Introduction
Gliomas are the most pervasive and aggressive major
type of primary tumour in the nervous system (1). The 5-year survival rate of
glioblastomas patients is 5% (1).
The development of the malignant phenotype is the result of
complicated processes that influence the expression of genes
associated with angiogenesis, invasion and proliferation of tumour
cells (2). Further studies on gene
regulation networks will lead to a better understanding of the
molecular mechanisms in glioma and eventually find novel biomarkers
and therapeutic targets for glioma (3).
Long non-coding RNAs (lncRNAs), which are a class of
transcripts >200 nucleotides in length, have been demonstrated
to serve important roles in gene transcription and
post-transcription regulation (4).
lncRNAs are frequently dysregulated in cancer progression; for
instance, targeting the microRNA (miRNA or
miR)-146b-5p/HuR/lincRNA-p21/β-catenin signalling pathway may be a
valuable therapeutic strategy against glioma (5); the expression of the
lncRNA-reprogramming was negatively correlated with Kruppel like
factor 4 and positively correlated with SRY-box (SOX)11 in glioma
(6). The present study focused on
the lncRNA X-inactive specific transcript (XIST), which is required
for transcriptional silencing of one X-chromosome during
development in female mammals (7).
It has been reported that the lncRNA XIST promotes glioma
tumourigenicity and angiogenesis (8). A previous study demonstrated that XIST
inhibited miR-133a in pancreatic cancer (PC) cell lines and further
revealed a XIST/miR-133a/EGFR signalling axis and provided a
potential mechanism for XIST in PC cell proliferation (9). miR-133a is a multicopy gene with two
copies, one on chromosome 18 and one on chromosome 20 (10). In tumourigenesis, miR-133a is
expressed at a low level in several cancer types, such as glioma
and other solid tumours, the epithelial-mesenchymal transition
(EMT)-related transcription factor SOX4 is a direct target gene of
miR-133a, and miR-133a directly binds to the 3′untranslated region
(UTR) of SOX4 (10).
SOX4 is a major member of the SOX family that is
located on 6p22.3 and encodes a protein of 474 amino acids with
three distinguishable domains: A high mobility group box, a
glycine-rich region, and a serine-rich region (11). SOX4 is associated with many
developmental processes, such as T cell differentiation and the
development of thymocytes, the nervous system, and the embryonic
cardiovascular system (12–14). In recent years, a number of studies
have suggested that SOX4 may be associated with tumour development
and progression (15–17).
In the present study, it was demonstrated that XIST
influenced glioma cell proliferation and migration by regulating
the miR-133a/SOX4 axis. These findings suggested that XIST is
potentially a therapeutic target for glioma.
Materials and methods
Cell culture
U251 glioma cell lines, control cell line HEB and
the 293 cells were obtained from Shanghai Cell Bank of Chinese
Academy of Sciences (Shanghai, China) and cultured at 37°C in
humidified 5% CO2. All cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All media contained 10% foetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). The 293 cells were used to investigate the regulatory
mechanism between miR-133a and XIST or between miR-133a and
SOX4.
XIST small hairpin RNA (shRNA) and
miRNA transfection
On the day prior to transfection, U251 cells were
digested by trypsin, counted and seeded in a 6-well plate at
1×105 cells/well. When the cell confluence reached 90%,
the medium was changed with serum-free DMEM and incubated at 37°C
overnight. An sh-XIST vector was used to achieve knockdown of XIST
(GeneCopoeia, Inc.). miR-133a mimics (5′-UUUGGUCCCCUUCAACCAGCUG-3′
and 5′-GCUGGUUGAAGGGGACCAAAU-3′) or an miR-133a inhibitor
(5′-CAGCUGGUUGAAGGGGACCAAA-3′; GeneCopoeia, Inc.) were used to
achieve miR-133a overexpression and inhibition, respectively.
Transient transfections were performed using Lipofectamine 2000
(Life Technologies; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Sh-negative control (NC; sense,
5′-GATCCGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAACTTTTTG-3′;
and antisense,
5′-AATTCAAAAAGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAACG-3′),
mimics NC (5′-UUCUCCGAACGUGUCACGUTT-3′ and
5′-ACGUGACACGUUCGGAGAATT-3′) and inhibitor NC
(5′-CAGUACUUUUGUGUAGUACAA-3′; GeneCopoeia, Inc.) were used as
respective controls. XIST shRNA (sense,
5′-CACCGCTCTTGAACAGTTAATTTGCTTCAAGAGAGCAAATTAACTGTTCAAGAGCTTTTTTG-3′
and antisense,
5′-GATCCAAAAAAGCTCTTGAACAGTTAATTTGCTCTCTTGAAGCAAATTAACTGTTCAAGAGC-3′)
or miRNA (hsa-miR-133a mimics, 5′-UUUGGUCCCCUUCAACCAGCUG-3′ and
5′-GCUGGUUGAAGGGGACCAAAUU-3′; and hsa-miR-133a inhibitor,
5′-CAGUACUUUUGUGUAGUACAA-3′) was transfected into the indicated
cell lines for 24 h at 37°C. Transfection efficiency was determined
via reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). Cells were transfected with mimics or inhibitors at a
final concentration of 100 nM.
