Introduction
According to the WHO data (1), cardiovascular diseases (CVD), such as
coronary heart disease (CHD) and stroke are globally the most
frequent causes of death. Hypertension, dyslipidemia, and
atherosclerosis are considered as key risk factors for CVD
(2,3).
The augmentation index (AI) is a parameter measured
by EndoPAT™ that characterizes arterial elasticity/stiffness
(4). As a pressure wave moves
through the arterial tree, it encounters impedance, resulting in a
reflected wave that moves back toward the heart and may augment
peak systolic pressure. Arterial stiffness increases pulse wave
velocity, causing early reflection of this waveform. Thus, lower AI
values reflect better arterial elasticity and a low peripheral
resistance of the arterial wall. Conversely, increased arterial
stiffness results in an altered AI, associated with high peripheral
resistance of the arterial wall (4).
AI in the Endo PAT™ is calculated from the PAT™
pulses at the baseline period of the occluded arm, by averaging
multiple valid pulses and finding the systolic peak (P1) and the
backward reflected peak (P2) and then using the following formula:
(P2-P1)/P1 (4). AI75 is
standardized to a heart rate of 75 beats per minute.
In 2008, a total of approximately 40% of adults age
25 and older were diagnosed with hypertension (5). However, the early stages of increased
blood pressure (BP) rarely cause symptoms, and thus remain
undiagnosed in many cases (2).
The classification for different levels of BP should
be based upon ≥2 readings at ≥2 separate occasions, separated by at
least 1 week. In individuals >50 years of age, a systolic blood
pressure (SBP) >140 mmHg is a much more important risk factor of
CVD than diastolic blood pressure (DBP) (6). The risk of CVD, beginning at 115/75
mmHg, doubles with each increment of 20/10 mmHg. Subjects who have
a normal BP at 55 years of age have a 90% lifetime risk of
developing hypertension (6). If an
individual's BP rises above the normal level, lifestyle
modifications without drug treatment are recommended in the first
line to prevent CVD, at least in the absence of <3 additional
risk factors (7). In The Seventh
Report of the Joint National Committee on Prevention, Detection,
Evaluation and Treatment of High Blood Pressure, Chobanian et
al (6) defined normal blood
pressure as a SBP <120 mmHg and DBP <80 mmHg,
pre-hypertension as a SBP 120–139 mmHg and DBP 80–89 mmHg, and
stage 1 hypertension as a SPB 140–159 mmHg and DBP 90–99 mmHg.
Various forms of dyslipidemia lead to an increased
risk of CVD, independently of e.g., known genetic variations or
lifestyle (8,9). Lipid parameters with known association
to CVD include elevated low-density lipoprotein cholesterol (LDL-C)
or triglyceride (TG) concentrations or high-density lipoprotein
cholesterol (HDL-C) below the lower limit of the normal range.
The determination of total cholesterol (TC) has been
established for decades in routine laboratory work as a first
screening step, with fractioning into HDL-C and LDL-C in the case
of elevated levels. Furthermore, a causal association between
increased circulating TG levels and CHD has now been established
(10).
Non-HDL-C is a parameter first proposed by Di
Angelantonio et al (11)
following the analysis of >300,000 individuals with lipid
assessments in vascular disease. The authors revealed that hazard
ratios (HR) were almost identical to those observed with Apo-B and
Apo-AI. Non-HDL-C levels are easier to assess than LDL-C levels,
which are calculated indirectly by subtracting HDL-C and fasting TG
levels from TC levels. Instead, non-HDL-C is calculated by the
subtraction of HDL-C from non-fasting TC levels, i.e. it does not
require a fasting state, but is also valid in fasted subjects
(12). As it also provides better
estimates of a treatment effect and of CVD risk reduction than
LDL-C, it is recommended in the most recent British NICE guidance
of July 2014 (13).
Garlic (Allium sativum L.) has been used in
traditional medicine for centuries. Aged garlic extract (AGE) is an
odorless garlic preparation that has been shown to contain various
water-soluble organic sulfur compounds, such as S-allylcysteine
(SAC) and S-1-propenylcysteine, which are formed during its unique
manufacturing process (14). AGE and
its components have been shown to display various health benefits
that have been described in >820 publications of in vitro and
animal studies, as well as in clinical trials, reporting the
cardiovascular protective effects against cholesterol levels
(15), hypertension (16,17),
homocysteine levels (18), LDL
oxidation (18), anti-platelet
aggregation and adhesion (15,19),
blood circulation (20), etc. It can
be concluded that the safety and beneficial effects of AGE in the
area of cardiovascular health have been well-established.
The aim of this randomized, double-blind,
placebo-controlled, parallel-group nutritional study was to
evaluate the benefit of AGE on arterial elasticity, as well as its
tolerability in overweight subjects with high normal and
hypertension grade 1 blood pressure. In addition, changes in blood
pressure and lipid profiles were explored.
Materials and methods
Participants
A total of 57 generally healthy male and female
subjects were randomised into the study (26 verum, 29 placebo)
according to the inclusion and exclusion criteria presented in
Table I. In total, 28 participiants
were administered the AGE, hereinafter referred to as verum, and 29
were administered a placebo. Following randomization, 2 subjects
completed the study before visit 3 (v3) without any measurement
values post baseline visit. These subjects were excluded from the
full analysis set (FAS) and valid case analysis set (VCAS), but
were be kept in the safety population. All subjects voluntarily
gave their written informed consent. The clinical trial was
approved by the Ethics Committee of Charité - Universitaetsmedizin
Berlin, Germany.
 | Table I.Inclusion and exclusion criteria. |
Table I.
