided by their parents/legal guardians.
Exclusion criteria
The following exclusion criteria were applied: i)
Acute and chronic viral infections and autoimmune diseases; ii)
rheumatic diseases and malignant tumors; iii) conditions including
severe respiratory infections, hepatobiliary infections, sepsis and
hyperpyrexia that are not directly associated with echinococcosis
infection; iv) long-term use of inflammatory inhibitors, including
steroids, non-steroidal anti-inflammatory drugs and opioids.
Blood samples
From each of the 30 patients with hepatic CE and the
healthy individuals, 3 ml venous blood was collected. Peripheral
blood was centrifuged at 1,008 × g for 10 min at 20°C; one aliquot
of the serum was used for liver function tests, including total
protein, albumin, alanine aminotransferase and aspartate
aminotransferase levels, while the other part was stored in a
refrigerator at −20°C for ELISA.
Hepatic tissue specimens
A total of 30 patients with hepatic CE were enrolled
in the present study. Each patient underwent biopsy of normal
hepatic tissue (the normal hepatic tissue 2 cm away from the
lesion) used as the control group and hepatic tissue adjacent to
the lesion, which was not directly part of the lesion, as the case
group (within 2 cm of the lesion) (17). The obtained specimens were kept in
10% formaldehyde and then embedded in paraffin for preparation of
3-µm slices, which were reserved for H&E staining, Masson's
trichrome staining and immunohistochemistry.
Histopathological analysis
H&E staining and Masson staining (Masson
Trichrome Staining kit; Maixin Biotechnologies, Inc.) of hepatic
tissue: All specimens were kept in 10% formaldehyde, embedded in
paraffin and then serially sectioned at a thickness 3 µm. After
H&E staining and Masson staining, pathological changes in
hepatic tissue were observed under a microscope (BX43; Olympus
Corp.), including the presence or absence of liver fibrosis and if
present, the degree of liver fibrosis was assessed (18).
Immunohistochemistry
Paraffin sections were subjected to gradient
dehydration. Diaminobenzidine color development was performed
according to the instructions of the two-step immunohistochemistry
kit (OriGene Technologies, Inc.). Rabbit anti-human TGF-β1 antibody
was from Santa Cruz Biotechnologies, Inc.). Images of 5 fields of
view were randomly captured under a high-power microscope (BX43;
Olympus Corp.) and the area occupied by positive and negative
staining was quantified using ipp6.0 software (Intel). The
percentage of positive area and standard deviation were
calculated.
ELISA
For detection of TGF-β1 in the serum, an ELISA kit
(Bender Med System) was used in accordance with the manufacturer's
protocol.
Statistical analysis
All experimental data were statistically analyzed
using SPSS software version 17.0 (SPSS, Inc.). Quantitative data
are expressed as the mean ± standard deviation. The Student's test
was used for comparison between the data of two groups. Spearman's
correlation coefficient was determined to assess the correlation
between WHO classification and severity of liver fibrosis. Ipp6.0
software (Intel.) was used for quantitative analysis of
morphological results. P<0.05 was considered to indicate a
statistically significant difference.
Results
General information
The cohort comprised 30 patients with hepatic CE.
The age ranged from 9 to 74 years. In accordance with the
classification diagnostic criteria (16) and their clinicopathological features,
30 hepatic CE patients were classified into stages of CL (0 cases),
CE1 (13 cases), CE2 (11 cases),
CE3a/b (0 cases), CE4 (5 cases) and
CE5 (1 case). The mean diameter of lesions was 7.78±4.43
cm and the median diameter of lesions was 7.35 cm.
Laboratory examination
The 30 patients with hepatic CE were diagnosed with
echinococcosis by imaging, surgery and pathology. Prior to the
operation, B-mode ultrasonography is the major examination method
for hepatic CE, which may determine the classification of
echinococcosis, as well as the location, number, size and structure
of hepatic CE lesions, and provide a reference for pre-operative
diagnosis, selection of surgical sites and observation of treatment
effects (19). On B-mode
ultrasonography, different degrees of liver fibrosis were detected.
