Introduction
Garlic (Allium sativum L.) is known to play
important roles in diet and traditional medicine for centuries
(1). A number of in vitro and in
vivo studies have demonstrated that garlic extracts have
numerous biological functions, such as antioxidant (1), anti-inflammatory (2), antimicrobial (3), anticancer (4), antidiabetic (5) and cardioprotective effects (6). Additionally, garlic extracts have been
shown to exert hepatoprotective effects against acute hepatic
injury (7), non-alcoholic
steatohepatitis (NASH) (8) and
hepatocellular carcinoma [HCC; International Classification of
Diseases for Oncology (ICD-O) code: 8170/3] (9), partly by targeting the LRP6/Wnt
signaling pathway (9).
Supplementation with allicin, a compound found in fresh aqueous
extracts of garlic, has been shown to exert protective effects
against alcoholic fatty liver disease by improving inflammatory
conditions and exerting antioxidant effects (10).
Lipid-associated inflammation, which leads to
repeated liver injury and compensatory proliferation, has recently
become the leading etiology of HCC (11). Non-alcoholic fatty liver disease has
been reported to contribute to 10–12% of HCC cases in Western
populations and 1–6% of HCC cases in Asian populations (12). We have previously reported that
arachidonic acid (AA), an unsaturated fatty acid known to be a
pro-inflammatory precursor and the levels of which are increased
during hepatic tumorigenesis, is a target for the chemoprevention
of HCC by acyclic retinoid, a novel anticancer agent (13,14). On
the other hand, we found that the nuclear accumulation of the
protein crosslinking enzyme transglutaminase (TG)2 under conditions
of oxidative stress plays a critical role in regulating cell death
in the liver, partly by crosslinking and inactivating the
transcription factor, Sp1 (15,16).
Recently, a mechanistic study by our group revealed that the
suppression of cell growth by AA accompanied the production of
reactive oxygen species (ROS) followed by the activation of nuclear
TG2 in hepatic cells, suggesting a critical role of the
ROS-mediated activation of nuclear TG2 by AA in chronic liver
injury and inflammation (17).
In this study, we investigated the protective effect
of garlic extracts against AA-induced cell death in hepatic cells
and aimed to elucidate the underlying mechanism associated with
ROS/TG2-dependent signaling pathways.
Materials and methods
Chemicals
Aged garlic extracts (AGEs) and the water-soluble
compound, S-allylmercaptocysteine (SAMC), were provided by
Wakunaga Pharmaceutical Co., Ltd. AA (A9673) and the ROS inhibitor,
N-acetyl-L-cysteine (NAC; A7250), were obtained from
Sigma-Aldrich. The irreversible TG inhibitor, Z-DON-Val-Pro-Leu-OMe
(ZDON; Z006), was purchased from Zedira, as previously described
(17).
Cell culture
The human liver cancer cell line, JHH7 (also known
as FLC7), was kindly supplied by Professor T. Matsuura of the Jikei
University School of Medicine, Tokyo, Japan (18). The cells were maintained in
Dulbecco's modified Eagle's medium (Wako Industries) containing 10%
fetal bovine serum (Mediatech), 100 U/ml penicillin/streptomycin
and 2 mmol/l L-glutamine (Mediatech) and were grown at 37°C in a
humidified incubator under 5% CO2 as previously
described (19). The cells were
seeded at a concentration of 100,000 cells/ml and cultured in
serum-containing medium for 24 h prior to chemical treatment.
H2O was used as the solvent control for SAMC. Ethanol
(EtOH) was used as the solvent control for AA. The doses and
treatment times of the chemical treatments are shown and explained
in detail in the figures and figure legends.
Cellular ROS detection
Cellular ROS levels were determined using the
chloromethyl derivative of 20,70-dichlorodihydrofluorescein
diacetate (CM-H2DCFDA; Life Technologies; Thermo Fisher
Scientific), a general oxidative stress probe, as previously
described (16). Following chemical
treatment for 16 h for AA treatment or 4 h for SAMC treatment, the
cells were monitored for FITC fluorescence signals using a plate
reader (ARVO MX; Perkin Elmer Inc.).
Determination of cell viability
The number of viable cells was determined using the
Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto,
Japan) in a plate reader (ARVO MX; Perkin-Elmer Inc.) at 450 nm as
previously described (19).
Determination of cellular TG2
activity
The cellular activity of TG2 was measured based on
the incorporation of 0.2 mM 5-biotinamidopentylamine (5-BAPA,
21345; Thermo Fisher Scientific) into the cells as previously
described (16). The cells were then
washed and stained with TRITC-conjugated secondary antibody (1:500,
016-020-084; Jackson ImmunoResearch Laboratories) for 20 min at
room temperature and cell nuclei were visualized using DAPI for 20
min at room temperature. Images were captured using an ImageXpress
Micro Confocal High-Content Imaging System (Molecular Devices). The
morphological analysis was performed using MetaXpress Image
Analysis software version 5.1 (Molecular Devices).