Inclusion and exclusion criteria.
| Inclusion
criteria | Exclusion
criteria |
|---|
| Age, 40–75
years | Known allergy or
hypersensitivity to the components of the investigational product,
genetic hyperlipidemia, secondary hypertension, white-coat
hypertension, type-1-diabetes or type-2-diabetes that was
uncontrolled or diagnosed within the last 6 months prior to first
visit, untreated or non-stabilized thyroid disorder |
| Body mass index
(BMI): 25–34,9 kg/m2 | History and/or
presence of clinically significant cardiovascular disease as per
investigator's judgement such as known congenital heart defects,
myocardial infarction, heart failure, angina pectoris,
life-threatening arrhythmia or stroke within the last 6 months
prior to first visit, existing thrombosis or disposition to
thrombosis, or any other known significant or serious
conditions/diseases that might render subjects ineligible, e.g., a
history of malignancy within the past 5 years prior to the first
visit, bleeding disorders and/or need for anticoagulants, current
psychiatric care and/or use of neuroleptics, bariatric surgery in
the last 12 months prior to the first visit, any known metabolic
disease, gastrointestinal disorder or other clinically significant
disease/disorder which in the investigator's opinion could
interfere with the results of the study or the safety of the
subject |
| High normal or
hypertension grade 1 blood pressure levels (130–159/85–99
mmHg) | Arm lymphedema
(e.g., due to mastectomy) |
| EndoPAT™ reactive
hyperemia index (RHI) score of <2.2 at first visit | Deviations of
laboratory parameter(s) at the first visit that were clinically
significant or 2× the upper limit of normal (ULN), unless the
deviation was justified by a previously known not clinically
relevant condition, e.g., Gilbert's syndrome) |
| Readiness to comply
with the study procedures, in particular with consumption of the
investigational product (IP) as instructed during the treatment
period | Dietary habits that
may interfere with the study objectives, such as eating disorders,
dietary restrictions that may affect the study outcome,
participation in a weight loss program or use of weight loss
treatment |
| Adhering to their
respective former diets (except consumption of max. 2 garlic cloves
per week) and physical activity, requirements for blood
pressure/EndoPAT™ measurements, and accepting blood draws | Making use of the
following medication/supplementation within the last 4 weeks prior
to first visit and during the study, according to investigator's
judgement: |
|
| • Drugs or
supplements that can influence SBP or DBP (e.g., ACE (angiotensin-
converting-enzyme) inhibitors, diuretics, calcium channel or
β-blockers, grape seed extract, coenzyme Q10 etc.) |
|
| • Lipid-lowering
drugs (affecting lipid metabolism, platelet function or antioxidant
status, etc.) |
|
| • Dietary or health
supplements (e.g., omega-3 fatty acids, green tea extract, calcium,
red yeast rice, phytosterols (incl. enriched products such as,
Becel), oat fiber, niacin, soy protein, psyllium seed husk,
glucomannan, chitosan or probiotics/prebiotics) |
|
| • Drugs that can
significantly influence cholesterol levels (e.g., corticosteroids,
amiodarone, anabolic steroids) |
|
| • Medications
(e.g., statins, renin angiotensin system inhibitors, nebivolol,
carvedilol, calcium channel blockers) |
|
| • Supplements
(e.g., cocoa) that can influence vascular endothelial function
and/or blood flow within the last 4 weeks prior to first visit and
during the study |
|
| • Antiplatelet
agents and/or anticoagulants (e.g., warfarin, acetylsalicylic
acid) |
| Non-smokers or,
respectively, smoking cessation in the last ≥12 months prior to the
first visit | Drug abuse or
alcohol abuse (males, ≥21 U/week; females, ≥14 U/week; 1 unit
equals approximately 250 ml of beer, 100 ml of wine or 35 ml of
spirits) |
| Stable body weight
over the past 3 months prior to the first visit (<3 kg
self-reported change) | Reported
participation in night-shift work 2 weeks prior to first visit
and/or during the study |
| If any allowed
concomitant medications stable at least during the last month prior
to the first visit | Participation in
another study or blood donation during the last 30 days prior to
the first visit and any other reason deemed suitable for exclusion
as per the investigator's judgment were taken as further exclusion
criteria |
| Negative pregnancy
testing (β human chorionic gonadotropin test in urine) at first
visit (women of childbearing potential) | For women of
child-bearing potential: being pregnant or breast-feeding |
Interventions
AGE is manufactured under ISO 9001 quality control
and Good Manufacturing Practices (GMP) under a license issued by
the Ministry of Health, Labor and Welfare of Japan and according to
the following steps: Organically grown raw garlic (Allium
sativum L.) was cut into slices, immersed in aqueous ethanol,
and aged over a period of 10 months at room temperature. The
procedure and specifications are described in the US
Pharmacopeia/National Formulary (USP/NF) monograph (21).
AGE was stored according to the manufacturer's
recommendations. The placebo was a liquid identical in color and
flavor to the verum. In order to maintain the ‘blind’ factor with
respect to the odour, the placebo contained 3% concentrated AGE
(drug-extract-ratio: 0.9–1.2:1), which is considered as inactive
with respect to any potential beneficial effect. The daily uptake
amount was 2 ml (with 1 ml taken twice per day at mealtimes) for a
duration of 84±3 days.
EndoPAT™
In this study, EndoPAT™ parameters AI75
and the reactive hyperemia index (RHI) were assessed as per
Endo-PAT 2000 according to standardized procedures provided by the
manufacturer (Itamar Medical Ltd.), at visits 1 (RHI only), 2 and
4.
Blood pressure measurements
Seated BP measurements were taken in a quiet
environment after the subjects had been seated for at least 15 min
upon arrival. The subjects were positioned in a comfortable manner
with their feet located on the floor, legs not crossed and back
placed against the back of a chair. The measurements were carried
out in triplicate according to a standardized method (22). The three BP measurements were
performed with 2- to 5-min intervals inbetween. The first
measurement was disregarded and the mean of the latter two was
calculated. If blood pressure varied in these determinations by ≥5
mmHg, 2 additional measurements were performed to measure SBP and
DBP. The final two measurements were then averaged to determine the
overall SBP and DBP for each subject.
