Introduction
Acute graft vs. host disease (aGVHD) is the most
common complication after allogeneic hematopoietic stem cell
transplantation (allo-HSCT). It is reported that the incidence of
aGVHD has reached 32±3% in several transplantation centers
(1), and severe aGVHD is the most
prevalent cause of death after transplantation. The first choice of
treatment for aGVHD is steroids. When poor clinical outcome is
shown, a combination of steroids with immunosuppressive drugs is
recommended. Given that some aGVHD cases persist even after
treatment with this combination, novel approaches to overcome aGVHD
have been explored in recent years.
Mesenchymal stem cells (MSCs) are multipotent cells
that can self-renew and differentiate into various somatic
lineages. MSCs have also been reported to regulate immunological
reactions by secreting soluble cytokines and/or by direct contact
with lymphocytes. MSCs were first identified and isolated from bone
marrow (2), and were subsequently
confirmed to exist in a variety of tissues such as adipose, muscle,
tendon, umbilical cord blood and amniotic fluid. Le Blanc et
al (3) reported that infusion of
bone marrow-derived MSCs (BMSCs) systemically attenuated aGVHD in a
mouse model, indicating the therapeutic potential of MSCs in
amelioration of aGVHD.
Adipose-derived mesenchymal stem cells (ADSCs),
which were first identified by Zuk et al in 2001 (4), share similar biological characteristics
and immunological phenotype with BMSCs. It is believed that ADSCs
confer more advantages in terms of proliferation and cause reduced
damages than BMSCs (5). The present
study was designed to ascertain whether ADSCs alleviate the
incidence and severity of aGVHD in a rat model. Hemopoiesis after
treatment with ADSCs was also observed.
Materials and methods
Animals
Specific-pathogen-free Sprague-Dawley (SD) and
Wistar rats (n=10 for each type of rat) were provided by the Animal
Center, Xinjiang Medical University, China [license no. SCXK (Xin)
2003-001]. The study was approved by the Ethics Committee of The
First Affiliated Hospital of Xinjiang Medical University, China
(lot no. 20080701017). The rats were housed in a
specific-pathogen-free laboratory as approved by the US Association
for Assessment and Accreditation of Laboratory Animal Care (AAALAC;
http://www.aaalac.org). Donor rats were male SD
rats and recipient rats were female Wistar rats aged 6–8 weeks and
weighing 180–210 g. Before sacrifice, all rats were given acidified
water containing erythromycin (250 mg/l) and gentamicin (pH
3.0–3.5) for bowel cleansing. Animal experiments were conducted in
the Animal Experimental Center of Clinical Research Institute of
the First Affiliated Hospital of Xinjiang Medical University. All
animal experiments were performed following the US Guidelines for
the Use and Management of Laboratory Animals (6). After intraperitoneal anesthesia with
10% chloral hydrate at a dose of 300 mg/kg body weight, the animals
were sacrificed by neck dislocation after losing consciousness. No
rats developed peritonitis due to the use of chloral hydrate. Then,
tissues and blood samples were obtained.
Culture and identification of ADSCs,
BMSCs and fibroblasts
After intraperitoneal anesthesia with 10% chloral
hydrate at a dose of 300 mg/kg body weight, the rats were
sacrificed by neck dislocation. Then, the rats were soaked in 75%
ethanol for 15 min. Bilateral inguinal skin was cut, bilateral
inguinal fat, femur and tibia were isolated and obtained, and the
required cells were obtained according to the experimental method
described below.
Bilateral inguinal fat was aseptically obtained,
washed with phosphate-buffered saline (PBS, pH 7.4) and cut into
small pieces. Following digestion with 0.1% type I collagenase
(Worthington Biochemical Corp.) for 30 min, the samples were
centrifuged at 1,200 × g for 10 min and the supernatant was
discarded. Cells were resuspended in low-glucose Dulbecco's
modified Eagle's medium (DMEM) supplemented with 100 U/ml
penicillin, 100 mg/ml streptomycin and 10% fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc.), and plated at a density of
4×104 cells/cm2 in 100-mm culture dishes
(Falcon, USA).