shRNA lentiviral particle
transduction
Stearoyl-CoA desaturase-1 (SCD1; sc-36464-V) and
control (sc-108080) short hairpin RNA (shRNA) lentiviral particles
were obtained from Santa Cruz Biotechnology. The cells were
transduced with lentiviral vectors expressing the shRNAs at
approximately 0.5 multiplicity of infection (MOI) using 5 µg/ml
Polybrene (Santa Cruz Biotechnology) and then selected with 2 µg/ml
puromycin-containing culture medium for >1 month for further
analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the cells using an
RNeasy kit (Qiagen) and quantified using a NanoDrop
spectrophotometer (NanoDrop Products) in accordance with the
manufacturer's instructions. cDNA was synthesized using a
PrimeScript RT Master Mix Kit (Takara Bio). The sequences of the
primers used were as follows: Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, CAATGACCCCTTCATTGACC and reverse,
GACAAGCTTCCCGTTCTCAG; and SCD1 forward, GTACCGCTGGCACATCAACTT and
reverse, TTGGAG ACTTTCTTCCGGTCAT. PCRs were performed using a
combination of the Roche LightCycler 96 Real-Time PCR System (Roche
Diagnostic Co., Ltd.) and SYBR Premix ExTaq II (Takara Bio) under
the following cycling conditions: 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec and 60°C for 10 sec. Relative gene
expression analysis calculation was performed using the ΔΔCq method
(20).
Statistical analysis
Quantitative data are expressed as the means ± SD of
at least 3 replicates. The significance of the differences between
the values was assessed using a Student's t-test or analysis of
variance (ANOVA) with the Bonferroni multiple comparison test.
Values of P<0.05 were considered to indicate statistically
significant differences.
Results and Discussion
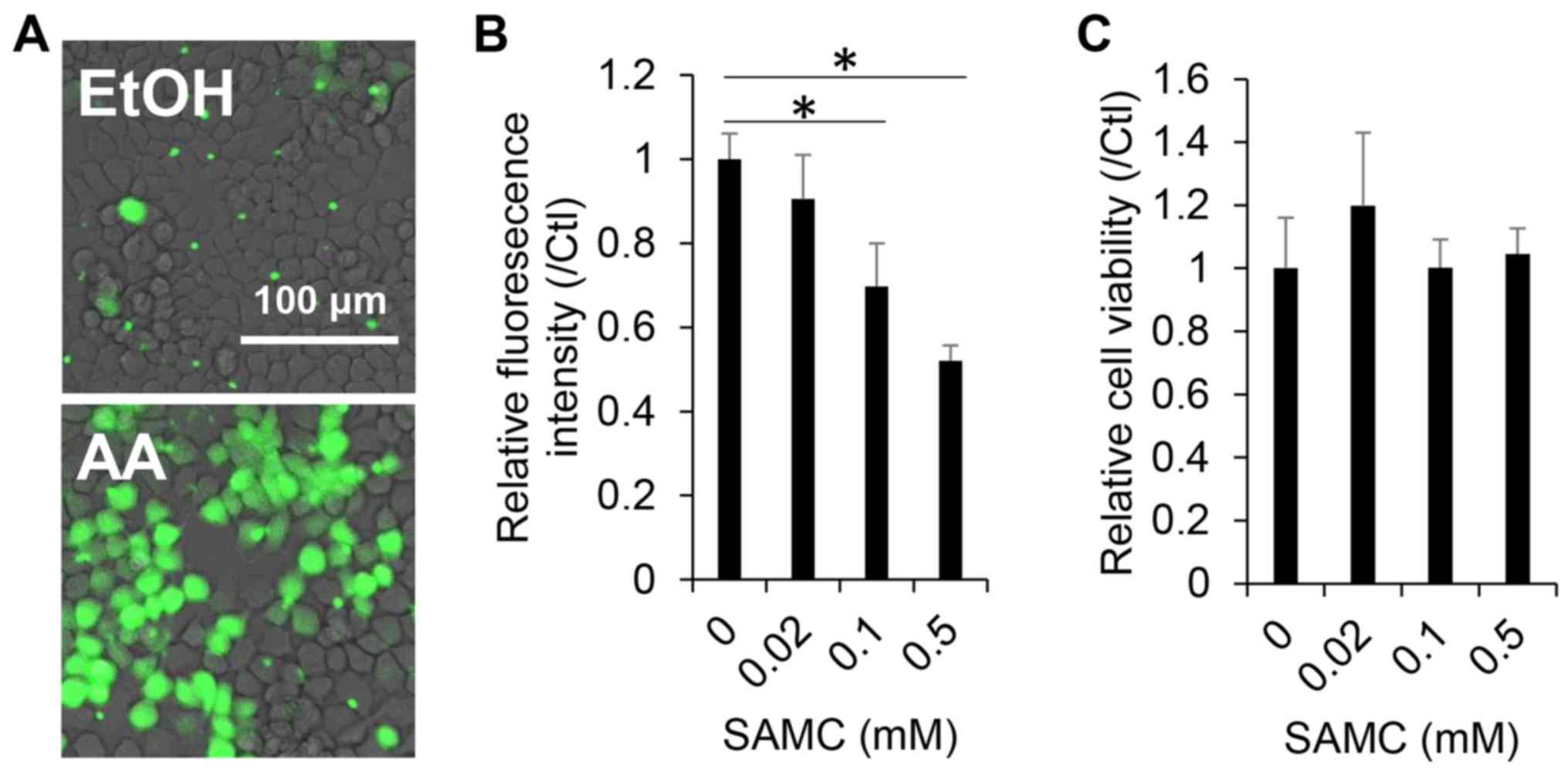
Antioxidant and cytotoxic effects of
SAMC on JHH7 cells
Both AGEs and their sulfur constituents, such as
SAMC and S-allyl cysteine (SAC), are recognized as potent
antioxidants (21–23). In this study, we first, we examined
the antioxidant effects of SAMC on the hepatic cells used in this
study. A dose-dependent decrease in cellular ROS levels in the JHH7
cells was observed upon SAMC treatment for 4 h, as detected using
CM-H2DCFDA (Fig. 1A and B), while no
obvious cytotoxic effect was observed in the JHH7 cells treated
with SAMC at concentrations as high as 0.5 mM for 24 h (Fig. 1C).