The time of day the measurements were taken was also
documented. A trained medical professional using a standardized
calibrated oscillometric device with a universal 22–42 cm cuff
performed the measurements. At visit 1, BP measurements were
performed on both arms; in the case of difference, the dominant arm
(with the higher BP) was used. The specification of which arm was
used for the BP measurements was documented. The measurements were
performed using the same method and equipment, the same arm (with
the arm supported at heart level and slightly flexed at the elbow)
at similar times of day per subject, preferably by the same medical
professional for individual subjects. The subject and the medical
professional were required to remain silent and not talk while the
measurements were being taken. The subjects were instructed and had
to comply with the following restrictions prior to any BP
assessment: i) To avoid any strenuous exercise and stimulants
(alcohol and caffeine) for at least last 24 h prior to the
assessment; ii) to refrain from extreme heat and cold exposure and
fluid and food intake for at least 1 h prior to the assessment;
iii) to empty their bladder and bowel prior to the assessment.
Determination of lipid parameters
Fasting blood samples were obtained for the
assessment of the serum TC, LDL-C, HDL-C and TG concentrations at
visits 1 through 4, with the results obtained from visit 1
considered as safety/eligibility assessments. Values from samples
at visit 2 were used as baseline. Measurements were performed in a
central laboratory using standard procedures.
SCORE value assessment
The SCORE value was assessed as described by the
European Society of Cardiology (23).
Global assessment
Both the subjects and investigator(s) evaluated
independently the benefit and the tolerability of the
Investigational Product (IP) by means of a global scaled evaluation
with ‘very good’, ‘good’, ‘moderate’ and ‘poor’.
Visit schedule
Visit 1 was conducted at day 0. Visit 2 (baseline)
was conducted 10–14 days after visit 1. Visit 3 (control) was
conducted 6 weeks ± 3 days after visit 2. Visit 4 (final) was
conducted 6 weeks ± 3 days after visit 3 (for the visit schedule,
please refer to Table II).
 | Table II.Visit schedule. |
Table II.
Visit schedule.
|
Procedure/assessment | Visit 1
screening | Visit 2 baseline
10+4 days after visit 1 | Visit 3 control 6
weeks ± 3 days after visit 2 | Visit 4 final 6
weeks ± 3 days after visit 3 |
|---|
| Subject
information | X |
|
|
|
| Written informed
consent | X |
|
|
|
| Anamnestic,
demographic data | X |
|
|
|
| Inclusion and
exclusion criteria | X |
Xa |
|
|
| Check eligibility
criteria |
| X |
|
|
| Randomization |
| X |
|
|
| Medical
history/concurrent diseases | X |
|
|
|
| Concurrent
treatment (incl. supplementation) | X | X | X | X |
| Physical
examination (incl. an electrocardiogram) | X |
|
|
|
| Blood pressure and
pulse rate | X | X | X | X |
| BMI, body weight
and heightb | X | X | X | X |
| Urine
analysisc | X |
|
| X |
| Blood draw safety
parameters (including glycated hemoglobin (HbA1c),
thyroid-stimulating hormone (TSH)d | X |
|
| X |
| Blood draws
(fasted) for lipids (TC, LDL-C, HDL-C, TG) | X | X | X | X |
| Changes in dietary
habits |
| X | X | X |
| Changes in physical
activity |
| X | X | X |
| EndoPAT™
measurements | X | X |
| X |
| Issue of
investigational product (IP) |
| X | X |
|
| Issue of IP
instructions |
| X |
|
|
| Collection of IP
and check of compliance |
|
| X | X |
| Adverse events | X | X | X | X |
| Global evaluation
of benefit and tolerability by subject and investigator |
|
|
| X |
Outcome measures
To characterize the benefits of AGE, the following
endpoints were analysed in comparison between the verum and
placebo: EndoPAT™ AI75 and RHI at visit 4 vs. visit 2:
i) SBP at visits 3 and 4 vs. visit 2, respectively; ii) DBP at
visits 3 and 4 vs. visit 2, respectively; iii) fasting LDL-C
concentrations and non-HDL-C at visits 3 and 4 vs. visit 2,
respectively; iv) fasting TC concentrations at visits 3 and 4 vs.
visit 2, respectively; v) fasting HDL-C concentrations at visits 3
and 4 vs. visit 2, respectively; vi) fasting TG concentrations at
visits 3 and 4 vs. visit 2, respectively; vii) fasting LDL-C/HDL-C
and TC/HDL-C ratio at visits 3 and 4 vs. visit 2, respectively;
viii) SCORE value at visits 3 and 4 vs. visit 2, respectively; ix)
global evaluation of benefit by the subjects/investigator at visit
4.
Statistical analysis
The benefit endpoints, as well as the safety and
tolerability and other concomitant variables received an
explorative examination and were descriptively assessed. For the
metric data (continuous data), the statistical characteristics are
given (number, mean, standard deviation, median, extremes,
quartiles). For ordinal data (discrete data), the frequency
distribution was performed. If suitable, ordinal data was
supplementary considered as metric data. All nominal data
(categorical data) was summarized using frequency tables. The
values of metric data could be merged in ordinal classes according
to clinical criteria to determine their frequency distribution.
Performing the explorative estimation the structural consistency of
the verum and placebo groups at baseline was proven in detail. If
there were differences or distinctive individual values
(‘outliers’), their possible influences on the study results was
considered.
All secondary, safety and tolerability, and
concomitant variables were evaluated primarily by exact
non-parametrics procedures as follows: i) The Mann-Whitney U test
for independent groups (comparison of groups or subgroups); ii) the
Wilcoxon test for dependent groups (comparison pre-post within
groups or subgroups); and iii) Fisher's exact test for the
comparison of percentages. Parametric procedures (t-tests)
supplemented the analysis if the scale of the observed values
justified this type of test.
