Introduction
Obesity is a global threat to the health of
individuals. Approximately 1.9 billion adults are overweight and
600 million are obese worldwide (1).
Due to the fact that the mechanisms of pathologies underlining
obesity differ among individuals, the discovery of novel compounds
from diverse sources and novel developments for new categories of
medications are urgently required for the prevention and treatment
of obesity and its related metabolic disorders, including diabetes
(2). Thus far, lifestyle
intervention has been a first choice for the treatment of obesity;
however, the lifestyle interventions are limited by low efficacy
and high drop-out rates.
Obesity is characterized by the excessive
accumulation of triglycerides in adipose tissues, including in
visceral and subcutaneous adipose tissues (3). Adipose tissue also acts as an endocrine
organ, which secretes a variety of cytokines, termed
adipocytokines. Adipocytokines are involved in the pathophysiology
of obesity, such as insulin resistance and metabolic syndrome.
Obesity is an independent risk factor for the promotion of
arteriosclerosis followed by the onset of thrombotic diseases, such
as myocardial infarction (4).
Adipose tissue can be categorized into white adipose
tissue (WAT) and brown adipose tissue (BAT) according to their
phenotype. The major cell type present in BAT is brown adipocytes.
Brown adipocytes contain small lipid droplets and have a high
density of mitochondria, which is a causative of the brown
appearance. On the other hand, WAT contains white adipocytes with
unilocular lipid droplets. BAT is involved in thermogenesis and
energy expenditure via uncoupling protein-1 (UCP1) located in the
mitochondrion, which uncouples oxidative phosphorylation from
adenosine triphosphate (ATP) production (5). WAT is involved in fat storage and the
production of adipocytokines in an endocrine manner. In response to
various stimuli, UCP1-expressing multilocular adipocytes develop
into WAT (6).
Garlic (Allium sativum L.) has long been used
as a medicinal food worldwide, as well as a spice (7). It has been reported that garlic has
various biological functions through which it procures medicinal
benefits to the human body, such as antibiotic (8–11),
antithrombotic (12), anticancer
(13–16), antioxidant (17), anti-hypertensive (18) and antilipidemic (19) effects. These effects are attributed
to organosulfur compounds derived from garlic (7). Garlic oil (GO) is produced by the steam
distillation of raw garlic homogenate; normally, 0.2–0.6 ml of
garlic oil is obtained from 100 g of garlic homogenate. The major
constituents of GO are allyl sulfides, including diallyl trisulfide
(DATS), diallyl disulfide (DADS), diallyl sulfide (DAS) and methyl
allyl trisulfide (MATS) (20). These
sulfide compounds are considered to be responsible for the potent
physiological functions of garlic.
In this study, we aimed to investigate the
anti-obesity effects of GO in a rat model of high-fat diet-induced
obesity. In addition, we aimed to elucidate the underlying
mechanisms of the anti-obesity effects of GO in terms of energy
metabolism.
Materials and methods
Rats and diets
All experiments in this study were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and were approved by the Nihon
University Animal Care and Use Committee (approval no. AP11B008).
The animals used in this study were 5-week-old male Sprague-Dawley
(SD) rats (Japan SLC). Ten of the SD rats were introduced and
housed individually in a stainless-steel wire-bottomed cage in a
temperature-controlled room (22–23°C) with a 12-h photoperiod. The
rats were provided with a high-fat diet (60% of total energy as
fat) prepared based on the composition of AIN-76 (21) and were provided with water ad
libitum. The diet contained 5% (wt:wt) corn oil, 31.9% (wt:wt)
lard, 28% (wt:wt) casein, 0.4% (wt:wt) D,L-methionine, 15% (wt:wt)
corn starch, 10% (wt:wt) sucrose, 1% (wt:wt) vitamins, 3.5%
minerals 0.2% (wt:wt) choline bitartrate and 5% (wt:wt) cellulose.
