Introduction
Neural stem and progenitor cells (NSPCs) have
self-renewal, proliferative and differentiation capabilities
(1). NSPCs can differentiate into
neurons, astrocytes or oligodendrocytes, all of which constitute
the brain tissue (2). Therefore, it
is possible that they have the potential to generate new neurons to
compensate for loss in neurological diseases and central nervous
system injuries, including Alzheimer's (AD) and Parkinson's disease
(PD) in addition to spinal cord and traumatic brain injury
(3,4). Therefore, strategies to promote the
neuronal differentiation of NSPCs are being investigated to allow
for NSPC-based therapies worldwide (3,4).
Accumulating evidence has demonstrated that neurogenesis is linked
to angiogenesis by numerous growth factors, including vascular
endothelial growth factor (VEGF), ephrins and angiogenic factors
(5,6).
Ephrin receptors (Ephs), which constitute the
largest receptor family within the receptor tyrosine kinase
superfamily, regulate numerous important physiological and
developmental processes (7–9). Both Ephs and ephrins are classified
into type A and B subclasses (7).
Ephrin-As bind with EphAs, while ephrin-Bs bind with EphBs.
However, EphB2 and EphA4 can bind to both ephrin-As and ephrin-Bs.
Ligand interaction with the cell membrane is through glycosyl
phosphatidylinositol linkage for ephrin-As, and through a short
cytoplasmic and transmembrane domain for ephrin-Bs. EphA4-mediated
forward signaling regulates neuroblast and astrocyte organization
in a neurogenic niche (10). EphA4
may protect against neuronal loss and reverse cellular aging
(5). EphA signaling commits NSPCs to
differentiate down a neuronal lineage (11).
Angiogenic growth factors such as VEGFs,
platelet-derived growth factors (PDGFs), and fibroblast growth
factors (FGFs) play important roles in the proliferation and
differentiation of NSPCs. Implantation of collagen
glycosaminoglycan has been reported to promote angiogenesis
accompanied by neurogenesis through VEGF, FGF2 and PDGF-BB
upregulation (12). As a major
angiogenic factor, VEGF promotes neurogenesis in NSPCs in
vitro and in the adult brain (13). VEGF also has neurotrophic and
neuroprotective effects (14). VEGF
exerts its function through the VEGF receptor (VEGFR) 2, which
mediates most neuron-specific effects (15). VEGF mediates positive neurogenic
effects of an enriched environment on the rate of adult rodent
de novo neurogenesis (16).
Previous research has shown that EphA4 and PDGF
receptors (PDGFRs) form a heterodimer, trans-phosphorylating each
other after stimulation with their ligands, and that their
interaction promotes mouse embryonic neural precursor cell
proliferation (17). It is therefore
important to examine whether EphA4 and VEGFR2 form a heterocomplex
and their role in NSPC differentiation. Due to their functions,
understanding the interactions between EphA4, angiogenic growth
factor receptors and the associated signaling pathways are critical
in de novo neurogenesis and neuroregeneration in the human
brain.
Materials and methods
Reagents
Recombinant human VEGF165 (cat no. 293-VE),
recombinant human ephrinA1-fragment crystallizable region (Fc; cat
no. 6417-A1), and recombinant human immunoglobulin G (IgG)-Fc (cat
no. MAB110) were used (all R&D systems, Inc.). Clustered
ephrin-A1-Fc was oligomerized according to the manufacturer's
instructions via incubation with recombinant human IgG-Fc for >1
h at 4°C, following previous protocols (18).
Mice and ethics statement
A total of three pregnant female C57BL/6 mice
(weight range, 25–35 g; age, 8 weeks) were supplied by the
Laboratory Animal Center of Shandong University (license no.
SYXK-2019-0005; Shandong, China), housed at an ambient temperature
of 22±2°C, 12-h light/dark cycle and 40–45% relative humidity.
Animals were allowed free access to food and water. All animal
experiments were performed in accordance with the guidelines of the
Liaocheng People's Hospital (Shandong, China), and were approved by
the Ethics Committee of Liaocheng People's Hospital (Shandong.
China).
Cell culture
293T cells (• Clontech Laboratories, Inc.) were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.). Mouse embryonic NSPCs were cultured as previously described
(19). Briefly, the NSPCs obtained
from dissected hippocampus on embryonic day 14.5 were passaged as
neurospheres in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with B27 (Gibco; Thermo Fisher Scientific, Inc.),
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
FGF2 (Gibco; Thermo Fisher Scientific, Inc.) and epidermal growth
factor (EGF; Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 for up to three generations (P3). For
experimentation with ligands, the P3 neurospheres were adherently
cultured and NSPCs were starved in serum-free medium containing
0.5% (m/v) BSA (Sigma-Aldrich; Merck KGaA) for 5 h prior to ligand
stimulation when a differentiation assay was performed without EGF
and FGF2.
Reverse transcription
(RT)-quantitative (q)PCR
Mouse embryonic NSPCs dissociated from P3
neurospheres were rinsed with PBS after 3-day culture. The cells
were homogenized using TRI reagent® (cat no. T9424;
Sigma-Aldrich; Merck KGaA) and total RNA was extracted according to
the manufacturer's protocol. RT and subsequent PCR or qPCR were
performed using the conditions as previously reported (20,21). The
forward and reverse primer sequences for RT-PCR and qPCR are shown
in Table I.
 | Table I.Sequences for each pair of PCR
primers. |
Table I.