The influence of baseline values was investigated
using analysis of variance with baseline value as covariate.
Changes in variables over time (repeated measurements) were
analyzed using analysis of variance (one-way ANOVA) with respect to
differences in groups and systematic changes over time within each
group, respectively. All tests were performed with a significance
level (type I error) of 5.0% (two-tailed test). The 95% confidence
interval was performed. Multiple tests were performed without
correction of significance level in the explorative analysis. All
P-values of statistical tests in the exploratory analysis, which go
beyond the examination of the primary endpoint, were also
understood only exploratory, meaning they did not serve to confirm
in advance proposed theses. A value of P<0.05 was considered to
indicate a statistically significant difference.
Analysis of datasets
The statistical analysis was carried out in the full
analysis set (FAS) population, which is defined as all subjects
enrolled in the study who have at least once taken the
investigational product and for whom benefit parameters can be
demonstrated. The primary endpoints were also evaluated in the
valid case analysis set (VCAS) population composed of all subjects
in the FAS population, for which there are no major protocol
violations. Secondary endpoints and other parameters can also be
evaluated in the VCAS population as a sensitivity analysis. The
evaluation of the safety endpoints is performed in the safety
population, for all the subjects who have at least once taken the
investigational product. No interim analysis was performed.
Results
Population characteristics
There were no differences in age or body mass index
(BMI) between the participants in the verum and placebo groups
(Table III). In addition, there
were no differences in sex between the participants in the verum
and placebo groups (Table IV).
 | Table III.Population characteristics. |
Table III.
Population characteristics.
| Characteristic | N | Mean | SD |
|---|
| Age (years) |
| V
group | 26 | 57.0 | 7.6 |
| P
group | 29 | 57.9 | 10.5 |
|
P-value |
| 0.479 |
|
| BMI (kg/m2) visit
1 |
| V
group | 26 | 28.07 | 2.63 |
| P
group | 29 | 27.69 | 2.03 |
|
P-value |
| 0.870 |
|
 | Table IV.Sex distribution in the verum and
placebo groups. |
Table IV.
Sex distribution in the verum and
placebo groups.
|
| V group (n=26) | P group (n=29) |
|---|
|
|
|
|
|---|
| Sex | No. (%) | No. (%) |
|---|
| Male | 12 (46.2) | 9
(31.0) |
| Female | 14 (53.8) | 20 (69.0) |
| P-value | 0.279 |
EndoPAT™: Arterial stiffness
reduction
Quantitative changes in arterial stiffness (measured
as AI75) from a mean of 12.86 to 10.08 were found from
baseline to the study end in the verum group, showing a
statistically significant result (P=0.028). The change in arterial
stiffness in the placebo group ranged from 8.10 to 5.59 during the
same time period (P=0.171) (Tables V
and VI). Satistically significant
qualitative changes in AI75 were observed from baseline
to study end in the verum group (P=0.041), with 69.2% of the
subjects responding to the AGE with an AI75 reduction
(Table VII).
 | Table V.Arterial stiffness reduction from
visit2 to visit 4. |
Table V.
Arterial stiffness reduction from
visit2 to visit 4.
|
|
| Visit 2 | Visit 4 |
|---|
|
|
|
|
|
|---|
| AI75
(%) | N | Mean | SD | Mean | SD |
|---|
| V group | 26 | 12.86 | 10.94 | 10.08 | 11.34 |
| P group | 29 |
8.10 | 18.26 |
5.59 | 15.11 |
 | Table VI.Arterial stiffness reduction:
Quantitative change. |
Table VI.
Arterial stiffness reduction:
Quantitative change.
| Change in
AI75 (%) Visit 2-visit 4 | N | Mean | SD | P-value |
|---|
| V group | 26 | 2.78 | 6.08 | 0.028 |
| P group | 29 | 2.51 | 9.62 | 0.171 |
 | Table VII.Arterial stiffness reduction:
Responders. |
Table VII.
Arterial stiffness reduction:
Responders.
|
| V group (n=26) | P group (n=29) |
|---|
|
|
|
|
|---|
| Changes in
AI75 Visit 2-visit 4 | No. (%) | No. (%) |
|---|
| Decreased | 18 (69.2) | 15 (51.7) |
| Unaltered | 0 (0.0) | 1 (3.4) |
| Increased | 8
(30.8) | 13 (44.8) |
| P-value | 0.041 | 0.295 |
EndoPAT™: RHI
No statistically significant differences were
observed in the RHI score between the study groups at the end of
the study (data not shown).