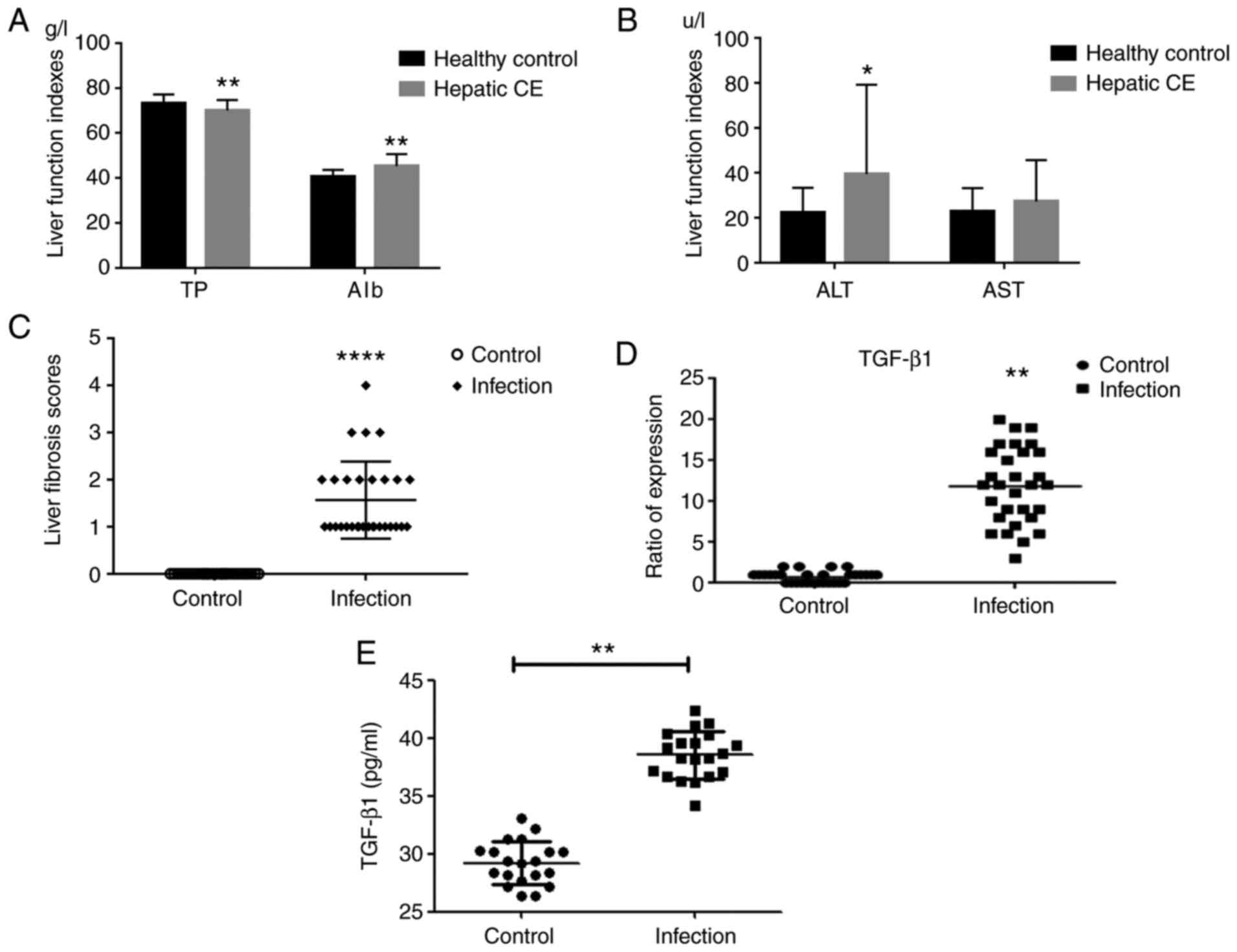
Furthermore, liver function test and assessment of total protein,
albumin, alanine aminotransferase and aspartate aminotransferase
levels were performed for these 30 patients. The results were
significantly different in comparison with those of the healthy
control group. It was clearly indicated that liver function damage
was present in patients with hepatic CE (Fig. 1A and B).
