Introduction
Small-for-gestational-age (SGA) infants are
10th-percentile infants that weigh less than the average birth
weight of a child of the same age (1). SGA can be divided into two types:
Uniform and non-uniform, based on the weight of the newborn and the
length/head circumference ratio (2).
SGA infants not only show a significantly higher perinatal
mortality than normal newborns, but also show a high probability of
cognitive dysfunction and decreased learning ability at school age
and in adulthood (3). In addition,
SGA infants are more likely to suffer from diseases during growth
to adulthood due to deficiencies in innate immunity, and their
final height is also likely to be significantly lower than that of
their peers by two standard deviations (4). According to statistics, SGA accounts
for 3–6% of all newborns worldwide (5). In countries with a large population and
fast population growth, such as China and India, the incidence of
SGA is as high as 10% (6). SGA has a
certain degree of influence on the intelligence, physical fitness
and neurodevelopment of newborns. Typically, the developmental
capacity of all aspects of SGA infants is significantly lower than
that of appropriate-for-gestational-age (AGA) infants (7). Some data also indicate that SGA may
decrease blood sugar, blood pressure and lipid regulation, which
will increase the risk of immune or metabolic diseases (8). Approximately 80% of SGA newborns
develop such diseases after delivery, and the current prevalence is
still on the rise (9,10). Moreover, SGA has a significantly
higher risk of death compared with AGA (11).
Currently, maternal hypertension during pregnancy,
multiple pregnancies and oligoamnios are considered as the main
causes of SGA in late preterm infants (12). Several studies have indicated that
the pathological changes associated with hypertension during
pregnancy, which include spasm of systemic arterioles of the
umbilical cord blood tube, which directly affects the blood
exchange between fetus and mother, can cause insufficient blood
supply to the fetus. Insufficient blood supply seriously affects
fetal growth and development, thereby leading to the occurrence of
SGA (13). It is clinically
recommended to intervene in pregnant women during the perinatal
period to prevent the occurrence of SGA (14). However, current B-ultrasound methods
cannot accurately distinguish whether or not the fetus in the
maternal uterus will be SGA (15).
Therefore, if relevant risk factors of SGA babies are found as
predictors, this could aid medical staff in assessing the
possibility of pregnant women giving birth to SGA infants. In
addition, relevant intervention measures may be carried out to
prevent the occurrence or reduce the risk of SGA. The present study
aimed to summarize the perinatal risk factors for SGA and provide
an effective reference and guidance for clinics.
Materials and methods
Ethics approval and patient
consent
The current study was approved by the Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University. The parents of all subjects gave their signed informed
consent.
Study subjects
In total, 100 cases of single term full-term SGA
delivered in the Department of Obstetrics were collected as
subjects and regarded as the SGA group between May 2017 to May
2018, comprising 64 males and 36 females. A total of 100 healthy
AGA patients who were born at the same time with the same
gestational age were randomly included as the control group,
comprising 68 males and 32 females.
The inclusion criteria for study subjects were as
follows: Full-term neonates, whose gestational age was 37–42 weeks
(260–293 days) with a body weight of >2.5 kg and body length of
>47 cm; the diagnosis of SGA and AGA met the reference standard
of birth weight for newborns of different gestational ages
published in 2015 (16). The
exclusion criteria for study subjects were as follows: Multiple
pregnancies; neonates with abnormal chromosomes or structure;
neonates with congenital malformations or inherited metabolic
diseases; neonates with a birth weight exceeding the 90th
percentile of average gestational age; uterine malformations;
placenta previa; family history of genetic disease; neonates with
fetal growth restriction according to prenatal B-ultrasound;
pregnant women who were transferred; and pregnant women with mental
illnesses.