Sequences for each pair of PCR
primers.
| Primer | Sequence
(5′-3′) |
|---|
| PDGFRα-F |
GACGCACGCCAGACTGTGTATAAG |
| PDGFRα-R |
TGCACCTCCACCACGAACTCTC |
| PDGFRβ-F |
TGGAGATTCGCAGGAGGTCACC |
| PDGFRβ-R |
GGCTTGCTTCTCGCTACTTCTGG |
| VEGFR1-F |
GCAGCACCTTGACCTTGGACAC |
| VEGFR1-R |
GACGGTGGCTTCGCAGTTCAG |
| VEGFR2-F |
TCAGACAACAACCATTGGCGAGAC |
| VEGFR2-R |
GCAGTGCCGACGAGGATAATGAC |
| GAPDH-F |
CAAGGAGTAAGAAACCCTGGACC |
| GAPDH-R |
CGAGTTGGGATAGGGCCTCT |
| Tuj1-F |
CCTTCATCGGGAACAGCACG |
| Tuj1-R |
ACTCCTCCTCGTCGTCTTCGTA |
| GFAP-F |
CCAAGATGAAACCAACCT |
| GFAP-R |
CGCTGTGAGGTCTGGCTT |
| β-actin-F |
AGATGTGGATCAGCAAGCAG |
| β-actin-R |
GCGCAAGTTAGGTTTTGTCA |
NSPC differentiation and
immunofluorescence
NSPC differentiation was performed based on a
previously published method with minor changes (21). Briefly, 2×105 cells were
plated on four-well chamber slides and incubated at 37°C in 5%
CO2 with vehicle control (PBS), VEGF165 (20 ng/ml)
and/or clustered ephrin-A1-Fc (0.5 µg/ml), dissolved in growth
factor-free DMEM/F12 medium for 7 days. For immunofluorescence
analysis, dissociated NSPCs or neurospheres were fixed with 4%
paraformaldehyde at room temperature for 1 h, and blocked in 1%
normal goat serum (Sigma-Aldrich; Merck KGaA) at room temperature
for 1 h. The cells were then incubated with mouse anti-β-tubulin
III (Tuj1) monoclonal antibody (cat. no. T8660; 1:1,000;
Sigma-Aldrich; Merck KGaA), rabbit anti- glial fibrillary acid
protein (GFAP) polyclonal antibody (cat. no. Z0334; 1:1,1000; DAKO;
Agilent Technologies, Inc.), rabbit anti-CD11 antigen-like family
member B (CD11b) (cat. no. NB110-89474; 1:500; Novus Biologicals,
Ltd.), mouse anti-Nestin monoclonal antibody (cat. no. ab22035;
1:500; Abcam), mouse anti-proliferation marker protein Ki-67 (Ki67;
cat. no. P6834; 1:500; Sigma-Aldrich; Merck KGaA) and rabbit
anti-transcription factor SOX-2 (SOX2; cat. no. BS-0523R; 1:500;
Bio-Connect B.V.) at 4°C overnight, followed by incubation with the
secondary antibodies, goat anti-mouse AlexaFluor®
488-conjugated (cat. no. 115-545-146; 1:1,000; Jackson
ImmunoResearch Laboratories, Inc.) and goat anti-rabbit cyanine
Cy3-conjugated IgG (cat. no. 711-165-152; 1:1,000; Jackson
ImmunoResearch Laboratories, Inc.) for 2 h at room temperature in
the dark. Nuclei were stained with Hoechst 33258 (1:10,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 5 min. Cells
(four fields of view/well) were examined (magnification, ×200)
under a Nikon fluorescence microscope (Nikon Ti-E; Nikon
Corporation) or a confocal microscope (Olympus Corporation).
Plasmid transfection
The plasmids encoding the EphA4 (deletion of
juxtamembrane domain, ΔJM; kinase dead, KD) mutant containing the
deletion of 591–602 amino acids + V635M mutation (KD) and the
VEGFR2 (KD) mutant containing K868R mutation were constructed using
the QuickChange Lightning Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.) as per the manufacturer's
protocols. Wild type (WT) EphA4-Flag, VEGFR2-HA, VEGFR2 (KD)-HA,
EphA4 (KD)-Flag, EphA4(ΔJM, KD)-Flag and EphA4(ΔJM, KD)-GFP
eukaryotic expression vectors were constructed as previously
described through subcloning into the pcDNA3.1 vector (18,22).
Plasmids, including 0.5 µg/ml EphA4-Flag together with increasing
concentrations (1.0, 2.0 and 3.0 µg/ml) of VEGFR2-HA, 0.5 µg/ml
EphA4-Flag together with increasing concentrations (0, 0.5 and 2.5
µg/ml) of VEGFR2-HA or 0.5 µg/ml EphA4-Flag, 1.5 µg/ml VEGFR2-HA
together with increasing concentrations (0, 0.5 and 1.5 µg/ml) of
EphA4 (ΔJM, KD)-GFP were transiently transfected into 293T cells
using PerFectin (Genlantis, Inc.). EphA4 (ΔJM, KD)-Flag and VEGFR2
(KD)-HA mutant eukaryotic expression vectors were subcloned into a
pMXs-IRES-GFP retroviral vector (obtained from Dr T. Kitamura,
University of Tokyo, Tokyo, Japan) and subsequently incorporated
into retroviral particules by co-transfection with pMXs-IRES-GFP
retroviral plasmids (2.1 µg/ml for EphA4 mutant and 2.7 µg/ml for
VEGFR2 mutant) and 0.4 µg/ml pCAGVSV-G vector (American Type
Culture Collection) into 293T cells using FuGENE6®
(Roche Diagnostics GmbH). Retroviruses for both mutants were
harvested for subsequent NSPC infection as previously described
(16,18).
Immunoprecipitation and
immunoblotting
Immunoprecipitation and immunoblotting were
performed as previously described (21). 293T cells were extracted using lysis
A buffer, containing 50 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, 5 mM
ethylene diamine tetraacetic acid, 1% Triton X-100, 50 mM sodium
chloride, protease inhibitors (1 µM pepstatin A, 1 mM
phenylmethylsulfonyl fluoride, 1 µM leupeptin, and 1 µM aprotinin)
and phosphatase inhibitors (50 mM sodium fluoride, 10 mM sodium
pyrophosphate, and 1 mM sodium orthovanadate) as previously
described (21). Following
immunoprecipitation with specific antibodies using protein A
agarose, the pellets were washed three times. Then immunoblotting
was performed with diluted antibodies for 2 h at room temperature
using a standard procedure (18).
The following primary antibodies were used: Mouse
anti-hemagglutinin (HA; 1:3,000; cat no. 11583816001, Roche
Diagnostics GmbH), mouse anti-FLAG® M2 (1:3,000; cat no.
F3165; Sigma-Aldrich; Merck KGaA), mouse anti-hemagglutinin (HA;
1:3,000, cat no. sc-7392; Santa Cruz Biotechnology, Inc.), rabbit
anti-EphA4 (1:3,000; cat no. sc-921; Santa Cruz Biotechnology,
Inc.), mouse anti-EphA4 (1:3,000; cat no. sc-365503; Santa Cruz
Biotechnology, Inc.), mouse anti-VEGFR2 (1:1,000; cat no.
sc-393163; Santa Cruz Biotechnology, Inc.), mouse
anti-phosphotyrosine (pY; clone 4G10®; 1:1,000; cat. no.