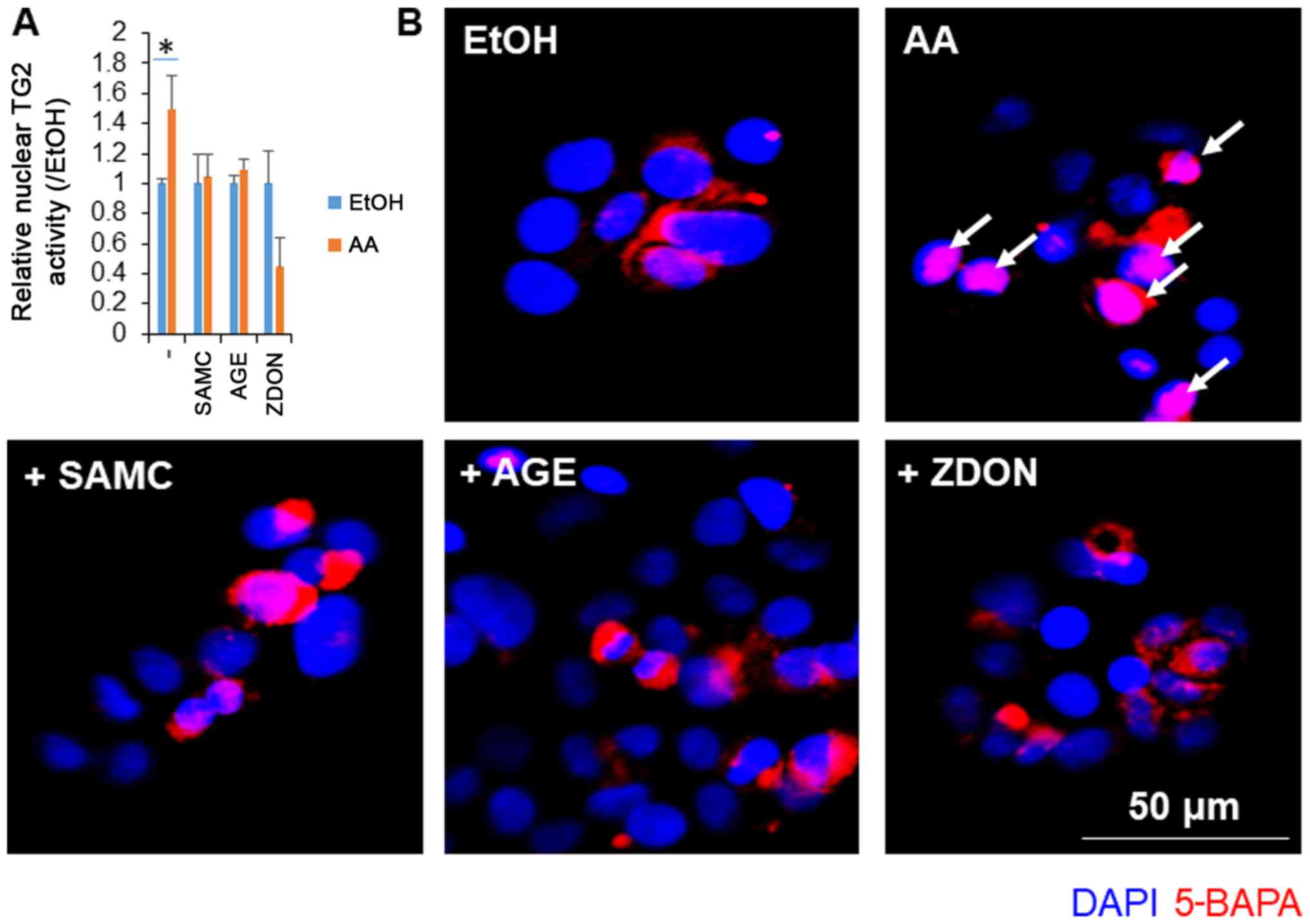
Garlic extracts suppressed the
AA-induced cellular activation of TG2 in JHH7 cells
We have previously reported that the AA-induced
suppression of cell growth accompanies the production of ROS
followed by the activation of nuclear TG2 in hepatic cells
(17). In this study, we examined
the effects of garlic extracts on the activation of TG2 induced by
AA (Fig. 2). In accordance with a
previous study (17), AA treatment
at a concentration of 10 µM for 24 h significantly increased the
nuclear activation of TG2 in the JHH7 cells. As the positive
control, TG2 activation induced by AA was significantly blocked by
the irreversible TG2 inhibitor, ZDON. Notably, co-treatment with AA
and either SMAC or AGEs almost completely inhibited the activity of
nuclear TG2 to basal levels.