Pathology of liver tissue
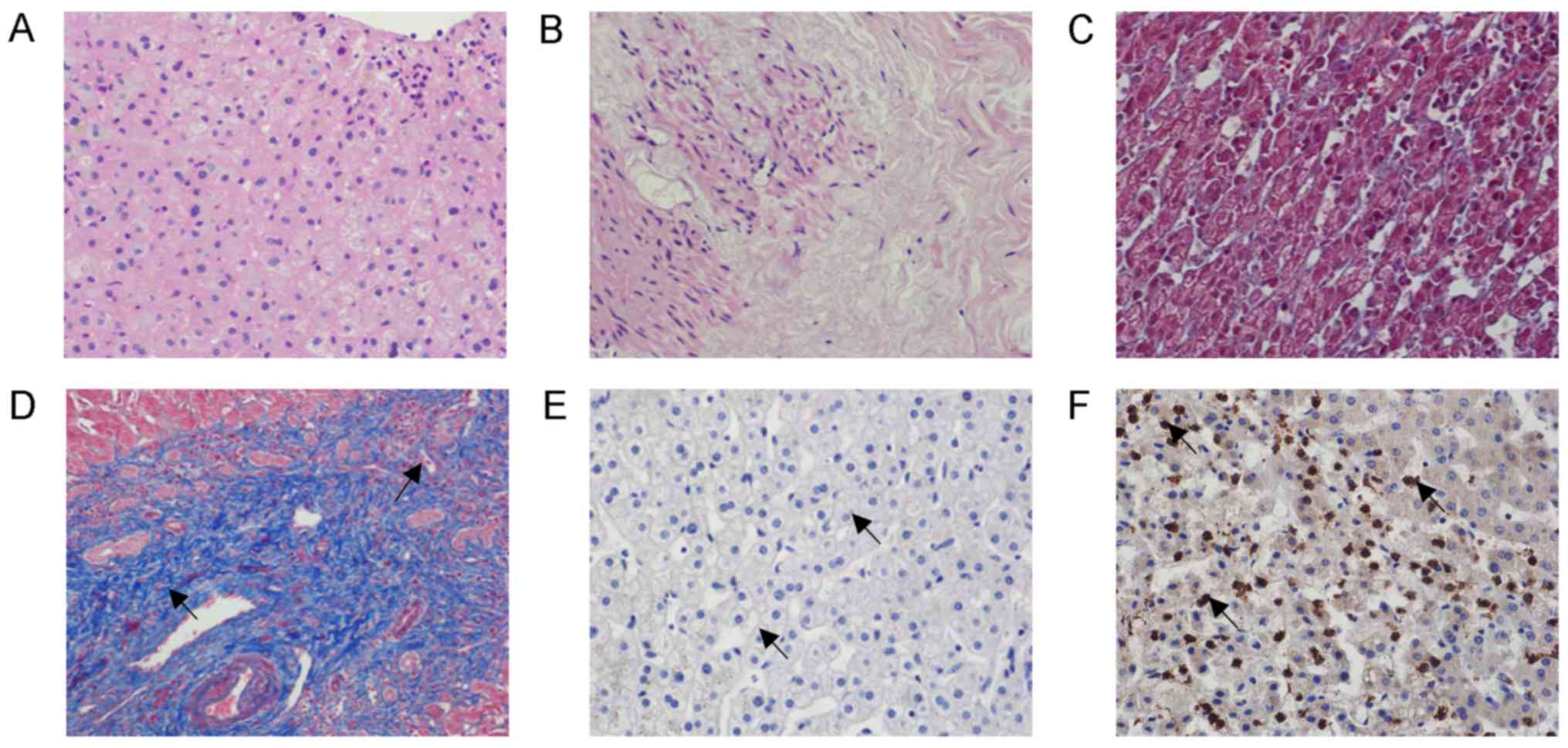
Microscopic observation of H&E-stained tissue
indicated that the boundary of hepatocytes in normal hepatic tissue
was clear and the structure of hepatic lobules was intact. Edema
appeared in the hepatic cells adjacent to the lesion in patients
with hepatic CE. The structure of certain hepatic lobules was
destroyed, collagen fiber deposition was observed and necrotic
hepatic cells were present (Fig. 2A and
B).
On Masson staining, the hepatic tissue of patients
with hepatic CE exhibited different degrees of hepatic steatosis
and spot-like necrosis of certain liver cells was observed. Fibrous
tissue hyperplasia and its extension into the lobular area were
observed in the portal area. Furthermore, fibrous tissue was
distributed in the portal area. In certain patients with hepatic
hydatid cyst, Masson staining resulted in blue staining in the
hepatic portal area and the outer area of the hydatid cyst. In the
control group, only a small amount of blue staining was observed
around the elastic fibers in the blood vessel wall, whereas no
obvious fibrous tissue was observed in other regions of hepatic
tissue (Fig. 2C and D). At the same
time, the liver fibrosis score was obtained from hepatic lesion
tissue of each patient with hepatic CE (Fig. 1C). The correlation between WHO
classification and severity of liver fibrosis was analyzed,
revealing that the WHO classification was positively correlated
with the severity of liver fibrosis (R=0.399, P<0.05; Table I).
 | Table I.Correlation analysis between WHO
classification and severity of liver fibrosis. |
Table I.