Outcomes measurement
Perinatal and postpartum adverse conditions such as
respiratory distress syndrome and infection in the two groups were
recorded. Based on the Apgar scoring criteria (17), Apgar tests were performed on all
newborns at 1 min (T1), 5 min (T2) and 10 min (T3) after birth. The
differences between the two groups of newborns were compared and
the hospital stays of the two groups were measured. All patients
underwent a 1-year follow-up survey. The follow-up included
hospital review and follow-up visits, which were performed at 6 and
12 months. The length and weight of the child were recorded. During
the second follow-up, the development quotient (DQ) of the children
was measured using Gesell Developmental Schedules (18). A DQ <85 indicated that the child
has physical damage; a DQ <65 indicated that the child showed
severe growth retardation. Logistic regression analysis was used to
study the maternal clinical situation and risk factors of SGA.
Statistical analysis
The data were analyzed and processed using SPSS 24.0
statistical software (IBM Corp.). The count data, such as the
percentage of pregnant women, are expressed in terms of (%), and
the χ2 test was used for comparison between groups.
Measurement data such as age are expressed in the form of the mean
± standard deviation, and Student's t-test was used for comparison
between groups. Repeated measures ANOVA and Bonferroni's post hoc
test were used for comparison between multiple time points.
Logistic regression analysis was used for risk factor analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline data
Maternal and newborn data for the two groups were
compared. No significant difference was found between the two
groups in terms of white blood cell count, red blood cell count,
platelet count, maternal age, maternal weight, gestational week,
delivery mode, living environment, neonatal gender, first delivery
status, maternal pregnancy-induced hypertension and gestational
diabetes (Table I), indicating that
the two groups were comparable.
 | Table I.Comparisons of clinical data. |
Table I.
Comparisons of clinical data.
| Parameter | SGA group
(n=100) | AGA group
(n=100) | χ2 or
t | P-value |
|---|
| WBC,
×109 | 16.24±2.25 | 16.58±2.51 | 1.009 | 0.314 |
| Maternal WBC,
×109 | 7.63±1.01 | 7.52±1.35 | 0.652 | 0.515 |
| RBC,
×109 | 4.27±1.12 | 4.05±1.34 | 1.260 | 0.209 |
| Maternal RBC,
×1012 | 4.05±1.36 | 4.12±1.07 | 0.405 | 0.686 |
| PLT,
×1012 | 269.53±40.59 | 262.16±42.88 | 1.248 | 0.213 |
| Maternal PLT,
×109 | 187.54±62.15 | 179.33±59.42 | 0.955 | 0.341 |
| Maternal age,
years | 24.86±3.22 | 25.04±3.50 | 0.379 | 0.706 |
| Maternal weight,
kg | 62.13±5.87 | 61.94±6.16 | 0.223 | 0.824 |
| Gestational week | 42.86±2.58 | 42.33±2.04 | 1.611 | 0.109 |
| Mode of delivery
(%) |
|
| 0.188 | 0.664 |
| Vaginal
birth | 62 (62.00) | 59 (59.00) |
|
|
| Caesarean
section | 38 (38.00) | 41 (41.00) |
|
|
| Living environment
(%) |
|
| 0.362 | 0.548 |
|
Urban | 69 (69.00) | 65 (65.00) |
|
|
|
Country | 31 (31.00) | 35 (35.00) |
|
|
| Neonatal sex
(%) |
|
| 0.357 | 0.551 |
|
Male | 64 (64.00) | 68 (68.00) |
|
|
|
Female | 36 (36.00) | 32 (32.00) |
|
|
| Number of births
(%) |
|
| 0.829 | 0.363 |
| First
birth | 79 (79.00) | 84 (84.00) |
|
|
| Two or
more births | 21 (21.00) | 16 (16.00) |
|
|
| Pregnancy-induced
hypertension (%) |
|
| 0.307 | 0.579 |
|
Yes | 8 (8.00) | 6 (6.00) |
|
|
| No | 92 (92.00) | 94 (94.00) |
|
|
| Gestational
diabetes (%) |
|
| 0.053 | 0.818 |
|
Yes | 11 (11.00) | 10 (10.00) |
|
|
| No | 89 (89.00) | 90 (90.00) |
|
|
Comparison of adverse conditions
between the SGA and AGA groups
No significant difference was found in the incidence
of neonatal pneumonia between the SGA and AGA groups. In the SGA
group, 13% (13 cases) of neonates had intrauterine distress, which
was significantly higher compared with the AGA group (4%, 4 cases;
P=0.023). In the SGA group, 10% (10 cases) of neonates developed
respiratory distress syndrome, which was significantly higher
compared with the AGA group (2.00%, 2 cases; P=0.017). In the SGA
group, 8% (8 cases) of neonates developed infectious disease, while
no cases in the AGA group developed infectious disease. The
difference between the two groups was found to be statistically
significant (P=0.004; Table
II).