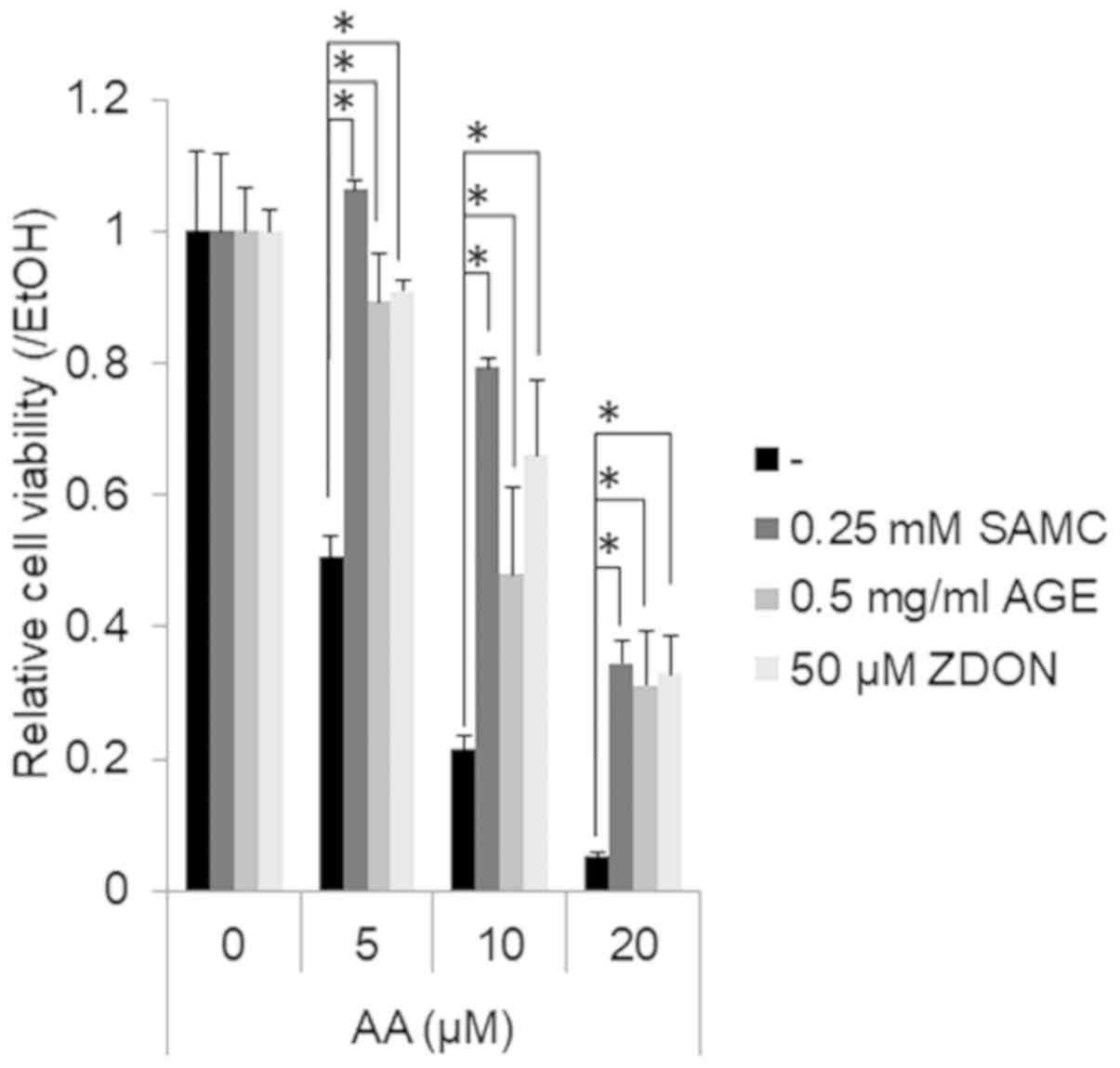
Garlic extracts prevent the AA-induced
suppression of the growth of JHH7 cells
Finally, we examined whether the growth-suppressive
effects of AA on hepatic cells may be prevented by garlic extracts
(Fig. 3). AA treatment for 24 h
suppressed the viability of the JHH7 cells in a dose-dependent
manner. Co-treatment with SMAC, AGEs and ZDON significantly
prevented the growth-suppressive effects of AA on the JHH7 cells.
Fatty acids are critical constituents of the membrane and serve as
energy sources and signal mediators of transduction signals
(24). Increasing attention has been
paid to the critical roles of unsaturated fatty acids in promoting
liver damage and tumorigenesis. SCD1 is a rate-limiting lipid
desaturase responsible for generating monounsaturated fatty acids,
such as palmitoleic acid and oleic acid. Recently, we reported that
the upregulation of SCD1 levels is observed in cancer stem cell
(CSC)-like populations compared to non-CSC liver populations within
human liver cancer cell lines (25).
