Introduction
Recently, the spread of drug-resistant bacteria has
become a serious global concern in the therapy of infectious
diseases. A number of antibiotics have been developed and used to
treat infectious diseases; however, the increased frequency in the
use of such antibiotics has led to changes in bacterial
characteristics, with bacteria acquiring drug-resistant ability
through the mutations of drug-target molecules, the overexpression
of efflux pumps, changes in the composition of the cell membrane,
the production of metabolizing enzymes and biofilm formation
(1). Among these, the biofilm
comprises a large community and aggregation of bacteria and its
formation protects microbial cells from antibiotics and immune
cells. The composition of biofilm is mainly water and extracellular
polymeric substances (EPS), such as proteins, DNA, RNA and
polysaccharides (1,2). The production of EPS is regulated by
quorum sensing (QS), which represents cell-to-cell communication in
bacteria and is controlled by chemical signaling molecules, such as
N-acyl-L-homoserine lactones (AHLs) (3,4). Thus,
QS plays a crucial role in biofilm formation. The biofilm formation
of bacteria has been reported to be associated with chronic
infections; therefore, an increase in biofilm-forming bacteria is a
serious issue, not only in the medical field, but also in a number
of industrial fields and facilities. Therefore, the discovery and
development of drugs to combat the formation of biofilm is an
important approach for the fight against drug-resistant
infections.
Garlic (Allium sativum L.) has been used as
not only a food, but also as a remedy for several diseases, such as
cardiovascular diseases and cancer (5–7). In
addition, garlic has long been used in the treatment of infectious
diseases, as described in the 9th century literary book entitled
ure ‘Bald's Leechbook’. A remedy termed Bald's eye salve for stye
that is caused by Staphylococcus aureus (S. aureus)
infection was prepared by the alcoholic extraction of garlic, onion
or leek in a brass pot (7,8). Fuchs et al demonstrated the
antimicrobial activity of Bald's eye salve against S. aureus
and Pseudomonas aeruginosa, including the
multidrug-resistant phenotype and identified allicin as the
principal antimicrobial compound in Bald's eye salve formulation.
Allicin may greatly contributed to the treatment of stye in that
era (7). On the other hand, allicin
is chemically unstable and rapidly disappears when it comes into
contact with body fluids (9,10). Therefore, it is difficult for allicin
to reach the infected sites of the body as an intact form. Recent
studies have demonstrated that sulfur-containing compounds derived
from garlic, such as diallyl disulfide (DAS2) and
ajoene, inhibit biofilm formation and the QS of bacteria, even
though the antimicrobial activities of these compounds are lower
than those in medical antibiotics used in clinical settings
(11–16). Furthermore, Slachmuylders et
al and others have demonstrated that some natural products,
which exert an inhibitory effect on biofilm formation, have
antibiotic-potentiating activity (17–20).
In this review, we focus on the antimicrobial
activity of sulfur-containig compounds derived from garlic and
describe their chemical and biological properties, including their
