Introduction
Garlic, which is well-known for its characteristic
odour, has various biological functions, such as antioxidant and
antimicrobial effects (1–3). It is also known to be able to prevent
atherosclerosis (4), diabetes
(5), cardiac hypertrophy (5), thrombosis, inflammation, hypertension
and even cancer (6–9). Garlic itself does not have any potent
odour; however, when garlic bulbs are cut or crushed, a sulfuric
odour is instantaneously produced. The major odour precursor is
S-(+)-allyl-L-cysteine sulfoxide (S-2-propenyl
cysteine sulfoxide; ACSO or alliin). When cells are damaged, ACSO
is decomposed to produce garlic odour compounds, such as diallyl
disulfide (di-2-propenyl disulfide; DADS) and diallyl trisulfide
(di-2-propenyltrisulfide; DATS). These sulfides are the functional
compounds that are responsible for the beneficial physiological
activities of garlic (10); however,
as they are lipophilic, volatile and have a strong odour, their use
in foods is limited. On the other hand, ACSO is hydrophilic and
odourless, rendering it useful as an additive in a wide range of
foods. In light of recent findings, this review summarizes the
characteristics and physiological functions of ACSO in
vivo.
Characteristics of ACSO
ACSO is an amino acid that contains an amino group
and carboxyl group in its structure with a molecular weight of
177.22. According to the substance information in Chemical
Abstracts (SciFinder Scholar), the physicochemical properties of
ACSO are estimated. The density, pKa, and boiling point of ACSO are
predicted to be 1.354 g/ml at 20°C, 1.88 at 25°C and 416°C,
respectively, according to calculations of the Advanced Chemistry
Development (ACD/Labs) software v11.02. Furthermore, the melting
point is 165°C according to PhysProp data obtained from the
Syracuse Research Corporation. The mass solubilities at pH 1, 2,
3–6, 7, 8, 9 and 10, as predicted by the ACD/Labs Software v11.02,
are 120, 35, 27, 32, 76, 535 and 1,000 g/l, respectively, and ACSO
is quite soluble in solutions above pH 9. ACSO itself is odourless
and enhances sweet, salty and umami tastes (11). Therefore, ACSO may be one of the key
components that produce a rich flavour in garlic-containing
foods.
Biosynthesis of ACSO
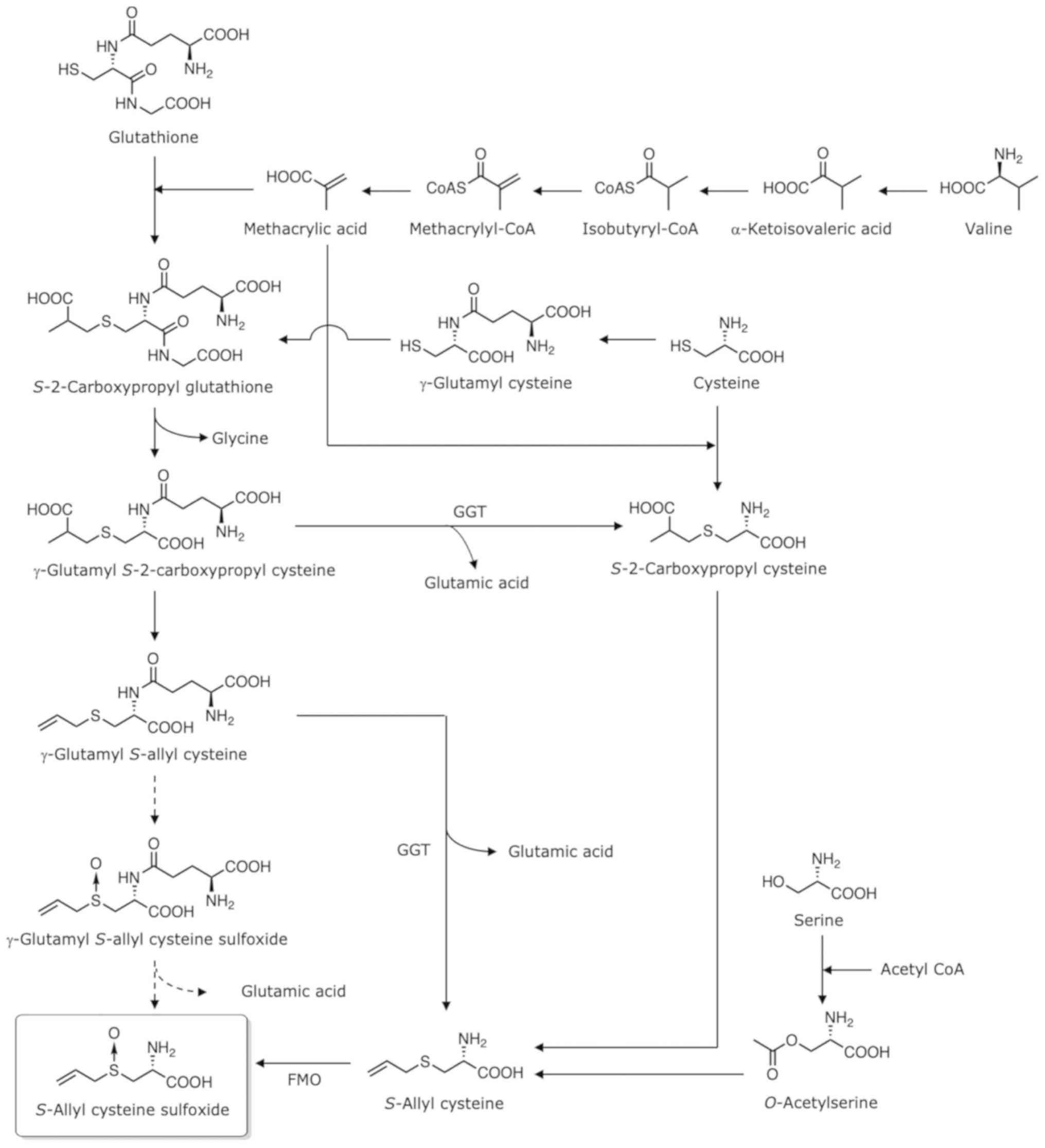
The biosynthesis of ASCO is presented in Fig. 1. Although several biosynthetic
pathways for ACSO have been proposed, its biosynthesis remains
under debate (12–14). Tracing the radioactivity of labelled
valine reveals that the radioactivity is located in the
carboxypropyl group of S-2-carboxypropyl glutathione and
S-2-carboxypropyl cysteine (15). S-2-Carboxypropyl glutathione
is biosynthesized from glutathione (a tripeptide comprised of
glycine, cysteine, and glutamic acid) with methacrylic acid
produced from valine. However, two possible pathways forming
S-2-carboxypropyl cysteine have been reported. One mechanism
involves γ-glutamyl S-2-carboxypropyl cysteine produced from
S-2-carboxypropyl glutathione via elimination of glycine,
while the other involves generation from cysteine and methacrylic
acid without passing through glutathione. These routes have also
been proposed for the biosynthesis of trans-1-propenyl
cysteine in onion (12).