SCD1-mediated ceramide synthesis has been reported to induce
mitochondrial dysfunction, ROS generation and cell apoptosis
(26). The protective effects of
SCD1 inhibition has been reported to ameliorate ethanol-induced
liver injury (27). A SCD1 inhibitor
has also been developed to attenuate lipid accumulation and liver
injury in a rat model of NASH (28).
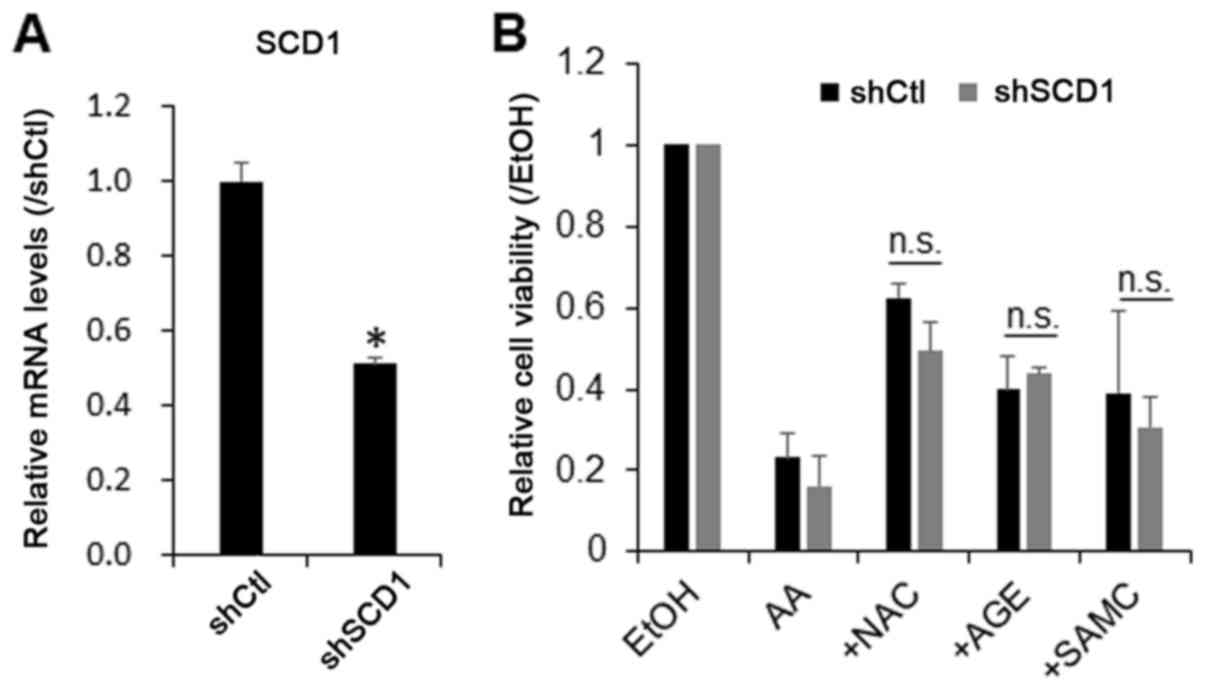
Therefore, in this study, we examined whether the knockdown of SCD1
prevents the growth-suppressive effect of AA on hepatic cells. For
this purpose, the cells were transduced with SCD1 shRNA lentiviral
particles, and endogenous SCD1 was stably knocked down by 60%
(Fig. 4A). The knockdown of SCD1 did
not significantly affect the growth-suppressive effect of AA or the
protective effects of AGEs, SAMC and ZDON against AA on the JHH7
cells (Fig. 4B). These data
suggested that AA may directly stimulate cellular ROS production
and initiate downstream signaling cascades, including nuclear TG2
activation, leading to the growth suppression of hepatic cells.