inhibitory effect on bacterial biofilm formation.
Antimicrobial activity of hydrophobic
compounds in garlic
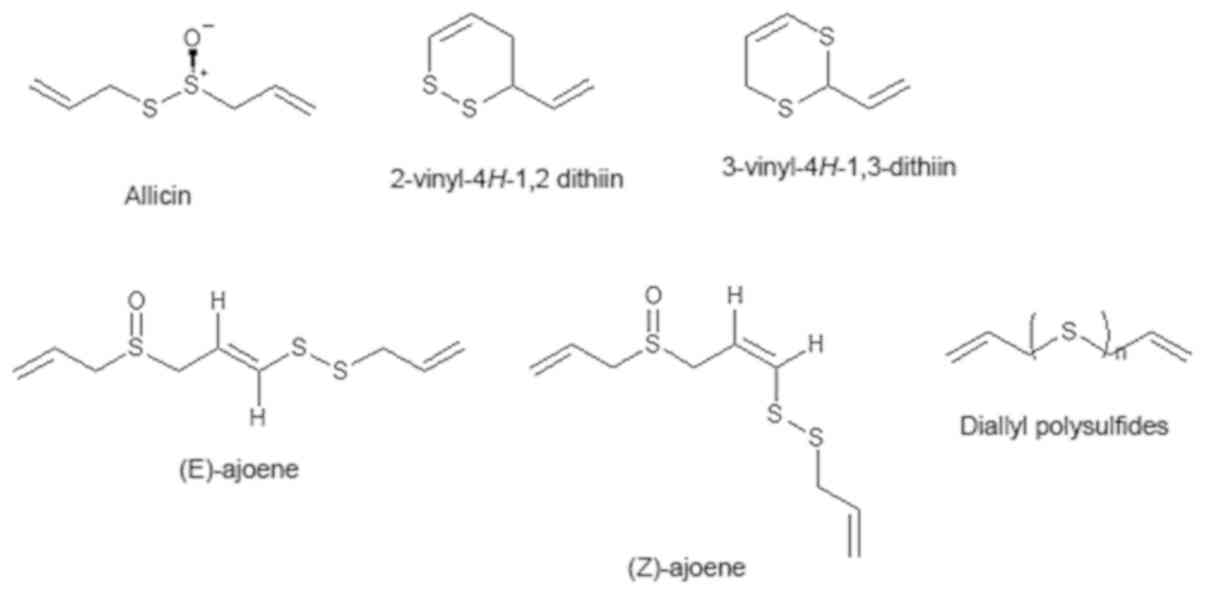
Various hydrophobic antimicrobial compounds have
been isolated from garlic and their structures are illustrated in
Fig. 1. Among these compounds,
allicin is considered to play a central role in the biological
activity of garlic. However, allicin is unstable and tends to be
converted into various compounds, such as ajoenes and diallyl
polysulfides (DASn), which have been reported to exhibit
antimicrobial activity. In this section, we describe the chemical
and biological properties of hydrophobic compounds in garlic and
its preparation, exhibiting antimicrobial activity.
Allicin
Allicin is the most abundant and characteristic
sulfur-containing compound in raw garlic. It is produced from
alliin (21). Allicin has been shown
to exhibit broad-spectrum antimicrobial activity against
Gram-positive and -negative bacteria, including multidrug-resistant
bacteria (22–26). In addition, allicin has been shown to
possess antiviral, anti-fungal and anti-parasitic activity
(27,28). It has been reported that allicin
exhibits antimicrobial activity by the S-allylmercapto
modification of thiol-containing proteins in bacteria, which leads
to lethal events, including the reduction of glutathione levels,
the induction of protein aggregation and the inactivation of
crucial enzymes (29). Reiter et
al reported that allicin vapour exhibited antimicrobial
activity against lung pathogenic bacteria (30). Additionally, topical treatment with
allicin has been shown to improve skin infection caused by
methicillin-resistant S. aureus (MRSA) (31). However, allicin is unstable and has
been shown to be decomposed or metabolized within a few seconds in
the blood (10). Therefore, the use
of allicin may be limited to direct inhalation or external medicine
due to its instability.
Vinyldithiins
Vinyldithiins that contain
2-vinyl-4H−1,2-dithiin and 3-vinyl-4H−1, 3-dithiin
are converted from one allicin molecule (32,33).
These compounds are characteristic sulfur-containing compounds in
garlic oil macerate products (33).
Vinyldithiins are known to have several biological activities, such
as anti-obesity activity (34);
however, they have no antimicrobial activity (35).
Ajoenes
Ajoenes (Z-ajoene and E-ajoene) are
also characteristic sulfur-containing compounds in garlic oil
macerate products. Both ajoenes are converted from 3 allicin
molecules (21). The antimicrobial
activity of ajoenes has been evaluated by several groups. Yoshida
et al examined activity of ajoenes against Gram-positive and
-negative bacteria and found that MIC values were 5–20 µg/ml for
Gram-positives and 100–160 µg/ml for Gram-negatives. They also
indicated that Z-ajoene had a slightly greater activity than
E-ajoene (36). An additional
antimicrobial study of ajoenes against 3 strains of H.
pylori demonstrated that the antimicrobial activities of both
forms were similar i.e., 15–20 µg/ml for the Z-form and 25
µg/ml for the E-form (37).
Ajoenes are also active against fungi, such as Aspergillus
niger and Candida albicans (38,39).
Thus, ajoenes seem to be potent antimicrobial compounds; however,
these compounds rapidly disappear after being mixed with the blood,
as the case with allicin (10).