RT-qPCR
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. cDNA was synthesized by reverse
transcription of total RNA using the All-in-One™ First-Strand cDNA
Synthesis kit (GeneCopoeia, Inc.) according to the manufacturers'
instruction at room temperature. Then, using cDNA as the template,
the gene expression levels were analysed by qPCR conducted using
iTaq™ Universal One-Step SYBR RT-qPCR kits on a real-time PCR
system (both Bio-Rad Laboratories, Inc.). The qPCR conditions were
95°C for 5 sec and 60°C for 15 sec, followed by 70°C for 15 sec,
for 45 cycles. The relative gene expression level was calculated
using the 2−ΔΔCq method (18). The primers used were as follows: XIST
forward, 5′-ACGCTGCATGTGTCCTTAG-3′ and reverse,
5′-GAGCCTCTTATAGCTGTTTG-3′; miR-133a forward,
5′-TTTGGTCCCCTTCAACCAGCTG-3′ and reverse,
5′-TAAACCAAGGTAAAATGGTCGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ and GAPDH forward,
5′-CATCACCATCTTCCAGGAGCG-3′ and reverse,
5′-TGACCTTGCCCACAGCCTTG-3′. All results were obtained from at least
three independent experiments.
Western blotting
Cells were washed with cold PBS twice and lysed with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) containing the protease inhibitor for 30 min on ice.
Following centrifugation at 12,000 × g and 4°C for 15 min, the
protein concentration was determined using the enhanced
bicinchoninic acid BCA protein assay kit (Beyotime Institute of
Biotechnology). The supernatant was loaded on a 10% SDS-PAGE gel
(10 µg total protein/lane) and proteins were separated. Proteins
were then transferred to a polyvinylidene difluoride membrane,
blocked with 5% bovine serum albumin (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature and
incubated with primary antibody at 4°C overnight. The primary
antibodies used in the present study were as follows: Anti-Sox4
antibody (1:1,000; sc-518016; Santa Cruz Biotechnology, Inc.),
anti-E-cadherin antibody (1:1,000; sc-59778; Santa Cruz
Biotechnology, Inc.), anti-vimentin antibody (1:1,000; sc-373717;
Santa Cruz Biotechnology, Inc.), anti-N-cadherin antibody (1:1,000;
sc-53488; Santa Cruz Biotechnology, Inc.), anti-α-catenin antibody
(1:1,000; sc-9988; Santa Cruz Biotechnology, Inc.) and anti-β-actin
antibody (1:5,000; #4970; Cell Signaling Technology, Inc.)
Membranes were washed with TBS containing 0.1% Tween-20 five times
and then incubated with donkey anti-rabbit horseradish
peroxidase-conjugated secondary antibody at room temperature
(1:10,000; sc-2313; Santa Cruz Biotechnology, Inc.) for 1 h.
Membranes were finally washed in TBS and developed with
diaminobenzidine horseradish peroxidase color development kit
(Beyotime Institute of Biotechnology) for chemiluminescence
substrate. Densitometric analyses were performed using a
chemiluminescence imaging system (model 5200; Tanon Science and
Technology Co., Ltd.) and built-in Tanon MP software (version
2014).
MTT assay
U251 cells were suspended in DMEM culture medium
containing 10% FBS and placed in a 96-well plate with approximately
2,000 cells/200 µl volume in each well. These cells were incubated
under the condition of 5% CO2 and 37°C for 24 h. Then,
20 µl MTT (5 mg/ml) was added in every well, and these cells
continued to be cultured for an additional 4 h. Culture medium was
removed, and 150 µl DMSO was added for the dissolution of the
crystals on the shaker for 10 min. Optical density values were
measured at 490 nm.
Cell migration analysis
Migration ability was determined using a
wound-healing assay. U251 cells were plated into 12-well plates
without antibiotics, and cells were transfected with sh-XIST or the
control or with miR-133a mimics, miR-133a inhibitor or the control.
Then, 24 h later, transfected cells were wounded with a sterile
plastic 100 µl micropipette tip, the floating debris were washed
with PBS, and the remaining cells were cultured in serum-free
medium at 37°C. The width of the wound was measured at 0 and 24
h.
Cell invasion analysis
First, 80 µl diluted Matrigel was put into the upper
chamber of a 24-well Transwell chamber (Corning Inc.) and incubated
at 37°C for 30 min for gelling. U251 cells were harvested, and the
cells were suspended in serum-free DMEM/F12. Then, all cells in
various groups were placed in the upper chamber with 200 µl
serum-free DMEM, and 600 µl DMEM culture medium containing 10% FBS
was placed in the bottom chamber. Cells were incubated at 37°C for
20 h, and cells that migrated or invaded the lower surface of the
membrane were removed from the 24-well plates. Cells adhering to
the membrane were stained with crystal violet for 10 min at room
temperature, and each insert was counted at ×100 magnification
using a bright field microscope (Model DC 300F; Leica Microsystems
GmbH).