BMSCs were harvested from bone marrow in the femur
and tibia by flushing with 5 ml low-glucose DMEM using a 21G
syringe. Cells were incubated at a density of 6–8×106/ml
for 48 h to allow adhesion. When reaching 70–80% confluency, the
cells were passaged and BMSCs before the 4th passage were used in
subsequent studies.
ADCSs and BMSCs at passage 3 were prepared into a
single-cell suspension after trypsinization with 0.25% trypsin.
After centrifugation at 1,000 × g for 10 min, the supernatant was
removed before washing with PBS twice. Cells (1×106)
were bound with monoclonal antibodies (100 µl system, the antibody
was 0.25 µg). The antibodies included: CD34-PE (cat. #119307;
Biolegend), HCAM-FITC (cat. #203906; Biolegend), CD106-PE (cat.
#200403; Biolegend), CD49-d-FITC (cat. #200103; Biolegend), and
CD29-PE (cat. #102207; Biolegend). At 4°C, the sample was incubated
for 30 min in the dark before flow cytometry using the CytoFLEX
V2-B4-R0 Flow Cytometer (C02944; Beckman Coulter). Then, EXPOTM32
MultiCOMP Software (Beckman Coulter, Inc.) was used for data
analysis. Adipogenic and osteogenic differentiation of cells was
identified by Oil red staining and Alizarin red staining,
respectively. Fibroblasts were obtained from rat dermis and
cultured according to previously described methods (7).
For Oil red staining, ADSCs and BMSCs in logarithmic
growth were mixed with low-glucose DMEM containing 10% FBS, 0.1
µmol/l dexamethasone, 200 µmol/l indometacin, and 0.5 mmol/l
3-isobutyl-1-methylxanthine. After culture for 8–10 days,
transparent lipid droplets appeared, and the cells were fixed with
4% paraformaldehyde before washing with PBS twice. Then, the cells
were stained with Oil red for 10 min before observation.
For Alizarin red staining, ADSCs and BMSCs in
logarithmic growth were mixed with low-glucose DMEM osteogenic
induction medium containing 10% FBS, 0.1 µmol/l dexamethasone, 50
µmol/l ascorbic acid, and 10 mmol/l sodium β-glycerophosphate.
After culture for 15 days, black sediments appeared, and the cells
were fixed with 4% paraformaldehyde before washing with PBS twice.
Then, the cells were stained with 1% Alizarin red (pH 4.2) for 3
min.
Coculture of ADSCs with hematopoietic
stem/progenitor cells
Bone marrow mononuclear cells (BMMNCs) were obtained
by density gradient centrifugation using rat lymphocyte separation
medium (1.083 g/cm3). After centrifugation, mononuclear
cells were resuspended and centrifuged at 290 × g for 5 min. Then,
the cells were collected and cocultured with ADSCs, BMSCs or
fibroblasts, respectively, which had been treated with mitomycin C
(0.5 µg/ml) for 24 h. The colony-forming ability of non-adherent
mononuclear cells cocultured with ADSCs, BMSCs or fibroblasts was
determined after 14 and 35 days using methylcellulose
colony-forming assay (Stem Cell Technologies, Canada). The number
and morphological characteristics of hematopoietic colony-forming
units (CFUs) were observed under an inverted microscope (×100
magnification; DMI4000B; Leica Microsystems). Each test was
performed 3 times.
Giemsa staining
The cells on the slides were fixed with methanol for
5 min, and Giemsa staining working solution (Sangon) was added onto
the slides before incubation at room temperature for 8 min. Then,
the slides were washed with distilled water and dried in the air
before observation under a microscope.
Infusion of ADSCs in the aGVHD rat
model
After sacrifice of the rats, splenic lymphocytes
were obtained by grinding the spleen into a single-cell suspension.