05-321, EMD Millipore) and mouse anti-GFP (1:3,000; cat no.
sc-9996; Santa Cruz Biotechnology, Inc.). Subsequently, membranes
were incubated with a goat anti-mouse IgG horseradish
peroxidase-conjugated (1:5,000; sc-2005, Santa Cruz Biotechnology,
Inc.) or goat anti-rabbit IgG (1:5,000; sc-2004, Santa Cruz
Biotechnology, Inc.) secondary antibody at room temperature for 2
h. For detecting VEGF165-mediated tyrosine phosphorylation of
VEGFR2, 293T cells were first starved for 5 h before stimulation
with VEGF165 (20 ng/ml) for 0 and 10 min. The immunoprecipitation
for VEGFR2 and immunoblotting for pY were performed as
aforementioned. To confirm reproducibility, experiments were
performed at least three times.
Statistical analysis
Data were analyzed using GraphPad Prism 6 software
(GraphPad Software, Inc.) using two-way ANOVA followed by Tukey's
post hoc test for multiple comparisons. Data are displayed as the
mean ± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Interactions and transphosphorylation
between EphA4 and VEGFR2 in a kinase-dependent manner
Transient transfection of expression vectors for WT
VEGFR2 and the expression vector for WT EphA4 or kinase-dead (KD)
EphA4, a kinase-inactivated mutant form of EphA4 in which a Met
residue was substituted for Val-653, was performed in 293T cells
(Fig. 1A and B). VEGFR2 was found to
interact with WT EphA4 but not with KD EphA4 from immunoblotting
with anti-VEGFR2 antibodies followed by immunoprecipitation with
anti-EphA4 antibodies. Phosphotyrosine analysis showed enhanced
phosphorylation of WT EphA4 but not KD EphA4 with increasing doses
of VEGFR2 (Fig. 1A and B). The
results also demonstrated the interaction between EphA4 and VEGFR2,
and the transphosphorylation in a protein dose-dependent manner
(Fig. 1A). Based on these results,
it was speculated that EphA4 interacts with VEGFR2 in a dose- and
kinase-dependent manner.
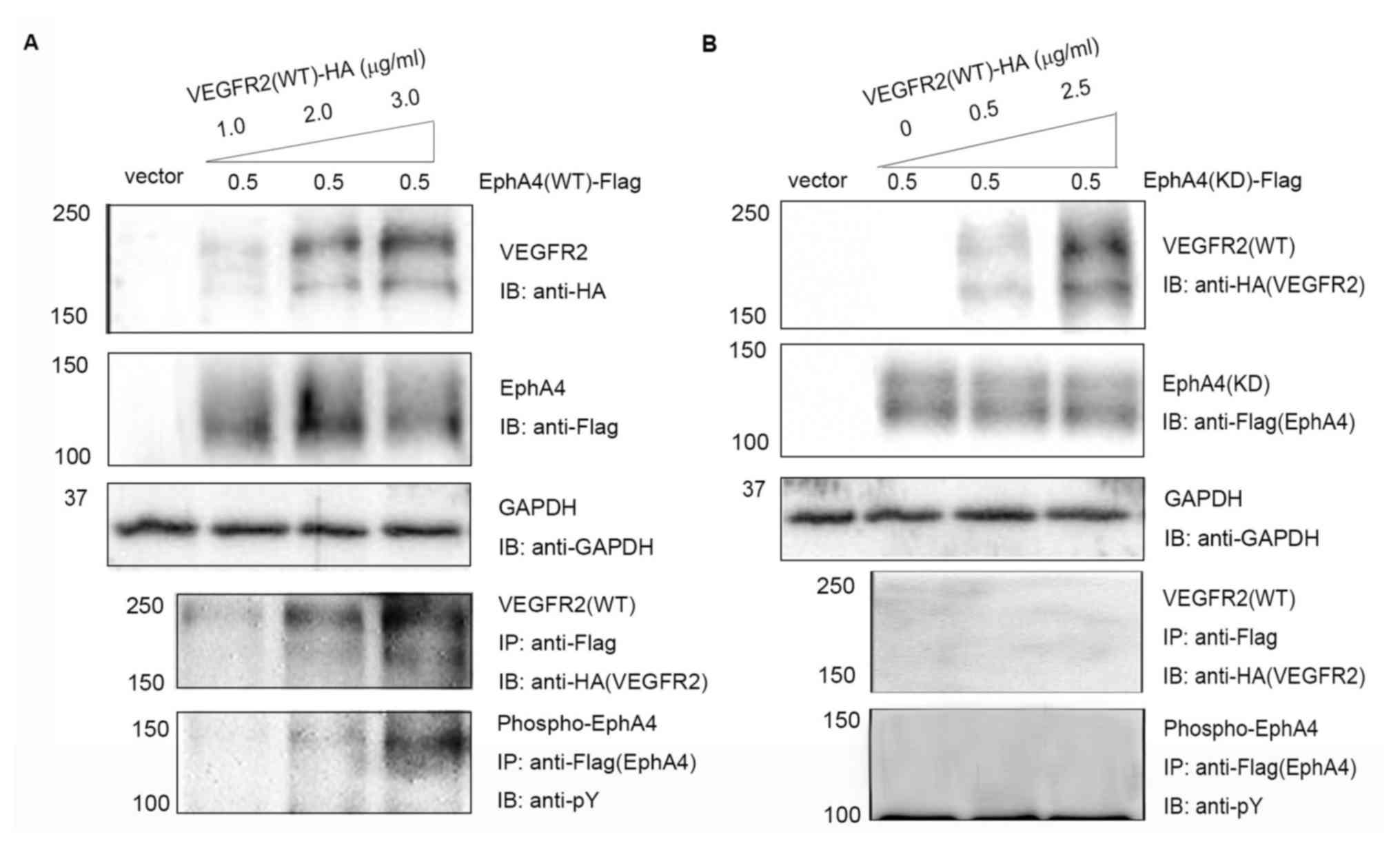 | Figure 1.Complex formation and
transphosphorylation of EphA4 and VEGFR2 in transfected 293T cells.