 | Table II.Comparison of adverse conditions
between the two newborn groups. |
Table II.
Comparison of adverse conditions
between the two newborn groups.
| Condition | SGA group
(n=100) | AGA group
(n=100) | χ2 | P-value |
|---|
| Fetal intrauterine
distress (%) | 13 (13.00) | 4 (4.00) | 5.207 | 0.023 |
| Respiratory
distress syndrome (%) | 10 (10.00) | 2 (2.00) | 5.674 | 0.017 |
| Neonatal pneumonia
(%) | 6 (6.00) | 2 (2.00) | 2.083 | 0.149 |
| Infectious disease
(%) | 8 (8.00) | 0 (0.00) | 8.333 | 0.004 |
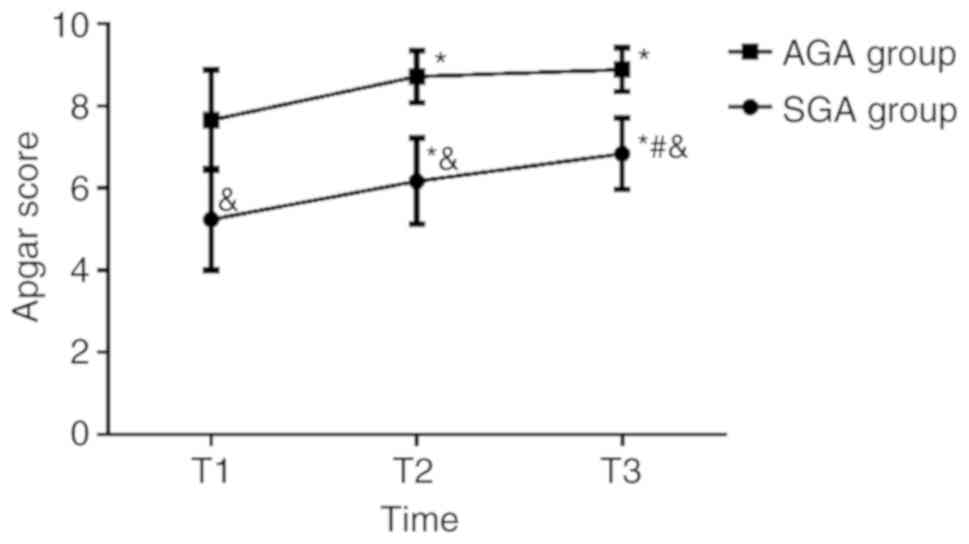
Comparison of Apgar score and hospital
stay
The Apgar scores of T1, T2 and T3 in the SGA group
were 5.24±1.24, 6.17±1.05 and 6.84±0.87, respectively. The Apgar
scores of T1, T2 and T3 in the AGA group were 7.66±1.22, 8.72±0.63
and 8.89±0.54, respectively. The comparison of Apgar scores at T1,
T2, and T3 between the two groups indicated that the SGA group
scores were significantly lower compared with the AGA group
(P<0.05). No significant difference was found in the Apgar score
of the AGA group between scores calculated at T2 and T3, while the
Apgar score at T1 was lower compared with the T2 and T3 scores
(P<0.05). In the SGA group, the Apgar score at T1 was lower