Then, inguinal adipose tissue was obtained for isolation and
culture of ADSCs. The femur of male rats was extracted, the
epiphysis was cut off, and the bone marrow cells of all the donors
were washed out with complete culture medium. Then, the single-cell
suspension of bone marrow cells was formed by passing the cells
through 200 meshes of metal mesh. Allogeneic hematopoietic stem
cells (HSCs) were obtained. According to previous reports (8,9),
infusion of allogeneic HSCs alone is not able to induce aGVHD.
Therefore, the present study established the model of aGVHD by
infusing a mixture of allogeneic HSCs and spleen lymphocytes.
Briefly, after receiving a total of 6 Gy body irradiation, the rats
were transplanted with a mixture of BMMNCs (2×108
cells/kg) and spleen cells (3×108/kg) (BM+spleen group)
by infusion via caudal vein of the tail.
To observe the suppressive effects of ADSCs on
aGVHD, ADSCs were infused at a dose of 1×107/kg, in
combination with either the mixture of BMMNCs and spleen
lymphocytes (ADSCs+BM+spleen group) or BMMNCs alone (BM group),
through the caudal vein of rats at 4–6 h after irradiation.
Occurrence of aGVHD was characterized by clinical manifestations
such as weight loss, abnormal hunched posture, decreased motion,
hair loss and skin ulcers (10).
Livers and small intestines were obtained from sacrificed rats for
pathological examinations. Life span exceeding 50 days was
considered a long-period of survival. Expression of the Sry
gene in recipient rats that survived longer than 21 days was
examined by real-time PCR to detect the presence of donor Y
chromosome. Briefly, the Blood & Cell Culture DNA Midi Kit
(cat. no. 13343; Qiagen) was used to extract DNA. Using the primers
5′-GAGGGTTATACTTTGCAGCGTGAA-3′ and 5′-CTGCTGTTTCTGCTGTAGTGGGT-3′,
the Sry gene on the Y chromosome of the rats was determined.
The reaction system (25 µl) was composed of 12.5 µl PCR mix, 0.5 µl
upstream primer, 0.5 µl downstream primer, 1 µl cDNA and 10.5 µl
ddH2O. PCR condition consisted of initial denaturation
at 95°C for 60 sec; 40 cycles of denaturation at 95°C for 15 sec,
annealing at 58°C for 15 sec and elongation at 72°C for 45 sec. The
reaction product underwent agarose gel electrophoresis.
Simultaneously, expression of serum interferon-γ (IFN-γ;
eBioscience; Thermo Fisher Scientific, Inc.) and interleukin-4
(IL-4; eBioscience; Thermo Fisher Scientific, Inc.) was detected by
ELISA at 0, 7, 14, 21 and 50 days after transplantation.
Statistical analysis
Data were analyzed using SPSS 13.0 software (IBM,
Corp.), and are expressed as means ± standard deviations. For
analysis of the survival rate, Kaplan-Meier method and the log-rank
method were used. For analysis of IL-4/INF-γ levels and cell
colonies, Student's t-test was used for analysis of the data. For
analysis of body weight and white blood cell count, one-way ANOVA
followed by Newman-Keuls test was utilized. A value of P<0.05
was considered statistically significant.