We have previously reported that i) the level of AA
is upregulated during liver tumorigenesis (13); ii) the nuclear accumulation of TG2 in
hepatic cells results in crosslinking and in the inactivation of
the transcription factor Sp1, which leads to the downregulation of
Sp1-responsive genes involved in cell survival, and thus resulting
in apoptosis (15); and iii) ROS
play critical roles in nuclear TG2-dependent AA-induced liver
injury (17). In this study, we
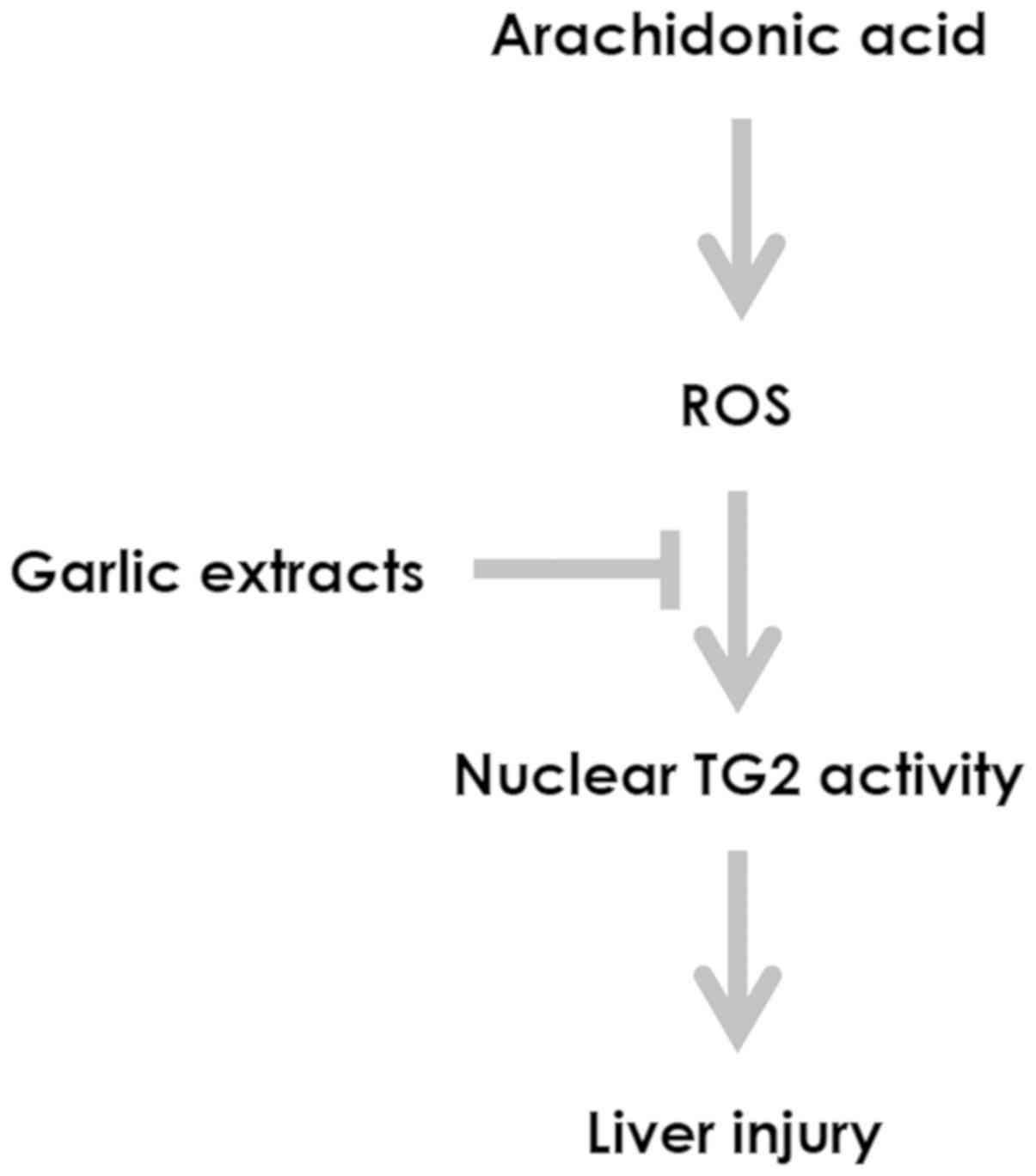
addressed a potential role of garlic extracts in preventing the
growth-suppressive effects of AA on hepatic cells by controlling
oxidative stress-mediated cellular TG2 activation (Fig. 5). Opposing roles of TG2 in the onset
of liver injury have been reported due to its dual function in the
regulation of cell survival and death (15,29,30).
Extracellular and cytoplasmic TG2 in the closed form exhibit
multiple functions, such as GTPase, cell adhesion and scaffold
activities, which are associated with cell growth and may prevent
liver injury by favoring tissue stability (31). By contrast, oxidative stress, the
Ca2+-dependent protein crosslinking activity of TG2 in
the open form and its subcellular location in the nucleus may lead
to crosslinking and the inactivation of proliferation-associated
transcription factors, such as Sp1, leading to the downregulation
of Sp1-responsive genes involved in cell survival and resulting in
apoptosis (31). This study provides
molecular evidence that the mechanism underlying the
hepatoprotective effects of garlic extract is associated with the
inhibition of the nuclear protein crosslinking activity of TG2.
Although the underlying mechanisms are not yet fully understood, it
is possible that the antioxidant garlic extracts may suppress the
transformation of TG2 from the closed form to the open form, which
is essential for the crosslinking activity of TG2 and likely
enhances the ability of TG2 to bind importins for nuclear
translocation (32).
Panyod et al reported that allicin, a
compound in fresh aqueous extracts of garlic, prevented alcohol
induced liver injury and inflammation, partly by increasing the
hepatic alcohol dehydrogenase activity (10). Colín-González et al (22) and Kodai et al (23) reported that the cytoprotective
effects of SAC were associated with the attenuation of oxidative
stress. However, this study further provided a mechanistic insight
regarding the association between the hepatoprotective effects of
garlic extract and the inhibition of the TG-related crosslinking of
nuclear proteins under oxidative stress conditions. Given the
critical roles of unsaturated fatty acids in the maintenance of
cancer cell stemness (33) and the
loss of cancer immune surveillance (34) in the context of chronic injury, we
propose that garlic extracts may serve as a therapeutic option for
preventing chronic liver injury and inflammation and the
carcinogenesis of fatty livers.