DASn
DASn are major components of garlic oil,
which are produced from allicin during the processing of garlic oil
by the steam distillation method (40). Sulfur atom numbers of DASn
in garlic oil vary from 1 to 9, depending on the production
conditions. Generally, tri- and tetra-sulfur compounds are
abundantly present (40).
DASn have limited antimicrobial activity against
Gram-positive bacteria, including drug-resistant bacteria (41). Their antimicrobial activities depend
on the number of sulfur atoms in the molecules and are in the order
of diallyl tetrasulfide (DAS4)> diallyl trisulfide
(DAS3)> DAS2> diallyl sulfide
(DAS1) (12). Therefore,
DASn containing a higher number of sulfur atom than 5
may have more potent activity against bacterial pathogens.
Antimicrobial activity of compounds
without sulfur atom derived from garlic
Matsuura et al isolated new furostanols
termed proto-eruboside-B and satiboside-B from a crude glycoside
fraction of garlic. They also found that these saponins transform
into spinostanol form by endogenous β-glucosidase during processing
period (42,43). Notably, spinostanol from eruboside-B
inhibits the growth of Candida albicans, whereas furostanol
from proto-eruboside-B, does not (44). Kodera et al isolated a
phenolic antimicrobial compound,
3-hydroxy-5-methoxy-6-methyl-2-n-pentyl-4H-pyran-4-one, termed
allixin (45). This compound was
phytoalexin; however, the antimicrobial activity was very low.
Effects of sulfur compounds on biofilm
formation and quorum sensing
Bacteria have a barrier system, biofilm formation,
which inhibits the entry of disinfectants, antibiotics and host
immune molecules into the bacterial cells and is a major cause of
the drug-resistance of bacteria (44). In addition, QS molecules, such as AHL
regulate biofilm formation, intercellular communication, bacterial
population and other processes (3,4,11,46). The
inhibition of biofilm formation and QS has been studied in various
scientific and technological fields. Certain natural products have
been reported to provide effective resources for the inhibition of
biofilm formation. Rasmussen et al performed screening to
identify QS inhibitors (QSIs) by using a novel genetic system and
found that toluene extract of garlic inhibited biofilm formation
(13). This result suggests that
hydrophobic compounds extracted from garlic might have activity as
a QSI (47). Allicin prevents
biofilm formation by inhibiting early bacterial adhesion and EPS
secretion (42,48,49). In
addition, allicin inhibits the secretion of virulence factors by
regulating QS (50). Ajoene
regulates biofilm formation by blocking the QS-induced production
of virulence factors (51,52). DAS2 inhibits the biofilm
formation of Pseudomonas spp by inhibiting the production of
virulence factors via the regulation of QS at the concentration of
0.16–1.28 mg/ml with no effects on microbial growth (44). In addition, DAS2 inhibits
the formation of biofilm by suppressing the expression of key
QS-related genes (44). Moreover, in
the S. aureus QS system, a peptidic compound having a
thioester group acts as an autoinducer (53). It is expected that DAS2
may also inhibit QS through the reaction with the thioester group
of the autoinducer. These hydrophobic compounds may contribute to
the reduction of undesirable impacts of microorganisms on humans
and they can be expected to suppress the development of drug
resistance due to biofilm formation of bacteria.
Conclusion
Various hydrophobic compounds derived from garlic
and its preparations have broad-spectrum antimicrobial activities.
In particular, allicin and its derivatives have been studied
extensively as antimicrobial active ingredients and have shown the
inhibitory activity of biofilm formation by inhibiting QS. However,
these compounds are unstable and could not be used against systemic
infections. Therefore, the development of allicin derivatives with
sufficient stability may lead to the development of superior
compounds with greater antimicrobial activity and more potent
inhibitory activity against biofilm formation for the treatment of
drug-resistant bacteria.
Acknowledgements
The authors would like to thank Dr Takami Oka of
Wakunaga Pharmaceutical Co., Ltd. for his helpful advice,
encouragement and critical reading of the manuscript.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MN and YK conceived this review. MN, KK and JIS
analyzed the relevant literature. MN wrote the first draft of the
manuscript and produced the figures. KK, JIS and YK critically
revised the manuscript. All authors have reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DASn
|
diallyl polysulfides
|
|
DAS1
|
diallyl sulfide
|
|
DAS2
|
diallyl disulfide
|
|
DAS3
|
diallyl trisulfide
|
|
QS
|
quorum sensing
|
References
|
1
|
López D, Vlamakis H and Kolter R:
Biofilms. Cold Spring Harb Perspect Biol. 2:a0003982010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muhsin J, Ufaq T, Tahir H and Saadia A:
Bacterial biofilm: its composition, formation and role in human
infections. J Microbiol Biotechnol. 4:1–14. 2015.