Results
Transplantation of ADSCs improves the
survival of aGVHD rats
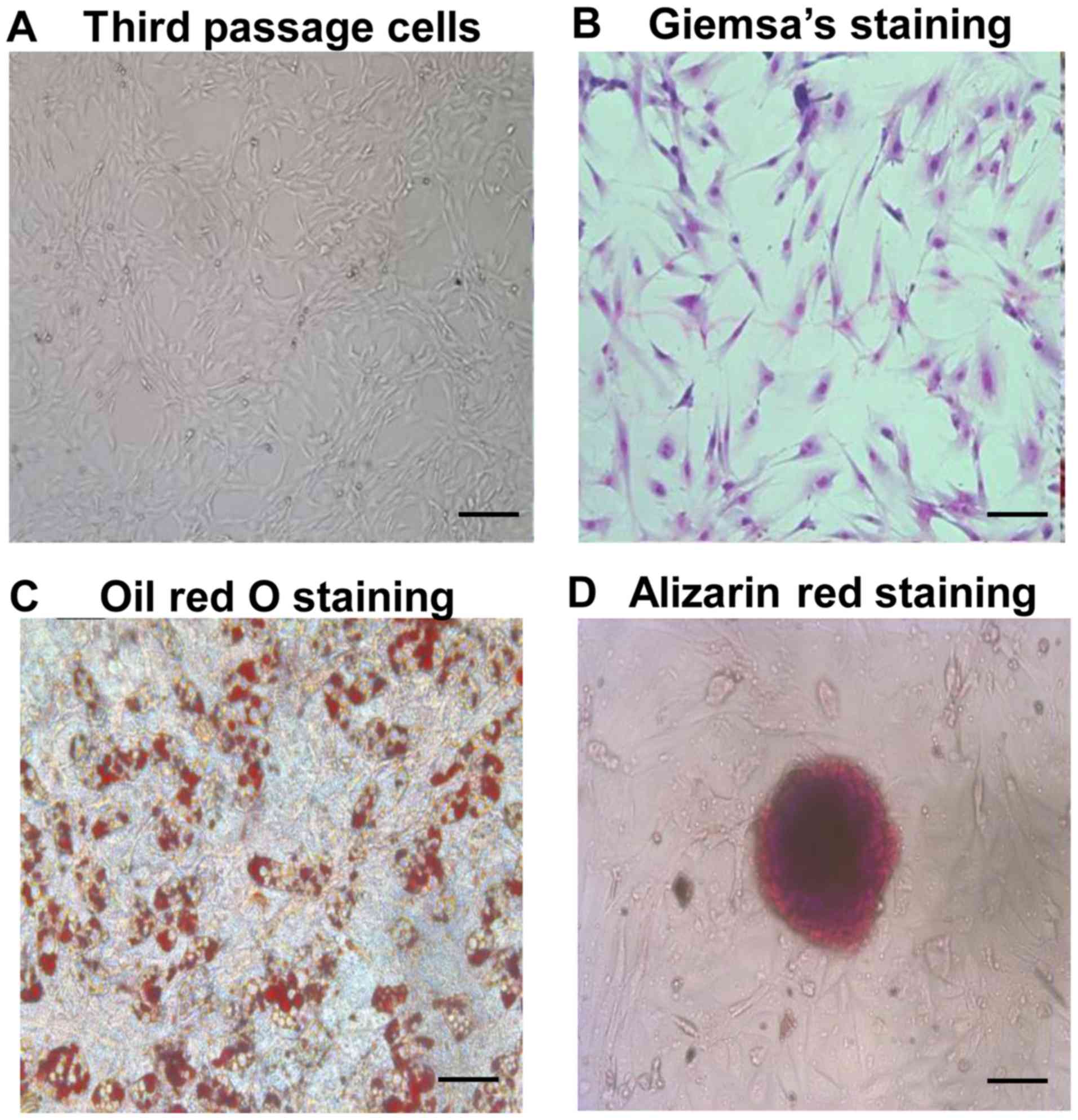
As shown in Fig. 1A and
B, ADSCs at passage 3 exhibited a typical spindle
fibroblast-like shape. Differentiation potential of ADSCs into
adipogenic and osteogenic lineages was determined by Oil red O and
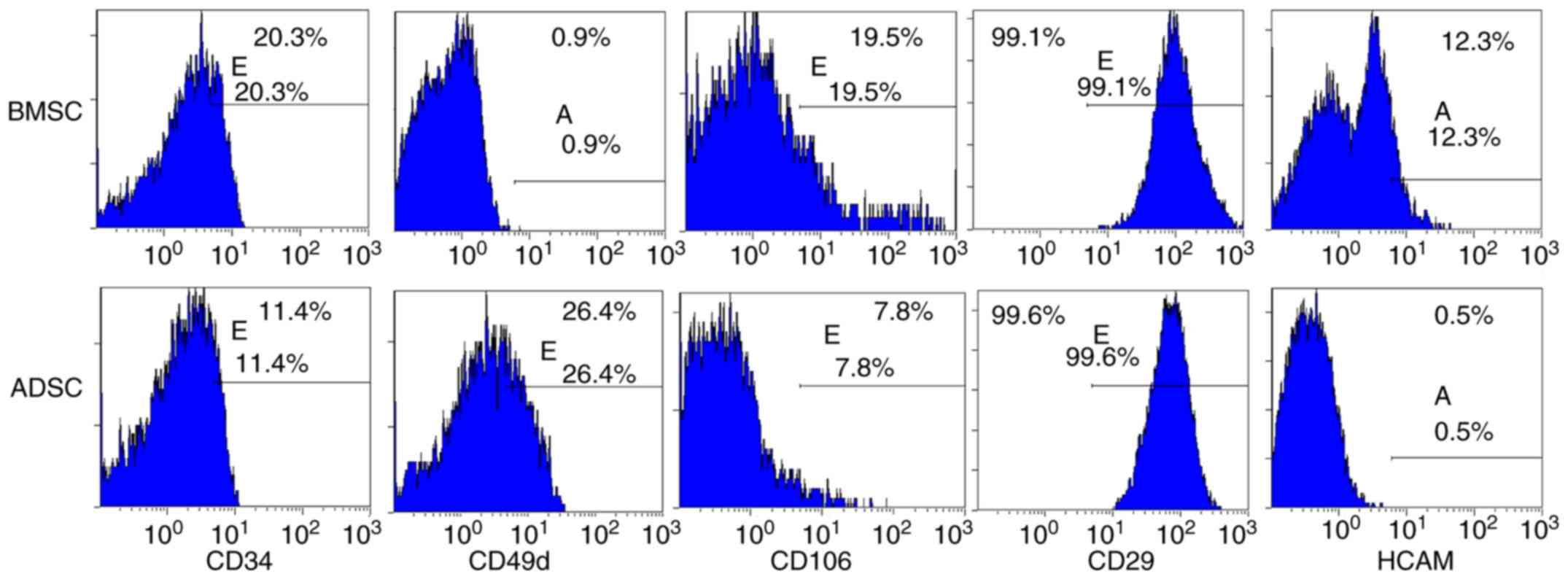
Alizarin red staining, respectively (Fig. 1C and D). Flow cytometry revealed
characteristic expression of CD markers, including CD34, CD49d,
CD29, CD44 (or HCAM) and CD106 (Fig.
2). The median survival time of rats that received a combined
infusion of BMMNCs and spleen lymphocytes (BM+spleen group) (14
days) was the same with that of rats that received BMMNCs alone (BM
group) (14 days). After transplanting ADSCs into the aGVHD model,
the median survival time of rats (ADSCs+BM+spleen group) was
significantly extended to 33.5 days, indicating that ADSCs improved
survival of the aGVHD rats (Fig.
3A). After transplanting BMMNCs and spleen lymphocytes, the
rats showed typical aGVHD manifestations, including hunched arched
posture, hair loss and decreased movement range of motion after 14
days. Body weight changes of rats from each group at different time
points after transplantation showed that ADSCs significantly
reduced the weight loss of the recipient rats on day 14 after
transplantation, and no difference between the BM+spleen group and
BM group was observed (Fig. 3B).
Moreover, pathological changes in liver and intestinal track such
as sinusoidal dilation and congestion, periportal inflammation,
intestinal epithelial necrosis and submucosal congestion and edema
in the aGVHD rats were markedly ameliorated after receiving ADSC
transplantation (Fig. 3C). The
results suggest that transplantation of ADSCs improves the survival
of aGVHD rats.