Luciferase reporter assay
Luciferase reporter gene assay was implemented using
the Dual-Luciferase Reporter Assay System (Promega Corporation)
according to the manufacturer's instructions. Cells were seeded
into 24-well plates and transfected with a wild-type (wt)-XIST
luciferase reporter gene vector, a mutant (mut)-XIST vector
containing a 6-bp mutation on the predicted miR-133a binding site
within XIST, a wt-SOX4 3′UTR vector, or a mut-SOX4 3′UTR vector
(all from Shanghai GenePharma Co., Ltd.) containing a mutation in
the predicted miR-133a binding site in the 3′UTR of SOX4, along
with the miR-133a mimic or mimic NC using Lipofectamine 3000 (Life
Technologies; Thermo Fisher Scientific, Inc.). Following 48 h,
cells were lysed using passive lysis buffer (Promega Corporation)
and the luciferase activity was detected. Luciferase activity was
normalized against Renilla. All experiments were performed
at least three times.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS software 16.0 (SPSS,
Inc.). Statistical significance was measured using Student's t-test
or one-way analysis of variance in conjunction with Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
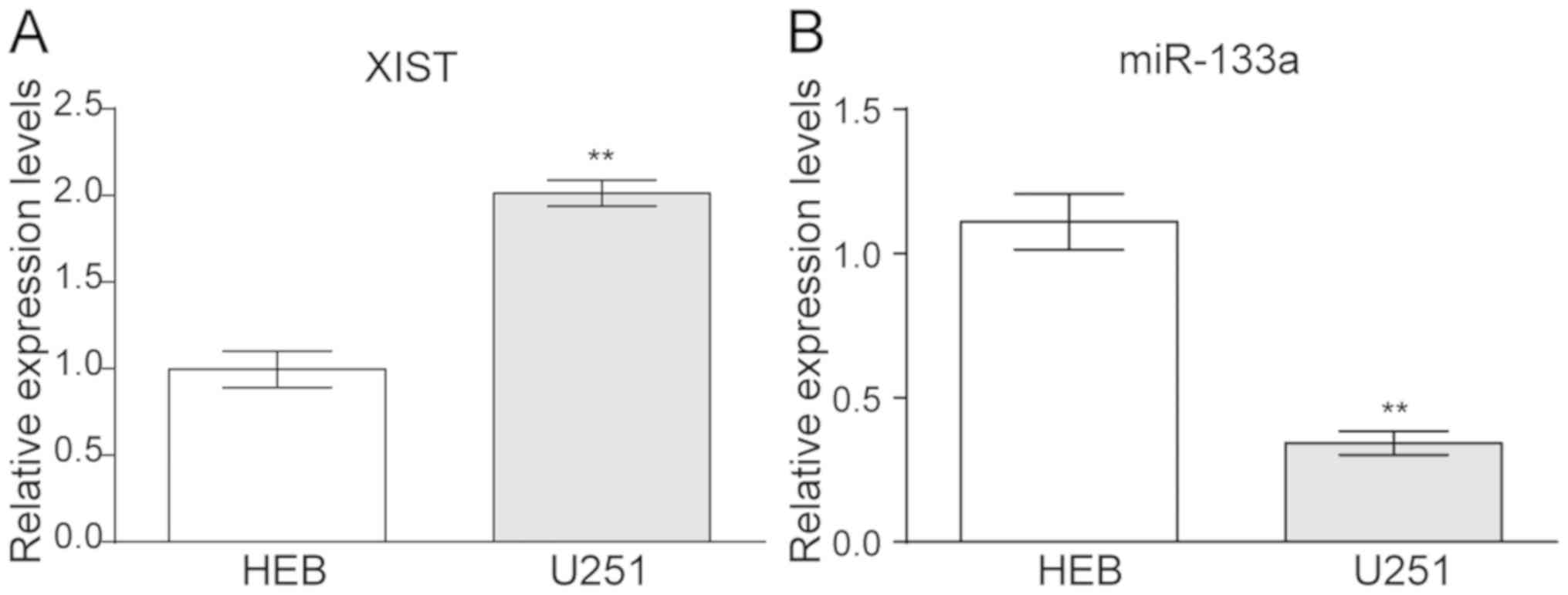
Upregulation of XIST and
downregulation of miR-133a in glioma cell lines
The relative expression levels of XIST and miR-133a
were detected via RT-qPCR in the glioma cell line U251. The data
revealed that XIST was upregulated in U251 compared with that in
the controlled HEB cells (Fig. 1A).
In contrast, miR-133a presented a lower expression in U251 compared
with HEB cells (Fig. 1B).
XIST suppresses miR-133a expression in
glioma cell lines and enhances cell proliferation and invasion
To evaluate the association of XIST expression with
glioma cells' proliferation and metastasis. XIST knockdown was
achieved by sh-XIST transfection, and the inhibitory efficiency was
verified by RT-qPCR (Fig. 2A). The
level of miR-133a was then detected by RT-qPCR. The results
revealed that miR-133a expression was significantly enhanced when
sh-XIST was transfected into U251 cells (Fig. 2A). These results indicated that XIST
negatively regulated miR-133a in glioma cell lines.
