Introduction
Ghrelin is a 28-amino acid peptide produced in the
stomach of most organisms, it is the endogenous ligand for the
growth hormone secretagogue receptor (GHSR) (1–3). Studies
have shown that ghrelin and growth hormone secretagogue receptor
are expressed in many tumors and may serve a role in cancer
progression (4,5). Ghrelin in particular has been
associated with tumor cell proliferation, survival and apoptosis
(6–9). Indeed, the biological function of
ghrelin in tumor cell proliferation and apoptosis is still
controversial.
The effects of ghrelin on cell proliferation and
apoptosis mainly depends on the cell type. For example, ghrelin can
regulate proliferation of pancreatic adenocarcinoma cells and HT-29
colon cancer cells (10,11). Studies have shown that ghrelin
inhibits lipopolysaccharide (LPS)-induced A549 cell apoptosis via
phosphoinositide 3-kinases (PI3K)/Akt and extracellular
signal-regulated kinases (ERK) signaling (12). On the other hand, ghrelin appears to
inhibit lung cancer cell proliferation (13) and another study reported that ghrelin
induces colon carcinoma cell apoptosis (14).
Ghrelin and GHSR have been observed to be expressed
in breast cancer and serve a role in breast cancer tumorigenesis
(15). Previous studies have
suggested ghrelin as a prognostic marker and potential therapeutic
target in breast cancer (16).
Ghrelin has also been reported to inhibit (17) and promote breast cancer cell
proliferation (15). The effects of
ghrelin on breast cancer cell apoptosis and its associated
molecular mechanisms remain unclear. The present study explores the
effects of ghrelin on cisplatin-induced apoptosis in human breast
cancer cells and the underlying mechanisms.
Materials and methods
Reagents
Ghrelin was acquired from Phoenix Pharmaceuticals,
Inc. (Burlingame, CA, USA). The Cell Counting Kit-8 (CCK-8) was
purchased from Dojindo Laboratory (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Cell culture reagents, including Dulbecco's
modified Eagle's medium (DMEM), glucose, foetal bovine serum (FBS),
penicillin and streptomycin, were acquired from Gibco (Invitrogen,
Thermo Fisher Scientific, Inc., Waltham, MA, USA). AnnexinV-FITC
was purchased from Pharmingen (San Diego, CA, USA).
Propidiumiodide, 4′,6-diamidino-2-phenylindole and cisplatin were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Antibodies against PI3K, phospho-PI3K, Akt, phospho-Akt, mammalian
target of rapamycin (mTOR), phospho-mTOR, B-cell lymphoma 2 (Bcl2),
Bcl2-associated X (Bax) and Caspase-3 were obtained from Cell
Signaling Technology, Inc., (Danvers, MA, USA). The in-situ
apoptosis detection kit was purchased from Roche Diagnostics
(Basel, Switzerland). Rapamycin (cat. no. sc-3504), LY 294002 (cat.
no. sc-201426), and β-actin (cat. no. sc-47778) were purchased from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA.
Cell culture and treatment
MDA-MB-231 cells were purchased from the China
Center for Type Culture Collection (Wuhan, China). The cells were
cultured in Dulbecco's modified Eagle's medium with glucose (4.5
mg/ml), L-glutamine (Invitrogen; Thermo Fisher Scientific, Inc.) (4
mM), 10% FBS, penicillin (100 U/ml) and streptomycin (100 mg/ml),
in a humidified atmosphere with 5% CO2 at 37°C. For
in vitro studies, MDA-MB-231 cells were pretreated with or
without rapamycin (20 nM; Santa Cruz Biotechnology, Inc.) or
LY294002 (10 µM; Santa Cruz Biotechnology, Inc.) for 30 min, and
then treated with ghrelin (50 nM) and/or cisplatin (25 µM) for 48 h
at 37°C and 5% CO2. MDA-MB-231 cells treated without
ghrelin and/or cisplatin were defined as the control group. Cell
apoptosis and western blot analyses were performed.
Cell viability assay
To determine a suitable concentration for cisplatin
intervention, a CCK-8 assay was used to monitor cell viability.
Briefly, MDA-MB-231 cells were seeded in 96-well culture plates
with 5×103 cells in 100 µl of culture medium per well.
MDA-MB-231 cells were treated with cisplatin at the concentrations
(0, 1, 10, 25 and 50 µM) described in the results for 48 h at 37°C.
Cells that did not receive cisplatin treatment were considered the
control group. Cell viability was measured using the CCK-8 and the
optical density was detected with a microculture plate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm. Each assay
was performed in triplicate.
Cell transfection
For GHSR silencing, MDA-MB-231 cells were transduced
with GHSR small interfering (si)RNA: forward,
5′-CCACAAACAGACAGUGAAGUU-3′ and reverse,
5′-CUUCACUGUCUGUUUGUGGUU-3′ and scrambled siRNA: forward,
5′-CAACAACGAAGCGACAUAAUC-3′ and reverse,
5′-UUAUGUCGCUUCGUUGUUGUC-3′; obtained from Ribobio (Guangzhou,
China). Cells were plated in 6-well plates and cultured for 24 h in
media without antibiotics, after which GHSR siRNA or the scrambled
siRNA was transfected at a final oligonucleotide concentration of
100 nM using Lipofectamine® 2000 (Invitrogen, Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The experiments were performed 48 h after transfection.