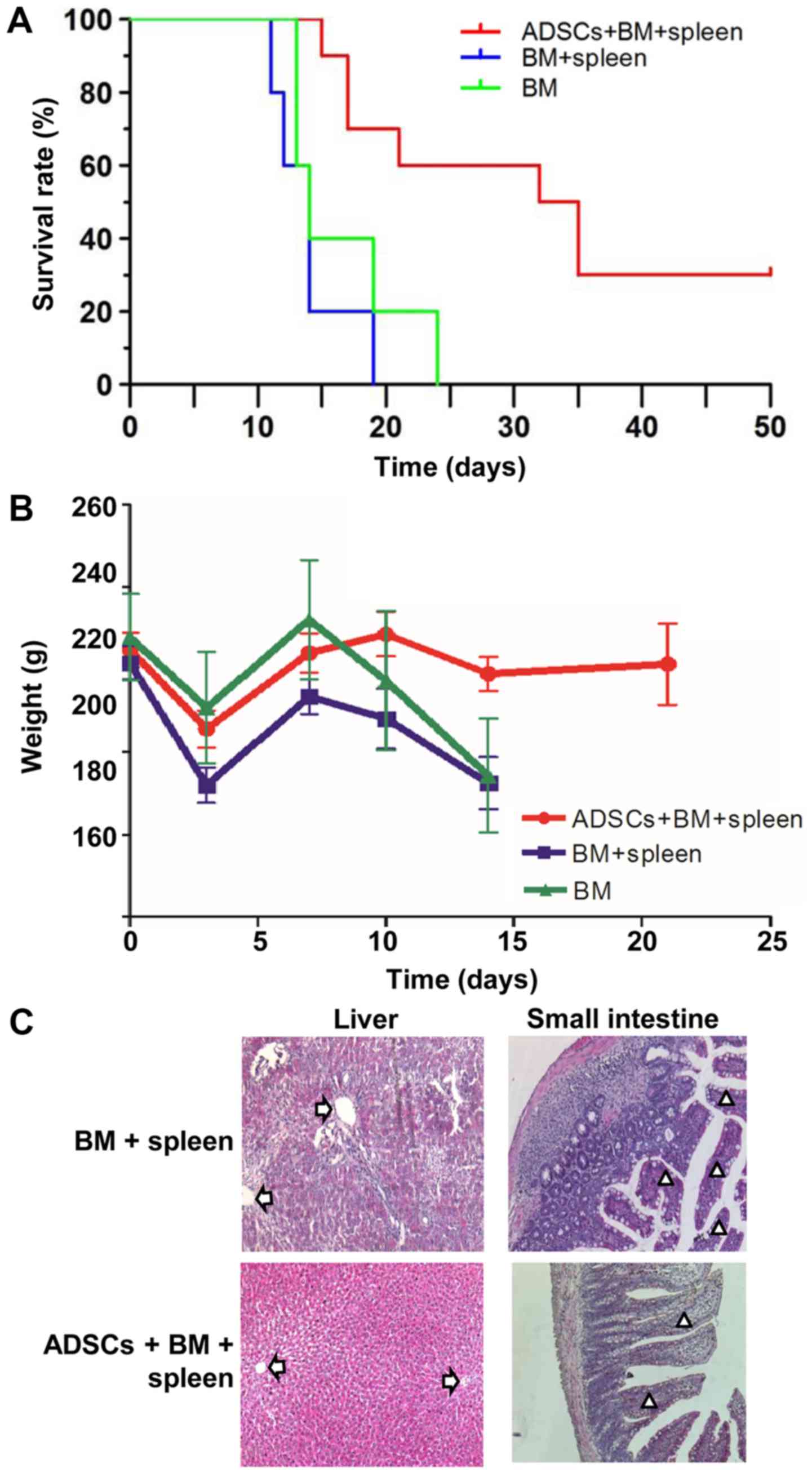 | Figure 3.ADSCs improve the survival of aGVHD
rats. (A) Overall survival rates of aGVHD rats in the BM group,
BM+spleen group and ADSCs+BM+spleen group. n=10. (B) Weight of the
aGVHD model rats in the BM group, BM+spleen group and
ADSCs+BM+spleen group. n=10. (C) Morphology of liver and small
intestinal tissues of the aGVHD model rats in the BM group,
BM+spleen group and ADSCs+BM+spleen group. Magnification, ×100.