S-2-Carboxypropyl glutathione may also be produced from
cysteine, as γ-glutamyl cysteine is detected in garlic (16). As decarboxylation and oxidation of
the carboxypropyl group produce an allyl group (13), γ-glutamyl S-allyl cysteine can
be produced from γ-glutamyl S-2-carboxypropyl cysteine, and
S-allyl cysteine can be produced from
S-2-carboxypropyl cysteine. The S-oxidation of
γ-glutamyl S-allyl cysteine forms γ-glutamyl S-allyl
cysteine sulfoxide, while that of S-allyl cysteine forms
ACSO. Flavin-containing monooxygenase (FMO) in the cytosol is
responsible for the S-oxidation in the biosynthesis of ACSO
in the presence of nicotinamide adenine dinucleotide phosphate
(NADPH) and flavin adenine dinucleotide (FAD) (17). On the other hand, the recombinant
γ-glutamyl transpeptidase (GGT) prepared by cloning genes from
garlic exhibits a high specificity for γ-glutamyl S-allyl
cysteine, but not for γ-glutamyl S-allyl cysteine sulfoxide
(18). Therefore, S-allyl
cysteine produced from the deglutamylation of γ-glutamyl
S-allyl cysteine forms ACSO via its S-oxidation, and
the pathway via γ-glutamyl S-allyl cysteine sulfoxide to
ACSO may not exist in garlic. The observations that ACSO can be
produced with the addition of S-allyl cysteine in garlic
callus (14) and that S-allyl
cysteine is found in garlic (19,20),
also support this biosynthetic route from S-allyl cysteine
to ACSO. As the addition of serine and allyl thiol produces ACSO
(12), the pathway from serine via
S-allyl cysteine to form ACSO is also probable.
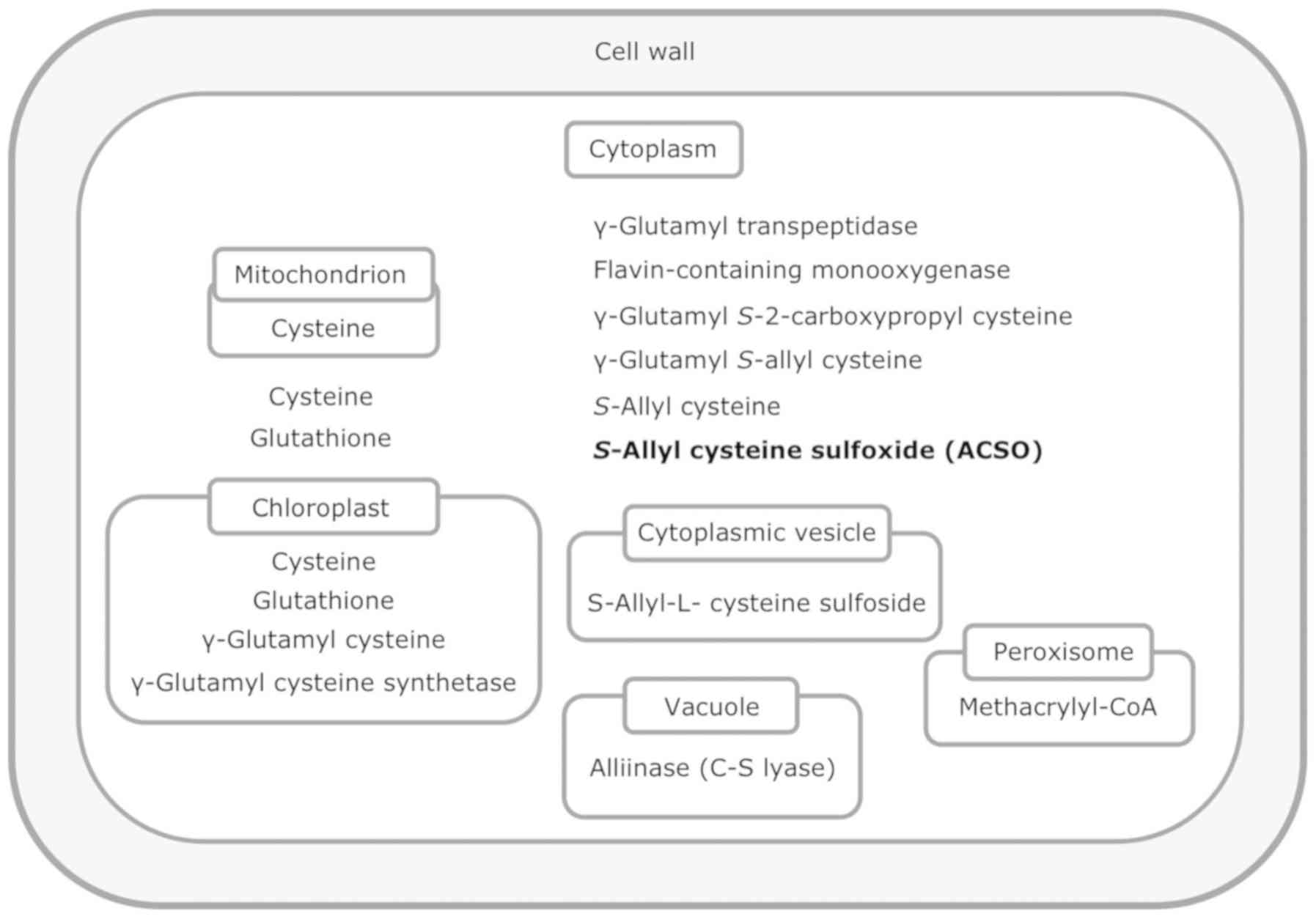
The location of ACSO biosynthesis has not yet been
convincingly identified. γ-Glutamyl peptides reside in the
cytoplasm and cysteine sulfoxides in the cytoplasm or cytoplasmic
vesicle, while γ-glutamyl cysteine resides in the chloroplast
(13) (Fig. 2). As for the enzymes required for
biosynthesis, γ-glutamyl cysteine synthetase is located in the
chloroplast, and γ-glutamyl transpeptidase and flavin-containing
monooxygenase are located in the cytoplasm (13).