Caspase-3 assay
Caspase-3 activation was detected using the
Caspase-3 Activity Assay Kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol. MDA-MB-231
cells were exposed to test substances for 48 h at 37°C.
Subsequently, the culture medium was removed, and cells were
resuspended in lysis buffer after washing with ice-cold PBS.
Incubation on ice followed for 15 min. After centrifugation at
14,000 × g for 15 min at 4°C, the supernatant was transferred to a
fresh tube. Caspase-3 activity was determined using a colorimetric
activity assay, which is based on spectrophotometric detection of
p-nitroaniline (pNA) after catalysis from the labeled substrate,
Ac-DEVD-pNA (Beyotime Institute of Biotechnology). Free pNA was
quantified at 405 nm using an enzyme-linked immunosorbent assay
reader (BioTek Instruments, Inc., Winooski, VT, USA).
Apoptosis assay by flow-cytometry
MDA-MB-231 cells were seeded in 6-well plates at a
density of 2×105 cells/well. Tumor cells were harvested
and incubated with AnnexinV-Fluorescein isothiocyanate and
propidiumiodide for 15 min in the dark at 25°C. Cell apoptosis was
analyzed using a FACScan flow cytometry device with BD CellQuest
Pro software 5.1 (Becton, Dickinson and Company, Franklin Lakes,
NJ, USA).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) assay
Apoptotic cells were detected in situ by the
TUNEL assay using an In Situ Cell Death Detection kit (Roche
Applied Science, Penzberg, Germany). Cells were fixed in 4%
paraformaldehyde for 30 min at room temperature and washed with
PBS. Following this, the cells were resuspended in permeabilisation
solution for 2 min on ice. Cells were washed by PBS three times,
resuspended in TUNEL reaction buffer mixture and incubated in the
dark at 37°C for 1 h. Subsequently, cells were washed with PBS
three times and were observed under a fluorescence microscope
(magnification, ×200). A total of 10 fields-of-view were randomly
selected for analysis.
Western blot analysis
Cells were lysed in the lysis buffer containing 20
mM Tris-HCl (pH 7.4), 1 mM EDTA, 140 mM NaCl, 1% (w/v) Nonidet
P-40, 1 mM Na3VO4 1 mM phenylmethylsulfonyl
fluoride, 50 mM NaF and 10 mg/ml aprotinin. The proteins were
separated by SDS-PAGE in an 8–12% gel and electrotransferred to
polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 5% skimmed milk
for 1 h at room temperature and incubated overnight at 4°C with the
primary antibodies against PI3K (cat. no. 4249, 1:1,000 dilution),
p-PI3K (cat. no. 4228; 1:1,000 dilution), Akt (cat. no. 4691;
1:1,000 dilution), p-Akt (cat. no. 4070; 1:1,000 dilution), mTOR
(cat. no. 2983; 1:1,000 dilution), p-mTOR (cat. no. 5536; 1:1,000
dilution), Bcl-2 (cat. no. 4223; 1:1,000 dilution), Bax (cat. no.
5023; 1:1,000 dilution) and cleaved caspase-3 (cat. no. 9661;
1:1,000 dilution) and β-actin (cat. no. sc-47778; 1:1,000 dilution;
all Cell Signaling Technology, Inc.) overnight at 4°C. After
washing with TBS with Tween, membranes were incubated with
appropriate Horse radish peroxidase-conjugated secondary antibodies
(anti-rabbit IgG; cat. no. sc-2357; 1:10,000 dilution; Santa Cruz
Biotechnology) at room temperature for 1 h. The bands were
visualized using a ChemicDoc XRS system (Bio-Rad Laboratories,
Inc.).