Acknowledgements
The authors would like to thank Dr T. Oka, Dr K.
Tamura and Dr K. Sakata of Wakunaga Pharmaceutical Co., Ltd. for
providing AGEs and SAMC.
Funding
This study was supported by Grants-in-Aid for Young
Scientists JP18K15833 (to XYQ) and a Grant-in-Aid for Scientific
Research (C) JP18K06976 (to SK) from the Ministry of Education,
Culture, Sports, Science and Technology of Japan, the Takeda
Science Foundation (to XYQ), and a Research on the Innovative
Development and the Practical Application of New Drugs for
Hepatitis B Grant JP19fk0310112 from the Japan Agency for Medical
Research and Development (to SK).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XYQ and SK designed the study. XYQ and TS conducted
the experiments. XYQ performed the statistical analysis and data
interpretation. XYQ, TS and SK wrote the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banerjee SK, Mukherjee PK and Maulik SK:
Garlic as an antioxidant: The good, the bad and the ugly.
Phytotherapy research. PTR. 17:97–106. 2003.PubMed/NCBI
|
|
2
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr. 131 (Suppl
3):1075S–1079S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fratianni F, Riccardi R, Spigno P, Ombra
MN, Cozzolino A, Tremonte P, Coppola R and Nazzaro F: Biochemical
Characterization and Antimicrobial and Antifungal Activity of Two
Endemic Varieties of Garlic (Allium sativum L.) of the
Campania Region, Southern Italy. J Med Food. 19:686–691. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao D, Pinto JT, Soh JW, Deguchi A,
Gundersen GG, Palazzo AF, Yoon JT, Shirin H and Weinstein IB:
Induction of apoptosis by the garlic-derived compound
S-allylmercaptocysteine (SAMC) is associated with
microtubule depolymerization and c-Jun NH(2)-terminal kinase 1
activation. Cancer Res. 63:6825–6837. 2003.PubMed/NCBI
|
|
5
|
Ghyasi R, Mohaddes G and Naderi R:
Combination effect of voluntary exercise and garlic (Allium
sativum) on oxidative stress, cholesterol level and
histopathology of heart tissue in type 1 diabetic rats. J
Cardiovasc Thorac Res. 11:61–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
García-Villalón AL, Amor S, Monge L,
Fernández N, Prodanov M, Muñoz M, Inarejos-García AM and Granado M:
In vitro studies of an aged black garlic extract enriched in
S-allylcysteine and polyphenols with cardioprotective
effects. J Funct Foods. 27:189–200. 2016. View Article : Google Scholar
|
|
7
|
Tsai JC, Chen YA, Wu JT, Cheng KC, Lai PS,
Liu KF, Lin YK, Huang YT and Hsieh CW: Extracts from Fermented
Black Garlic Exhibit a Hepatoprotective Effect on Acute Hepatic
Injury. Molecules. 24(pii): E11122019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu ZR, Peng-Chen, Yang-Li, Li JY,
Xin-Wang, Yong-Wang, Guo DD, Lei-Cui, Guan QG and Li HY: Two
cinnamoyloctopamine antioxidants from garlic skin attenuates
oxidative stress and liver pathology in rats with non-alcoholic
steatohepatitis. Phytomedicine. 22:178–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao J, Xing F, Liu Y, Lv Y, Wang X, Ling
M-T, Gao H, Ouyang S, Yang M, Zhu J, et al: Garlic-derived compound
S-allylmercaptocysteine inhibits hepatocarcinogenesis
through targeting LRP6/Wnt pathway. Acta Pharm Sin B. 8:575–586.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panyod S, Wu WK, Ho CT, Lu KH, Liu CT, Chu
YL, Lai YS, Chen WC, Lin YE, Lin SH and Sheen LY: Diet
Supplementation with Allicin Protects against Alcoholic Fatty Liver
Disease in Mice by Improving Anti-inflammation and Antioxidative
Functions. J Agric Food Chem. 64:7104–7113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Starley BQ, Calcagno CJ and Harrison SA:
Nonalcoholic fatty liver disease and hepatocellular carcinoma: A
weighty connection. Hepatology. 51:1820–1832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong SW, Ting YW and Chan WK: Epidemiology
of non-alcoholic fatty liver disease-related hepatocellular
carcinoma and its implications. JGH Open. 2:235–241. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin XY, Tatsukawa H, Hitomi K, Shirakami
Y, Ishibashi N, Shimizu M, Moriwaki H and Kojima S: Metabolome
Analyses Uncovered a Novel Inhibitory Effect of Acyclic Retinoid on
Aberrant Lipogenesis in a Mouse Diethylnitrosamine-Induced Hepatic
Tumorigenesis Model. Cancer Prev Res (Phila). 9:205–214. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin XY, Suzuki H, Honda M, Okada H, Kaneko
S, Inoue I, Ebisui E, Hashimoto K, Carninci P, Kanki K, et al:
Prevention of hepatocellular carcinoma by targeting MYCN-positive
liver cancer stem cells with acyclic retinoid. Proc Natl Acad Sci
USA. 115:4969–4974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tatsukawa H, Fukaya Y, Frampton G,
Martinez-Fuentes A, Suzuki K, Kuo TF, Nagatsuma K, Shimokado K,
Okuno M, Wu J, et al: Role of transglutaminase 2 in liver injury
via cross-linking and silencing of transcription factor Sp1.