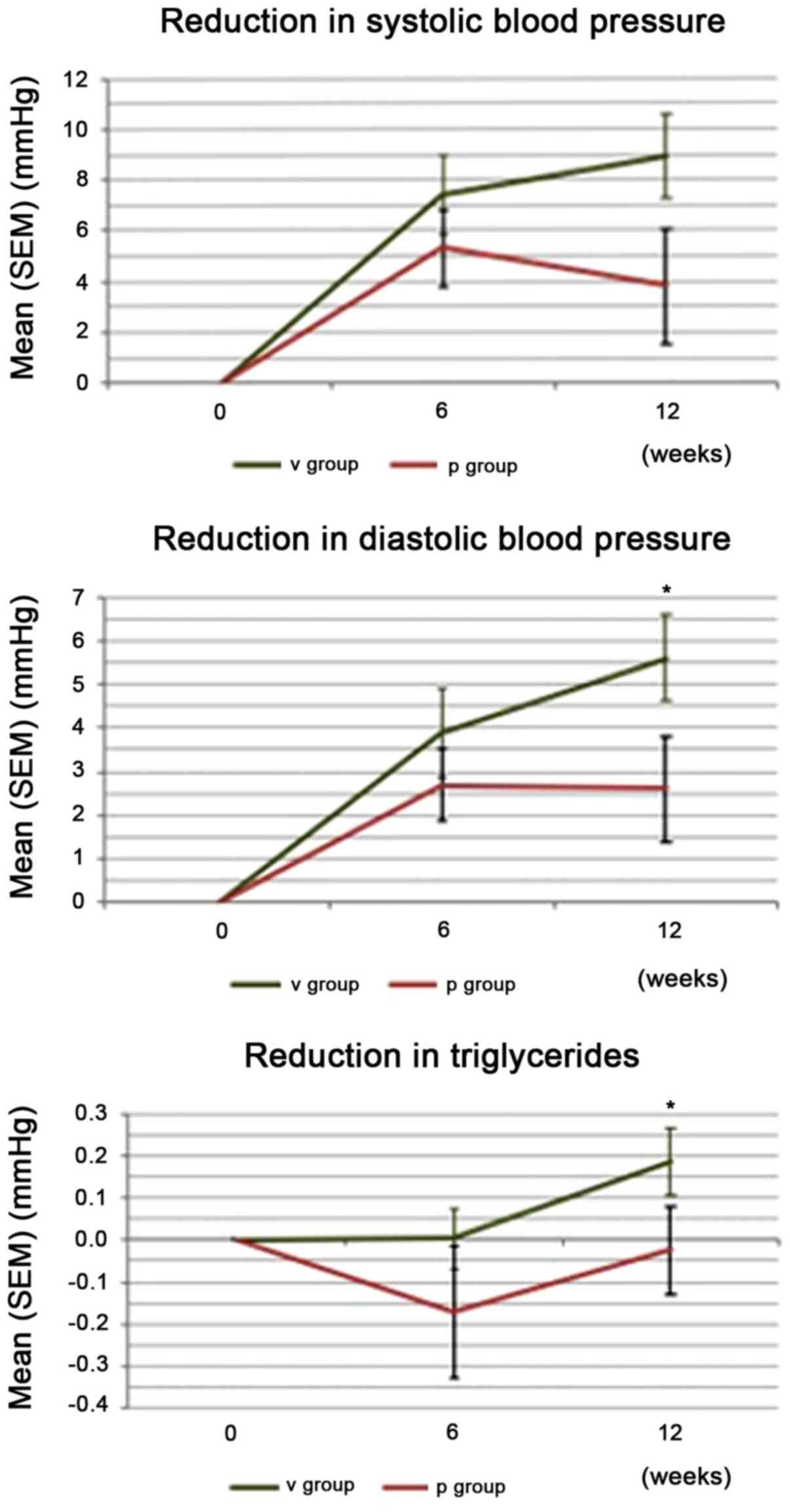
Changes in SBP
A strong trend towards differences in the
quantitative change of SBP from baseline to study end was observed
between the groups in favor of verum. The mean SBP decreased from
142.9 mmHg at visit 2 to 134.0 mmHg at visit 4 in the verum group,
corresponding to a mean change of 8.9 mmHg (Tables VIII and IX). This differed from the placebo group
(strong trend, P=0.056). In the verum group, 21 of the 26 subjects
(80.8%) responded to AGE with a decrease in SBP (data not
shown).
 | Table VIII.Systolic blood pressure. |
Table VIII.
Systolic blood pressure.
|
|
| Visit 2 | Visit 4 |
|---|
|
|
|
|
|
|---|
| SBP (mmHg) | N | Mean | SD | Mean | SD |
|---|
| V group | 26 | 142.9 | 8.4 | 134.0 | 10.7 |
| P group | 29 | 141.9 | 7.5 | 138.1 | 11.5 |
 | Table IX.Reduction in systolic blood
pressure. |
Table IX.
Reduction in systolic blood
pressure.
|
| Reduction in SBP
(mmHg) v2 - v4 |
|---|
|
|
|
|---|
| Group | N | Mean | SD |
|---|
| V group | 26 | 8.9 |
8.2 |
| P group | 29 | 3.8 | 12.3 |
| P-value |
| 0.056 |
|
Changes in DBP
Similar to the SBP, a difference in quantitative
changes (P=0.038) in DBP was observed from baseline to study end
between the groups in favor of verum. The mean DBP decreased in the
verum group from 91.0 to 85.3 mmHg, corresponding to a mean change
of 5.6 mmHg (Tables X and XI). In the verum group, 22 of the 26
participants (85%) responded to the AGE with a DBP reduction.
 | Table X.Diastolic blood pressure. |
Table X.
Diastolic blood pressure.
|
|
| Visit 2 | Visit 4 |
|---|
|
|
|
|
|
|---|
| DBP (mmHg) | N | Mean | SD | Mean | SD |
|---|
| V group | 26 | 91.0 | 3.8 | 85.3 | 6.8 |
| P group | 29 | 89.2 | 3.8 | 86.6 | 7.0 |
 | Table XI.Reduction in diastolic blood
pressure. |
Table XI.
Reduction in diastolic blood
pressure.
|
| Reduction in DBP
(mmHg) v2 - v4 |
|---|
|
|
|
|---|
| Group | N | Mean | SD |
|---|
| V group | 26 | 5.6 | 5.1 |
| P group | 29 | 2.6 | 6.5 |
| P-value |
| 0.038 |
|
Decrease in the TG level
A statistically significant difference in
quantitative changes was found for the TG levels from baseline to
study end between the groups in favor of verum (1.33 mmol/l at
visit 2 to 1.15 mmol/l at visit 4, P=0.022). Qualitative changes in
the TG levels were statistically significant (P=0.022), with 77% of
the responders observed with decreased values in the verum group
(Table XII). Changes in CVD
parameters SBP, DBP, and TG are summarized in Fig. 1.
 | Table XII.Changes in observed CVD
parameters. |
Table XII.