Statistical analyses
All data are presented as the mean ± standard error
of the mean. Various treatment groups were compared with analysis
of variance, followed by the Least Significant Difference test for
differences. All statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software, Inc., USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Ghrelin inhibits cisplatin-induced
apoptosis of MDA-MB-231 cells
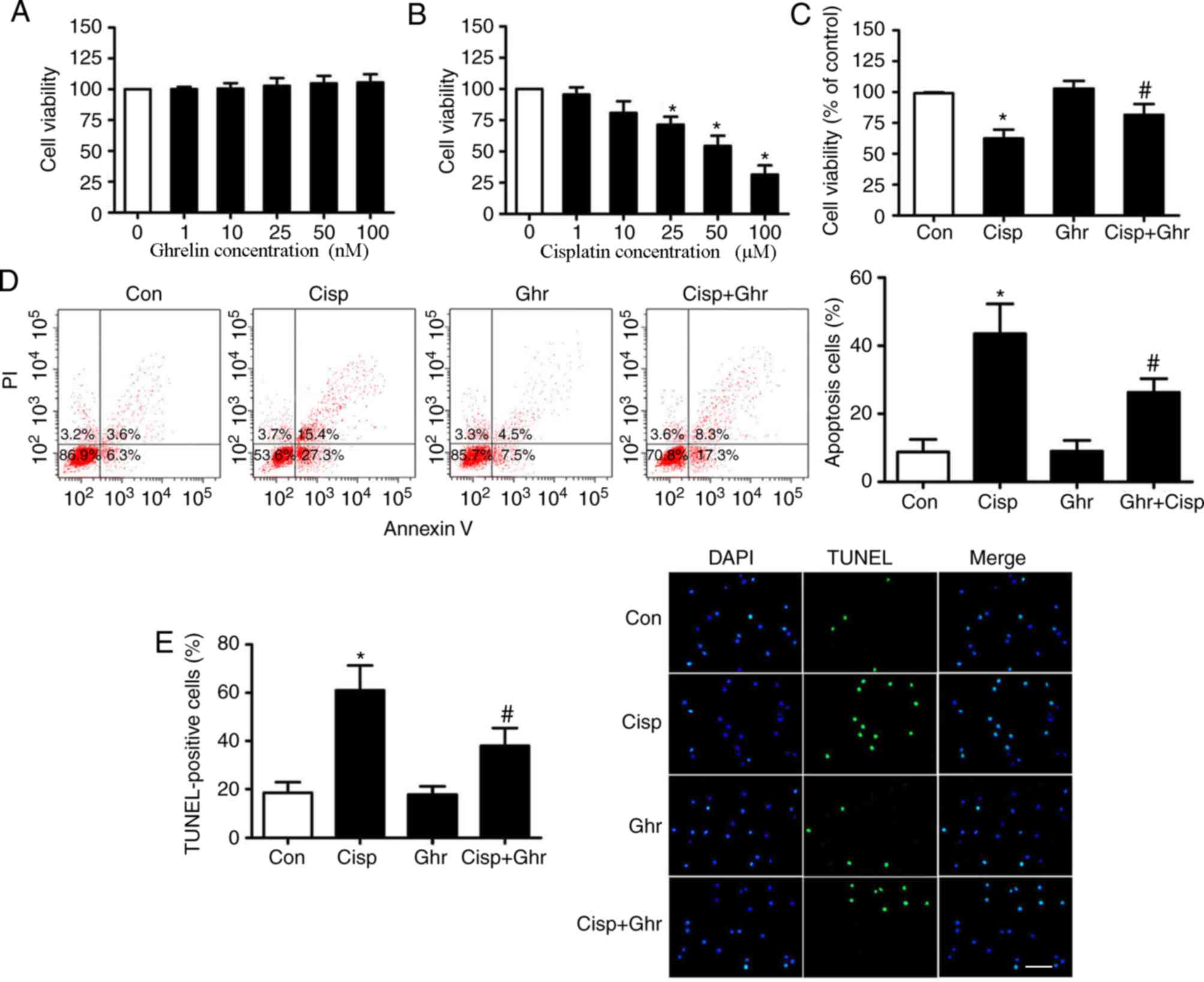
To determine the working concentrations of ghrelin,
MDA-MB-231 cells were treated with 1, 10, 25, 50 and 100 nM of
ghrelin. The CCK-8 assay showed that none of the concentrations
tested had marked effects on cell viability (Fig. 1A). In addition, dose-dependent assays
were performed to assess the cytotoxicity of cisplatin on
MDA-MB-231 cells. Cells were treated with 1, 10, 25, 50 and 100 µM
of cisplatin and cell viability was determined at 48 h. It was
observed that cisplatin treatment induced a dose-dependent
reduction of cell viability (Fig.
1B), which was inhibited by ghrelin (Fig. 1C). A previous study reported that
10–100 nM ghrelin did not affect MDA-MB-231 cells viability
(15). Based on these results, 25 µM
cisplatin and 50 nM ghrelin was chosen for subsequent experiments.
Subsequently, whether ghrelin affected apoptosis of MDA-MB-231
cells after cisplatin treatment was examined. Results showed
ghrelin significantly inhibited apoptosis in MDA-MB-231 cells in
comparison with cells treated with cisplatin only (Fig. 1D and E).