Gastroenterology. 136:1783–1795.e10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shrestha R, Shrestha R, Qin XY, Kuo TF,
Oshima Y, Iwatani S, Teraoka R, Fujii K, Hara M, Li M, et al:
Fungus-derived hydroxyl radicals kill hepatic cells by enhancing
nuclear transglutaminase. Sci Rep. 7:47462017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin XY, Lu J, Cai M and Kojima S:
Arachidonic acid suppresses hepatic cell growth through
ROS-mediated activation of transglutaminase. FEBS Open Bio.
8:1703–1710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujise K, Nagamori S, Hasumura S, Homma S,
Sujino H, Matsuura T, Shimizu K, Niiya M, Kameda H, Fujita K, et
al: Integration of hepatitis B virus DNA into cells of six
established human hepatocellular carcinoma cell lines.
Hepatogastroenterology. 37:457–460. 1990.PubMed/NCBI
|
|
19
|
Qin XY, Wei F, Tanokura M, Ishibashi N,
Shimizu M, Moriwaki H and Kojima S: The effect of acyclic retinoid
on the metabolomic profiles of hepatocytes and hepatocellular
carcinoma cells. PLoS One. 8:e828602013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rose P, Moore PK and Zhu YZ: Garlic and
Gaseous Mediators. Trends Pharmacol Sci. 39:624–634. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colín-González AL, Santana RA, Silva-Islas
CA, Chánez-Cárdenas ME, Santamaria A and Maldonado PD: The
antioxidant mechanisms underlying the aged garlic extract- and
S-allylcysteine-induced protection. Oxid Med Cell Longev.
2012:9071622012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodai S, Takemura S, Minamiyama Y, Hai S,
Yamamoto S, Kubo S, Yoshida Y, Niki E, Okada S, Hirohashi K, et al:
S-allyl cysteine prevents CCl(4)-induced acute liver injury
in rats. Free Radic Res. 41:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eyster KM: The membrane and lipids as
integral participants in signal transduction: Lipid signal
transduction for the non-lipid biochemist. Adv Physiol Educ.
31:5–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin XY, Dohmae N and Kojima S: Reply to
Yoshida: Liver cancer stem cells: Identification and lipid
metabolic reprogramming. Proc Natl Acad Sci USA. 115:E6390–E6391.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Ren J, Yang L, Li Y, Fu J, Li Y,
Tian Y, Qiu F, Liu Z and Qiu Y: Stearoyl-CoA desaturase-1 mediated
cell apoptosis in colorectal cancer by promoting ceramide
synthesis. Sci Rep. 6:196652016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lounis MA, Escoula Q, Veillette C,
Bergeron KF, Ntambi JM and Mounier C: SCD1 deficiency protects mice
against ethanol-induced liver injury. Biochim Biophys Acta.
1861:1662–1670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurikawa N, Takagi T, Wakimoto S, Uto Y,
Terashima H, Kono K, Ogata T and Ohsumi J: A novel inhibitor of
stearoyl-CoA desaturase-1 attenuates hepatic lipid accumulation,
liver injury and inflammation in model of nonalcoholic
steatohepatitis. Biol Pharm Bull. 36:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nardacci R, Lo Iacono O, Ciccosanti F,
Falasca L, Addesso M, Amendola A, Antonucci G, Craxì A, Fimia GM,
Iadevaia V, et al: Transglutaminase type II plays a protective role
in hepatic injury. Am J Pathol. 162:1293–1303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piacentini M, Baiocchini A, Del Nonno F,
Melino G, Barlev NA, Rossin F, D'Eletto M and Falasca L:
Non-alcoholic fatty liver disease severity is modulated by
transglutaminase type 2. Cell Death Dis. 9:2572018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tatsukawa H, Furutani Y, Hitomi K and
Kojima S: Transglutaminase 2 has opposing roles in the regulation
of cellular functions as well as cell growth and death. Cell Death
Dis. 7:e22442016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shrestha R, Tatsukawa H, Shrestha R,
Ishibashi N, Matsuura T, Kagechika H, Kose S, Hitomi K, Imamoto N
and Kojima S: Molecular mechanism by which acyclic retinoid induces
nuclear localization of transglutaminase 2 in human hepatocellular
carcinoma cells. Cell Death Dis. 6:e20022015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Condello S, Thomes-Pepin J, Ma X,
Xia Y, Hurley TD, Matei D and Cheng JX: Lipid Desaturation Is a
Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem
Cells. Cell Stem Cell. 20:303–314.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma C, Kesarwala AH, Eggert T,
Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor
V, ElGindi M, et al: NAFLD causes selective CD4(+) T lymphocyte
loss and promotes hepatocarcinogenesis. Nature. 531:253–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|