Content of ACSO and related compounds in
garlic
The majority of the organosulfur compounds in garlic
are γ-glutamyl S-alkenyl cysteines and cysteine sulfoxides
(21,22), with ACSO being the major component.
The ACSO content in fresh garlic is 3–14 mg/g fresh weight
(20,23,24) and
20–30 mg/g dry weight (25,26). As regards other cysteine sulfoxides,
the contents of S-methyl-L-cysteine sulfoxide (MCSO or
methiin), trans-S-1-propenyl-L-cysteine sulfoxide (PeCSO or
isoalliin) and cycloalliin are 0.5–2, 0.1–1.2 and 0.5–1.5 mg/g
fresh weight, respectively (24).
γ-L-Glutamyl-S-allyl-L-cysteine content in garlic bulbs is
2–7 mg/g fresh weight (21,24) and 3–20 mg/g dry weight (26), while the
γ-L-glutamyl-S-(trans-1-propenyl)-L-cysteine content
is 3–9 mg/g fresh weight (21,24) and
5–15 mg/g dry weight (26).
γ-L-Glutamyl-S-methyl-L-cysteine is present at 0.1–0.4 mg/g
fresh weight (24).
Sulfur fertilization increases the ACSO content in
garlic cloves, while nitrogen fertilization decreases this content
(27). Irrespective of the
fertilization conditions, the ACSO content increases during storage
at 20°C and decreases during storage at temperatures >35°C
(28). Drying at 60°C does not
affect the ACSO content (29).
ACSO accumulates in leaves during early growth
stages and translocates to the bulbs during bulb formation
(30). Approximately 85% of ACSO is
present in the bulbs, with approximately 12% in the leaves and 2%
in the roots, while γ-glutamyl cysteines are found only in the
bulbs (24). The γ-glutamyl-cysteine
content in the bulbs increases a few weeks prior to harvest
(25,31). The ACSO content in the bulbs also
begins to increase a few weeks prior to harvest (25,31) but
also further increases during the curing process (drying process
after harvest for storage) (21).
The content of ACSO in the leaves, however, remains relatively
stable from 6 weeks to 1 week prior to harvest and subsequently
decreases.
Decomposition of ACSO and other
sulfoxides
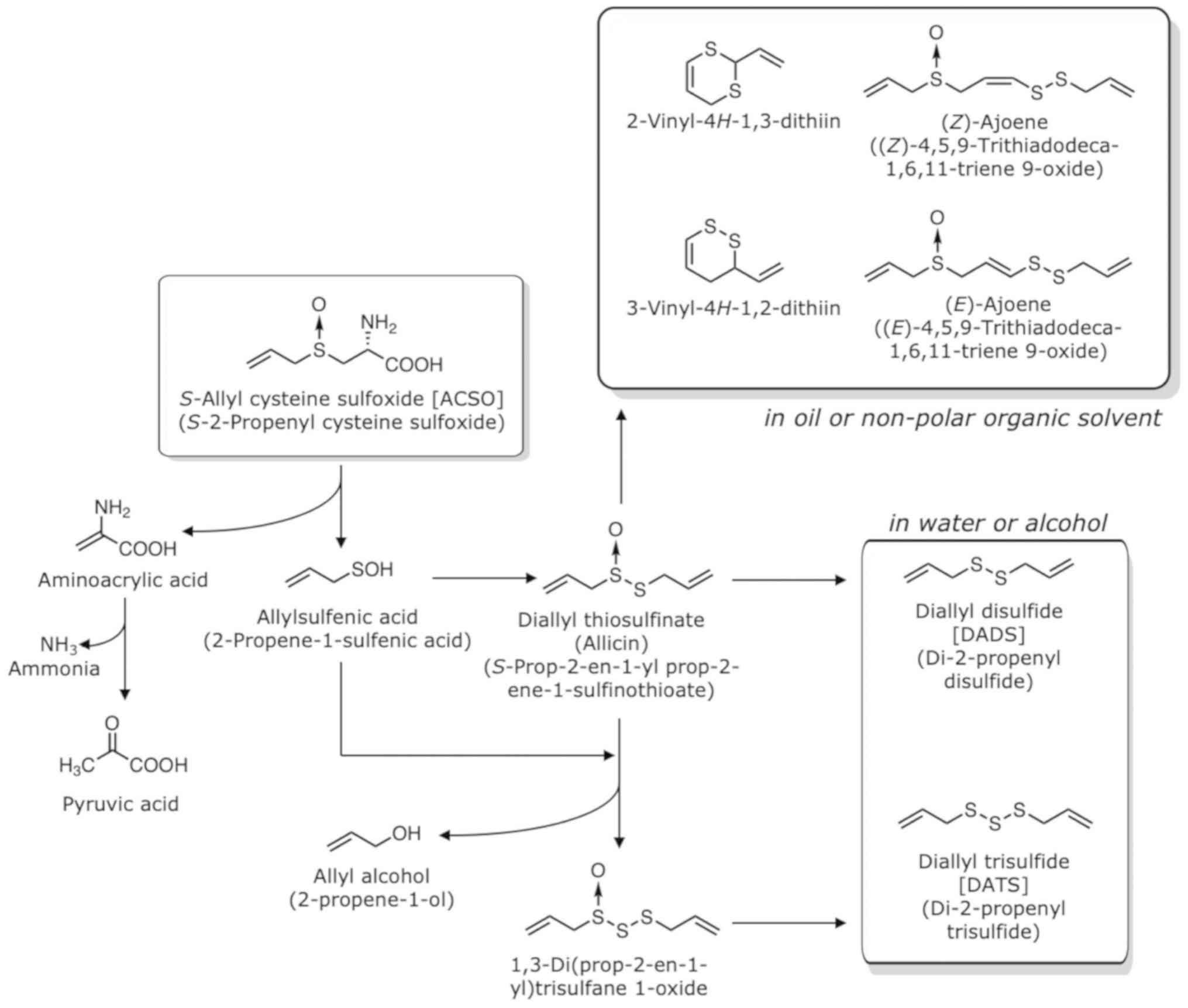
The decomposition of ASCO and other sulfoxides is
illustrated in Figs. 3 and 4. When garlic bulbs are sliced or ground
during cooking, ACSO that resides in the cytoplasm or cytoplasmic
vesicle of all cells (13) binds
alliinase (C-S lyase; EC 4.4.1.4) that localizes in the vacuoles of
vascular bundle sheath cells located around the veins or phloem
(32). Alliinase belongs to a class
1 family of pyridoxal-5′-phosphate-dependent enzymes (2) and is a homodimeric glycoprotein,
consisting of 448-amino-acid subunits with a molecular weight of
51,500 Da each (2). Two alliinase
isozymes have been identified in garlic: One is specific for ACSO
and PeCSO, and the other is specific for MCSO (22). ACSO is converted to allylsulfenic
acid (2-propene-1-sulfenic acid; CH2=CHCH2SOH) (33) and aminoacrylic acid by alliinase.