Ghrelin inhibits cisplatin-induced
mitochondria-dependent apoptosis of MDA-MB-231 cells
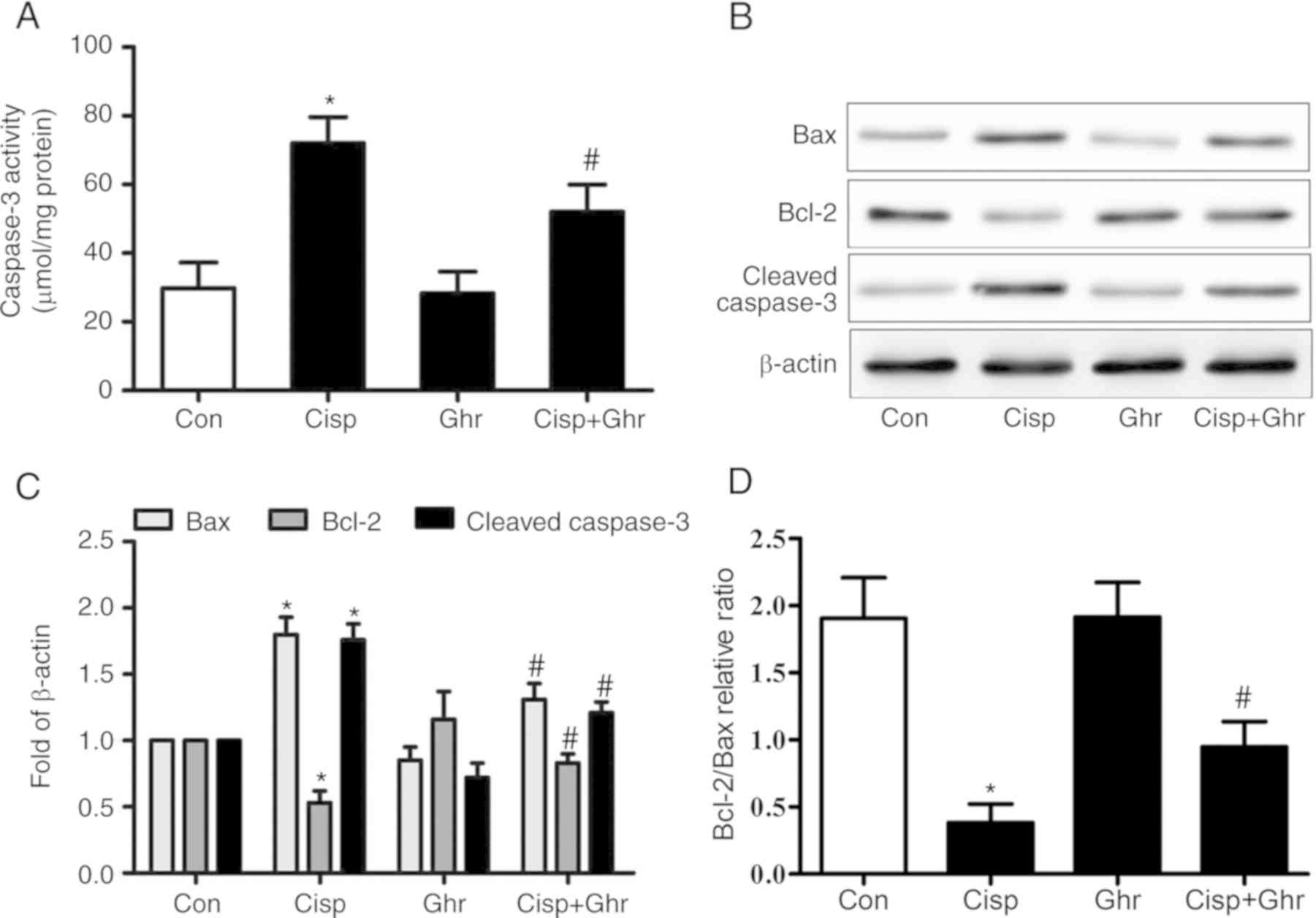
Caspase-3 activity is a pivotal biomarker of
apoptosis. It was found that cisplatin increased caspase-3 activity
in MDA-MB-231 cells in comparison with the control, while ghrelin
reduced caspase-3 activity (Fig.
2A). As expected, it was observed that the protein levels of
cleaved caspase-3 increased after cisplatin treatment (Fig. 2B), while ghrelin significantly
inhibited caspase-3 activation induced by cisplatin. Furthermore,
ghrelin inhibited the cisplatin-induced inhibition of Bcl-2
expression and upregulation of Bax, which resulted in an increased
Bcl-2/Bax ratio (Fig. 2B-D). These
results indicate ghrelin inhibits cisplatin-induced apoptosis in
MDA-MB-231 cells through mitochondria-dependent processes.
The inhibitory effect of ghrelin is
mediated by PI3K/Akt/mTOR signaling
Activation of PI3K/Akt and mTOR is known to be
associated with cell proliferation, apoptosis and survival
(12,18,19).
Whether ghrelin regulates the expression of p-PI3K/p-Akt and p-mTOR
in MDA-MB-231 cells was investigated. It was observed that the
level of p-PI3K/p-Akt and p-mTOR decreased after cisplatin
treatment in comparison with control, whereas ghrelin increased
levels of p-PI3K/p-Akt and p-mTOR (Fig.
3A). It was estimated that ghrelin could promote MDA-MB-231
cell survival by activating PI3K/Akt/mTOR signaling. To understand
this effect, PI3K inhibitor LY294002 and mTOR inhibitor rapamycin
to block PI3K/Akt and mTOR signaling in MDA-MB-231 cells were
studied. In the presence of LY294002 or rapamycin, the effect of
ghrelin on cisplatin-induced Bax activation, and the induction of
Bcl-2 expression were reversed (Fig. 3B
and C). LY294002 or rapamycin partially reduced the
pro-survival effects of ghrelin on MDA-MB-231 cells treated with
cisplatin (Fig. 3D). In summary,
these results suggest PI3K/Akt/mTOR signaling mediates the
inhibitory effects of ghrelin on cisplatin-induced apoptosis in
MDA-MB-231 cells.