Changes in observed CVD
parameters.
| Parameter | Group | N decreased
(%) | N unaltered
(%) | N increased
(%) | P-value |
|---|
| SBP | Verum | 21 (80.8) | 0 (0.0) | 5
(19.2) | <0.001 |
|
| Placebo | 18 (62.1) | 0 (0.0) | 11 (37.0) |
0.091 |
| DBP | Verum | 22 (84.6) | 0 (0.0) | 4
(15.4) | <0.001 |
|
| Placebo | 17 (58.6) | 2 (6.9) | 10 (34.5) |
0.051 |
| TG | Verum | 20 (76.9) | 0 (0.0) | 6
(23.1) |
0.022 |
|
| Placebo | 12 (41.4) | 0 (0.0) | 17 (58.6) |
0.408 |
| SCORE | Verum | 8
(30,8) | 17 (65.4) | 1 (3.8) |
0.039 |
|
| Placebo | 4
(13.8) | 23 (79.3) | 2 (6.9) |
0.688 |
SCORE value of CVD risk
The SCORE value indicating a risk of developing CVD
from baseline to study end showed stastistically significant
qualitative changes in the verum group (31% responders with
reduction) (Table XII).
Global assessments of benefit and
tolerability
Differences between the study groups in the global
assessment of benefit were in favor of the verum both in the rating
by subjects (P=0.006) and by the investigator (P=0.007); close
comparability between verum and placebo was observed in the global
assessment of tolerability (data not shown).
Adverse events
In total, 9% of the subjects reported an adverse
event (AE; there were 6 AEs in 5 subjects (5/57; 8.8%) (Table XIII). No differences in the
percentage of subjects with AE was observed between the groups. In
all cases, there was an ‘unlikely’ causal association to the
investigational product. None of the AEs were considered
serious.
 | Table XIII.Adverse events. |
Table XIII.
Adverse events.
| Group | N | Adverse event | Intensity |
|---|
| V | 1 | Back pain | Moderate |
| V | 1 | Common cold | Light |
| P | 1 | Common cold | Moderate |
| V | 1 | Alanine
transaminase increased | Light |
| Pa | 1 | Knee joint
mobilization | Moderate |
| Pa | 1 | Vomiting | Light |
No differences were observed between the groups with
regards to the examined safety laboratory parameters. Pulse rate
and body weight exhibited no changes. In addition, no changes in
dietary habits or level of physical activity were observed during
the study (data not shown).
Discussion
The present placebo-controlled, randomized,
double-blind, parallel-group nutritional study in subjects with a
high BMI and elevated blood pressure provides further clinical
evidence for the cardiovascular benefits of AGE, a specialized
garlic extract. As well as showing, for the first time, at least to
the best of our knowledge, indications of improved arterial
elasticity (measured by EndoPAT™ as AI), this study also supports
efficacy regarding common cardiovascular targets (TG levels, and
SBP and DBP), which all exhibited significant changes from baseline
to study end in favor of verum.
Arterial stiffness reduction. In spite of the small
sample size, the present study demonstrated a significant
improvement in arterial elasticity, as measured by AI75
using EndoPAT™ technology. The majority of the subjects in the
verum group (69,2%) exhibited decreased values of arterial
stiffness from baseline to study end; furthermore, significant
quantitative changes were observed (reduction by 21.6%). This
direct significant effect of AGE on arterial elasticity has not
previously been reported, at least to the best of our knowledge,
further cementing its benefits in the area of CVD prevention. Ried
et al (24) measured arterial
stiffness using the Mobil-O-Graph device (Millar Instruments) and
found non-significant tendencies towards a decrease of arterial
stiffness as measured by the AI, which are supported by the first
significant results of the present study.
The results of the present study indicate that
EndoPAT™ AI measurements may be a valuable method for measuring
AGE-mediated improvements of arterial elasticity. Further studies
on larger sample sizes are required to substantiate these results.
The results obtained in the present study support the numerous
published studies on AGE that have previously shown improvements in
cardiovascular targets (15–17).
Reduction of hypertension. In a pre-clinical trial,
Harauma and Moriguchi (25) compared
the effects of AGE and raw garlic (RG) in spontaneaously
hypertensive rats. Their findings indicated an improvement of the
pliability of the artery for AGE and a reduction in SBP compared to
the controls for both AGE and RG. These findings are in line with
the results of the present study on human subjects.
Ried et al (24) observed a reduction in peripheral and
central blood pressure in patients with uncontrolled hypertension
by 11.5±1.9 mmHg systolic and by 6.3±1.1 mmHg diastolic BP in
responders, compared to the placebo. This is in line with the
results found in the present study (8.9 mean SBP reduction and 5.6
mean DPB reduction). Ried et al (24) also reported a percentage of garlic
non-responders (30%), comparable to the 20% of non-responders in
the present study. The authors speculated the incidence of these
non-responders among their study population as being linked to
underlying vitamin B6, vitamin B12, or folate
deficiency, or to genetic polymorphisms. Further investigation into
this phenomenon is warranted.
Nitric oxide (NO) production may play a role in the
observed BP reduction. Morihara et al (26) observed an increase of NO of 30–40% in
mice from 15 to 60 min following the administration of AGE, leading
to a possible vasorelaxant effect, supporting the findings of an ex
vivo trial conducted by Takashima et al (27), who found that AGE induced a
concentration-dependant vasorelaxation in isolated, precontracted
rat aortic rings with their endotheliums intact. This NO-dependent
effect may account for the short-term BP-lowering effects of
AGE.
Another indication of the possible mode of action of
AGE on hypertension was discovered by Ushijima et al
(28). The authors found a
significant and dose-dependent reduction in SBP in hypertensive
rats treated with S-1-propenylcysteine, one of the key compounds of
AGE. Other AGE compounds did not exert the same effect. The authors
concluded that S-1-propenylcysteine was responsible for the
anti-hypertensive effects of AGE.