Correlation analysis between WHO
classification and severity of liver fibrosis.
|
|
| Sex | Liver fibrosis scores
in patients with hepatic CE |
|---|
|
|
|
|
|
|---|
| WHO
classification | Cases | Male | Female | 0 | 1 | 2 | 3 | 4 |
|---|
| CL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CE1 | 13 | 7 | 6 | 0 | 10 | 3 | 0 | 0 |
| CE2 | 11 | 6 | 5 | 0 | 6 | 4 | 1 | 0 |
|
CE3a/b | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CE4 | 4 | 4 | 0 | 0 | 1 | 0 | 2 | 1 |
| CE5 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 |
Expression of cytokine TGF-β1 in liver
tissue of patients with hepatic CE
Cytokine TGF-β1 was not substantially expressed in
normal hepatic tissues. The expression of TGF-β1 in the hepatic
lesion tissue of patients with hepatic CE was mainly located in the
cytoplasm of hepatocytes (Figs. 1D,
2E and F). The relative levels of
TGF-β1 in hepatic lesion tissue were 11.87±4.64 and 0.73±0.69 in
the control group (P<0.01).
Changes in serum TGF-β1 levels in
patients with hepatic CE
In order to determine the cytokine TGF-β1 expression
in peripheral blood of patients with hepatic CE, the serum levels
of TGF-β1 in each group were determined by ELISA. It was indicated
that the expression of TGF-β1 in serum was significantly increased
in the group of patients with hepatic CE in comparison with that in
the healthy control group (Fig.
1E).
Discussion
On the basis of extensive necrosis of hepatocytes,
liver fibrosis produces diffuse hyperplasia of liver fibrous
tissue. Upon further progression, cirrhosis may occur and
subsequently, regenerated nodules and pseudolobules are formed,
resulting in a normal structural and vascular anatomy of the liver.
Hepatic CE refers to a cystic change caused by the invasion of the
parasite Eg into the liver. Hepatic fibrosis occurs when the worm
body causes damage to the liver through direct erosion, toxic
damage and mechanical pressure. Hepatic fibrosis affects the
function of the liver. Further progression may cause cirrhosis and
even hepatic failure. Early hepatic fibrosis is reversible, whereas
cirrhosis is basically irreversible. Therefore, prevention and
treatment of early hepatic fibrosis are of particular importance.
TGF-β1 is a multifunctional cytokine involved in various biological
processes, including tumors, inflammatory cell differentiation and
tissue repair (20,21) High expression of TGF-β1 was reported
in lymphocytes surrounding hepatic lesions and high expression of
inflammatory cytokine TGF-β1 was observed around hepatic lesions of
patients with alveolar echinococcosis (AE) and in a mouse Em
infection model (22,23). AE is a severe chronic helminthic
disease caused by the intra-hepatic tumor-like growth of the
metacestode of Echinococcus multilocularis. Gottstein et
al (23) pointed out that
cytokine TGF-β1 is involved in the parasite-host interaction in AE.
AE and CE are two types of echinococcosis, i.e., echinococcosis
caused by inoculation of the eggs of Echinococcus
multilocularis or Eg, respectively. The above studies have
elucidated the immune effect of cytokine TGF-β1 in AE, and the
present study aimed to elucidate whether cytokine TGF-β1 has a
similar role in CE. Of note, liver fibrosis is part of the two
pathologies; therefore, liver fibrosis in hepatic AE is the next
research goal of our group. In the present study, cytokine TGF-β1
levels were markedly increased in diseased tissues and organs,
particularly in areas of fibrosis. Exogenous TGF-β1 may cause
fibrosis of tissues and organs and excessive deposition of ECM of
cells if used in experimental animals and the treatment of
experimental anti-TGF-β1 may inhibit the formation of fibrosis. In
a previous study by our group, the degree of fibrosis in the livers
of mice infected with Eg gradually increased with the prolongation
of parasite infection; in line with this, the TGF-β1 levels in the
mice were gradually increased and were positively correlated with
the degree of liver fibrosis (25).
This may indicate that the cytokine TGF-β1 is the core substance
regulating the development of liver fibrosis (26). Therefore, understanding the dynamic
changes and effects of the most important cytokine, TGF-β1, in the
development of liver fibrosis diseases is of great significance for
the interpretation of the mechanism and treatment of hepatic
fibrosis.