|
|
3
|
Bassler BL: Small talk. Cell-to-cell
communication in bacteria. Cell. 109:421–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nadell CD, Xavier JB, Levin SA and Foster
KR: The evolution of quorum sensing in bacterial biofilms. PLoS
Biol. 6:e142008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahman K and Lowe GM: Garlic and
cardiovascular disease: A critical review. J Nutr. 136:736S–740S.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roy N, Davis S, Narayanankutty A, Nazeem
P, Babu T, Abida P, Valsala P and Raghavamenon AC: Garlic
phytocompounds possess anticancer activity by specifically
targeting breast cancer biomarkers-an in silico study. Asian Pac J
Cancer Prev. 17:2883–2888. 2016.PubMed/NCBI
|
|
7
|
Fuchs AL, Weaver AJ Jr, Tripet BP, Ammons
MCB, Teintze M and Copié V: Characterization of the antibacterial
activity of Bald's eyesalve against drug resistant
Staphylococcus aureus and Pseudomonas aeruginosa.
PLoS One. 13:e02081082018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zwergal A: Beitrag zur Kenntnis der
Inhaltsstoffe des Knoblauchs, Allium sativum L. Chem Abstr.
47:32241952.(In German).
|
|
9
|
Lawson LD and Wang ZJ: Pre-hepatic fate of
the organosulfur compounds derived from garlic (Allim
sativum). Planta Med. 59((S1)): A688–A689. 1993. View Article : Google Scholar
|
|
10
|
Freeman F and Kodera Y: Garlic chemistry:
Stability of S-(2-propyl) 2-propen-1-sulfinothioate (allicin) in
blood, solvents, and stimulated physiological fluids. J Agric Food
Chem. 43:2332–2338. 1995. View Article : Google Scholar
|
|
11
|
Li WR, Ma YK, Shi QS, Xie XB, Sun TL, Peng
H and Huang XM: Diallyl disulfide from garlic oil inhibits
Pseudomonas aeruginosa virulence factors by inactivating key
quorum sensing genes. Appl Microbiol Biotechnol. 102:7555–7564.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsao S and Yin M: In vitro activity
of garlic oil and four diallyl sulphides against
antibiotic-resistant Pseudomonas aeruginosa and Klebsiella
pneumoniae. J Antimicrob Chemother. 47:665–670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rasmussen TB, Bjarnsholt T, Skindersoe ME,
Hentzer M, Kristoffersen P, Köte M, Nielsen J, Eberl L and Givskov
M: Screening for quorum-sensing inhibitors (QSI) by use of a novel
genetic system, the QSI selector. J Bacteriol. 187:1799–1814. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gowrishankar S, Kamaladevi A, Balamurugan
K and Pandian SK: In vitro and in vivo biofilm
characterization of methicillin-resistant staphylococcus
aureus from patients associated with pharyngitis infection.
BioMed Res Int. 2016:12891572016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corrigan RM, Rigby D, Handley P and Foster
TJ: The role of Staphylococcus aureus surface protein SasG
in adherence and biofilm formation. Microbiology. 153:2435–2446.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alabdullatif M and Ramirez-Arcos S:
Biofilm-associated accumulation-associated protein (Aap): A
contributing factor to the predominant growth of Staphylococcus
epidermidis in platelet concentrates. Vox Sang. 114:28–37.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slachmuylders L, Van Acker H, Brackman G,
Sass A, Van Nieuwerburgh F and Coenye T: Elucidation of the
mechanism behind the potentiating activity of baicalin against
Burkholderia cenocepacia biofilms. https://doi.org/10.1371/journal.pone.0190533
View Article : Google Scholar
|
|
18
|
Yu C, Li X, Zhang N, Wen D, Liu C and Li
Q: Inhibition of biofilm formation by D-tyrosine: Effect of
bacterial type and D-tyrosine concentration. Water Res. 92:173–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kariu T, Nakao R, Ikeda T, Nakashima K,
Potempa J and Imamura T: Inhibition of gingipains and Porphyromonas
gingivalis growth and biofilm formation by prenyl flavonoids. J
Periodontal Res. 52:89–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hasibul K, Nakayama-Imaohji H, Hashimoto
M, Yamasaki H, Ogawa T, Waki J, Tada A, Yoneda S, Tokuda M, Miyake
M and Kuwahara T: D-Tagatose inhibits the growth and biofilm
formation of Streptococcus mutans. Mol Med Rep. 17:843–851.