Reduction of TG levels. The reduction in the TG
levels observed in the present study is also in line with the
results from previous studies. Lau et al (29) observed a decrease in cholesterol, TG,
low density and very low density lipoprotein levels, together with
an increase in HDL levels in the majority of subjects after 6
months of taking AGE. The authors observed an initial significant
increase in cholesterol and TG levels after 1 month and attributed
this to a mobilization of lipids from the tissue deposits, pointing
out previous studies that had observed the same phenomenon. The
present study with a duration of 12 weeks demonstrated a
significant decrease in TG levels by study end, supporting the
findings of the study by Lau et al (29) with a duration of 24 weeks, that also
showed the first significant decrease in TG levels after 12
weeks.
The observed reduction in arterial stiffness due to
the consumption of AGE, together with lowered blood pressure, as
well as improved TG values, leads to a significant reduction in CVD
risk in subjects with elevated BMI and blood pressure, as shown by
the SCORE value (31% of subjects with decreased values).
To evaluate the mechanisms of the long-term effects
of AGE on cardiovascular targets observed in numerous studies, Ried
et al (17) investigated
changes in gut microbial diversity in subjects receiving AGE over a
period of 12 weeks, observing a small, yet significant increase in
diversity, with the increase in numbers of Clostridia and
Lactobacillus species and the decrease in numbers of
Firmicutes prausnitzii being significantly associated with a
concommittant decrease in BP. While the present study did not
monitor the gut microbiota composition of the study participants,
this effect of AGE may still be one of the mechanisms responsible
for its cardioprotective effects. Further investigation into this
matter is warranted.
Tolerability of AGE. AGE was generally very well
tolerated. While some AEs were reported, none of them involved
gastrointestinal (GI) disturbances, which are the expected AEs
linked to garlic products. Nakagawa et al (30) observed that raw garlic juice (5
ml/kg) administered to Wistar rats led to stomach injury, swelling
of the liver, hypertrophy of the spleen and adrenal glands, and to
the decrease of erythrocytes with various morphological changes.
Harauma and Moriguchi (25) reported
that animals in the RG group exhibited several harmful effects that
were not present in the AGE group, indicating that RG contains
compounds that exert a harmful effect, which are not present in
AGE. In contrast to these findings, Sumiyoshi et al
(31) reported no toxic symptoms in
Wistar rats due to AGE even at dose levels of 2,000 mg/kg for 5
times a week over a period of 6 months.
This is in line with the fact that AGE does not
contain the harsh organosulfur constituents present in fresh garlic
that can lead to GI disturbances in humans (32). This generally high tolerability of
AGE is frequently reported in other studies. For example, Ried
et al (24) reported a
compliance of 96.6% of participants, with minor side-effects that
abated after the first week of the trial and were considered
bothersome by few participants.
As regards the study limitations, it should be noted
that the main limitation of this study was the small sample
size.
In conclusion, the present study supports the
cardiovascular benefits of AGE that have been previously reported.
In addition, at least to the best of our knowledge, for the first
time, an effect on arterial stiffness as measured by EndoPAT™
AI75 in healthy subjects was observed. AGE was
well-tolerated, further cementing its usefulness as a long-term
preventative measure against cardiovascular conditions.
Acknowledgements
The authors thank Dr Norman Bitterlich and Irene
Wohlfahrt for their valuable assistance in this study.
Funding
This clinical study was funded by Wakunaga of
America Co., Ltd. and conducted by Analyze & Realize GmbH.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG was responsible for the study initiation and
conception, and scientific supervision, UB was the study
investigator, GB contributed to the study planning and design, and
RU was the study medical expert and principal investigator.
Ethics approval and consent to
participate
All subjects voluntarily gave their written informed
consent. The clinical trial was approved by the Ethics Committee of
Charité - Universitaetsmedizin Berlin, Germany. Participation was
based upon written informed consent by the participant following
written and oral information by the investigator regarding nature,
purpose, consequences, and possible risks of the clinical
study.
Patient consent for publication
Not applicable.
Competing interests
Wakunaga of America Co., Ltd. was not involved in
the conduct of this clinical study. The authors declare that they
have no competing interests.
References
|
1
|
World Health Organization (WHO), .
Cardiovascular diseases (CVDs): Fact sheet No. 317. WHO; Geneva:
2013
|
|
2
|
World Health Organization (WHO), . World
Health Day 2013: Control your blood pressure. WHO; Geneva: 2013
|
|
3
|
Dieterle T: Blood pressure measurement -
an overview. Swiss Med Wkly. 142:w135172012.PubMed/NCBI
|
|
4
|
Itamar Medical: PAT® Technology. Itamar
Medical Ltd., Caesarea, . 2019, https://www.itamar-medical.com/pat_technology/March.
2019
|
|
5
|
World Health Organization (WHO), . Global
status report on noncommunicable diseases. WHO; Geneva: 2010,
https://www.who.int/nmh/publications/ncd_report2010/en/March.
2019
|
|
6
|
Chobanian AV, Bakris GL, Black HR, Cushman
WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright
JT Jr, et al Joint National Committee on Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure. National Heart,
Lung, and Blood Institute; National High Blood Pressure Education
Program Coordinating Committee, : Seventh report of the Joint
National Committee on Prevention, Detection, Evaluation, and
Treatment of High Blood Pressure. Hypertension. 42:1206–1252. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mancia G, Fagard R, Narkiewicz K, Redon J,
Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G,
Dominiczak A, et al: 2013 ESH/ESC guidelines for the management of
arterial hypertension: The Task Force for the Management of
Arterial Hypertension of the European Society of Hypertension (ESH)
and of the European Society of Cardiology (ESC). Eur Heart J.