Aminoacrylic acid is then spontaneously converted to pyruvic acid
and ammonia. Two molecules of allylsulfenic acid produce diallyl
thiosulfinate (S-prop-2-en-1-yl prop-2-ene-1-sulfinothioate;
allicin), which exerts strong antimicrobial activity (34–37). The
amount of allicin produced is approximately 0.1 to 7 mg/g fresh
weight due to the abundance of ACSO in garlic (7,38). The
incorporation of an additional allylsulfenic acid and the removal
of allyl alcohol yield 1,3-di(prop-2-en-1-yl)trisulfane 1-oxide.
When garlic bulbs are homogenized in water or in an alcohol
solution, DADS and DATS are mainly produced from the diallyl
thiosulfinate and 1,3-di(prop-2-en-1-yl)trisulfane 1-oxide.
Volatile oil obtained from garlic products by steam distillation
contains DADS and DATS as the major components (38). These sulfides are known to have
various physiological activities (10,39);
however, they are lipophilic and have a potent garlic odour. On the
other hand, when garlic bulbs are homogenized in oil or in
non-polar solvent, dithiins become dominant,
2-vinyl-4H−1,3-dithiin and 3-vinyl-4H−1,2-dithiin
being about 0.4 mg/g and 0.17 mg/g, respectively (38). Other components produced are
(E)-ajoene (0.07 mg/g), (Z)-ajoene (0.04 mg/g), DATS
(0.07 mg/g), methyl allyl trisulfide (MATS) (0.06 mg/g) and DADS
(0.03 mg/g).
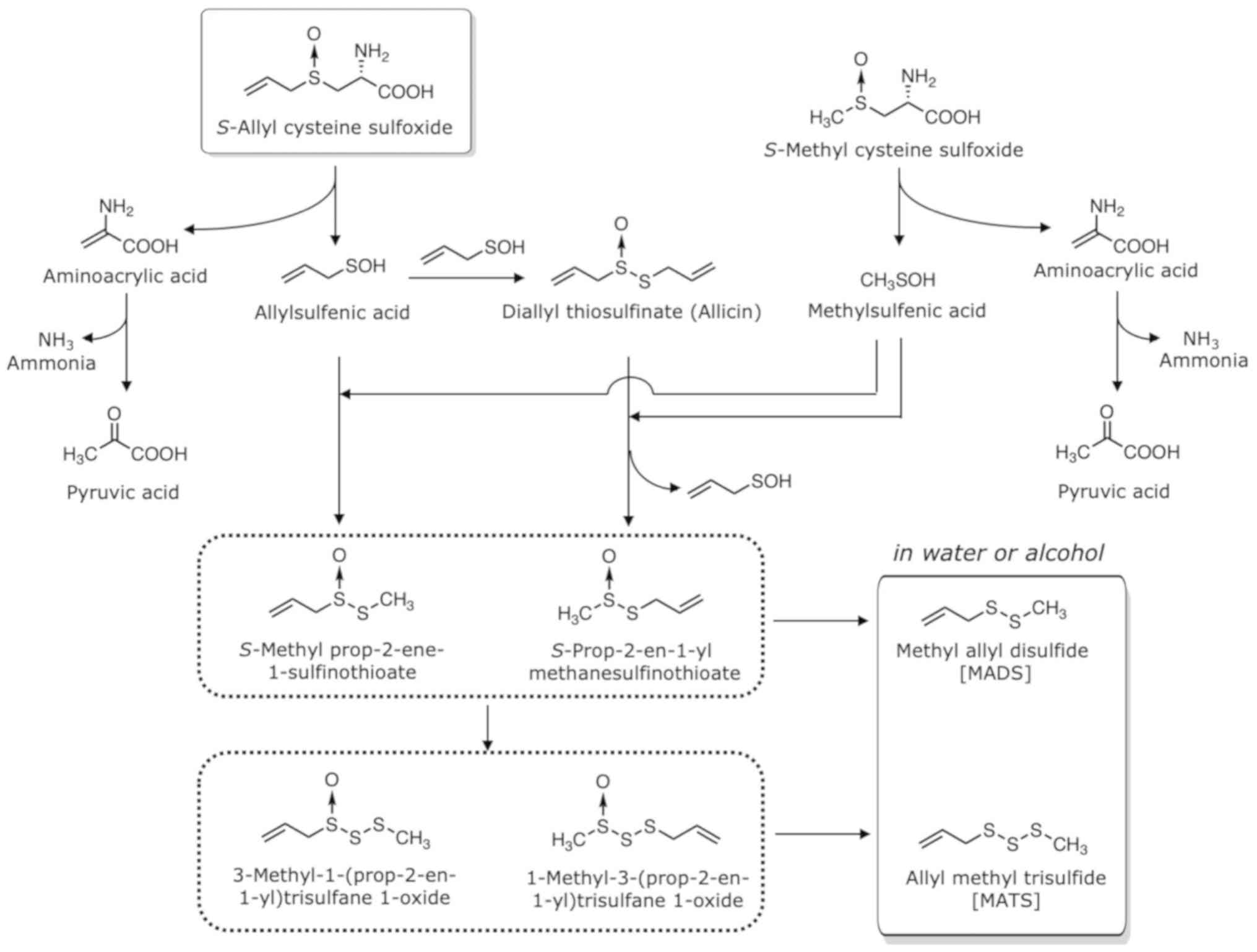
As MCSO is the second most abundant cysteine
sulfoxide in garlic, representing approximately 10% of the total
cysteine sulfoxides, the compounds produced from MCSO contribute to
the composition of volatile oil. MCSO is also decomposed by
alliinase when garlic is sliced or grated to yield methylsulfenic
acid (Fig. 4). Allylsulfenic acid
produced from ACSO and methylsulfenic acid produced from MCSO form
S-methyl prop-2-ene-1-sulfinothioate and
S-prop-2-en-1-yl methanesulfinothioate, the latter being
twice as abundant at the former, though allicin accounts for 60–90%
of the total thiosulfinates. The S-methyl
prop-2-ene-1-sulfinothioate and S-prop-2-en-1-yl
methanesulfinothioate form methyl allyl disulfide (MADS) and MATS.