(A) 293T cells were co-transfected with pcDNA/EphA4-Flag (0.5
µg/ml) and increasing concentrations (1.0, 2.0 and 3.0 µg/ml)
pcDNA/VEGFR2-HA. (B) 293T cells were co-transfected with
pcDNA/EphA4(KD)-Flag (0.5 µg/ml) and increasing concentrations (0,
0.5 and 2.5 µg/ml) of pcDNA/VEGFR2(WT)-HA. Interactions were
detected using SDS-PAGE and IB using anti-Flag antibodies following
with IP using anti-HA antibodies. Tyrosine phosphorylation of EphA4
was detected using immunoprecipitation with anti-Flag antibodies
followed by immunoblotting with anti-pY anitbodies. Eph, ephrin
receptor; HA, hemagglutinin; IB, immunoblotting; IP,
immunoprecipitation; KD, kinase-dead; pY, phosphotyrosine; VEGFR,
vascular endothelial growth factor receptor; WT, wild-type. |
Inhibition of the interaction between
EphA4 and VEGFR2 by an EphA4 dominant-negative mutant, EphA4 (ΔJM,
KD)
EphA4 missing the juxtamembrane domain can bind to
FGFR but loses kinase activity (18). To further confirm the interaction
between EphA4 and VEGFR2, whether EphA4 (ΔJM, KD), a dominant
negative mutant of EphA4, could inhibit binding of EphA4 to VEGFR2
was examined. Fixed amounts of VEGFR2 (1.5 µg/ml per 6-cm plate in
2 ml culture medium) and WT EphA4 (0.5 µg/ml per 6-cm plate in 2 ml
culture medium) were co-expressed with incremental concentrations
of EphA4 (ΔJM, KD) in 293T cells, and the binding of EphA4 to
VEGFR2 was investigated. It was found that EphA4 (ΔJM, KD)
inhibited the interaction between EphA4 and VEGFR2 in a
dose-dependent manner (Fig. 2A).
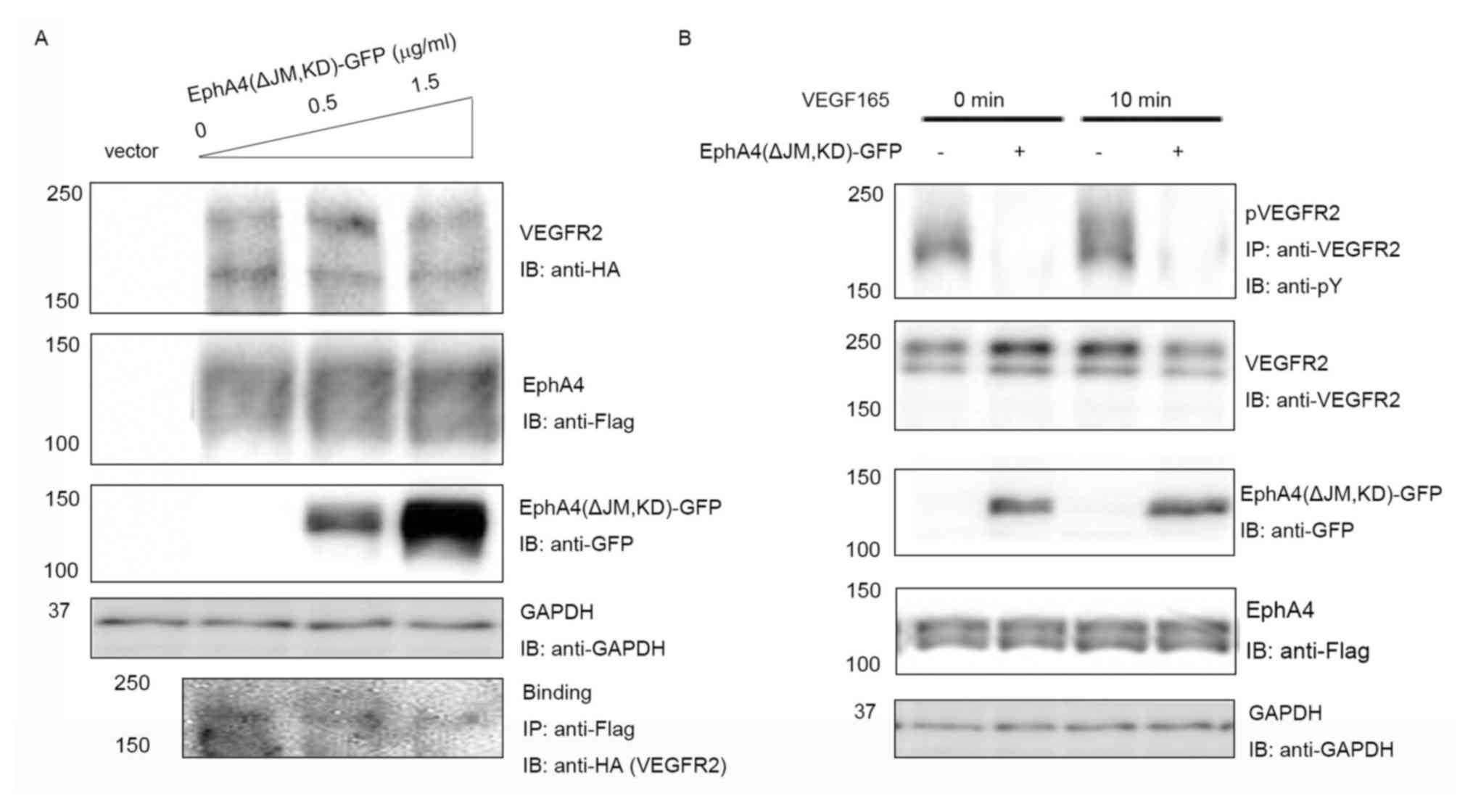 | Figure 2.Inhibition of EphA4-VEGFR2 binding by
EphA4(ΔJM, KD)-GFP. (A) EphA4-Flag and VEGFR2-HA were co-expressed
with increasing doses of EphA4(ΔJM, KD)-GFP in 293T cells, and the
binding of EphA4-Flag and VEGFR2-HA was examined using IB with or
without IP using the antibodies shown following SDS-PAGE. (B)
Inhibition of ligand-mediated receptor phosphorylation by
EphA4(ΔJM, KD) tagged with GFP. EphA4-Flag and VEGFR2-HA were
co-expressed in 293T cells with or without EphA4(ΔJM, KD)-GFP, the
phosphorylation of VEGFR2 was examined using IP with anti-VEGFR2
followed by IB with anti-pY following 0 or 10 min stimulation with
20 ng/ml VEGF165. ΔJM, juxtamembrane domain deleted; Eph, ephrin
receptor; GFP, green fluorescent protein; HA, hemagglutinin; IB,
immunoblotting; IP, immunoprecipitation; KD, kinase-dead; pY,
phosphotyrosine; VEGF, vascular endothelial growth factor; VEGFR,
VEGF receptor; WT, wild-type. |
To further analyze whether there was a
dominant-negative effect, the expression vector for EphA4 (ΔJM, KD)
was transfected into 293T cells together with the expression
vectors for EphA4 and VEGFR2. When fixed amounts of WT EphA4 and WT
VEGFR2 were co-transfected in 293T cells, as shown in Fig. 2B, EphA4 (ΔJM, KD) significantly
suppressed VEGF165 mediated tyrosine phosphorylation of WT VEGFR2
both at 0 and 10 min (the peak of ligand stimulation). The results
showed that the binding of EphA4 to VEGFR2 is important for both
the EphA4 and VEGFR2 signaling pathways.