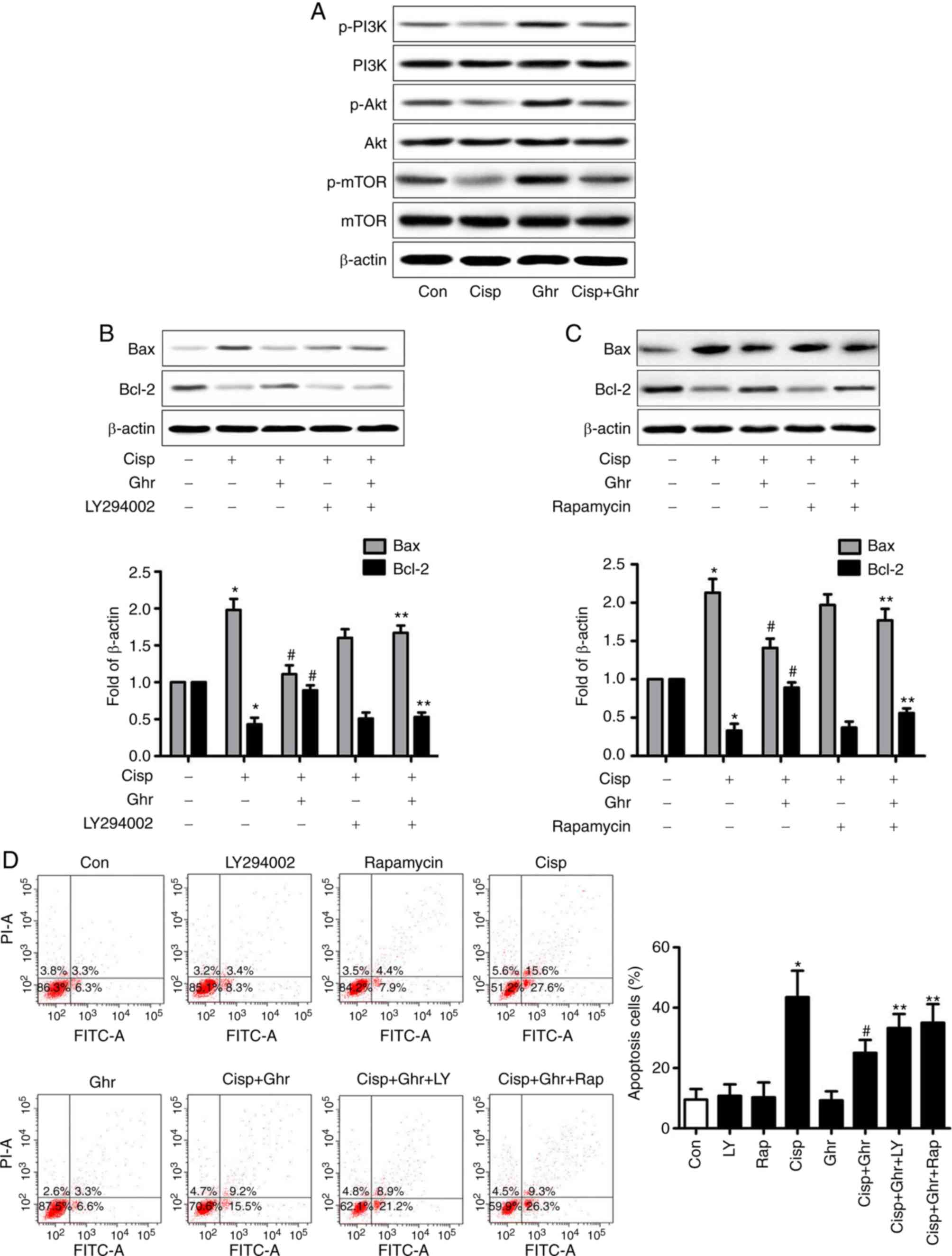 | Figure 3.Involvement of p-PI3K/p-Akt and p-mTOR
on the effects of cisplatin and ghrelin. (A) Phosphorylation of
PI3K/Akt/mTOR was detected by western blotting. (B and C)
Expression of Bax and Bcl-2 was detected by western blotting, PI-3K
inhibitor, LY294002 (10 µM) or rapamycin (20 nM) was added 30 min
before ghrelin and/or cisplatin treatment. (D) Apoptosis of
MDA-MB-231 cells was analysed by flow cytometry. *P<0.05 vs.