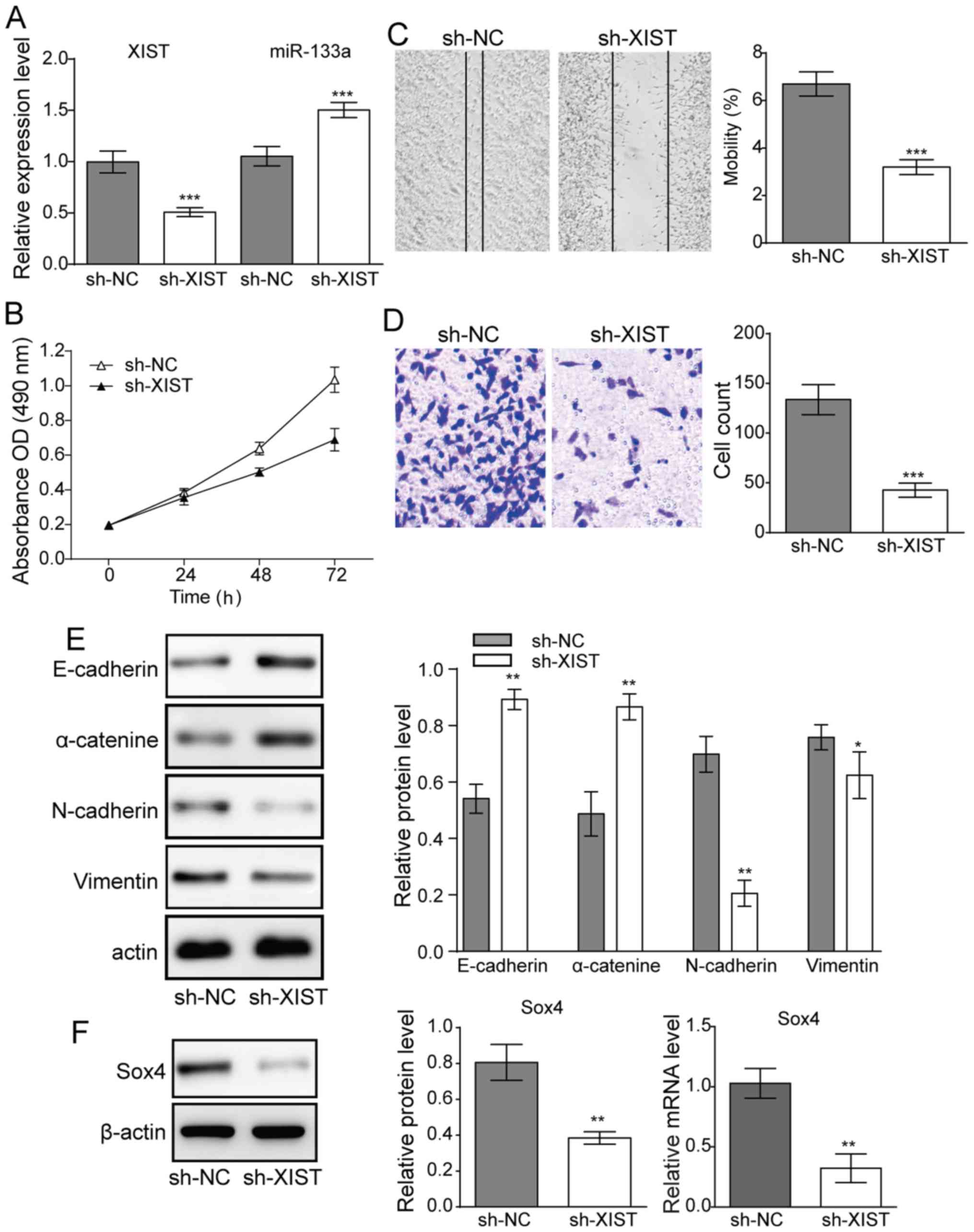 | Figure 2.Effect of downregulation of XIST on
glioma cell proliferation and metastasis. (A) Following
transfection with sh-XIST, XIST was significantly downregulated.
The transfection of sh-XIST further potentiated miR-133a in U251
cells. (B) Cell viability of U251 lines following sh-XIST
transfection. (C) Cell migration (magnification, ×16) and (D) cell
invasion assay of glioma cells (magnification, ×100). (E) Western
blot results demonstrated the expression levels of E-cadherin
N-cadherin, α-catenin and vimentin in glioma cells following
sh-XIST transfection. (F) Expression levels of SOX4 transcription
factor. n=3, *P<0.05, **P<0.01 and ***P<0.001 vs. sh-NC.
XIST, X-inactive specific transcript; sh, small hairpin RNA; miR,
microRNA; SOX, SRY-box; NC, negative control; OD, optical
density. |
MTT assays revealed that knocking down XIST reduced
cell proliferation of U251 cell lines (Fig. 2B). Meanwhile, in the migration and
invasion experiments, XIST knockdown also suppressed cell migration
(Fig. 2C) and invasion activity
(Fig. 2D) in glioma cells.
Furthermore, the expression of EMT-related proteins E-cadherin,
vimentin, N-cadherin and α-catenin, was investigated. Western blot
results demonstrated that E-cadherin and α-catenin were
significantly upregulated, whereas N-cadherin and vimentin were
downregulated when XIST was knocked down in U251 cells (Fig. 2E), suggesting a suppression of EMT.
Both mRNA and protein expression of the EMT-related transcription
factor Sox4 were also significantly decreased upon knockdown of
XIST (Fig. 2F), further supporting a
significant reduction in EMT upon XIST knockdown.
Role of miR-133a in glioma cell
proliferation, migration and EMT
Mimics or an inhibitor of miR-133a were used to
assess the role of miR-133a in glioma cell behaviour. It was first
demonstrated that elevated miR-133a expression could lower XIST
expression in U251 glioma cells. In contrast, inhibitor of miR-133a
significantly suppressed the level of miR-133a and elevated the
level of XIST (Fig. 3A). Using the
MTT assay, it was demonstrated that when a mimic of miR-133a was
used for transfection, the cell proliferation of U251 cells was
inhibited compared with that of the control group; in contrast,
U251 cells transfected with an inhibitor of miR-133a demonstrated
increased proliferation (Fig. 3B).
Consequently, in the migration and invasion test, the mimic of
miR-133a notably decreased both migration and invasion abilities;
whereas the inhibitor of miR-133a markedly increased migration and
invasion (Fig. 3C and D).
Furthermore, the EMT-related proteins E-cadherin, N-cadherin,
α-catenin and vimentin were also detected when a mimic of miR-133a
or inhibitor of miR-133a was transfected into U251 cell. Western
blotting demonstrated that the mimic of miR-133a could increase the
expression of E-cadherin and α-catenin but decrease the expression
of N-cadherin and vimentin. The inhibitor of miR-133a exerted the
opposite effect (Fig. 3E). These
results collectively indicated that miR-133a acts as a tumour
suppressor in glioma. The expression of the EMT-related
transcription factor SOX4 was also detected by western blotting.
The results revealed that both mRNA and protein expression of SOX4
was significantly reduced when the mimic of miR-133a was
transfected and was significantly increased when the inhibitor of
miR-133a was transfected (Fig.