NSPC differentiation under ephrin-A1
and/or VEGF165 stimulation
To analyze the functional role of the interactions
between EphA4 and VEGFR2, hippocampal cells from embryonic day 14.5
mice were cultured in DMEM/F12 serum-free medium supplemented with
B27, FGF2 and EGF. Following one week of culture, the cells
aggregated and formed spheroid neurospheres (Fig. 3A). Immunofluorescence staining
revealed that cells within the neurospheres were immunoreactive to
markers of neural stem cells nestin, Ki67 and SOX2 (Fig. 3B). Cells derived from the
neurospheres were also immunoreactive to Tuj1, which is an immature
neuronal marker, GFAP, an astrocyte marker and CD11b, an
oligodendrocyte marker (1,23) (Fig.
3C). These observations suggested that neurospheres derived
from the hippocampus of embryonic mice exhibited active
proliferative, self-renewal and multipotent properties in
vitro.
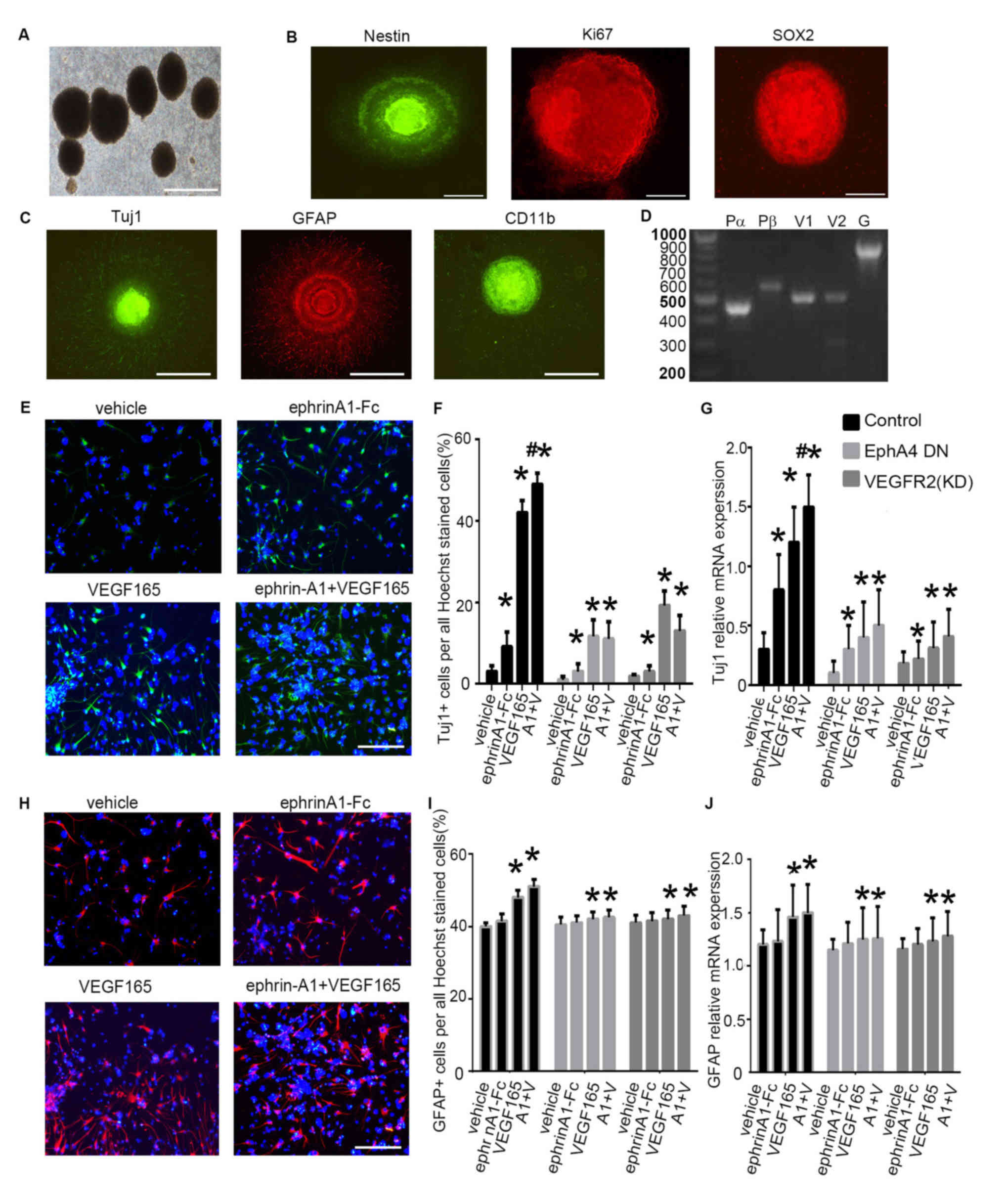 | Figure 3.Differentiation of mouse embryonic
NSPCs under clustered ephrin-A1-Fc and/or VEGF165 stimulation. (A)
Cultured NSPCs grew as neurospheres. (B) Immunofluorescence of the
neurospheres show that the cells were nestin+,
Ki67+ and SOX2+. (C) NSPCs differentiated
into neurons (Tuj1+), astrocytes (GFAP+), and
oligodendrocytes (CD11b+). Scale bars, 100 µm. (D)
Expression of VEGFRs and PDGFRs in mouse embryonic NSPCs. Reverse
transcription PCR was performed with equal amounts of total RNA
isolated from mouse embryonic NSPCs. Fragment lengths are indicated
on the left in base pairs. (E) NSPC differentiation was induced
under ephrin-A1 stimulation. Tuj1+ cells in the
different groups were stained after culturing for 7 days in normal
medium or medium containing clustered ephrin-A1-Fc (0.5 µg/ml)
and/or VEGF165 (20 ng/ml). (F) The proportion of Tuj1+
cells per all Hoechst-stained cells in the different treatment
groups was analyzed. (G) The mRNA expression levels of Tuj1 in
NSPCs cultured in normal medium or medium containing ephrin-A1
and/or VEGF165 was also examined. (H) GFAP+ cells in the
different groups were stained after culturing for 7 days in normal
medium or medium containing clustered ephrin-A1-Fc (0.5 µg/ml)
and/or VEGF165 (20 ng/ml). (I) The proportion of GFAP+
cells and (J) mRNA expression levels of GFAP were calculated. NSPCs
were transfected with dominant-negative EphA4 mutant or with
kinase-negative VEGFR2 mutant prior to stimulation. Data are
presented as the mean ± standard deviation. For (F) and (I), n=3 in
three separate experiments. For (G) and (J), n=5 in three separate
experiments. *P<0.05 vs. the control group;
#P<0.05 vs. VEGF165 treatment alone. DN, dominant
negative; Eph, ephrin receptor; fc, fragment crystallizable region;
GFAP, glial fibrillary acidic protein; PDGFR, platelet-derived
growth factor receptor; NSPC, neural stem and progenitor cell; Pα,
PDGFRα; Pβ, PDGFRβ; Tuj1, β-tubulin III; VEGF, vascular endothelial
growth factor; VEGFR, VEGF receptor; KD, kinase-dead. A1 + V,
ephrin-A1-Fc + VEGF165 stimulation. |
The expression patterns of Ephs, VEGFRs and PDGFRs
were investigated in the mouse embryonic NSPCs using RT-PCR. VEGFR
family members (VEGFR1 and VEGFR2) and PDGFR family members (PDGFRα
and PDGFRβ) were detected in NSPCs (Fig.
3D).
Subsequently, VEGF165 (20 ng/ml) and/or clustered
ephrin-A1-Fc (0.5 µg/ml) were added to the NSPC culture medium, and