Importantly, the ratio of allyl groups and methyl groups in total
thiosulfinates almost directly coincides with that in total
S-alk(en)yl-L-cysteine sulfoxides in garlic (40). Among the sulfides, DATS (0.8–1.1
mg/g) and DADS (0.5–1 mg/g) are the most abundant, with MATS
(0.2–0.7 mg/g) and MADS (0.08–0.5 mg/g) as the next most abundant.
Dimethyl trisulfide (DMTS; 0.01–0.1 mg/g) and dimethyl disulfide
(DMDS; 0.001–0.06 mg/g) occur in smaller quantities.
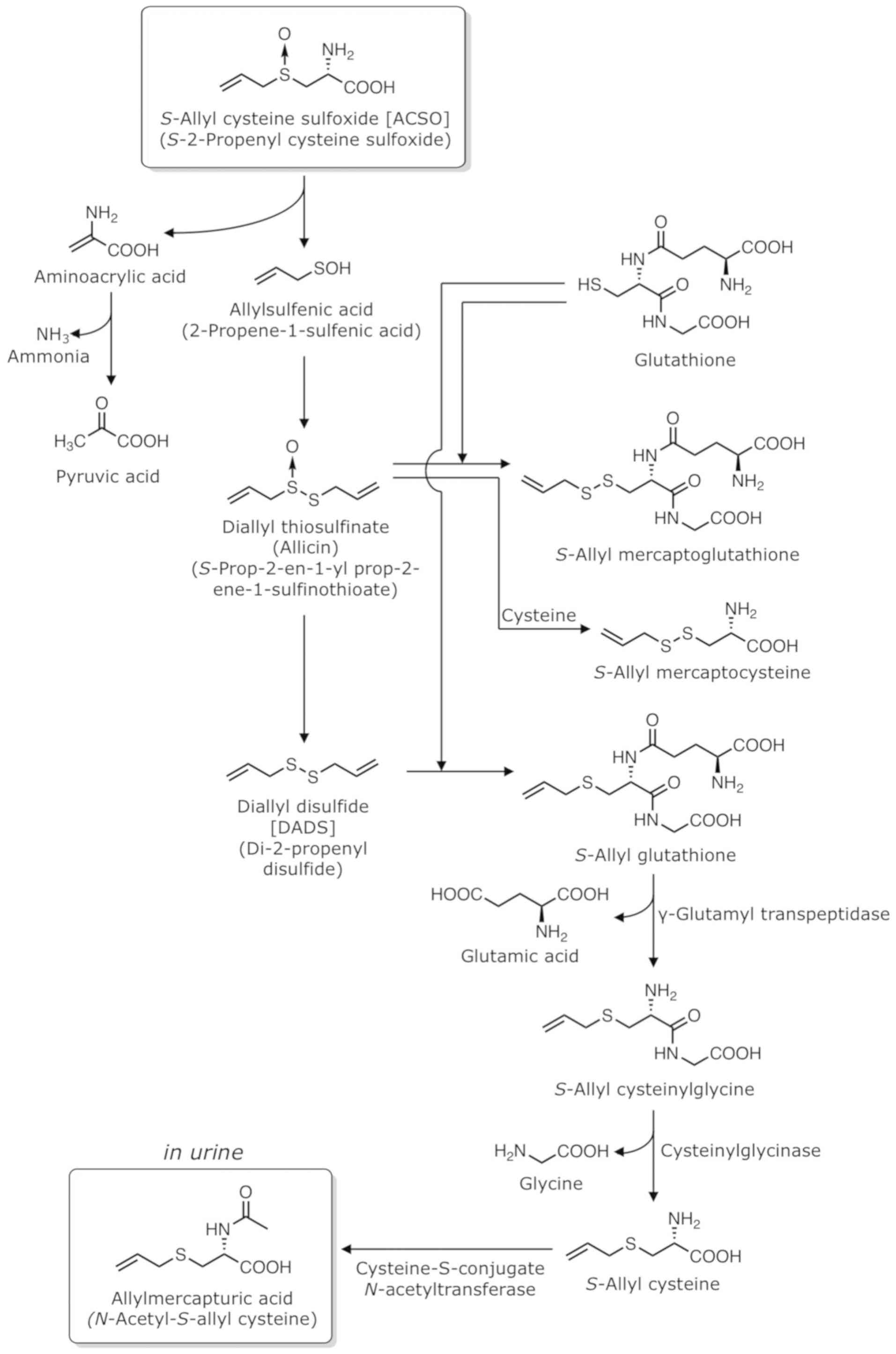
Metabolism of ACSO
The metabolism of ASCO is illustrated in Fig. 5. When 10 ml of 100 mM ACSO/kg body
weight is injected into the ligated loop of the small intestine of
rats, both ACSO and pyruvic-acid concentrations in the portal vein
increase to approximately 4 and 0.2 mM, respectively, after 30 min
(41), indicating that ACSO is
mostly absorbed from the small intestine into the blood in the
intact form; however, it is also partly converted to allylsulfenic
acid, pyruvic acid and ammonia. In addition, mixing ACSO with crude
proteins extracted from the small intestine of rats yields DADS and
DAS as volatile compounds (41),
supporting the assumption that ACSO is decomposed to allylsulfenic
acid and two molecules of allylsulfenic acid produce DADS and DAS
via allicin. Therefore, ACSO may follow the same fate as allicin
and DADS in the body. When garlic oil is consumed, DADS, DAS and
DMDS are detected in urine (42).