34:2159–2219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conroy RM, Pyörälä K, Fitzgerald AP, Sans
S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti
P, Keil U, et al SCORE project group, : Estimation of ten-year risk
of fatal cardiovascular disease in Europe: The SCORE project. Eur
Heart J. 24:987–1003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacobson TA, Ito MK, Maki KC, Orringer CE,
Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al:
National Lipid Association recommendations for patient-centered
management of dyslipidemia: Part 1 - executive summary. J Clin
Lipidol. 8:473–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarwar N, Sandhu MS, Ricketts SL,
Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W,
Watkins H, Samani NJ, Saleheen D, et al Triglyceride Coronary
Disease Genetics Consortium and Emerging Risk Factors
Collaboration, : Triglyceride-mediated pathways and coronary
disease: Collaborative analysis of 101 studies. Lancet.
375:1634–1639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Angelantonio E, Sarwar N, Perry P,
Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N,
Packard CJ, et al Emerging Risk Factors Collaboration, : Major
lipids, apolipoproteins, and risk of vascular disease. JAMA.
302:1993–2000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Virani SS: Non-HDL cholesterol as a metric
of good quality of care: Opportunities and challenges. Tex Heart
Inst J. 38:160–162. 2011.PubMed/NCBI
|
|
13
|
Duerden M, O'Flynn N and Qureshi N:
Cardiovascular risk assessment and lipid modification: NICE
guideline. Br J Gen Pract. 65:378–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kodera Y, Ushijima M, Amano H, Suzuki JI
and Matsutomo T: Chemical and Biological Properties of
S-1-Propenyl-l-Cysteine in Aged Garlic Extract. Molecules.
22:222017. View Article : Google Scholar
|
|
15
|
Budoff MJ, Takasu J, Flores FR, Niihara Y,
Lu B, Lau BH, Rosen RT and Amagase H: Inhibiting progression of
coronary calcification using Aged Garlic Extract in patients
receiving statin therapy: A preliminary study. Prev Med.
39:985–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ried K, Frank OR and Stocks NP: Aged
garlic extract lowers blood pressure in patients with treated but
uncontrolled hypertension: A randomised controlled trial.
Maturitas. 67:144–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ried K, Travica N and Sali A: The Effect
of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and
Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front
Nutr. 5:1222018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Budoff MJ, Ahmadi N, Gul KM, Liu ST,
Flores FR, Tiano J, Takasu J, Miller E and Tsimikas S: Aged garlic
extract supplemented with B vitamins, folic acid and L-arginine
retards the progression of subclinical atherosclerosis: A
randomized clinical trial. Prev Med. 49:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steiner M and Lin RS: Changes in platelet
function and susceptibility of lipoproteins to oxidation associated
with administration of aged garlic extract. J Cardiovasc Pharmacol.
31:904–908. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kikuchi N, Nishimura Y, Tsukamoto C,
Kawashima Y, Ochiai H, Hayashi Y and Fujisaki I: Shinyaku to
Rinsho. Jpn J New Remedies Clin. 43:146–158. 1994.
|
|
21
|
Pharmacopoeia US, . US Pharmacopeial Conv
I. 38:6052–6055. 2015.
|
|
22
|
Pickering TG, Hall JE, Appel LJ, Falkner
BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG and Roccella EJ:
Recommendations for blood pressure measurement in humans and
experimental animals: part 1: blood pressure measurement in humans:
a statement for professionals from the Subcommittee of Professional
and Public Education of the American Heart Association Council on
High Blood Pressure Research. Circulation. 111:697–716. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perk J, De Backer G, Gohlke H, Graham I,
Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R,
et al European Association for Cardiovascular Prevention &
Rehabilitation (EACPR), : European guidelines on cardiovascular
disease prevention in clinical practice (version 2012): The fifth
joint task force of the European society of cardiology and other
societies on cardiovascular disease prevention in clinical practice
(constituted by representatives of nine societies and by invited
experts). Int J Behav Med. 19:403–488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at Heart trial.
Integr Blood Press Control. 9:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harauma A and Moriguchi T: Aged garlic
extract improves blood pressure in spontaneously hypertensive rats
more safely than raw garlic. J Nutr. 136 (Suppl):769S–773S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takashima M, Kanamori Y, Kodera Y,
Morihara N and Tamura K: Aged garlic extract exerts
endothelium-dependent vasorelaxant effect on rat aorta by
increasing nitric oxide production. Phytomedicine. 24:56–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ushijima M, Takashima M, Kunimura K,
Kodera Y, Morihara N and Tamura K: Effects of S-1-propenylcysteine,
a sulfur compound in aged garlic extract, on blood pressure and
peripheral circulation in spontaneously hypertensive rats. J Pharm
Pharmacol. 70:559–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lau BH, Lam F and Wang-Cheng R: Effect of
an odor-modified garlic preparation on blood lipids. Nutr Res.
7:139–149. 1987. View Article : Google Scholar
|
|
30
|
Nakagawa S, Masamoto K, Sumiyoshi H,
Kunihiro K and Fuwa T: Effect of raw and extracted-aged garlic
juice on growth of young rats and their organs after peroral
administration (author's transl). J Toxicol Sci. 5:91–112. 1980.(In
Japanese). View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sumiyoshi H, Kanezawa A, Masamoto K,
Harada H, Nakagami S, Yokota A, Nishikawa M and Nakagawa S: Chronic
toxicity test of garlic extract in rats. J Toxicol Sci. 9:61–75.
1984.(In Japanese). View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aged Garlic Extract™, . Research Excerpts
from Peer Reviewed Scientific Journals & Scientific Meetings.
Wakunaga of America Co., Ltd.; Mission Viejo, CA: 2015, https://www.kyolic.ca/wp-content/uploads/2016/03/Aged-Garlic-Research-Excerpts.pdfMarch.
2019
|