2018.PubMed/NCBI
|
|
21
|
Block E: The chemistry of garlic and
onions. Sci Am. 252:114–119. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cavallito CJ and Bailey JH: Allicin,
antibacterial principle of Allium sativum. I. Islation,
physical properties, and antibacterial action. J Am Chem Soc.
66:1950–1951. 1944. View Article : Google Scholar
|
|
23
|
Wu X, Santos RR and Fink-Gremmels J:
Analyzing the antibacterial effects of food ingredients: Model
experiments with allicin and garlic extracts on biofilm formation
and viability of Staphylococcus epidermidis. Food Sci Nutr.
3:158–168. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wallock-Richards D, Doherty CJ, Doherty L,
Clarke DJ, Place M, Govan JR and Campopiano DJ: Garlic revisited:
Antimicrobial activity of allicin-containing garlic extracts
against Burkholderia cepacia complex. PLoS One.
9:e1127262014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feldberg RS, Chang SC, Kotik AN, Nadler M,
Neuwirth Z, Sundstrom DC and Thompson NH: In vitro mechanism of
inhibition of bacterial cell growth by allicin. Antimicrob Agents
Chemother. 32:1763–1768. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loi VV, Huyen NTT, Busche T, Tung QN,
Gruhlke MCH, Kalinowski J, Bernhardt J, Slusarenko AJ and Antelmann
H: Staphylococcus aureus responds to allicin by global
S-thioallylation-Role of the Brx/BSH/YpdA pathway and the
disulfide reductase MerA to overcome allicin stress. Free Radic
Biol Med. 139:55–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weber ND, Andersen DO, North JA, Murray
BK, Lawson LD and Hughes BG: In vitro virucidal effects of
Allium sativum (garlic) extract and compounds. Planta Med.
58:417–423. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Getti GTM and Poole PL: Allicin causes
fragmentation of the peptidoglycan coat in Staphylococcus
aureus by effecting synthesis and aiding hydrolysis: A
determination by MALDI-TOF mass spectrometry on whole cells. J Med
Microbiol. 68:667–677. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Müller A, Eller J, Albrecht F, Prochnow P,
Kuhlmann K, Bandow JE, Slusarenko AJ and Leichert LI: Allicin
induces thiol stress in bacteria through S-alylmercapto
modification of protein cysteines. J Biol Chem. 291:11477–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reiter J, Levina N, van der Linden M,
Gruhlke M, Martin C and Slusarenko AJ: Diallylthiosulfinate
(allicin), a volatile antimicrobial from garlic (Allium
sativum), kills human lung pathogenic bacteria, including MDR
strains, as a vapor. Molecules. 22:E17112017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sharifi-Rad J, Hoseini Alfatemi S, Sharifi
Rad M and Iriti M: Antimicrobial synergic effect of allicin and
silver nanoparticles on skin infection caused by
methicillin-resistant staphylococcus aureus spp. Ann Med
Health Sci Res. 4:863–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136 (Suppl):716S–725S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Block E: Garlic and Other Alliums: The
Lore and the Science. The Royal Society of Chemistry; Cambridge,
UK: 2010
|
|
34
|
Keophiphath M, Priem F, Jacquemond-Collet
I, Clément K and Lacasa D: 1,2-Vinyldithiin from garlic inhibits
differentiation and inflammation of human preadipocytes. J Nutr.
139:2055–2060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hermes Robert E: Antithrombogenic and
antibiotic composition and methods of preparation thereof. US
Patent 4917921. Filed April 20, 1988; issued January 25, 1990.
|
|
36
|
Yoshida H, Iwata N, Katsuzaki H, Naganawa
R, Ishikawa K, Fukuda H, Fujino T and Suzuki A: Antimicrobial
activity of a compound isolated from an oil-macerated garlic
extract. Biosci Biotechnol Biochem. 62:1014–1017. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohta R, Yamada N, Kaneko H, Ishikawa K,
Fukuda H, Fujino T and Suzuki A: In vitro inhibition of the growth
of Helicobacter pylori by oil-macerated garlic constituents.