When garlic extract is consumed,
N-acetyl-S-allyl-L-cysteine (allylmercapturic acid)
is identified in urine (43,44). N-Acetyl-L-cysteine
(mercapturic acid; MA) conjugates are commonly formed from
glutathione conjugates. The glutathione conjugates are first
transformed into L-cysteine conjugates via the elimination of
glutamic acid by γ-glutamyl transpeptidase and glycine by
cysteinylglycinase. Subsequently, L-cysteine conjugates are
N-acetylated by cysteine-S-conjugate
N-acetyltransferase to form MA conjugates (43). Allicin reacts with glutathione to
produce S-allyl-mercaptoglutathione or with L-cysteine to
produce S-allyl-mercaptocysteine in vivo (45). DADS undergoes nucleophilic
substitution at the α-carbon in a reaction with glutathione to
yield S-allyl-glutathione and allyl perthiol. This
S-allyl-glutathione is then converted to S-allyl
cysteine by γ-glutamyl transpeptidase and cysteinylglycinase. The
S-allyl cysteine is further transformed into
N-acetyl-S-allyl-L-cysteine via N-acetylation
by the cysteine-S-conjugate N-acetyltransferase (46).
Physiological functions of ACSO
Orally administered ACSO ameliorates the diabetic
condition (47,48). When diabetes is induced in rats by an
injection of alloxan monohydrate, fasting blood glucose levels are
reduced, and insulin secretion is increased by the daily oral
administration of 200 mg ACSO/kg body weight. In addition, orally
administered ACSO reduces the generation of reactive aldehydes
produced by lipid peroxidation, increasing catalase activity and
glutathione content (47). Similar
effects have been observed in mice with diet-induced obesity (DIO)
(48). When these mice are fed a
high-fat (60% kcal from fat) diet for 8 weeks and provided with
water containing 0.1 mg ACSO/ml for a further 8 weeks, glucose
homeostasis and insulin sensitivity are enhanced. The increase in
blood glucose levels following the injection of glucose is
suppressed, and the decrease in blood glucose level following the
injection of insulin is promoted. In addition, ACSO administration
improves the lipid profile and liver functions of the mice with
DIO. ACSO treatment reduces the levels of triglycerides,
low-density lipoprotein and non-esterized free fatty acid. It also
decreases the activities of aspartate aminotransferase and alkaline
phosphatase, and the levels of total protein and albumin in the
blood (48).
Furthermore, ACSO has been shown to prevent
myocardial ischaemia induced by isoproterenol in rats (49). When 40 or 80 mg ACSO/kg body weight
is administered to rats for 5 weeks, the increase in the activities
of creatine kinase, lactate dehydrogenase, aspartate transaminase
and alanine transferase in serum, and their decrease in the heart
due to the induction of myocardial ischaemia are both suppressed.
In addition, ACSO administration suppresses the increase in
3-hydroxy 3-methyl glutaryl coenzyme A (HMG CoA) reductase activity
in the liver and the decrease in lecithin cholesterol acyl
transferase activity in the liver and heart in these
isoproterenol-treated rats (49).
The increase in the levels of plasma thiobarbituric acid reactive
substances and hydroperoxide in mice with
myocardial-ischaemia-induced is also suppressed by ACSO
administration.
ACSO has also been shown to suppress oxidative
stress in rats with carbon tetrachloride (CCl4)-induced
hepatic injury (41). In rats with
hepatic injury, following the oral administration of 50 µmol ACSO
for 7 days, the increased aspartate transaminase, alanine
transaminase and lactate dehydrogenase activities as well as the
amount of thiobarbituric acid reactive substances are suppressed.
In addition, the decrease in glutathione levels and the activities
of glutathione S-transferase and glutathione peroxidase are
also suppressed. These observations indicate that ACSO prevents
hepatic injury by enhancing the activities of phase II detoxifying
enzymes and reducing oxidative stress. As MCSO,
S-ethyl-L-cysteine sulfoxide (ECSO) and
S-allyl-L-cysteine (ACS) are not effective for the
prevention of hepatic injury, the allyl and sulfoxide groups in
ACSO are essential for this preventive effect. Moreover, ACSO
promotes nuclear factor erythroid 2-related factor 2 (Nrf2) nuclear
translocation in HepG2 cells, leading to the induction of
antioxidant and detoxifying enzymes, and contributing to these
preventive effects (41).
In garlic, some of the sulfides, such as MATS and
DMTS, exhibit inhibitory activity against platelet aggregation
in vitro (50). As thrombosis
is triggered by platelet aggregation, these sulfides may be the
factors responsible for the prevention of thrombosis following
garlic consumption. The administration of ACSO to rats, however,
results in greater inhibitory activity against platelet aggregation
than DADS (51). The effect of ACSO
is most potent at a dosage of 10 mg/kg body weight, and the
duration of the effect persists for 16–24 h following
administration. Although DADS is produced during the absorption of
ACSO from the small intestine (41),
the functional component is assumed to be different, suggesting the
in vivo production of potent ACSO metabolites that confer
inhibitory activity against platelet aggregation.
Finally, ACSO suppresses the increase in blood
ethanol concentration following the oral administration of ethanol
and ACSO together (52). Although
the supply of NAD+ is the rate-limiting factor for the metabolism
of ethanol to acetaldehyde, and NAD+ is produced from NADH during
the conversion of pyruvic acid to lactic acid by lactate
dehydrogenase, pyruvate production during the absorption of ACSO
from the small intestine is not responsible for this suppressive
effect on blood ethanol elevation. The administration of pyruvic
acid at levels equivalent to those of ACSO does not exert the
suppressive effect on blood ethanol elevation. The mechanisms
underlying the reduction in blood ethanol concentrations by ACSO
involve the promotion of ethanol metabolism in the liver and the
suppression of ethanol absorption from the gut (52). Specifically, ACSO induces the
activities of alcohol dehydrogenase, which converts ethanol into
acetaldehyde, and aldehyde dehydrogenase, which converts
acetaldehyde into acetic acid. In addition, the injection of ACSO
into the ligated stomach or small intestine together with ethanol
results in the suppression of the increase in ethanol concentration
in the portal vein, indicating overall suppression of ethanol
absorption. Garlic extract rich in ACSO (Garlic-H) exhibits an
effect similar to ACSO itself, while garlic extract that does not
contain ACSO (Garlic-N) exerts less-potent effects compared to
Garlic-H (52).
Conclusions
On the whole, as discussed in this review article,
the physiological functions of ACSO are extensive and can be
applied to promote human health. As ACSO is water-soluble and
odourless and enhances taste, the addition of this garlic component
to food products will provide both a rich flavour and may confer
numerous health benefits.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YY and HK conceived and designed this review article
and wrote the manuscript. Both authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goncagul G and Ayaz E: Antimicrobial
effect of garlic (Allium sativum) and traditional medicine.