Following 2 weeks of acclimatization, the rats were divided into 2
groups with matched body weight; i.e., i) The vehicle-administered
control group (Vehicle group, 5 rats); and ii) the GO-administered
group (GO group, 5 rats). GO (Riken Chemical Ind. Co., Ltd.; 80
mg/kg body weight) was administered per os (p.o.) with corn
oil (2 ml/kg body weight; Wako Pure Chemical Industries, Ltd) every
other day for 10 weeks. The same amount of corn oil without GO was
administered to the Vehicle group. The dose of GO was decided upon
according to a previous study (17);
the effective dose of DATS (500 µmol/kg body weight) in the study
was considered to determine the dose of GO.
Expired gas analysis
To clarify the mechanisms responsible for the
anti-obesity effects of garlic, we performed expired gas analysis
by measuring the oxygen consumption (VO2) and validation
of carbon dioxide production (VCO2) at 9 weeks following
GO administration. The respiratory metabolism was analyzed by the
use of Oxymax equal flowTM (Columbus Instruments). The rats were
placed in the instrument chamber for 24 h prior to the expired gas
analysis for the purpose of acclimatization, and the analyses were
then performed every 10 min. The respiratory exchange ratio (RER),
energy expenditure (EE) and fuel oxidations were calculated using
the following equations as previously described (22,23): RER
= VCO2/VO2; EE (kcal/h) = (3.815+1.232 ×
VO2) × RER; fat oxidation (kcal/h) = (1 - RER)/0.3 × EE;
glucose oxidation (kcal/h) = EE - fat oxidation.
Western blot analysis
Following 10 weeks of GO administration, the rats
were fasted for 4 h and then sacrificed by CO2
euthanasia (fill rate of approximately 20% of the chamber volume
per minute with CO2). Following the verification of the
death of rats by observations for lack of respiration, absence of
heartbeat and faded eye color, the epididymal, perirenal,
mesenteric, subcutaneous WAT and interscapular BAT were collected
and then frozen immediately by flashing the samples with liquid
nitrogen. These tissue samples were stored at −80°C until analysis.
Western blot analysis was then performed as previously described
(24). Briefly, the mitochondrial
fraction prepared from BAT was dissolved in 0.5% protease inhibitor
cocktail (Sigma) containing STE buffer (0.25 M sucrose, 5 mM
Tris-HCl, 2 mM EGTA, pH 7.4) and the protein concentration was
measured using the Pierce™ BCA Protein Assay kit (Thermo Fisher
Scientific). The protein samples (1 µg/lane) were subjected to
SDS-PAGE (10% gel), and the proteins migrated were electrically
transferred to a polyvinylidene fluoride (PVDF) microporous
membrane (Millipore Corp.). The membrane was incubated with 10%
skimmed milk (Yukijirushi Milk Products Co., Ltd.) in 0.1%
Tween-20, 100 mM NaCl, 10 mM Tris-HCl (pH 7.4), at room temperature
for 10 min followed by the reaction with anti-UCP1 rabbit antibody
(1:5,000; cat. no. ab10983, poly clonal, Abcam) and anti-cytochrome
c oxidase subunit 4 (COX-4) mouse monoclonal antibody
(1:5,000; sc-376731, Santa Cruz Biotechnology, Inc.) at 4°C for 18
h. Following incubation, peroxidase-AffiniPure goat anti-rabbit IgG
(HL) (1:20,000; cat. no. 111-035-003, Jackson ImmunoResearch
Laboratories, Inc.) or goat anti-mouse IgG (H+L) HRP conjugate
(1:20,000; cat. no. 115-035-003, Jackson ImmunoResearch
Laboratories, Inc.) were added and allowed to stand for 30 min at
room temperature. The antigenic proteins on the membrane were
visualized by chemiluminescence using a Lumi-LightPLUS Western
blotting kit (Roche Diagnostics), and the images were analyzed
using an Image Analyzer LAS-4000 (Fujifilm).