compared with the T2 score (P<0.05), and the Apgar score at T2
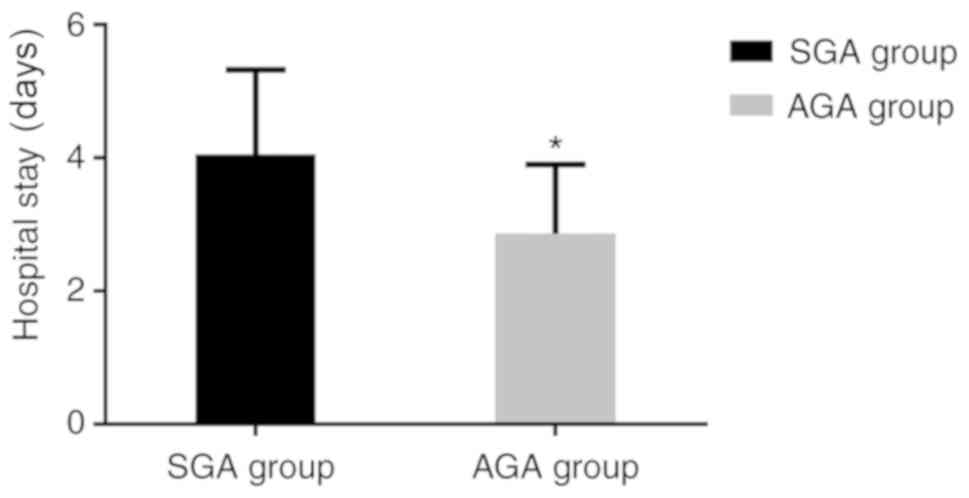
was lower compared with the T3 score (P<0.05). The length of
hospital stay of the SGA group was 4.05±1.27 days, which was
significantly longer compared with the AGA group (2.86±1.04 days;
P<0.05) (Figs. 1 and 2).
Comparison of prognosis
The follow-up success rate was 100%. The body length
and body weight at the 6th month of the SGA group were measured to
be at 62.33±2.34 cm and 7.28±0.34 kg, respectively, which was
significantly lower compared with AGA group measurements
(67.25±1.06 cm and 8.07±0.12 kg; P<0.05). At the 12th month, the
SGA group body length and body weight were measured to be at
70.24±2.38 cm and 8.62±0.42 kg, respectively. This was also
significantly lower compared with AGA group measurements
(74.86±0.95 cm and 9.08±0.24 kg; P<0.05). The body length and
body weight at the 12th month significantly increased (P<0.05)
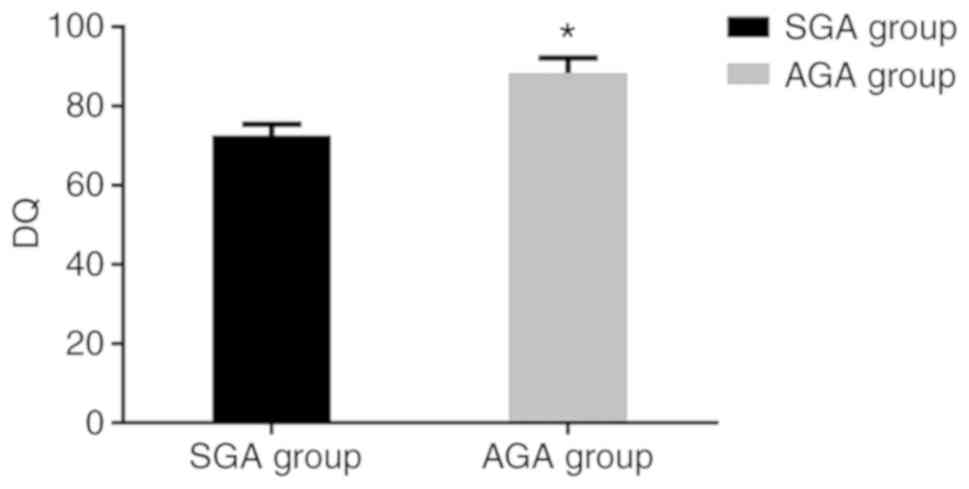
compared with the measurements at the 6th month. The DQ of the SGA
group was 72.62±2.87, which was significantly lower compared with
that of the AGA group (88.36±3.87; P<0.05) (Figs. 3–5).