J Anim Vet Adv. 9:1–4. 2010. View Article : Google Scholar
|
|
2
|
Touloupakis E and Ghanotakis DF:
Nutraceutical use of garlic sulfur-containing compounds. Adv Exp
Med Biol. 698:110–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahman MS, Al-Sheibani HI, Al-Riziqi MH,
Mothershaw A, Guizani N and Bengtsson G: Assesment of the
anti-microbial activity of dried garlic powders produced by
different methods of drying. Int J Food Prop. 9:503–513. 2006.
View Article : Google Scholar
|
|
4
|
Durak I, Aytaç B, Atmaca Y, Devrim E, Avci
A, Erol C and Oral D: Effects of garlic extract consumption on
plasma and erythrocyte antioxidant parameters in atherosclerotic
patients. Life Sci. 75:1959–1966. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Padiya R, Chowdhury D, Borkar R, Srinivas
R, Pal Bhadra M and Banerjee SK: Garlic attenuates cardiac
oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in
fructose-fed diabetic rat. PLoS One. 9:e942282014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bongiorno PB, Fratellone PM and LoGiudice
P: Potential health benefits of garlic (Allium sativum): A
narrative review. J Complement Integr Med. 5:1–26. 2008. View Article : Google Scholar
|
|
7
|
Rose P, Whiteman M, Moore PK and Zhu YZ:
Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the
genus Allium: The chemistry of potential therapeutic agents.
Nat Prod Rep. 22:351–368. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zawistowski J, Kopec A, Jedrszczyk E,
Francik R and Bystrowska B: Garlic grown from air bulbils and its
potential health benefits. ACS Symp Ser. 1286:315–328. 2018.
View Article : Google Scholar
|
|
9
|
Mithen R: Sulphur-containing compounds.
Plant secondary metabolites: occurrence, structure and role in the
human diet. Crozier A, Clifford N and Ashida H: Blackwell
Publishing; Hoboken, NJ: pp. 25–46. 2006, View Article : Google Scholar
|
|
10
|
Palani S, Joseph NM, Tegene Y and Zacharia
A: Medical properties of garlic - A concise review. Curr Res Pharm
Sci. 4:92–98. 2014.
|
|
11
|
Ueda Y, Sakaguchi M, Hirayama K, Miyajima
R and Kimizuka A: Characteristic flavor constituents in water
extract of garlic. Agric Biol Chem. 54:163–169. 1990. View Article : Google Scholar
|
|
12
|
Granroth B: Biosynthesis and decomposition
of cysteine derivatives in onion and other Allium species.
Ann Acad Sci Fenn, Ser A2. Chem. 154:1–71. 1970.
|
|
13
|
Jones MG, Hughes J, Tregova A, Milne J,
Tomsett AB and Collin HA: Biosynthesis of the flavour precursors of
onion and garlic. J Exp Bot. 55:1903–1918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hughes J, Tregova A, Tomsett AB, Jones MG,
Cosstick R and Collin HA: Synthesis of the flavour precursor,
alliin, in garlic tissue cultures. Phytochemistry. 66:187–194.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki T, Sugii M and Kakimoto T:
Metabolic incorporation of L-valine-[14C] into
S-(2-carboxypropyl) glutathione and
S-(2-carboxypropyl) cysteine in garlic. Chem Pharm Bull
(Tokyo). 10:328–331. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lancaster JE and Shaw ML: γ-Glutamyl
peptides in the biosynthesis of S-alk(en)yl-L-cysteine
sulfoxides (flavor precursors) in Allium. Phytochemistry.
28:455–460. 1989. View Article : Google Scholar
|
|
17
|
Yoshimoto N, Onuma M, Mizuno S, Sugino Y,
Nakabayashi R, Imai S, Tsuneyoshi T, Sumi S and Saito K:
Identification of a flavin-containing S-oxygenating
monooxygenase involved in alliin biosynthesis in garlic. Plant J.
83:941–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshimoto N, Yabe A, Sugino Y, Murakami S,
Sai-Ngam N, Sumi S, Tsuneyoshi T and Saito K: Garlic γ-glutamyl
transpeptidases that catalyze deglutamylation of biosynthetic
intermediate of alliin. Front Plant Sci. 5:7582015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki T, Sugii M, Kakimoto T and Tsuboi
N: Isolation of (−)-S-allyl-L-cysteine from garlic. Chem
Pharm Bull (Tokyo). 9:251–252. 1961. View Article : Google Scholar
|
|
20
|
Ziegler SJ and Sticher O: HPLC of
S-alk(en)yl-L-cysteine derivatives in garlic including
quantitative determination of (+)-S-allyl-L-cysteine
sulfoxide (alliin). Planta Med. 55:372–378. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lawson LD, Wang Z-Y and Hughes BG:
γ-Glutamyl-S-alkylcysteines in garlic and other Allium spp.:
Precursors of age-dependent trans−1-propenyl thiosulfinates.
J Nat Prod. 54:436–444. 1991. View Article : Google Scholar
|
|
22
|
Farooqui AA and Farooqui T: Garlic and its
effects in neurological disorders. Neuroprotective effects of
phytochemicals in neurological disorders. John Wiley & Sons;
Hoboken, NJ: pp. 113–131. 2017, View Article : Google Scholar
|
|
23
|
Hirata S, Abdelrahman M, Yamauchi N and
Shigyo M: Characteristics of chemical components in genetic
resources of garlic Allium sativum collected from all over
the world. Genet Resour Crop Evol. 63:35–45. 2016. View Article : Google Scholar
|
|
24
|
Lawson LD: Garlic: A review of its
medicinal effects and indicated active compounds. ACS Symp Ser.
691:176–209. 1998. View Article : Google Scholar
|
|
25
|
Ueda Y, Kawajiri H, Miyamura N and
Miyajima R: Content of some sulfur-containing components and free
amino acids in various strains of garlic. Nippon Shokuhin Kogyo
Gakkaishi (J Jpn Soc Food Sci Technol). 38:429–434. 1991.
View Article : Google Scholar
|
|
26
|
Mütsch-Eckner M, Sticher O and Meier B:
Reversed-phase high-performance liquid chromatography of
S-alk(en)yl-L-cysteine derivatives in Allium sativum
including the determination of (+)-S-allyl-L-cysteine
sulphoxide, γ-L-glutamyl-S-allyl-L-cysteine and
γ-L-glutamyl-S-(trans−1-propenyl)-L-cysteine. J
Chromatogr A. 625:183–190. 1992. View Article : Google Scholar
|
|
27
|
Bloem E, Haneklaus S and Schnug E: Storage
life of field-grown garlic bulbs (Allium sativum L.) as
influenced by nitrogen and sulfur fertilization. J Agric Food Chem.