The clinical data of the present cohort indicated
that patients with hepatic CE may have different degrees of liver
fibrosis; however, the degree of fibrosis is not significant, which
may be associated with the current development of imaging diagnosis
(27). An association with infection
is frequently present at the early stage, at the beginning of the
disease caused by infection by the parasite (28). In the present study, patients with
hepatic CE exhibited no ethnic differences and the disease was
frequently accompanied by hepatic damage. In addition, hepatic
tissue specimens from patients with hepatic CE were observed to
include vesicle tissue. On H&E staining, pathological features
including inflammatory cell infiltration, steatosis and necrosis
were observed in the hepatic tissue, while Masson staining
indicated different degrees of fibrosis in the hepatic tissue. It
was suggested that hepatic CE causes pathological damage to the
liver, as well as different degrees of hepatic fibrosis. In the
present study, the WHO classification was positively correlated
with the severity of liver fibrosis. It is possible that cytokine
TGF-β1 activates hepatic stellate cells when the parasite infects
the liver of the patient, which promotes hepatic fibrosis and is
accompanied by infiltration of inflammatory neutrophils and
fibroblasts. A future study by our group will investigate whether
hepatic stellate cell activation is associated with the cytokine
TGF-β1. With the growth of the hydatid sac, severe inflammatory
reaction leads to the formation of a fibrous layer around the
hydatid cyst to separate it from the host tissue, effectively
avoiding the host's immune response, which is conducive to the
growth and erosion of the parasite. In the present study,
immunohistochemistry and serum ELISA were used to detect the
expression of cytokine TGF-β1 in patients with hepatic CE,
indicating that cytokine TGF-β1 has an important role in liver
fibrosis in hepatic CE. Determination of serum cytokine TGF-β1
levels may contribute to the diagnosis of liver fibrosis,
particularly in early liver fibrosis, suggesting that anti-TGF-β1
treatment may help to treat Eg infection. For analysis of serum
levels, the healthy control group of the present study was matched
with the case group in terms of age and sex. Liver lesion tissue
and normal tissue were paired from the same patient. There was no
influence of age or sex on the data analysis. It is also required
to evaluate liver fibrosis in hepatic CE based on protein
expression levels and serological levels, such as liver fibrosis
activation indictors including α-smooth muscle actin, collagen I
and III. In addition, in future studies, the sample size requires
to be expanded. It is crucial to identify novel methods to improve
the treatment of echinococcosis in order to enhance the quality of
life and survival rates of patients with hepatic CE.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation (grant nos. 81760372 and 81260253) and the State
Key Laboratory of Pathogenesis, Prevention and Treatment of
High-Incidence Diseases in Central Asia (grant no.
SKL-HIDCA-2018-27).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM, JG and FT made substantial contributions to
conception and design. YL, XS were responsible for designing the
clinical and experimental studies, drafting the manuscript, and
revising it critically for important intellectual content. NY, DB
and JL made substantial contributions to collection of samples and
patients' general information. XZ and CZ made substantial
contributions to analysis and interpretation of data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocols were approved by the ethics
committee of the First Affiliated Hospital of Xinjiang Medical
University (Urumqi, China; approval nos. ZACUS-201304255002 and
20160218-14) and received informed consent from all subjects. For
the minors (<18 years of age) that participated in the study,
written informed consent was provided by their parents/legal
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou
X, Wen H and McManus D: Epidemiology and control of echinococcosis
in central Asia, with particular reference to the People's Republic
of China. Acta Trop. 141:235–243. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi P, Tamarozzi F, Galati F, Pozio E,
Akhan O, Cretu CM, Vutova K, Siles-Lucas M, brunetti E and Casuli
A; HERACLES extended network, : The first meeting of the European
register of cystic echinococcosis (ERCE). Parasit Vectors.
9:2432016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Devi AM, Venumadhav T, Sukanya B, Manmada
TR, Gopal P and Rammurti S: Role of imaging in diagnosis,
predicting biological activity and in treatment plan of hydatid
disease. Open J Intern Med. 8:177–195. 2018. View Article : Google Scholar
|
|
4
|
Ramachandran P and Iredale JP: Liver
fibrosis: A bidirectional model of fibrogenesis and resolution.
QJM. 105:813–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clin Gastroenterol.
25:195–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kong X, Horiguchi N, Mori M and Gao B:
Cytokines and STATs in liver fibrosis. Front Physiol. 3:692012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman SL: Evolving challenges in
hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 7:425–436. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorelik L and Flavell RA: Transforming
growth factor-beta in T-cell biology. Nat Rev Immunol. 2:46–53.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perumal N, Perumal M, Halagowder D and
Sivasithamparam N: Morin attenuates diethylnitrosamine-induced rat
liver fibrosis and hepatic stellate cell activation by co-ordinated
regulation of Hippo/Yap and TGF-β1/Smad signaling. Biochimie.