3F).
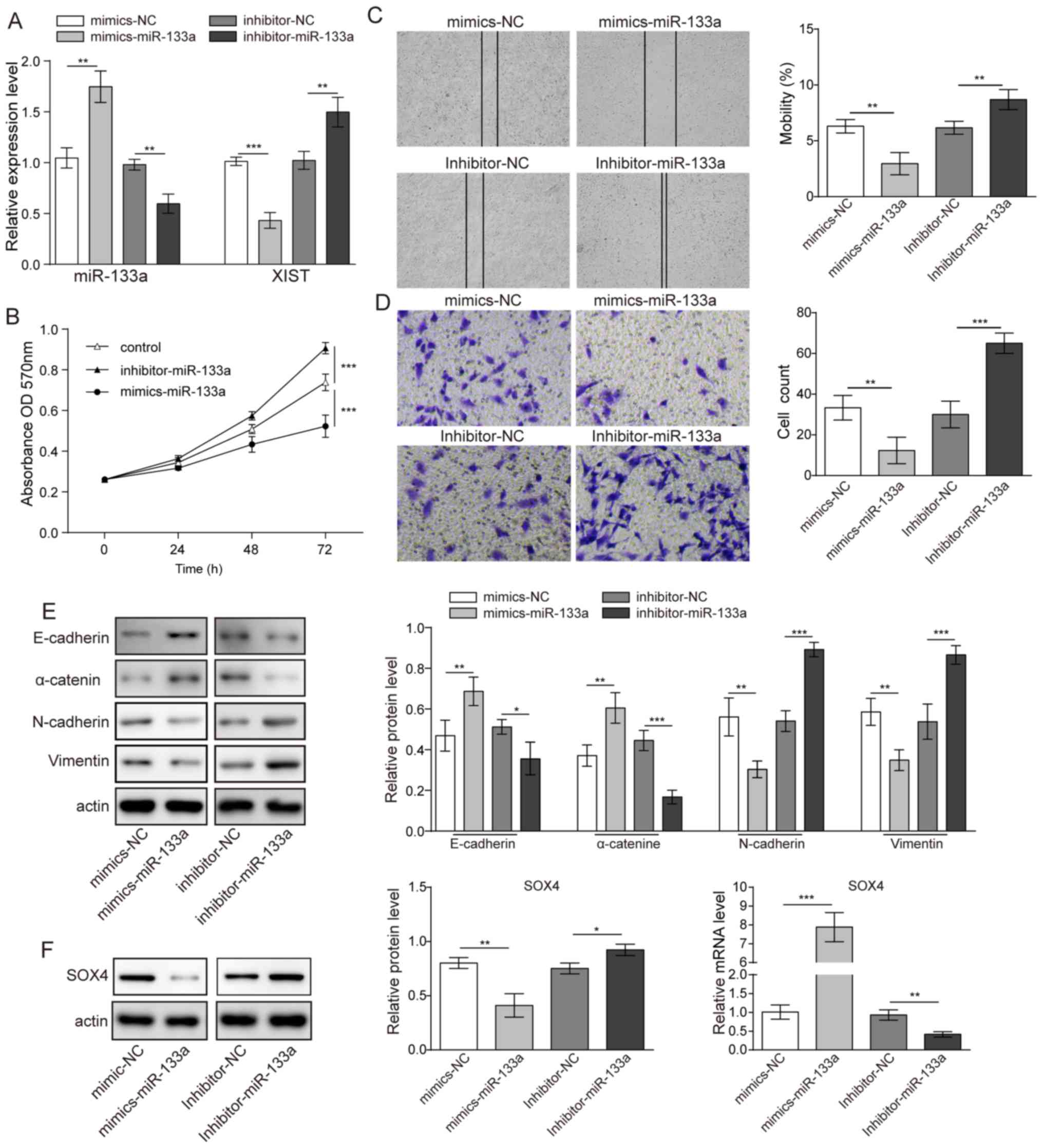 | Figure 3.Effects of miR-133a on glioma cell
proliferation and metastasis. (A) Relative expression levels of
miR-133a and XIST in glioma cells following transfection with
miR-133a mimics or inhibitor. (B) Cell viability following
transfection with miR-133a mimics or inhibitor assessed by MTT
assay. (C) Migration (magnification, ×16) and (D) invasion
(magnification, ×100) of cell lines detected by scratch-wound
healing and Transwell migration assays, respectively. (E) Western
blotting showing the expression of E-cadherin, N-cadherin,
α-catenin and vimentin in glioma cells following transfection with
miR-133a inhibitor or mimics. (F) Expression level of SOX4
transcription factor. n=3. *P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; XIST, X-inactive specific transcript;
SOX, SRY-box; NC, negative control; OD, optical density. |
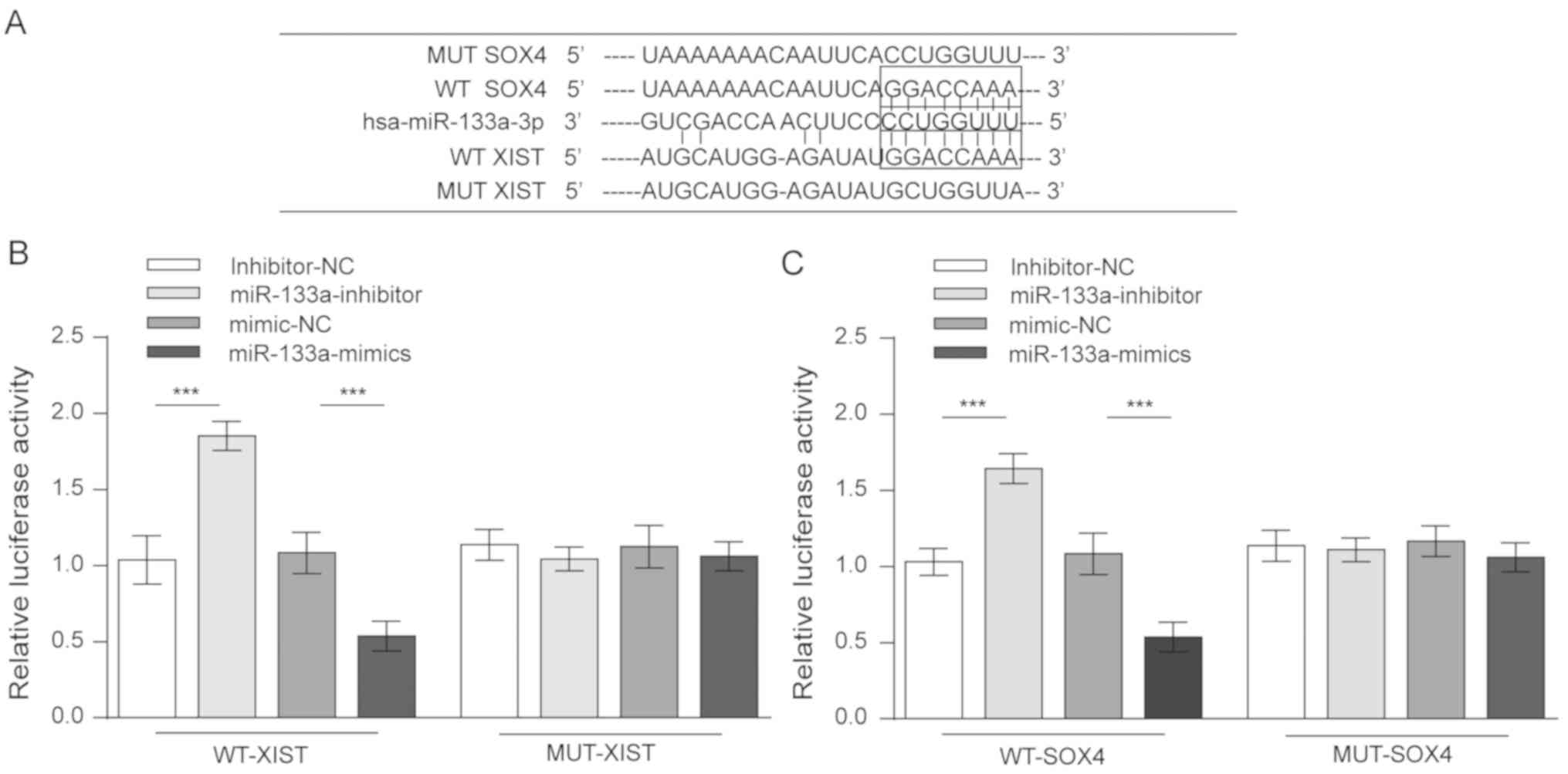
XIST competes with SOX4 for miR-133a
binding
The above results collectively revealed that XIST
and miR-133a served opposite roles in glioma cell proliferation,
migration and invasion, as well as in the expression of SOX4 and
EMT behaviour. A possible model was proposed in which XIST competed
with SOX4 for miR-133a binding. To validate this hypothesis, four
luciferase reporter gene vectors were constructed (wt-XIST
luciferase reporter, mut-XIST vector containing a 6-bp mutation
within the predicted miR-133a binding site within XIST, wt-SOX4
3′UTR, and mut-SOX4 3′UTR vector containing a 6-bp mutation within
the predicted miR-133a binding site in the 3′UTR of SOX4; Fig. 4A). These indicated vectors were
co-transfected into 293 cells along with the miR-133a mimics or the
miR-133a inhibitor, and the luciferase activity was then determined
by dual luciferase assays. The results demonstrated that the