Arrows indicate portal vein of the liver, and triangles indicate
small intestinal villi. ADSCs, adipose-derived stem cells; aGVHD,
acute graft vs. host disease; BMMNCs, bone marrow mononuclear
cells. Groups: BM, 2×108/kg bone marrow cells were
infused; BM+spleen, 2×108/kg bone marrow cells +
3×108/kg spleen cells were infused; ADSCs+BM+spleen,
2×108/kg bone marrow cells + 3×108/kg spleen
cells + 1×107/kg ADSCs were infused. |
Infusion of ADSCs promotes
hematopoietic reconstitution in the aGVHD rats
The number of leukocytes in the aGVHD models was
observed to decrease from 9×109 to 1×109 g/l
on day 3 after irradiation. On day 10 after transplantation, the
number of leukocytes in rats that received BMMNCs and a mixture of
BMMNCs and lymphocytes decreased to 2.7±0.65 and 1.03±0.51,
respectively, being significantly lower than that in the
ADSCs+BM+spleen group (7.36±3.47) (P<0.05) (Fig. 4A). To further trace the surviving
ADSCs in the recipient rats, the expression of the Sry gene
in the bone marrow was determined using RT-PCR. Expression of
Sry was detected even on day 14 after transplantation
(Fig. 4B). The results indicate that
the surviving ADSCs participated in the hematopoietic
reconstitution in the aGVHD rats.
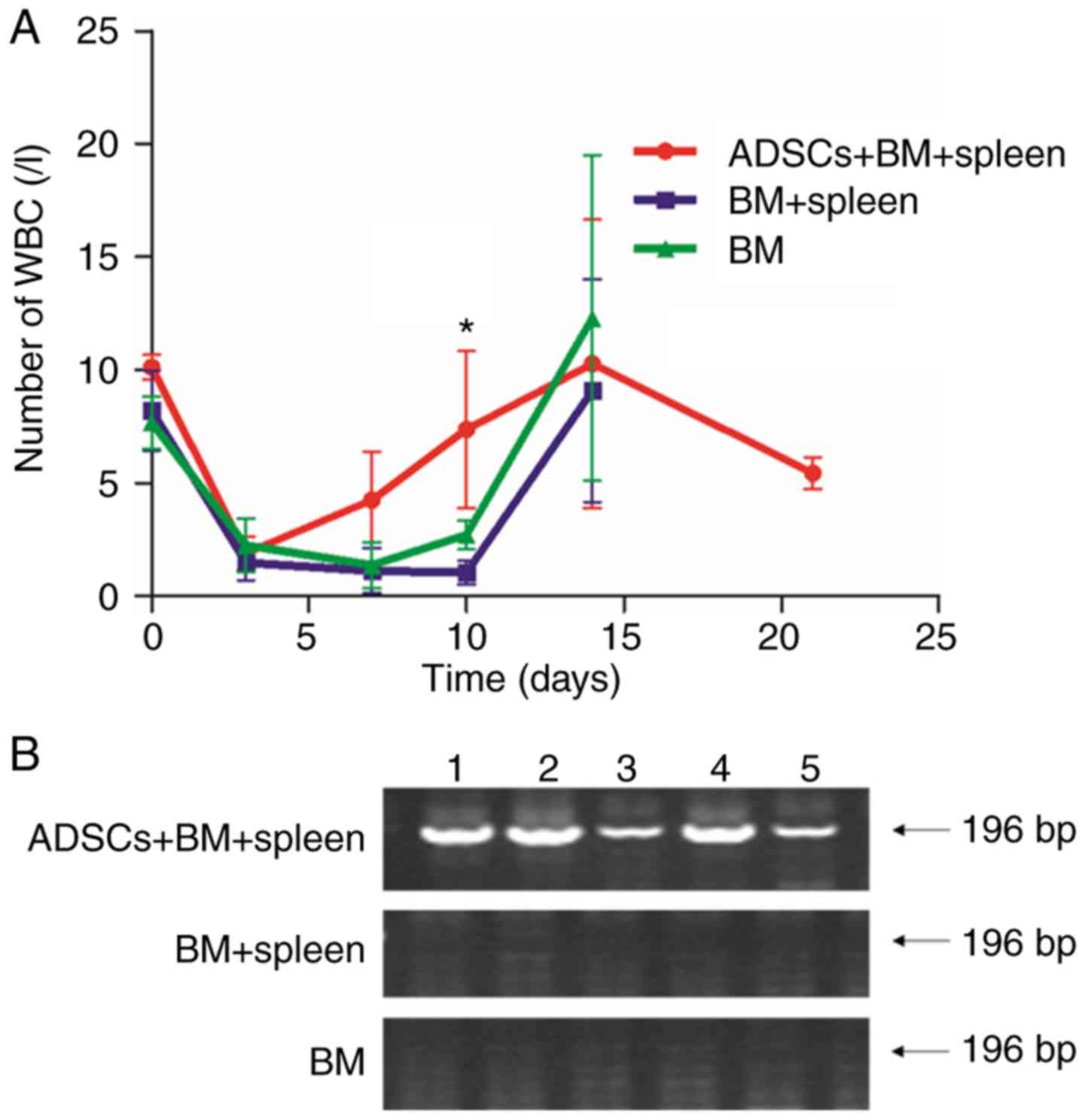 | Figure 4.ADSCs promotes hematopoietic
reconstitution. (A) Leukocyte count of aGVHD model rats in the BM
group, BM+spleen group and ADSCs+BM+spleen group. n=10. *P<0.05.