Statistical analysis
Each result is expressed as the mean ± SE and was
analyzed using Prism 6 software (GraphPad Software). The
statistical comparison between two groups was analyzed using a
Student's t-test. One-way analysis of variance followed by Tukey's
post hoc test was used to analyze differences among multiple
groups. All experiments were performed duplicate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight gain, food intake, energy
efficiency, fat accumulation
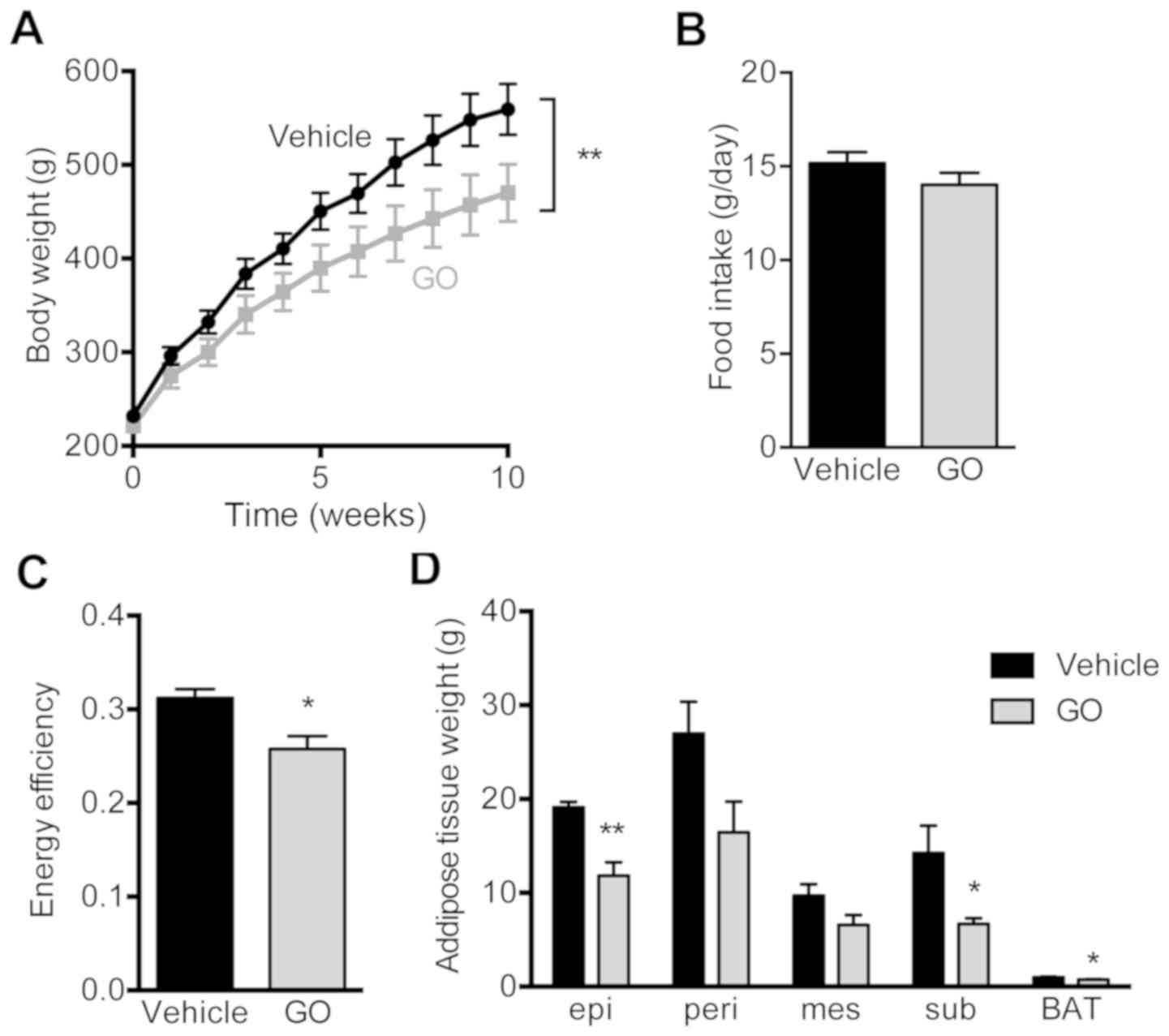
The body weights gain of the rats administered GO
for 10 weeks were significantly lower than those of the control
rats administered corn oil (Vehicle group) (Fig. 1A). On the other hand, there were no
marked differences in food intake between the Vehicle and GO groups
(Fig. 1B). Food efficiency (body
weight gain/food intake) was also significantly lower in the GO
group than in the Vehicle group (Fig.