140:10–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kocabayoglu P and Friedman SL: Cellular
basis of hepatic fibrosis and its role in inflammation and cancer.
Front Biosci (Schol Ed). 5:217–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsolaki E, Athanasiou E, Gounari E, Zogas
N, Siotou E, Yiangou M, Anagnostopoulos A and Yannaki E:
Hematopoietic stem cells and liver regeneration: Differentially
acting hematopoietic stem cell mobilization agents reverse induced
chronic liver injury. Blood Cells Mol Dis. 53:124–132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu X, Rui W, Wu C, He S, Jiang J, Zhang X
and Yang Y: Compound astragalus and salvia miltiorrhiza extracts
suppress hepatocarcinogenesis by modulating transforming growth
factor-β/Smad signaling. J Gastroenterol Hepatol. 29:1284–1291.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta signaling through the Smad pathway: Role in
extracellular matrix gene expression and regulation. J Invest
Dermatol. 118:211–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pang N, Zhang F, Ma X, Zhu Y, Zhao H, Xin
Y, Wang S, Chen Z, Wen H and Ding J: TGF-β/Smad signaling pathway
regulates Th17/Treg balance during Echinococcus
multilocularis infection. Int Immunopharmacol. 20:248–257.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma X, Wang L, Zhao H, Pang N, Zhang F,
Jiang T, Liu X, Mamuti W, Wen H and Ding J: Th17 cells are
associated with the Th1/Th2-cell balance during Echinococcus
multilocularis infection. Mol Med Rep. 10:236–240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
WHO Informal Working Group, :
International classification of ultrasound images in cystic
echinococcosis for application in clinical and field
epidemiological settings. Acta Trop. 85:253–261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Wang L, Ali T, Li L, Bi X, Wang
J, Lü G, Shao Y, Vuitton DA, Wen H and Lin R: Hydatid cyst fluid
promotes peri-cystic fibrosis in cystic echinococcosis by
suppressing miR-19 expression. Parasit Vectors. 9:2782016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali M, Zhang T and Jing H: The clinical
value and significance of B type ultrasound in liver cystic
echinococcosis. Prog Mod Biomed. 13:5483–5485. 2013.
|
|
20
|
Dong C: TH17 cells in development: An
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin-Martin N, Slattery C, McMorrow T
and Ryan MP: TGF-β1 mediates sirolimus and cyclosporine A-induced
alteration of barrier function in renal epithelial cells via a
noncanonical ERK1/2 signaling pathway. Am J Physiol Renal Physiol.
301:F1281–F1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S, Hüe S, Sène D, Penfornis A,
Bresson-Hadni S, Kantelip B, Caillat-Zucman S and Vuitton DA:
Expression of major histocompatibility complex class I
chain-related molecule A, NKG2D, and transforming growth
factor-beta in the liver of humans with alveolar echinococcosis:
New actors in the tolerance to parasites? J Infect Dis.
197:1341–1349. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gottstein B and Hemphill A:
Echinococcus multilocularis: The parasite-host interplay.
Exp Parasitol. 119:447–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Zhang C, Wei X, Blagosklonov O, Lv
G, Lu X, Mantion G, Vuitton DA, Wen H and Lin R: TGF-β and
TGF-β/Smad signaling in the interactions between Echinococcus
multilocularis and its hosts. PLoS One. 8:e553792013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Abudounnasier G, Zhang T, Liu X,
Wang Q, Yan Y, Ding J, Wen H, Yimiti D and Ma X: Increased
expression of TGF-β1 in correlation with liver fibrosis during
Echinococcus granulosus infection in mice. Korean J
Parasitol. 54:519–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Hu H and Yin J: Therapeutic
strategies against TGF-beta signaling pathway in hepatic fibrosis.
Liver Int. 26:8–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petitclerc L, Sebastiani G, Gilbert G,
Cloutier G and Tang A: Liver fibrosis: Review of current imaging
and MRI quantification techniques. J Magn Reson Imaging.
45:1276–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cruz A, Cooper P, Figueiredo C,
Alcantara-Neves N, Rodrigues L and Barreto M: Global issues in
allergy and immunology: Parasitic infections and allergy. J Allergy
Clin Immunol. 140:1217–1228. 2017. View Article : Google Scholar : PubMed/NCBI
|