Analysis of SGA-related risk
factors
Logistic regression analysis showed that there was
no correlation between SGA and maternal age, regardless of first
child status, neonatal sex, mode of delivery and living
environment. SGA was significantly associated with umbilical cord
abnormalities, maternal pregnancy-induced hypertension, gestational
diabetes, pregnancy infection and intrauterine distress
(P<0.05); therefore, these are risk factors for SGA (OR>1;
Table III).
 | Table III.Logistic regression analysis of SGA
risk factors. |
Table III.
Logistic regression analysis of SGA
risk factors.
| Risk factor | OR | 95% CI | P-value |
|---|
| Umbilical cord
abnormality | 2.29 | 1.36–3.82 | 0.002 |
| Maternal age | 0.93 | 0.65–1.33 | 0.698 |
| Number of
births | 1.09 | 0.66–1.81 | 0.736 |
| Neonatal sex | 0.83 | 0.25–2.66 | 0.752 |
| Pregnancy-induced
hypertension | 1.36 | 1.04–1.77 | 0.024 |
| Living
environment | 1.08 | 0.79–1.47 | 0.648 |
| Fetal intrauterine
distress | 1.80 | 1.31–2.47 | <0.001 |
| Pregnancy
infection | 1.59 | 1.07–2.37 | 0.022 |
| Mode of
delivery | 1.05 | 0.84–1.31 | 0.660 |
Discussion
SGA is presently one of the key factors that
threaten the healthy growth of newborns (19,20). The
clinical prevention of SGA is currently advocated to pregnant women
in the perinatal period. However, the only current testing
available is mainly the B-ultrasound method (21), and this method is still unable to
correctly diagnose whether or not the fetus will develop SGA
(22,23). Therefore, clinical research is
constantly looking for effective means to distinguish the
occurrence of SGA in newborns. However, to the best of our
knowledge, no breakthrough research results have been obtained.
Therefore, summarizing the risk factors that may affect SGA through
representative clinical data is the most traditional and the most
effective way. A review of relevant literature found that articles
on the study of SGA risk factors were generally outdated and
because the hospital conditions at that time were quite different
from modern conditions, it is not suitable for clinical guidance
(24–26). Therefore, the present study aimed to
summarize the influencing factors affecting SGA through the study
of infants with SGA and AGA admitted to the Department of
Obstetrics, The First Affiliated Hospital of Chongqing Medical
University in 2017. Advanced statistical software and a random
experimental design were used to ensure that the experimental
results were more authentic and reliable, and possible high-risk
events were analyzed in detail in the present study for clinical
reference.
The results of this experiment showed that the risk
of intrauterine distress, respiratory distress syndrome and
infectious diseases in the SGA group was significantly higher than
that in the AGA group, suggesting that SGA can increase the risk of
neonatal disease. Studies have shown that SGA has an impact on the
normal development of neonatal body function (27,28),
The significant increase in the incidence of SGA in
this study also confirms the view that SGA will affect the normal
development of neonatal body function. The pathogenesis of SGA
mainly includes maternal umbilical blood vessels spasms and
systemic small arterial spasms, which affects blood exchange
between the fetus and the mother (29). This causes insufficient blood supply
to the fetus and affects fetal growth and development, leading to
SGA (30). Therefore, SGA causes
incomplete development of organ function, due to insufficient blood
supply in the maternal uterus. Furthermore, the immune capacity of
the fetus may be relatively low, hence the risk of disease greatly
increased after birth (31). No
significant difference was found in the incidence of neonatal
pneumonia between the two groups, although this may be due to the
small number of subjects. The SGA group demonstrated lower Apgar
scores and a longer hospital stay compared with the AGA group.