59:4442–4447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamazaki Y and Okuno T: Accumulation of
S-allyl-L-cysteine in garlic bulbs by warming. Nippon
Shokuhin Kagaku Kogaku Kaishi. 55:410–415. 2008. View Article : Google Scholar
|
|
29
|
Rahman MS: Allicin and other functional
active components in garlic: Health benefits and bioavailability.
Int J Food Prop. 10:245–268. 2007. View Article : Google Scholar
|
|
30
|
Martins N, Petropoulos S and Ferreira
ICFR: Chemical composition and bioactive compounds of garlic
(Allium sativum L.) as affected by pre- and post-harvest
conditions: A review. Food Chem. 211:41–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuura H, Inagaki M, Maeshige K, Ide N,
Kajimura Y and Itakura Y: Changes in contents of γ-glutamyl
peptides and fructan during growth of Allium sativum. Planta
Med. 62:70–71. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ellmore GS and Feldberg RS: Allin lyase
localization in bundle sheaths of the garlic clove (Allium
sativum). Am J Bot. 81:89–94. 1994. View Article : Google Scholar
|
|
33
|
Penn RE, Block E and Revelle LK: Flash
vacuum pyrolysis studies. 5. Methanesulfenic acid. J Am Chem Soc.
100:3622–3623. 1978. View Article : Google Scholar
|
|
34
|
Cavallito CJ, Buck JS and Suter CM:
Allicin, the antibacterial principle of Allium sativum. II.
Determination of the chemical structure. J Am Chem Soc.
66:1952–1954. 1944. View Article : Google Scholar
|
|
35
|
Borlinghaus J, Albrecht F, Gruhlke MC,
Nwachukwu ID and Slusarenko AJ: Allicin: Chemistry and biological
properties. Molecules. 19:12591–12618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi MK: CHae K-Y, Lee J-Y and Kyung KH:
Antimicrobial activity of chemical substances derived from
S-alk(en)yl-L-cysteine sulfoxide (alliin) in garlic,
Allium sativum L. Food Sci Biotechnol. 16:1–7. 2007.
|
|
37
|
Rabinkov A, Miron T, Konstantinovski L,
Wilchek M, Mirelman D and Weiner L: The mode of action of allicin:
Trapping of radicals and interaction with thiol containing
proteins. Biochim Biophys Acta. 1379:233–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lawson LD, Wang ZJ and Hughes BG:
Identification and HPLC quantitation of the sulfides and
dialk(en)yl thiosulfinates in commercial garlic products. Planta
Med. 57:363–370. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh VK and Singh DK: Pharmacological
effects of garlic (Allium sativum L.). Ann Rev Biomed Sci.
10:6–26. 2008.
|
|
40
|
Block E, Naganathan S, Putman D and Zhao
SH: Allium chemistry: HPLC analysis of thiosulfinates from
onion, garlic, wild garlic (ramsoms), leek, scallion, shallot,
elephant (great-headed) garlic, chive, and Chinese chive. Uniquely
high allyl to methyl ratios in some garlic samples. J Agric Food
Chem. 40:2418–2430. 1992. View Article : Google Scholar
|
|
41
|
Yamaguchi Y, Honma R, Yazaki T, Shibuya T,
Sakaguchi T, Uto-Kondo H and Kumagai H: Sulfuric odor precursor
S-allyl-L-cysteine sulfoxide in garlic induces detoxifying
enzymes and prevents hepatic injury. Antioxidants. 8:3852019.
View Article : Google Scholar
|
|
42
|
Bartzatt R, Blum D and Nagel D: Isolation
of Garlic Derived Sulfur Compounds from Urine. Anal Lett.
25:1217–1224. 1992. View Article : Google Scholar
|
|
43
|
de Rooij BM, Boogaard PJ, Rijksen DA,
Commandeur JN and Vermeulen NP: Urinary excretion of
N-acetyl-S-allyl-L-cysteine upon garlic consumption
by human volunteers. Arch Toxicol. 70:635–639. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jandke J and Spiteller G: Unusual
conjugates in biological profiles originating from consumption of
onions and garlic. J Chromatogr A. 421:1–8. 1987. View Article : Google Scholar
|
|
45
|
Trio PZ, You S, He X, He J, Sakao K and
Hou D-X: Chemopreventive functions and molecular mechanisms of
garlic organosulfur compounds. Food Funct. 5:833–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Verhagen H, Hageman GJ, Rauma A-L,
Versluis-de Haan G, van Herwijnen MHM, de Groot J, Törrönen R and
Mykkänen H: Biomonitoring the intake of garlic via urinary
excretion of allyl mercapturic acid. Br J Nutr. 86 (Suppl
1):S111–S114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Augusti KT and Sheela CG: Antiperoxide
effect of S-allyl cysteine sulfoxide, an insulin
secretagogue, in diabetic rats. Experientia. 52:115–120. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhai B, Zhang C, Sheng Y, Zhao C, He X, Xu
W, Huang K and Luo Y: Hypoglycemic and hypolipidemic effect of
S-allyl-cysteine sulfoxide (alliin) in DIO mice. Sci Rep.
8:35272018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sangeetha T and Darlin Quine S: Preventive
effect of S-allyl cysteine sulphoxide (Alliin) on
mitochondrial dysfunction in normal and isoproterenol induced
cardiotoxicity in male Wistar rats: A histopathological study. Mol
Cell Biochem. 328:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nishimura H and Ariga T: Vinyldithiins in
garlic and Japanese domestic Allium (A. victorialis).
CS Symp Ser. 546:128–143. 1993.
|
|
51
|
Akao M, Shibuya T, Shimada S, Sakurai H
and Kumagai H: In vivo production of bioactive compounds from
S-allyl-L-cysteine sulfoxide, garlic odor precursor, that
inhibit platelet aggregation. J Clin Biochem Nutr. (Suppl 43):1–3.
2008.
|
|
52
|
Uto-Kondo H, Hase A, Yamaguchi Y, Sakurai
A, Akao M, Saito T and Kumagai H: S-Allyl-L-cysteine
sulfoxide, a garlic odor precursor, suppresses elevation in blood
ethanol concentration by accelerating ethanol metabolism and
preventing ethanol absorption from gut. Biosci Biotechnol Biochem.
82:724–731. 2018. View Article : Google Scholar : PubMed/NCBI
|