luciferase activity of either wt-XIST- or wt-SOX4-transfected cells
was significantly suppressed by miR-133a mimics and was remarkably
amplified by a miR-133a inhibitor. However, when the miR-133a
binding site was mutated, such modulating effects on luciferase
activity were abolished (Fig. 4B and
C). These data strongly suggest that XIST competed with SOX4
for miR-133a binding.
Discussion
A previous study revealed that ~18% of lncRNAs are
associated with human tumours, and that only 9% of human
protein-coding genes perform this function (19). Thus, it is important to clarify the
role of lncRNAs in tumours.
The lncRNA XIST is upregulated in many cancers
(20), and a high expression level
of XIST is associated with poor clinical outcome (9). For example, the expression level of
lncRNA-XIST was significantly increased in both colorectal cancer
tissues samples and colorectal cancer cells and promoted colorectal
cancer cell proliferation by affecting the cell cycle (21); lncRNA-XIST was specifically
upregulated in lung cancer cell lines and promoted lung cancer cell
growth (22); lncRNA-XIST was
specifically upregulated in pancreatic cancer tissues and cell
lines, and high XIST expression in pancreatic cancer was associated
with poorer prognosis (9). In the
present study, it was demonstrated that XIST demonstrated high
expression in the glioma cell line U251. Furthermore, when XIST was
knocked down, the proliferation, migration and invasion of these
cells were reduced. These results revealed that XIST may serve as a
potential therapeutic target in glioma. For the mechanism through
which XIST affects glioma, these assays demonstrated that lnc-XIST
knockdown significantly upregulated E-cadherin and α-catenin,
whilst N-cadherin and vimentin were downregulated. More in
vitro and in vivo evidence is required to substantiate
the regulation of XIST on glioma metastasis.