Other reasons are speculated to be consistent with the above
points. Due to the decreased oxygen saturation of the mother, the
fetal tissue is hypoxic, causing dyspnea, and this is consistent
with the results of Kiely et al (32), who demonstrated that children with
SGA have lower Apgar scores than children with AGA.
The prognosis of the two groups of newborns was
further compared. The growth and development of the SGA group was
significantly lower compared with the AGA group. This suggested
that SGA has a great negative impact on the healthy growth of
newborns, and should be paid more attention in the clinic. It is
necessary to conduct a careful and complete prenatal examination in
pregnant women to prevent the occurrence of SGA. Logistic
regression analysis showed that umbilical cord abnormalities,
maternal pregnancy-induced hypertension, gestational diabetes,
pregnancy infection and intrauterine distress were all risk factors
for SGA.
Khalil et al (33) found that the older the mother, the
higher the likelihood of SGA in the newborn. However, no difference
was observed with respect to maternal age in the present study.
Moreover, the research subjects of Khalil et al were mostly
Caucasian, and the present study focused on Asians. Regional and
race differences may also be one of the reasons for the difference
in results. The sample size in future studies will be enlarged for
subsequent analysis and verification.
This study analyzed the risk factors of SGA by
comparing the differences in the growth and development, as well as
the risk of diseases between SGA and AGA newborns. However, due to
limited experimental conditions, there are still some limitations.
For example, the number of subjects in the study is too small to
perform a larger statistical analysis. Moreover, the risk factors
may be influenced by other variables such as ethnicity, region and
living habits. However, since the limitations of this study are
relatively high, this may have resulted in some accidental error
data. Future studies could be conducted with several hospitals to
expand the sample size of the study and obtain more comprehensive
and representative case data for analysis. In addition, the
pathogenesis of SGA has not been completely defined, and a possible
correlation between fetal anthropometric data, usually registered
during prenatal medical check-ups, and the aforementioned pregnancy
risk factors was not analyzed herein, which will be studied in
future research.
In summary, an abnormal umbilical cord, maternal
pregnancy-induced hypertension, gestational diabetes, infection
during pregnancy and intrauterine distress are all perinatal risk
factors of SGA. Effective interventions are needed in the clinic to
prevent the occurrence of SGA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Hospital
Training Fund of The First Affiliated Hospital of Chongqing Medical
University (grant no. PYJJ2017-37).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC conceived and designed the research and
interpreted the results of the experiments. JL contributed to the
design of the study and the interpretation of experimental results.
XT performed experiments, analyzed data and drafted the initial
manuscript. JL and XT approved the final version of manuscript. JC
edited and revised the manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of The First Affiliated Hospital of Chongqing Medical
University. The parents of all subjects gave their signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mendez-Figueroa H, Truong VT, Pedroza C,
Khan AM and Chauhan SP: Small-for-gestational-age infants among
uncomplicated pregnancies at term: A secondary analysis of 9
maternal-fetal medicine units network studies. Am J Obstet Gynecol.
215:628 e621–628 e627. 2016. View Article : Google Scholar
|
|
2
|
Roberge S, Sibai B, McCaw-Binns A and
Bujold E: Low-dose aspirin in early gestation for prevention of
preeclampsia and small-for-gestational-age neonates: Meta-analysis
of large randomized trials. Am J Perinatol. 33:781–785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin D and Song WO: Prepregnancy body mass
index is an independent risk factor for gestational hypertension,
gestational diabetes, preterm labor, and small-and
large-for-gestational-age infants. J Matern Fetal Neonatal Med.