control; #P<0.05 vs. cisplatin treated alone;
**P<0.05 vs. cisplatin + Ghrelin. Bax; B-cell lymphoma
2-associated X; Bcl2, B-cell lymphoma 2; Cisp, cisplatin; Con,
control; FITC-A, Annexin V-Fluorescein isothiocyanate; Ghr,
ghrelin; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of
rapamycin; siRNA, small interfering RNA. |
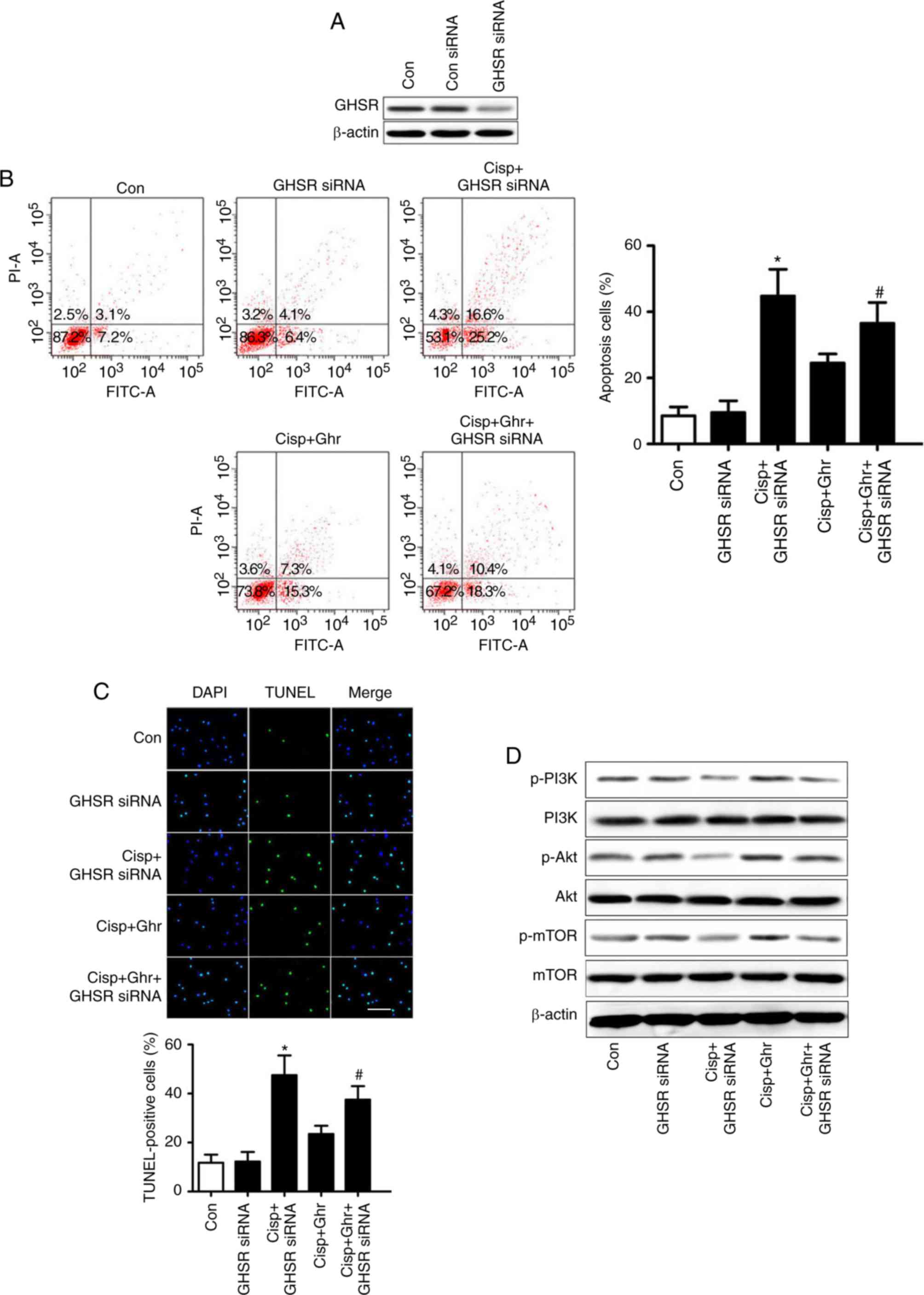
Ghrelin inhibits cisplatin-induced
apoptosis through GHSR
The effects of ghrelin appear to be mainly mediated
by GHSR (20). To test this,
MDA-MB-231 cells were transfected with GHSR siRNA, and the
expression of GHSR was validated by western blotting (Fig. 4A). As expected, knockdown of GHSR
inhibited the effects of ghrelin on cisplatin-induced apoptosis in
MDA-MB-231 cells (Fig. 4B and C). In
addition, GHSR siRNA reduced ghrelin-induced phosphorylation of
PI3K/Akt/mTOR (Fig. 4D). These
results suggest that GHSR mediates the effects of ghrelin on
cisplatin-induced apoptosis in MDA-MB-231 cells.
Discussion
The effects of ghrelin on tumor cell apoptosis are
controversial. The present study investigated the potential effects
of ghrelin on cisplatin-induced apoptosis in MDA-MB-231 cells and
the underlying mechanisms involved. It was observed that ghrelin
inhibited the cisplatin-induced apoptosis in vitro and
reduced MDA-MB-231 cell sensitivity to cisplatin in vivo.
Furthermore, ghrelin-mediated effects were accompanied by
activation of PI3K/Akt/mTOR signaling, which was inhibited by
cisplatin. Moreover, the effect of ghrelin was significantly
attenuated by GHSR siRNA, indicating ghrelin inhibits
cisplatin-induced apoptosis in MDA-MB-231 cells through GHSR and
activation of PI3K/Akt/mTOR signaling.
Ghrelin has been reported to regulate tumor cell
proliferation, apoptosis and survival (21–23). In
cancer studies, ghrelin has shown different proliferative and
anti-proliferative effects in various colon cancer cell lines
(14,24). Ghrelin has been reported to induce
HT-29 cell proliferation via GHSR and the Ras/PI3K/AKT/mTOR pathway
(24). Studies have also reported
ghrelin induces apoptosis in human colorectal carcinoma HCT116
cells (14). Previous findings have
also indicated ghrelin inhibits LPS-induced apoptosis in A549 cells
(12). In addition, ghrelin appears
to induce proliferation of A549 cells via PI3K/Akt/mTOR/P70S6K and
ERK signaling. Indeed, ghrelin seems to serve an important role in
cancer cell proliferation and apoptosis. The effects of ghrelin on
human breast cancer cell apoptosis and its associated mechanisms
remain unclear. The present study identified that cisplatin reduced
tumor cell viability in a dose-dependent manner, with ghrelin
markedly reducing the death rate of MDA-MB-231 cells. In addition,
flow cytometry and TUNEL analysis indicated ghrelin inhibited
cisplatin-induced apoptosis in MDA-MB-231 cells.