1C). In good accordance with the suppression of body weight
gain, total WAT weight including epididymal adipose tissue,
perirenal adipose tissue, mesenteric adipose tissue, and
subcutaneous adipose tissue against body weight (WAT %) in the GO
group (8.8±0.8) was significantly lower than that in the Vehicle
group (12.3±0.8; P<0.05). Among the WATs, epididymal and
subcutaneous adipose tissues were significantly decreased; however,
perirenal (P=0.07) and mesenteric (P=0.28) adipose tissues tended
to decrease by GO administration, although not significantly. The
weight of BAT was also influenced by GO administration (Fig. 1D).
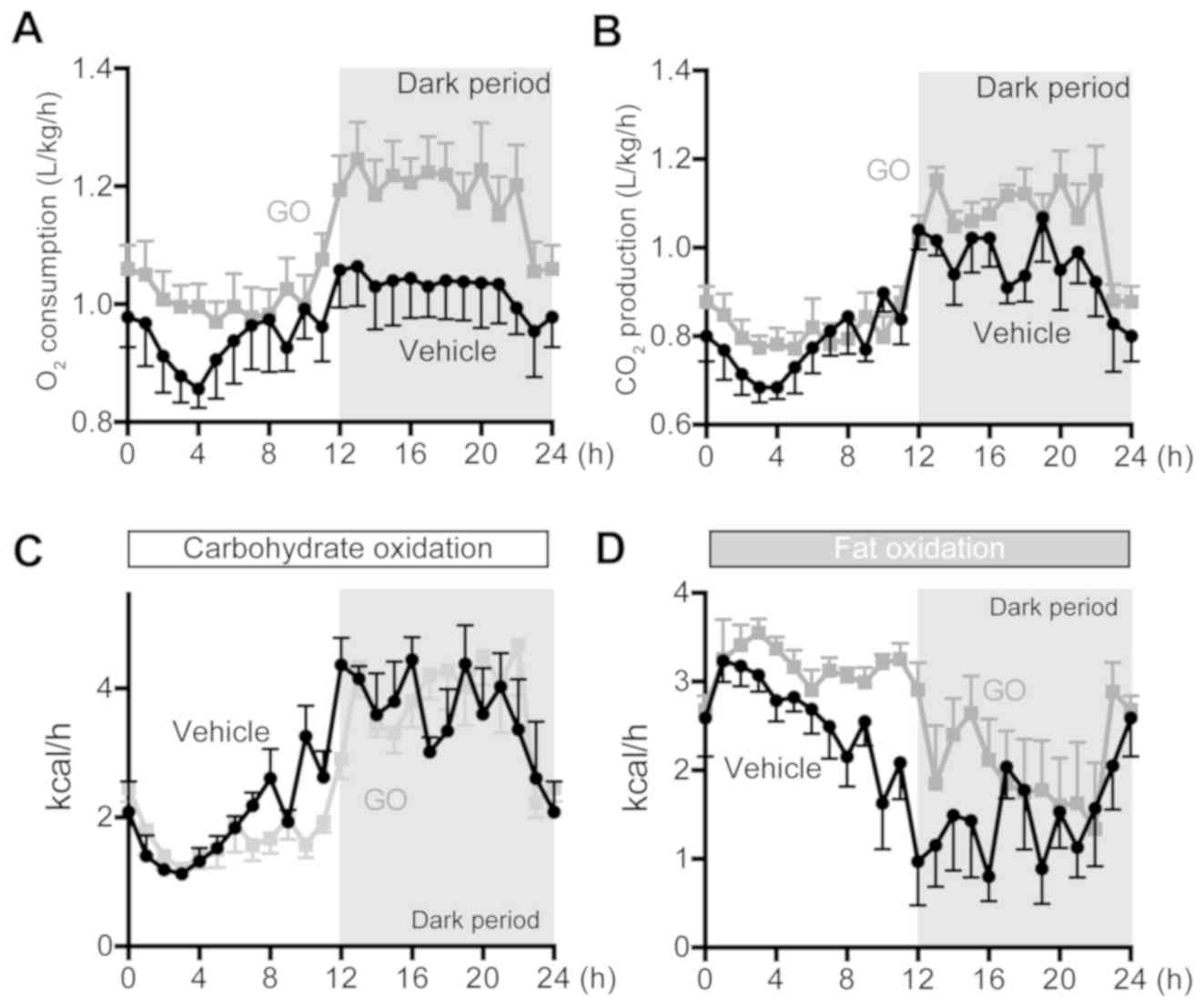
Expired gas analyses
Expired gas analyses were performed at 9 weeks
following GO administration. GO was precisely administered at
Zeitgeber time (ZT) 0 (8:00 a.m.; Fig.