The SOX4 transcription factor belongs to a large
family of proteins that serves a fundamental role during
embryogenesis and controls cell fate and differentiation (23). SOX4 expression is increased in a wide
variety of cancer types, including colorectal, breast and
glioblastoma, and correlates with poor prognosis and disease
progression (15,24). Several cancer-associated signalling
pathways have been implicated in the activation of SOX4, including
transforming growth factor-β, Wnt, and tumour necrosis factor-α
(25–27). SOX4 activation controls various
aspects of tumour development and progression, such as inhibition
of apoptosis, induction of cell migration and metastasis, and the
generation and maintenance of cancer stem cells (11,24). In
the present study, it was demonstrated that knockdown of XIST could
reduce the expression of SOX4, which revealed that XIST regulates
the expression of SOX4.
Recently, an increasing number of studies (8–10) have
focused on miRNA, as these small molecules affect many genetic
pathways, including cell cycle checkpoint, cell proliferation,
apoptosis, and altering the expression of miRNAs correlated with
cancers by acting as tumour suppressors and oncogenes (28–30).
Recently, miR-133a has been reported as a tumour suppressor in lung
and osteosarcoma cancers, as well as head and neck squamous cell
carcinomas, in which miR-133a reduces cell proliferation,
migration, and invasion (31–33).
However, previous study has explored the role of miR-133a in glioma
(34). In the present study, it was
demonstrated that overexpression of miR-133a promotes glioma
proliferation, migration and invasion but reduces the expression of
SOX4; in contrast, knockdown of miR-133a induces the opposite
effect, revealing that miR-133a negatively regulates SOX4.
lncRNAs are widely associated with regulating gene
expression networks at epigenetic, transcriptional and
post-transcriptional levels, which may affect the growth,
proliferation, differentiation, metabolism, apoptosis and other
important physiological processes of cells (35). Sometimes, lncRNAs have miRNA response
elements and act as natural miRNA sponges to reduce the binding of
endogenous miRNAs to target genes (36). Following the indication that XIST and
miR-133a had different effects on glioma cell proliferation,
migration and invasion, it was further investigated whether XIST
could mutually regulate miR-133a expression. In the present study,
dual negative regulation between XIST and miR-133a in glioma cell
lines was observed: Following XIST knock-down, miR-133a expression
increased in glioma cell lines; XIST expression could be
downregulated by miR-133a overexpression while being upregulated by
miR-133a inhibition. Similar mechanisms were implicated when XIST
was demonstrated to be regulated by miR-101, although further
studies such as a binding assay are required for substantiation of
this finding (37). In
post-transcriptional regulation, lncRNAs act as competing
endogenous RNAs to sponge miRNAs, consequently modulating the
repression of miRNA targets (38,39).
Using luciferase assays, it was demonstrated that XIST and SOX4
could bind miR-133a in the predicted binding site; thus XIST
competed with SOX4 for miR-133a binding.
The present study still has some limitations at its
current stage. For example, in vivo evidence was not sought
to demonstrate the modulation of tumour metastasis by XIST.
Furthermore, miR-133a mimic and inhibitor regulated XIST
expression, however, the detailed mechanism requires further assays
for elucidation. In conclusion, an XIST/miR-133a/SOX4 axis and a
potential mechanism of XIST in glioma cell proliferation and
metastasis were revealed. These findings suggest that XIST is
potentially a therapeutic target for glioma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
CL substantially contributed to the conception of
this study and drafting the manuscript. CT, ZQ and BaZ were
involved in the study conception and design. MZ performed the
literature research and experimental studies. BoZ and ZQ performed
data acquisition and edited the manuscript. SW and XL performed
data and statistical analysis. CT was involved in revising it
critically for important intellectual content. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prados MD and Levin V: Biology and
treatment of malignant glioma. Semin Oncol. 27 (Suppl 6):S1–S10.
2000.
|
|
3
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yarmishyn AA and Kurochkin IV: Long
noncoding RNAs: A potential novel class of cancer biomarkers. Front
Genet. 6:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang W, Yu H, Shen Y, Liu Y, Yang Z and
Sun T: MiR-146b-5p overexpression attenuates stemness and
radioresistance of glioma stem cells by targeting
HuR/lincRNA-p21/β-catenin pathway. Oncotarget. 7:41505–41526.
2016.PubMed/NCBI
|
|
6
|
Feng S, Yao J, Chen Y, Geng P, Zhang H, Ma
X, Zhao J and Yu X: Expression and functional role of
reprogramming-related long noncoding RNA (lincRNA-ROR) in glioma. J
Mol Neurosci. 56:623–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wutz A: Gene silencing in X-chromosome
inactivation: Advances in understanding facultative heterochromatin
formation. Nat Rev Genet. 12:542–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Yuan J, Li L, Yang Y, Xu X and
Wang Y: Long non-coding RNA XIST exerts oncogenic functions in
human glioma by targeting miR-137. Am J Transl Res. 9:1845–1855.
2017.PubMed/NCBI
|
|
9
|
Wei W, Liu Y, Lu Y, Yang B and Tang L:
LncRNA XIST promotes pancreatic cancer proliferation through
miR-133a/EGFR. J Cell Biochem. 118:3349–3358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Qin X, Li Y, Zhang X, Niu R, Zhang
H, Cui A, An W and Wang X: MiR-133a suppresses the migration and
invasion of esophageal cancer cells by targeting the EMT regulator
SOX4. Am J Transl Res. 7:1390–1403. 2015.PubMed/NCBI
|
|
11
|
Jafarnejad SM, Ardekani GS, Ghaffari M and
Li G: Pleiotropic function of SRY-related HMG box transcription
factor 4 in regulation of tumorigenesis. Cell Mol Life Sci.