It has been reported that PI3K/Akt and mTOR
signaling pathways are activated by ghrelin, serving important
roles in cell growth, apoptosis and survival (25–27). The
present study assessed whether the PI3K/Akt and mTOR pathways were
activated by ghrelin in MDA-MB-231 cells. The results demonstrated
that ghrelin reversed cisplatin-induced inhibition of PI3K/Akt and
mTOR activity in MDA-MB-231 cells. The results suggested
PI3K/Akt/mTOR signaling participates in the effects of ghrelin on
cisplatin-induced apoptosis in MDA-MB-231 cells. Nevertheless,
other signaling pathways may be also activated by ghrelin in
MDA-MB-231 cells, and further studies are required to examine
whether other pathways intervene in the effects of ghrelin on
breast cancer cell apoptosis.
The Bcl-2 family includes several important
regulators of intracellular apoptotic signal transduction, and the
Bcl-2/Bax ratio determines cell survival or apoptosis (26). Bcl-2 protein levels decreased
following treatment with cisplatin, whereas ghrelin increased Bcl-2
expression and inverted the Bcl-2/Bax ratio. Inhibition of PI3K and
mTOR largely attenuated the ghrelin-mediated changes of the Bcl-2
and Bax expression. This provides strong evidence that
PI3K/Akt/mTOR signaling mediates ghrelin-induced anti-apoptosis in
MDA-MB-231 cells treated with cisplatin. Furthermore, ghrelin
inhibited cisplatin-induced caspase-3 activation, consistent with
precious observations in A549 and INS-1 cells (12,28).
Ghrelin exerts its physiological function by binding
to GHSR. This receptor is expressed in many cancers, including lung
cancer, breast cancer, renal cell carcinoma, colorectal cancer and
ovarian cancer (12,17,29–31).
Previous studies have reported the use of GHSR siRNA or
(D-Lys3)-GHRP-6 to block the biological functions of ghrelin
(22,26,27). The
present study determined the involvement of GHSR in the
ghrelin-mediated anti-apoptotic effects in MDA-MB-231 cells through
the knockdown of GHSR with GHSR siRNA. This method reversed the
inhibitory effect of ghrelin on cisplatin-induced apoptosis in
MDA-MB-231 cells. The results suggested the anti-apoptotic effects
of ghrelin to be GHSR-dependent. However, previous studies have
implied the effects of ghrelin on intestinal epithelial cell
survival and apoptosis may be dependent on an uncharacterized GHSR
subtype. These discrepancies may be attributed to different GHSR
subtype expression in cancer cells.
In summary, the present study provides evidence that
ghrelin inhibits cisplatin-induced apoptosis in MDA-MB-231 cells
through mechanisms dependent on GHSR and PI3K/Akt/mTOR signaling,
but this needs further investigation.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX designed the experiments and wrote the
manuscript. JZ carried out the experiments and analysed the data.
All authors read and approved the final manuscript, and each author
believes that the manuscript represents honest work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang QH, Jiang Y, Zhu X, Cui RR, Liu GY,
Liu Y, Wu SS, Liao XB, Xie H, Zhou HD, et al: Ghrelin attenuates
the osteoblastic differentiation of vascular smooth muscle cells
through the ERK pathway. PLoS One. 7:e331262012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Chen Q, Ke D and Li G: Ghrelin
inhibits atherosclerotic plaque angiogenesis and promotes plaque
stability in a rabbit atherosclerotic model. Peptides. 90:17–26.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin TC and Hsiao M: Ghrelin and cancer
progression. Biochim Biophys Acta Rev Cancer. 1868:51–57. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chopin L, Walpole C, Seim I, Cunningham P,
Murray R, Whiteside E, Josh P and Herington A: Ghrelin and cancer.
Mol Cell Endocrinol. 340:65–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waseem T, Javaid-Ur-Rehman, Ahmad F, Azam
M and Qureshi MA: Role of ghrelin axis in colorectal cancer: A
novel association. Peptides. 29:1369–1376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeffery PL, Herington AC and Chopin LK:
Expression and action of the growth hormone releasing peptide
ghrelin and its receptor in prostate cancer cell lines. J
Endocrinol. 172:R7–R11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh AH, Jeffery PL, Duncan RP, Herington
AC and Chopin LK: Ghrelin and a novel preproghrelin isoform are
highly expressed in prostate cancer and ghrelin activates
mitogen-activated protein kinase in prostate cancer. Clin Cancer
Res. 11:8295–8303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Díaz-Lezama N, Hernández-Elvira M,
Sandoval A, Monroy A, Felix R and Monjaraz E: Ghrelin inhibits
proliferation and increases T-type Ca2+ channel expression in PC-3
human prostate carcinoma cells. Biochem Biophys Res Commun.
403:24–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bustin SA and Jenkins PJ: The growth
hormone-insulin-like growth factor-I axis and colorectal cancer.