2). Oxygen consumption in both groups was markedly higher
during the dark period (active time for rats) than during the light
period (inactive, sleeping time for rats), and it increased by GO
administration in comparison with the Vehicle group during the dark
period (Fig. 2A). Similarly,
CO2 production was markedly higher in the GO group than
the Vehicle group during the dark period (Fig. 2B). As regards fuel oxidation, no
significant differences were observed in carbohydrate oxidation
between the GO and Vehicle groups (Fig.
2C). On the other hand, fat oxidation was high during the light
period than the dark period in both the GO and Vehicle groups
(Fig. 2D). In contrast to
carbohydrate oxidation, fat oxidation was higher in the GO group
than the Vehicle group (Fig. 2C and
D).
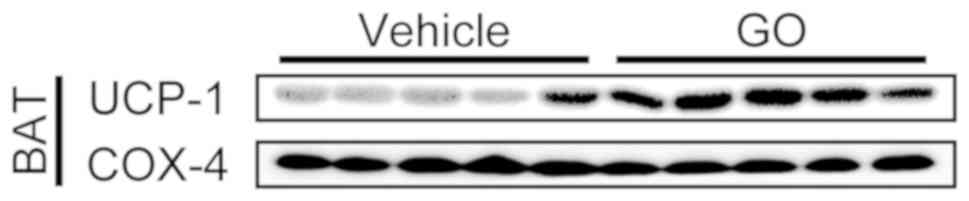
UCP-1 protein expression
UCP-1 protein expression in BAT was measured by
western blot analysis using the mitochondrial fraction prepared
from BAT (Fig. 3). COX-4 was
employed as the loading control in this analysis. UCP-1 protein
expression in BAT was markedly increased in the GO group in
comparison with the Vehicle group (Fig.
3).
Discussion
Garlic has been known to possess a variety of
compounds which affects our body functions. In this study, we
demonstrated the anti-obesity effects of GO in a rat model of
high-fat diet-induced obesity. The body weight gain and the total
mass of WAT were significantly reduced in the rats administered GO
in comparison with the vehicle-administered control rats. On the
other hand, no differences in energy intake were observed between
the GO group and the Vehicle group, indicating that the
anti-obesity effects were not due to the anorectic effect induced
by GO administration. It has been reported that garlic exerts
anti-obesity effects in animal models of obesity by using garlic
powder (25) and garlic extracts
(26,27). In these studies, water-soluble
compounds, such as allicin, phenolic compounds, or fiber
compositions were suggested to be responsible for the anti-obesity
effects (25–27). In this study, we focused on the
effects of GO, which contains a variety of oil soluble organosulfur
compounds. The major components of the GO used in this study were
as follows (20): DATS (31.5%), DADS
(21.5%), DAS (16.3%) and MATS (7.8%). These sulfides, particularly
DATS, are considered to be responsible for the physiological
functions of garlic (7,13–15,16).