neuronal differentiation of NSPCs was evaluated using Tuj1
immunofluorescence (Fig. 3E). When
compared with cells that were treated with PBS, the proportion of
Tuj1+ cells increased significantly following
stimulation with VEGF165 (P<0.05) or clustered ephrin-A1-Fc
(P<0.05). The percentage of Tuj1+ cells exhibited a
further increase under stimulation with clustered ephrin-A1-Fc +
VEGF165 compared to cells with no stimulation (P<0.05) or
stimulation with VEGF165 alone (P<0.05), suggesting enhanced
neuronal differentiation of NSPCs when the cells were induced by
simultaneous stimulation with the two ligands (Fig. 3E and F). Furthermore, the expression
of Tuj1 in the vehicle-treated cells in the presence of VEGF165
and/or clustered ephrin-A1-Fc was markedly decreased in NSPCs after
expression of a dominant-negative EphA4 mutant or expression of a
kinase-negative VEGFR2 mutant. These data confirmed that ephrin-A1
promoted VEGF165-mediated neuronal differentiation of NSPCs
(Fig. 3F).
The qPCR analysis indicated that the mRNA levels of
Tuj1 were markedly upregulated following stimulation with VEGF165
(P<0.05), clustered ephrin-A1-Fc (P<0.05) and VEGF165 +
clustered ephrin-A1-Fc (P<0.05) as compared to that of
unstimulated control cells. The increased Tuj1 mRNA levels in the
presence of VEGF165 and/or clustered ephrin-A1-Fc in the control
cells were markedly inhibited with transfection of a
dominant-negative EphA4 mutant or expression of a kinase-negative
VEGFR2 mutant (Fig. 3G).
Glial differentiation from NSPCs was also analyzed
using GFAP immunofluorescence (Fig.
3H). When compared with no stimulation, the proportion of
GFAP+ cells increased significantly following
stimulation with VEGF165 (P<0.05) but not with clustered
ephrin-A1-Fc stimulation. The percentage of GFAP+ cells
exhibited a slight but not significant increase under stimulation
with clustered ephrin-A1-Fc + VEGF165 as compared to stimulation
with VEGF165 alone (Fig. 3H and I).
Furthermore, the expression of GFAP was markedly decreased in NSPCs
after expression of a dominant-negative EphA4 mutant or expression
of a kinase-dead VEGFR2 mutant. These results confirmed that
ephrin-A1 promoted VEGF165-mediated glial differentiation of NSPCs
(Fig. 3I).
The qPCR analysis indicated that the mRNA levels of
GFAP were significantly upregulated following stimulation with
VEGF165 (P<0.05) and VEGF165 + clustered ephrin-A1-Fc
(P<0.05) as compared to that of unstimulated control cells. The
increased GFAP mRNA levels in the presence of VEGF165 and/or
clustered ephrin-A1-Fc were markedly reduced when cells were
transfected with a dominant-negative EphA4 mutant or a kinase-dead
VEGFR2 mutant (Fig. 3J). These
results are consistent with the findings aforementioned in
non-neuronal cells using ectopically expressed molecules (Figs. 1 and 2).
Discussion
The present study showed that EphA4 and VEGFR2 bind
together in a dose and kinase-dependent manner when transiently
co-expressed in the same cells. A dominant-negative molecule of
EphA4 can inhibit the interaction between EphA4 and VEGFR2.
Ephrin-A1 alone only produced a minor effect on NSPC
differentiation. However, when ephrin-A1 and VEGF165 were added
together, ephrin-A1 could potentiate VEGF-165-induced NSPC
differentiation, revealing that ephrin-A1-stimulated EphA4 and
VEGFR2 interactions may mediate the signaling pathway.