Trends Mol Med. 7:447–454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duxbury MS, Waseem T, Ito H, Robinson MK,
Zinner MJ, Ashley SW and Whang EE: Ghrelin promotes pancreatic
adenocarcinoma cellular proliferation and invasiveness. Biochem
Biophys Res Commun. 309:464–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang C, Zheng H, He W, Lu G, Li X, Deng Y
and Zeng M: Ghrelin ameliorates the human alveolar epithelial A549
cell apoptosis induced by lipopolysaccharide. Biochem Biophys Res
Commun. 474:83–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghè C, Cassoni P, Catapano F, Marrocco T,
Deghenghi R, Ghigo E, Muccioli G and Papotti M: The
antiproliferative effect of synthetic peptidyl GH secretagogues in
human CALU-1 lung carcinoma cells. Endocrinology. 143:484–491.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonfili L, Cuccioloni M, Cecarini V,
Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M and Eleuteri AM:
Ghrelin induces apoptosis in colon adenocarcinoma cells via
proteasome inhibition and autophagy induction. Apoptosis.
18:1188–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeffery PL, Murray RE, Yeh AH, McNamara
JF, Duncan RP, Francis GD, Herington AC and Chopin LK: Expression
and function of the ghrelin axis, including a novel preproghrelin
isoform, in human breast cancer tissues and cell lines. Endocr
Relat Cancer. 12:839–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grönberg M, Ahlin C, Naeser Y, Janson ET,
Holmberg L and Fjällskog ML: Ghrelin is a prognostic marker and a
potential therapeutic target in breast cancer. PLoS One.
12:e01760592017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cassoni P, Papotti M, Ghè C, Catapano F,
Sapino A, Graziani A, Deghenghi R, Reissmann T, Ghigo E and
Muccioli G: Identification, characterization, and biological
activity of specific receptors for natural (ghrelin) and synthetic
growth hormone secretagogues and analogs in human breast carcinomas
and cell lines. J Clin Endocrinol Metab. 86:1738–1745. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rak-Mardyla A and Gregoraszczuk EL: ERK
1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin
action on cell proliferation and apoptosis in the porcine ovarian
follicular cells. J Physiol Pharmacol. 61:451–458. 2010.PubMed/NCBI
|
|
19
|
Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei
M, Wang L and Zhong M: Leptin regulates proliferation and apoptosis
of colorectal carcinoma through PI3K/Akt/mTOR signaling pathway. J
Biosci. 37:91–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sever S, White DL and Garcia JM: Is there
an effect of ghrelin/ghrelin analogs on cancer? A systematic
review. Endocr Relat Cancer. 23:R393–R409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Györffy B and Schäfer R: Meta-analysis of
gene expression profiles related to relapse-free survival in 1,079
breast cancer patients. Breast Cancer Res Treat. 118:433–441. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barzon L, Pacenti M, Masi G, Stefani AL,
Fincati K and Palù G: Loss of growth hormone secretagogue receptor
1a and overexpression of type 1b receptor transcripts in human
adrenocortical tumors. Oncology. 68:414–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu J, Yao J, Huang R, Wang Y, Jia M and
Huang Y: Ghrelin promotes human non-small cell lung cancer A549
cell proliferation through PI3K/Akt/mTOR/P70S6K and ERK signaling
pathways. Biochem Biophys Res Commun. 498:616–620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lien GS, Lin CH, Yang YL, Wu MS and Chen
BC: Ghrelin induces colon cancer cell proliferation through the
GHS-R, Ras, PI3K, Akt, and mTOR signaling pathways. Eur J
Pharmacol. 776:124–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang D, Liu Z, Zhang H and Luo Q: Ghrelin
inhibits human pulmonary artery endothelial cells against
hypoxia-induced injury via PI3-kinase/Akt. Peptides. 42:112–117.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu J, Zheng C, Chen J, Luo J, Su B, Huang
Y, Su W, Li Z and Cui T: Ghrelin inhibits human umbilical vein
endothelial cells against high glucose-induced apoptosis via
mTOR/P70S6K signaling pathway. Peptides. 52:23–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Chen S, Ren J, Li B and Qin B:
Ghrelin inhibits retinal ganglion cells against rotenone via
inhibiting apoptosis, restoring mitochondrial function, and
activating AKT-mTOR signaling. Neuropeptides. 67:63–70. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Li L, Zhao B, Jiao A, Li X, Sun N
and Zhang J: Ghrelin protects against dexamethasone-induced INS-1
cell apoptosis via ERK and p38MAPK signaling. Int J Endocrinol.
2016:45130512016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Korbonits M, Bustin SA, Kojima M, Jordan
S, Adams EF, Lowe DG, Kangawa K and Grossman AB: The expression of
the growth hormone secretagogue receptor ligand ghrelin in normal
and abnormal human pituitary and other neuroendocrine tumors. J
Clin Endocrinol Metab. 86:881–887. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin TC, Liu YP, Chan YC, Su CY, Lin YF,
Hsu SL, Yang CS and Hsiao M: Ghrelin promotes renal cell carcinoma
metastasis via snail activation and is associated with poor
prognosis. J Pathol. 237:50–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai RX, Wang WP, Zhao PW and Li CB:
Ghrelin attenuates the growth of HO-8910 ovarian cancer cells
through the ERK pathway. Braz J Med Biol Res. 49(pii):
S0100-879X2016000300602. 2016.
|