In relation to the anti-obesity effects of garlic,
DATS has been reported to inhibit adipogenesis in vitro.
DATS has been shown to downregulate CCAAT/enhancer-binding protein
(C/EBP) α and β and peroxisome proliferator-activated receptor
(PPAR)γ leading to a decrease in fatty acid synthase and lipid
accumulation in 3T3-L1 adipocytes (28). Another lipophilic component,
1,2-vinyldithiin, which is mainly found in the oily macerate of
crushed garlic, leading to the degradation of allicin, has also
been shown to inhibit the differentiation and inflammation of human
preadipocytes by decreasing C/EBPα, PPARγ, interleukin-6 and
monocyte chemoattractant protein-1 expression (29). These inhibitory effects of garlic
compounds on adipocyte differentiation and lipid accumulation, as
well as on adipocytokine production may also be responsible for the
anti-obesity effects of garlic.
The energy efficiency of the rats administered GO in
this study was significantly decreased in comparison with the
vehicle-administered control rats (Fig.
1C). Thus, we performed an expired gas analyses to measure
energy expenditure in the GO-administered rats. In the
GO-administered rats, an overall higher O2 consumption
was observed compared with the vehicle-administered control rats
(Fig. 2A); O2 consumption
by the rats administered GO, particularly during the dark period,
was significantly higher than that by the vehicle-administered
rats. This increase in O2 consumption seems more
obvious, as it was measured at 9 weeks following GO administration
with significantly different body weights observed between the
GO-administered group and the vehicle-administered group (Fig. 1A). We further calculated the
carbohydrate and fat oxidation rate of these rats, according to the
method described in the study by Lusk (22). No differences were observed in
carbohydrate oxidation between the GO-administered rats and the
vehicle-administered control rats (Fig.
2C); however, fat oxidation was higher in the GO-administered
rats than that in the control rats (Fig.
2D). These data indicate that GO enhances O2
consumption during the dark period and stimulates fat oxidation
rather than carbohydrate oxidation during the light period.
UCP1 plays important roles in non-shivering heat
production by BAT. Thermogenesis in BAT is activated by the
sympathetic nerve system through norepinephrine, thyroid hormone
and the signals produced by β-adrenergic receptor (30). UCP1 in BAT is an important target for
the development of anti-obesity nutraceuticals. The UCP1 expression
in BAT was potently upregulated by GO administration. We previously
reported that DAS, DADS, and DATS were agonists of both transient
receptor potential cation channel, subfamily A, member 1 (TRPA1)
and transient receptor potential cation channel subfamily V member
1 (TRPV1) (31). Surprisingly, DATS
(EC50, 0.49 µmol/l) was shown to be a much more potent
agonist than allyl isothiocyanate (EC50, 1.47 µmol/l),
which is a representative agonist of TRPA1 in the assay system,
which employs TRPA1-overexpressing CHO cells (31). Taken together, all these data suggest
that GO upregulates UCP1 expression through norepinephrine and
β-adrenergic receptor signaling by the activation of both TRPA1 and
TRPV1. UCP1 also contributes to energy expenditure and
O2 consumption in animal models. Previously, the
upregulation of O2 consumption by specific agonist for
β3-adrenegic receptor was not observed in UCP1-deficient mice
(32,33). These data indicate that β3-adrenegic
stimuli increase energy expenditure via the upregulation of UCP1
protein expression.
In conclusion, the findings of the study
demonstrated that GO suppressed body weight gain and WAT mass in
the rat model of high fat diet-induced obesity. To the best of our
knowledge, in this study, it is demonstrated, for the first time,
that GO administration can alter the fuel oxidation rate and
increase fat oxidation, leading to a decrease in body weight gain.
The anti-obesity effects of GO are due to, at least in part, the
upregulation of energy expenditure by UCP1.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from Nihon
University (to TS) and the programs Grants-in-Aid for Scientific
Research (B) (to TS) from the Japan Society for the Promotion of
Science (JSPS).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YK, TH, TS conceived and designed the study. YK
conducted the research. YK, YOM, TH, TS analyzed the data and wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments in this study were performed in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and were approved by the Nihon
University Animal Care and Use Committee (approval no.