28:1679–1686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Savchev S, Sanz-Cortes M, Cruz-Martinez R,
Arranz A, Botet F, Gratacos E and Figueras F: Neurodevelopmental
outcome of full-term small-for-gestational-age infants with normal
placental function. Ultrasound Obstet Gynecol. 42:201–206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mericq V, Martinez-Aguayo A, Uauy R,
Iniguez G, Van der Steen M and Hokken-Koelega A: Long-term
metabolic risk among children born premature or small for
gestational age. Nat Rev Endocrinol. 13:50–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho WK and Suh BK: Catch-up growth and
catch-up fat in children born small for gestational age. Korean J
Pediatr. 59:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castanys-Muñoz E, Kennedy K,
Castañeda-Gutierrez E, Forsyth S, Godfrey KM, Koletzko B, Ozanne
SE, Rueda R, Schoemaker M, van der Beek EM, et al: Systematic
review indicates postnatal growth in term infants born
small-for-gestational-age being associated with later
neurocognitive and metabolic outcomes. Acta Paediatr.
106:1230–1238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vazquez-Benitez G, Kharbanda EO, Naleway
AL, Lipkind H, Sukumaran L, McCarthy NL, Omer SB, Qian L, Xu S,
Jackson ML, et al: Risk of preterm or small-for-gestational-age
birth after influenza vaccination during pregnancy: Caveats when
conducting retrospective observational studies. Am J Epidemiol.
184:176–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendez-Figueroa H, Truong VT, Pedroza C
and Chauhan SP: Morbidity and mortality in
small-for-gestational-age infants: A secondary analysis of nine
MFMU network studies. Am J Perinatol. 34:323–332. 2017.PubMed/NCBI
|
|
10
|
Faienza MF, Brunetti G, Delvecchio M, Zito
A, De Palma F, Cortese F, Nitti A, Massari E, Gesualdo M, Ricci G,
et al: Vascular function and myocardial performance indices in
children born small for gestational age. Circ J. 80:958–963. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meher S, Duley L, Hunter K and Askie L:
Antiplatelet therapy before or after 16 weeks' gestation for
preventing preeclampsia: An individual participant data
meta-analysis. Am J Obstet Gynecol. 216:121–128 e122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bukowski R, Davis KE and Wilson PW:
Delivery of a small for gestational age infant and greater maternal
risk of ischemic heart disease. PLoS One. 7:e330472012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luyckx VA, Bertram JF, Brenner BM, Fall C,
Hoy WE, Ozanne SE and Vikse BE: Effect of fetal and child health on
kidney development and long-term risk of hypertension and kidney
disease. Lancet. 382:273–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaiworapongsa T, Romero R, Whitten AE,
Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Yeo L and
Hassan SS: The use of angiogenic biomarkers in maternal blood to
identify which SGA fetuses will require a preterm delivery and
mothers who will develop pre-eclampsia. J Matern Fetal Neonatal
Med. 29:1214–1228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Triunfo S, Crispi F, Gratacos E and
Figueras F: Prediction of delivery of small-for-gestational-age
neonates and adverse perinatal outcome by fetoplacental Doppler at
37 weeks' gestation. Ultrasound Obstet Gynecol. 49:364–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chauhan SP, Beydoun H, Chang E, Sandlin
AT, Dahlke JD, Igwe E, Magann EF, Anderson KR, Abuhamad AZ and
Ananth CV: Prenatal detection of fetal growth restriction in
newborns classified as small for gestational age: Correlates and
risk of neonatal morbidity. Am J Perinatol. 31:187–194.
2014.PubMed/NCBI
|
|
17
|
Dalili H, Nili F, Sheikh M, Hardani AK,
Shariat M and Nayeri F: Comparison of the four proposed Apgar
scoring systems in the assessment of birth asphyxia and adverse
early neurologic outcomes. PLoS One. 10:e01221162015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meinzen-Derr J, Wiley S, Phillips J,
Altaye M and Choo DI: The utility of early developmental
assessments on understanding later nonverbal IQ in children who are
deaf or hard of hearing. Int J Pediatr Otorhinolaryngol.