Previous research showed that EphA4 and PDGFRs or
EphA4 and FGFRs could form a heterodimer, trans-phosphorylating
each other when overexpressed in 293T cells or after stimulation
with their ligands (17,18). In the present study, a
kinase-dependent interaction between EphA4 and VEGFR2 was found.
Specifically, no interaction and trans-activation between the two
molecules was detected when their kinase-dead mutants were
overexpressed in 293T cells. In addition to the Ephs measured in
the NSPCs in a previous report (21), VEGFRs (VEGFR1 and VEGFR2) and PDGFRs
(PDGFRα and PDGFRβ) were also found to be expressed in the NSPCs.
It was also found that NSPCs derived from the embryonic mouse brain
respond to ephrin-A1 and VEGF165, promoting differentiation, and
the effect of these two ligands was inhibited by the expression of
a dominant-negative mutant of EphA4. The kinase-dead EphA4
abrogates the effect of VEGF165 and/or ephrin on NSPC
differentiation by constitutively binding to VEGFR2. VEGF and
ephrin-A1 enhanced the multipotent differentiation ability of NSPCs
into neurons and astrocytes; however, limited detection of
oligodendrocyte differentiation of NSPCs was observed using the
routine differentiation protocol due to the absence of additional
essential supplements that are required for oligodendrocyte
differentiation (24). Together with
the finding that the EphA4 dominant-negative mutant inhibits VEGFR2
phosphorylation at both the basal and the ligand-stimulated phases,
these results support the presence of molecular interactions
between EphA4 and VEGFR2.
The possibility that EphA4 may crosstalk with VEGFR2
through other growth factor receptors, such as FGFR or PDGFR,
cannot be excluded as cross-family interactions have been reported
between VEGF/EGFR, PDGF/VEGFR, VEGF-A/PDGFR, PDGFR/FGFR and
EphA4/FGFR (25–29). Preliminary immunoprecipitation and
immunoblotting experiments also demonstrated that EphA4 and EGFR
could interact and transactivate with each other when overexpressed
in 293T cells (data not shown). For future experiments, the aim is
to investigate the downstream signaling pathways associated with
the signals mediated by the EphA/FGFR/EGFR/PDGFR/VEGFR complex.
Ephrin-A1 may stimulate dopaminergic neurogenesis
and angiogenesis in a 6-hydroxydopamine (6-OHDA) lesioned PD rat
model through activating the EphA4 signaling pathway (5). Activating EphA receptors also alters
the fate of NSPCs to a neuronal commitment in vitro and
in vivo (11). EphA4/ephrin-A
signaling serves an important role in establishing the brain
vascular system which supports the adult neurogenic niche (30). EphA4 regulates hippocampal
neurogenesis via d-serine-regulated N-Methyl-D-aspartic acid
receptor signaling in the adult mouse brain (31). As a major angiogenic factor, VEGF
promotes neurogenesis in NSPCs in vitro and in the adult
brain through the VEGFR2 signaling pathway (13). The proliferative effects of
VEGF/VEGFR2 require the ERK and Akt signaling cascades in cultured
hippocampal neuronal progenitor cells and in the adult rat
hippocampus (32). NSPCs maintain
their stem cell proliferative and differentiation ability via
self-secreted VEGF interacting with VEGFR2 and VEGF-expressing
cells, which in turn provide an enriched environment. This activity
may restore functions following brain injuries or in
neurodegenerative diseases (16,33).
VEGF may trigger spinal cord NSPC proliferation and self-renewal
in vitro and the VEGF/VEGFR2/EGFR signaling plays an
important role in NSPC activation in vivo (26). Hippocampal administration of VEGF
enhances neurogenesis and alleviates the cognitive deficits in
immature rats after status epilepticus (34). To the best of the authors' knowledge,
the present study is the first demonstration of a potential
function of the interaction of Ephs and VEGFRs in NSPC
differentiation. Further studies should be designed investigating
the efficacy of transplanted NSPCs, treated with combined ephrin-A1
and VEGF165, into several disease animal models in order to
identify potential therapies for neurodegenerative diseases such as
AD and PD.
Acknowledgements
The authors are grateful to Professor Paul Lu from
the University of California San Diego for his suggestions in
preparing the manuscript.
Funding
QC is a recipient of a Grant for Doctor from the
Shandong Province Scientific Foundation (grant no.
ZR2017BH094).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QFC and FBH conceived and designed the experiments.
QFC, JL, TS, CFW and SCW performed the experiments and analyzed the
data. QFC and FH wrote the manuscript. All authors approved the
final version of this manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the guidelines of the Liaocheng People's Hospital (Shandong,
China), and were approved by the Ethics Committee of Liaocheng
People's Hospital (approval no. LUACC201604).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGrath E, Gao J and Wu P: Proliferation
and differentiation of human fetal brain neural stem cells in
vitro. J Neurorestoratol. 6:19–27. 2018. View Article : Google Scholar
|
|
3
|
Baptista P and Andrade JP: Adult
hippocampal neurogenesis: Regulation and possible functional and
clinical correlates. Front Neuroanat. 12:442018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S and Chen Z: Employing endogenous
NSCs to promote recovery of spinal cord injury. Stem Cells Int.
2019:19586312019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jing X, Miwa H, Sawada T, Nakanishi I,
Kondo T, Miyajima M and Sakaguchi K: Ephrin-A1-mediated
dopaminergic neurogenesis and angiogenesis in a rat model of
Parkinson's disease. PLoS One. 7:e320192012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Wei M, Lu L, Chu T, Li X, Fu Z,
Liu J, Kang Y, Liu L, Lou Y, et al: Angiopoietin-2 induces the
neuronal differentiation of mouse embryonic NSCs via
phosphatidylinositol 3 kinase-Akt pathway-mediated phosphorylation
of mTOR. Am J Transl Res. 11:1895–1907. 2019.PubMed/NCBI
|
|
7
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murai KK and Pasquale EB: Eph receptors,
ephrins, and synaptic function. Neuroscientist. 10:304–314. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilkinson DG: Multiple roles of EPH
receptors and ephrins in neural development. Nat Rev Neurosci.
2:155–164. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Todd KL, Baker KL, Eastman MB, Kolling FW,
Trausch AG, Nelson CE and Conover JC: EphA4 regulates neuroblast
and astrocyte organization in a neurogenic niche. J Neurosci.