70:2677–2696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schilham MW, Oosterwegel MA, Moerer P, Ya
J, de Boer PA, van de Wetering M, Verbeek S, Lamers WH, Kruisbeek
AM, Cumano A and Clevers H: Defects in cardiac outflow tract
formation and pro-B-lymphocyte expansion in mice lacking Sox-4.
Nature. 380:711–714. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ya J, Schilham MW, de Boer PA, Moorman AF,
Clevers H and Lamers WH: Sox4-deficiency syndrome in mice is an
animal model for common trunk. Circ Res. 83:986–994. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung M, Abu-Elmagd M, Clevers H and
Scotting PJ: Roles of Sox4 in central nervous system development.
Brain Res Mol Brain Res. 79:180–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Ju HL, Yuan XY, Wang TJ and Lai
BQ: SOX4 is a potential prognostic factor in human cancers: A
systematic review and meta-analysis. Clin Transl Oncol. 18:65–72.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi S, Cao X, Gu M, You B, Shan Y and You
Y: Upregulated expression of SOX4 is associated with tumor growth
and metastasis in nasopharyngeal carcinoma. Dis Markers.
2015:6581412015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foronda M, Martínez P, Schoeftner S,
Gómez-López G, Schneider R, Flores JM, Pisano DG and Blasco MA:
Sox4 links tumor suppression to accelerated aging in mice by
modulating stem cell activation. Cell Rep. 8:487–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khachane AN and Harrison PM: Mining
mammalian transcript data for functional long non-coding RNAs. PLoS
One. 5:e103162010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Cao X, Zhang L, Zhang X, Sheng H
and Tao K: UCA1 overexpression predicts clinical outcome of
patients with ovarian cancer receiving adjuvant chemotherapy.
Cancer Chemother Pharmacol. 77:629–634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song H, He P, Shao T, Li Y, Li J and Zhang
Y: Long non-coding RNA XIST functions as an oncogene in human
colorectal cancer by targeting miR-132-3p. J BUON. 22:696–703.
2017.PubMed/NCBI
|
|
22
|
Tang Y, He R, An J, Deng P, Huang L and
Yang W: lncRNA XIST interacts with miR-140 to modulate lung cancer
growth by targeting iASPP. Oncol Rep. 38:941–948. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai W, Xu X, Li S, Ma J, Shi Q, Guo S, Liu
L, Guo W, Xu P, He Y, et al: SOX4 promotes proliferative signals by
regulating glycolysis through AKT activation in melanoma cells. J
Invest Dermatol. 137:2407–2416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vervoort SJ, van Boxtel R and Coffer PJ:
The role of SRY-related HMG box transcription factor 4 (SOX4) in
tumorigenesis and metastasis: Friend or foe? Oncogene.
32:3397–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vervoort SJ, Lourenco AR, van Boxtel R and
Coffer PJ: SOX4 mediates TGF-β-induced expression of mesenchymal
markers during mammary cell epithelial to mesenchymal transition.
PLoS One. 8:e532382013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L,
Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional
regulation of vascular endothelial cell responses to hypoxia by
HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Banno T, Gazel A and Blumenberg M: Effects
of tumor necrosis factor-alpha (TNF alpha) in epidermal
keratinocytes revealed using global transcriptional profiling. J
Biol Chem. 279:32633–32642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mishra P and Chan DC: Metabolic regulation
of mitochondrial dynamics. J Cell Boil. 212:379–387. 2016.
View Article : Google Scholar
|
|
29
|
Mei Q, Li X, Guo M, Fu X and Han W: The
miRNA network: Micro-regulator of cell signalling in cancer. Expert
Rev Anticancer Ther. 14:1515–1527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nohata N, Hanazawa T, Kikkawa N, Mutallip
M, Fujimura L, Yoshino H, Kawakami K, Chiyomaru T, Enokida H,
Nakagawa M, et al: Caveolin-1 mediates tumor cell migration and
invasion and its regulation by miR-133a in head and neck squamous
cell carcinoma. Int J Oncol. 38:209–217. 2011.PubMed/NCBI
|
|
32
|
Xu M and Wang YZ: miR133a suppresses cell
proliferation, migration and invasion in human lung cancer by
targeting MMP14. Oncol Rep. 30:1398–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen G, Fang T, Huang Z, Qi Y, Du S, Di T,
Lei Z, Zhang X and Yan W: MicroRNA-133a inhibits osteosarcoma cells
proliferation and invasion via targeting IGF-1R. Cell Physiol
Biochem. 38:598–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakr M, Takino T, Sabit H, Nakada M, Li Z
and Sato H: miR-150-5p and miR-133a suppress glioma cell
proliferation and migration through targeting membrane-type-1
matrix metalloproteinase. Gene. 587:155–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye ZQ, Wang T and Song W: Long noncoding
RNAs in prostate cancer. Zhonghua Nan Ke Xue. 20:963–968. 2014.(In
Chinese). PubMed/NCBI
|
|
36
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen DL, Ju HQ, Lu YX, Chen LZ, Zeng ZL,
Zhang DS, Luo HY, Wang F, Qiu MZ, Wang DS, et al: Long non-coding
RNA XIST regulates gastric cancer progression by acting as a
molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin
Cancer Res. 35:1422016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sarver AL and Subramanian S: Competing
endogenous RNA database. Bioinformation. 8:731–733. 2012.
View Article : Google Scholar : PubMed/NCBI
|