AP11B008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAT
|
brown adipose tissue
|
|
C/EBP
|
CCAAT/enhancer-binding protein
|
|
COX-4
|
cytochrome c oxidase subunit
4
|
|
DATS
|
diallyl trisulfide
|
|
DADS
|
diallyl disulfide
|
|
DAS
|
diallyl sulfide
|
|
EE
|
energy expenditure
|
|
GO
|
garlic oil
|
|
MATS
|
methyl allyl trisulfide
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
TRPA
|
transient receptor potential cation
channel, subfamily A, member 1
|
|
UCP
|
uncoupling protein
|
|
WAT
|
white adipose tissue
|
|
ZT
|
Zeitgeber time
|
References
|
1
|
Ng M, Fleming T, Robinson M, Thomson B,
Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF,
et al: Global, regional, and national prevalence of overweight and
obesity in children and adults during 1980–2013: A systematic
analysis for the Global Burden of Disease Study 2013. Lancet.
384:766–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi F, Punzo F, Umano GR, Argenziano M
and Miraglia Del Giudice E: Role of cannabinoids in obesity. Int J
Mol Sci. 19:E26902018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golbidi S and Laher I: Exercise induced
adipokine changes and the metabolic syndrome. J Diabetes Res.
2014:7268612014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seki T and Hosono T: Prevention of
cardiovascular diseases by garlic-derived sulfur compounds. J Nutr
Sci Vitaminol (Tokyo). 61 (Suppl):S83–S85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García MDC, Pazos P, Lima L and Diéguez C:
Regulation of energy expenditure and brown/beige thermogenic
activity by interleukins: New roles for old actors. Int J Mol Sci.
19:E25692018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartelt A and Heeren J: Adipose tissue
browning and metabolic health. Nat Rev Endocrinol. 10:24–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ariga T and Seki T: Antithrombotic and
anticancer effects of garlic-derived sulfur compounds: A review.
Biofactors. 26:93–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petropoulos S, Fernandes Â, Barros L,
Ciric A, Sokovic M and Ferreira ICFR: Antimicrobial and antioxidant
properties of various Greek garlic genotypes. Food Chem. 245:7–12.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujisawa H, Suma K, Origuchi K, Kumagai H,
Seki T and Ariga T: Biological and chemical stability of
garlic-derived allicin. J Agric Food Chem. 56:4229–4235. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujisawa H, Suma K, Origuchi K, Seki T and
Ariga T: Thermostability of allicin determined by chemical and
biological assays. Biosci Biotechnol Biochem. 72:2877–2883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujisawa H, Watanabe K, Suma K, Origuchi
K, Matsufuji H, Seki T and Ariga T: Antibacterial potential of
garlic-derived allicin and its cancellation by sulfhydryl
compounds. Biosci Biotechnol Biochem. 73:1948–1955. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ariga T, Tsuj K, Seki T, Moritomo T and
Yamamoto JI: Antithrombotic and antineoplastic effects of
phyto-organosulfur compounds. Biofactors. 13:251–255. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seki T, Tsuji K, Hayato Y, Moritomo T and
Ariga T: Garlic and onion oils inhibit proliferation and induce
differentiation of HL-60 cells. Cancer Lett. 160:29–35. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hosono T, Fukao T, Ogihara J, Ito Y, Shiba
H, Seki T and Ariga T: Diallyl trisulfide suppresses the
proliferation and induces apoptosis of human colon cancer cells
through oxidative modification of beta-tubulin. J Biol Chem.
280:41487–41493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosono T, Hosono-Fukao T, Inada K, Tanaka
R, Yamada H, Iitsuka Y, Seki T, Hasegawa I and Ariga T: Alkenyl
group is responsible for the disruption of microtubule network
formation in human colon cancer cell line HT-29 cells.