37:3331–3341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aoki M, Yamashita T and Tohyama M: EphA
receptors direct the differentiation of mammalian neural precursor
cells through a mitogen-activated protein kinase-dependent pathway.
J Biol Chem. 279:32643–32650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang KF, Hsu WC, Hsiao JK, Chen GS and
Wang JY: Collagen-glycosaminoglycan matrix implantation promotes
angiogenesis following surgical brain trauma. Biomed Res Int.
2014:6724092014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin K, Zhu Y, Sun Y, Mao XO, Xie L and
Greenberg DA: Vascular endothelial growth factor (VEGF) stimulates
neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA.
99:11946–11950. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosenstein JM, Mani N, Khaibullina A and
Krum JM: Neurotrophic effects of vascular endothelial growth factor
on organotypic cortical explants and primary cortical neurons. J
Neurosci. 23:11036–11044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao T and Rockwell P: Signaling through
the vascular endothelial growth factor receptor VEGFR-2 protects
hippocampal neurons from mitochondrial dysfunction and oxidative
stress. Free Radic Biol Med. 63:421–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM,
Young D and During MJ: VEGF links hippocampal activity with
neurogenesis, learning and memory. Nat Genet. 36:827–835. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Sawada T, Sakaguchi K and Han F:
Direct interaction of receptor tyrosine kinases, EphA4 and PDGFRβ,
plays an important role in the proliferation of neural stem cells.
J Neurorestoratol. 5:133–141. 2017. View Article : Google Scholar
|
|
18
|
Yokote H, Fujita K, Jing X, Sawada T,
Liang S, Yao L, Yan X, Zhang Y, Schlessinger J and Sakaguchi K:
Trans-activation of EphA4 and FGF receptors mediated by direct
interactions between their cytoplasmic domains. Proc Natl Acad Sci
USA. 102:18866–18871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang S, Mao J, Ding K, Zhou Y, Zeng X,
Yang W, Wang P, Zhao C, Yao J, Xia P and Pei G: Polysaccharides
from Ganoderma lucidum promote cognitive function and neural
progenitor proliferation in mouse model of Alzheimer's disease.
Stem Cell Reports. 8:84–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Jia D, Fu J, Zhao S, He G, Ling EA,
Gao J and Hao A: Effects of granulocyte colony-stimulating factor
on the proliferation and cell-fate specification of neural stem
cells. Neuroscience. 164:1521–1530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sawada T, Arai D, Jing X, Furushima K,
Chen Q, Kawakami K, Yokote H, Miyajima M and Sakaguchi K:
Trans-activation between EphA and FGFR regulates self-renewal and
differentiation of mouse embryonic neural stem/progenitor cells via
differential activation of FRS2α. PLoS One. 10:e01288262015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanlandewijck M, Lebouvier T, Andaloussi
Mäe M, Nahar K, Hornemann S, Kenkel D, Cunha SI, Lennartsson J,
Boss A, Heldin CH, et al: Functional characterization of germline
mutations in PDGFB and PDGFRB in primary familial brain
calcification. PLoS One. 10:e01434072015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deierborg T, Roybon L, Inacio AR, Pesic J
and Brundin P: Brain injury activates microglia that induce neural
stem cell proliferation ex vivo and promote differentiation of
neurosphere-derived cells into neurons and oligodendrocytes.
Neuroscience. 171:1386–1396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raff MC, Miller RH and Noble M: A glial
progenitor cell that develops in vitro into an astrocyte or an
oligodendrocyte depending on culture medium. Nature. 303:390–396.
1983. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mamer SB, Chen S, Weddell JC, Palasz A,
Wittenkeller A, Kumar M and Imoukhuede PI: Discovery of
high-affinity PDGF-VEGFR interactions: Redefining RTK dynamics. Sci
Rep. 7:164392017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu SM, Xiao ZF, Li X, Zhao YN, Wu XM, Han
J, Chen B, Li JY, Fan CX, Xu B, et al: Vascular endothelial growth
factor activates neural stem cells through epidermal growth factor
receptor signal after spinal cord injury. CNS Neurosci Ther.
25:375–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen PY, Simons M and Friesel R: FRS2 via
fibroblast growth factor receptor 1 is required for
platelet-derived growth factor receptor beta-mediated regulation of
vascular smooth muscle marker gene expression. J Biol Chem.
284:15980–15992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Arai D, Kawakami K, Sawada T, Jing
X, Miyajima M, Hirai S, Sakaguchi K and Furushima K: EphA4
regulates the balance between self-renewal and differentiation of
radial glial cells and intermediate neuronal precursors in
cooperation with FGF signaling. PLoS One. 10:e01269422015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ball SG, Shuttleworth CA and Kielty CM:
Vascular endothelial growth factor can signal through
platelet-derived growth factor receptors. J Cell Biol. 177:489–500.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara Y, Nomura T, Yoshizaki K, Frisén J
and Osumi N: Impaired hippocampal neurogenesis and vascular
formation in ephrin-A5-deficient mice. Stem Cells. 28:974–983.
2010.PubMed/NCBI
|
|
31
|
Zhao J, Taylor CJ, Newcombe EA, Spanevello
MD, O'Keeffe I, Cooper LT, Jhaveri DJ, Boyd AW and Bartlett PF:
EphA4 regulates hippocampal neural precursor proliferation in the
adult mouse brain by d-Serine modulation of N-Methyl-d-Aspartate
receptor signaling. Cereb Cortex. 29:4381–4397. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fournier NM, Lee B, Banasr M, Elsayed M
and Duman RS: Vascular endothelial growth factor regulates adult
hippocampal cell proliferation through MEK/ERK- and
PI3K/Akt-dependent signaling. Neuropharmacology. 63:642–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirby ED, Kuwahara AA, Messer RL and
Wyss-Coray T: Adult hippocampal neural stem and progenitor cells
regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci
USA. 112:4128–4133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han W, Song X, He R, Li T, Cheng L, Xie L,
Chen H and Jiang L: VEGF regulates hippocampal neurogenesis and
reverses cognitive deficits in immature rats after status
epilepticus through the VEGFR2 signaling pathway. Epilepsy Behav.
68:159–167. 2017. View Article : Google Scholar : PubMed/NCBI
|

