92:136–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sung KU, Roh JA, Eoh KJ and Kim EH:
Maternal serum placental growth factor and pregnancy-associated
plasma protein A measured in the first trimester as parameters of
subsequent pre-eclampsia and small-for-gestational-age infants: A
prospective observational study. Obstet Gynecol Sci. 60:154–162.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuhle S, Maguire B, Ata N, MacInnis N and
Dodds L: Birth weight for gestational age, anthropometric measures,
and cardiovascular disease markers in children. J Pediatr.
182:99–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kullinger M, Haglund B, Kieler H and
Skalkidou A: Effects of ultrasound pregnancy dating on neonatal
morbidity in late preterm and early term male infants: A
register-based cohort study. BMC Pregnancy Childbirth. 16:3352016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duan SY, Kong XY, Xu FD, Lv HY, Ju R, Li
ZK, Zeng SJ, Wu H, Zhang XF, Liu WP, et al: Impact of premature
rupture of membranes on neonatal complications in preterm infants
with gestational age <37 weeks. Nan Fang Yi Ke Da Xue Xue Bao.
36:887–891. 2016.(In Chinese). PubMed/NCBI
|
|
23
|
Olsen JE, Brown NC, Eeles AL, Einspieler
C, Lee KJ, Thompson DK, Anderson PJ, Cheong JL, Doyle LW and
Spittle AJ: Early general movements and brain magnetic resonance
imaging at term-equivalent age in infants born <30 weeks'
gestation. Early Hum Dev. 101:63–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCowan LM, Roberts CT, Dekker GA, Taylor
RS, Chan EH, Kenny LC, Baker PN, Moss-Morris R, Chappell LC and
North RA; SCOPE consortium, : Risk factors for
small-for-gestational-age infants by customised birthweight
centiles: Data from an international prospective cohort study.
BJOG. 117:1599–1607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monk C, Georgieff MK and Osterholm EA:
Research review: Maternal prenatal distress and poor
nutrition-mutually influencing risk factors affecting infant
neurocognitive development. J Child Psychol Psychiatry. 54:115–130.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lampi KM, Lehtonen L, Tran PL, Suominen A,
Lehti V, Banerjee PN, Gissler M, Brown AS and Sourander A: Risk of
autism spectrum disorders in low birth weight and small for
gestational age infants. J Pediatr. 161:830–836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller SL, Huppi PS and Mallard C: The
consequences of fetal growth restriction on brain structure and
neurodevelopmental outcome. J Physiol. 594:807–823. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nordman H, Voutilainen R, Laitinen T,
Antikainen L, Huopio H, Heinonen S and Jaaskelainen J: Growth and
cardiovascular risk factors in prepubertal children born large or
small for gestational age. Horm Res Paediatr. 85:11–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Block HS: Neurological complications of
pregnancy. Curr Neurol Neurosci Rep. 16:672016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laptook AR, Bell EF, Shankaran S,
Boghossian NS, Wyckoff MH, Kandefer S, Walsh M, Saha S and Higgins
R; Generic and Moderate Preterm Subcommittees of the NICHD Neonatal
Research Network, : Admission temperature and associated mortality
and morbidity among moderately and extremely preterm infants. J
Pediatr. 192:53–59.e52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muszynski JA, Spinella PC, Cholette JM,
Acker JP, Hall MW, Juffermans NP, Kelly DP, Blumberg N, Nicol K,
Liedel J, et al: Transfusion-related immunomodulation: Review of
the literature and implications for pediatric critical illness.
Transfusion. 57:195–206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kiely ME, Zhang JY, Kinsella M, Khashan AS
and Kenny LC: Vitamin D status is associated with uteroplacental
dysfunction indicated by pre-eclampsia and
small-for-gestational-age birth in a large prospective pregnancy
cohort in Ireland with low vitamin D status. Am J Clin Nutr.
104:354–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khalil A, Syngelaki A, Maiz N, Zinevich Y
and Nicolaides KH: Maternal age and adverse pregnancy outcome: A
cohort study. Ultrasound Obstet Gynecol. 42:634–643. 2013.
View Article : Google Scholar : PubMed/NCBI
|