Antimicrob Agents Chemother. 43:1811–1812. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maluf ML, Takahachi G, Svidzinski TI,
Xander P, Apitz-Castro R, Bersani-Amado CA and Cuman RK: Antifungal
activity of ajoene on experimental murine paracoccidioidomycosis.
Rev Iberoam Micol. 25:163–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida S, Kasuga S, Hayashi N,
Ushiroguchi T, Matsuura H and Nakagawa S: Antifungal activity of
ajoene derived from garlic. Appl Environ Microbiol. 53:615–617.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Satyal P, Craft JD, Dosoky NS and Setzer
WN: The chemical compositions of the volatile oils of garlic
(allium sativum) and wild garlic (Allium vineale).
Foods. 6:e632017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koch HP and Lawson LD: The Science and
Therapeutic Application of Allium Sativum L and Related
Species. 2nd. Williams and Wilkins; Baltimore, MD, USA: 1996
|
|
42
|
Matsuura H, Ushiroguchi T, Itakura Y,
Hayashi N and Fuwa T: A furostanol glycoside from garlic, bulbs of
Allium sativum L. Chem Pharm Bull (Tokyo). 36:3659–3663.
1988. View Article : Google Scholar
|
|
43
|
Matsuura H, Ushiroguchi T, Itakura Y and
Fuwa T: Further studies on steroidal glycosides from bulbs, root
and leaves of Allium sativum L. Chem Pharm Bull (Tokyo).
37:2741–2743. 1989. View Article : Google Scholar
|
|
44
|
Høiby N: A short history of microbial
biofilms and biofilm infections. APMIS. 125:272–275. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kodera Y, Matsuura H, Yoshida S, Sumida T,
Itakura Y, Fuwa T and Nishino H: Allixin, a stress compound from
garlic. Chem Pharm Bull (Tokyo). 37:1656–1658. 1989. View Article : Google Scholar
|
|
46
|
Novick RP: Autoinduction and signal
transduction in the regulation of staphylococcal virulence. Mol
Microbiol. 48:1429–1449. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Persson T, Hansen TH, Rasmussen TB,
Skindersø ME, Givskov M and Nielsen J: Rational design and
synthesis of new quorum-sensing inhibitors derived from acylated
homoserine lactones and natural products from garlic. Org Biomol
Chem. 3:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lihua L, Jianhuit W, Jialini Y, Yayin L
and Guanxin L: Effects of allicin on the formation of
Pseudomonas aeruginosa biofinm and the production of
quorum-sensing controlled virulence factors. Pol J Microbiol.
62:243–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ranjbar-Omid M, Arzanlou M, Amani M,
Shokri Al-Hashem SK, Amir Mozafari N and Peeri Doghaheh H: Allicin
from garlic inhibits the biofilm formation and urease activity of
Proteus mirabilis in vitro. FEMS Microbiol Lett.
362:fnv0492015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu Z, Zhang H, Yu H, Dai Q, Xiong J, Sheng
H, Qiu J, Jiang L, Peng J, He X, et al: Allicin inhibits
Pseudomonas aeruginosa virulence by suppressing the
rhl and pqs quorum-sensing systems. Can J Microbiol.
65:563–574. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jakobsen TH, van Gennip M, Phipps RK,
Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen
TB, Friedrich K, Uthe F, et al: Ajoene, a sulfur-rich molecule from
garlic, inhibits genes controlled by quorum sensing. Antimicrob
Agents Chemother. 56:2314–2325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jakobsen TH, Warming AN, Vejborg RM,
Moscoso JA, Stegger M, Lorenzen F, Rybtke M, Andersen JB, Petersen
R, Andersen PS, Nielsen TE, Tolker-Nielsen T, Filloux A, Ingmer H
and Givskov M: A broad range quorum sensing inhibitor working
through sRNA inhibition. Sci Rep. 7:98572017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vasquez JK, Tal-Gan Y, Cornilescu G, Tyler
KA and Blackwell HE: Simplified AIP-II peptidomimetics are potent
inhibitors of Staphylococcus aureus AgrC quorum sensing
receptors. Chembiochem. 18:413–423. 2017. View Article : Google Scholar : PubMed/NCBI
|