Carcinogenesis. 29:1400–1406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iitsuka Y, Tanaka Y, Hosono-Fukao T,
Hosono T, Seki T and Ariga T: Relationship between lipophilicity
and inhibitory activity against cancer cell growth of nine kinds of
alk(en)yl trisulfides with different side chains. Oncol Res.
18:575–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosono-Fukao T, Hosono T, Seki T and Ariga
T: Diallyl trisulfide protects rats from carbon
tetrachloride-induced liver injury. J Nutr. 139:2252–2256. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ried K, Frank OR, Stocks NP, Fakler P and
Sullivan T: Effect of garlic on blood pressure: A systematic review
and meta-analysis. BMC Cardiovasc Disord. 8:132008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ried K, Toben C and Fakler P: Effect of
garlic on serum lipids: An updated meta-analysis. Nutr Rev.
71:282–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukao T, Hosono T, Misawa S, Seki T and
Ariga T: The effects of allyl sulfides on the induction of phase II
detoxification enzymes and liver injury by carbon tetrachloride.
Food Chem Toxicol. 42:743–749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reeves PG: Components of the AIN-93 diets
as improvements in the AIN-76A diet. J Nutr. 127 (Suppl):838S–841S.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lusk G: Analysis of the oxidation of
mixtures of carbohydrate and fat: A Correction. J Biol Chem.
59:21924.
|
|
23
|
Bruss MD, Khambatta CF, Ruby MA, Aggarwal
I and Hellerstein MK: Calorie restriction increases fatty acid
synthesis and whole body fat oxidation rates. Am J Physiol
Endocrinol Metab. 298:E108–E116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen Y, Fukushima M, Ito Y, Muraki E,
Hosono T, Seki T and Ariga T: Verification of the antidiabetic
effects of cinnamon (Cinnamomum zeylanicum) using
insulin-uncontrolled type 1 diabetic rats and cultured adipocytes.
Biosci Biotechnol Biochem. 74:2418–2425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee MS, Kim IH, Kim CT and Kim Y:
Reduction of body weight by dietary garlic is associated with an
increase in uncoupling protein mRNA expression and activation of
AMP-activated protein kinase in diet-induced obese mice. J Nutr.
141:1947–1953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Joo H, Kim CT, Kim IH and Kim Y:
Anti-obesity effects of hot water extract and high hydrostatic
pressure extract of garlic in rats fed a high-fat diet. Food Chem
Toxicol. 55:100–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim I, Kim HR, Kim JH and Om AS:
Beneficial effects of Allium sativum L. stem extract on
lipid metabolism and antioxidant status in obese mice fed a
high-fat diet. J Sci Food Agric. 93:2749–2757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lii CK, Huang CY, Chen HW, Chow MY, Lin
YR, Huang CS and Tsai CW: Diallyl trisulfide suppresses the
adipogenesis of 3T3-L1 preadipocytes through ERK activation. Food
Chem Toxicol. 50:478–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keophiphath M, Priem F, Jacquemond-Collet
I, Clément K and Lacasa D: 1,2-vinyldithiin from garlic inhibits
differentiation and inflammation of human preadipocytes. J Nutr.
139:2055–2060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yonezawa T, Kurata R, Hosomichi K, Kono A,
Kimura M and Inoko H: Nutritional and hormonal regulation of
uncoupling protein 2. IUBMB Life. 61:1123–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koizumi K, Iwasaki Y, Narukawa M, Iitsuka
Y, Fukao T, Seki T, Ariga T and Watanabe T: Diallyl sulfides in
garlic activate both TRPA1 and TRPV1. Biochem Biophys Res Commun.
382:545–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szentirmai É and Kapás L: The role of the
brown adipose tissue in β3-adrenergic receptor activation-induced
sleep, metabolic and feeding responses. Sci Rep. 7:9582017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hankir MK, Kranz M, Keipert S, Weiner J,
Andreasen SG, Kern M, Patt M, Klöting N, Heiker JT, Brust P, et al:
Dissociation between brown adipose tissue 18F-FDG uptake and
thermogenesis in uncoupling protein 1-deficient mice. J Nucl Med.
58:1100–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|