(B) Images of agarose gel electrophoresis of the Sry gene of
male Wistar rat as detected by PCR. The numbers 1, 2, 3, 4, and 5
represent the five rats. The expression of the Sry gene was
detected in the ADSC group, suggesting that rats in the
ADSCs+BM+spleen group reached complete donor engraftment. ADSCs,
adipose-derived stem cells; aGVHD, acute graft vs. host disease.
Groups: BM, 2×108/kg bone marrow cells were infused;
BM+spleen, 2×108/kg bone marrow cells +
3×108/kg spleen cells were infused; ADSCs+BM+spleen,
2×108/kg bone marrow cells + 3×108/kg spleen
cells + 1×107/kg ADSCs were infused. |
ADSCs decrease aGVHD severity by
immunomodulation
An imbalance in Th1 and Th2 cytokines is suggested
to play an important role in the pathogenesis of aGVHD (11). IL-4 and INF-γ can influence the
balance of Th1 and Th2. As determined by ELISA analysis, serum
concentration of IL-4 in rats that received BMMNCs and spleen
lymphocytes (BM+spleen) was 1.19±0.06 µg/ml on day 7.
Administration of ADSCs significantly reduced IL-4 concentration to
0.75±0.3 µg/ml (P<0.05) (Fig.
5A), while the concentration of IFN-γ showed no significant
change when ADSCs were transplanted on day 7 (Fig. 5B). However, on day 14 after infusion,
transplantation of ADSCs resulted in significant increase in IL-4
concentration and a decrease in IFN-γ concentration (P<0.05)
(Fig. 5C and D). The results suggest
that ADSCs decrease aGVHD severity by immunomodulation.
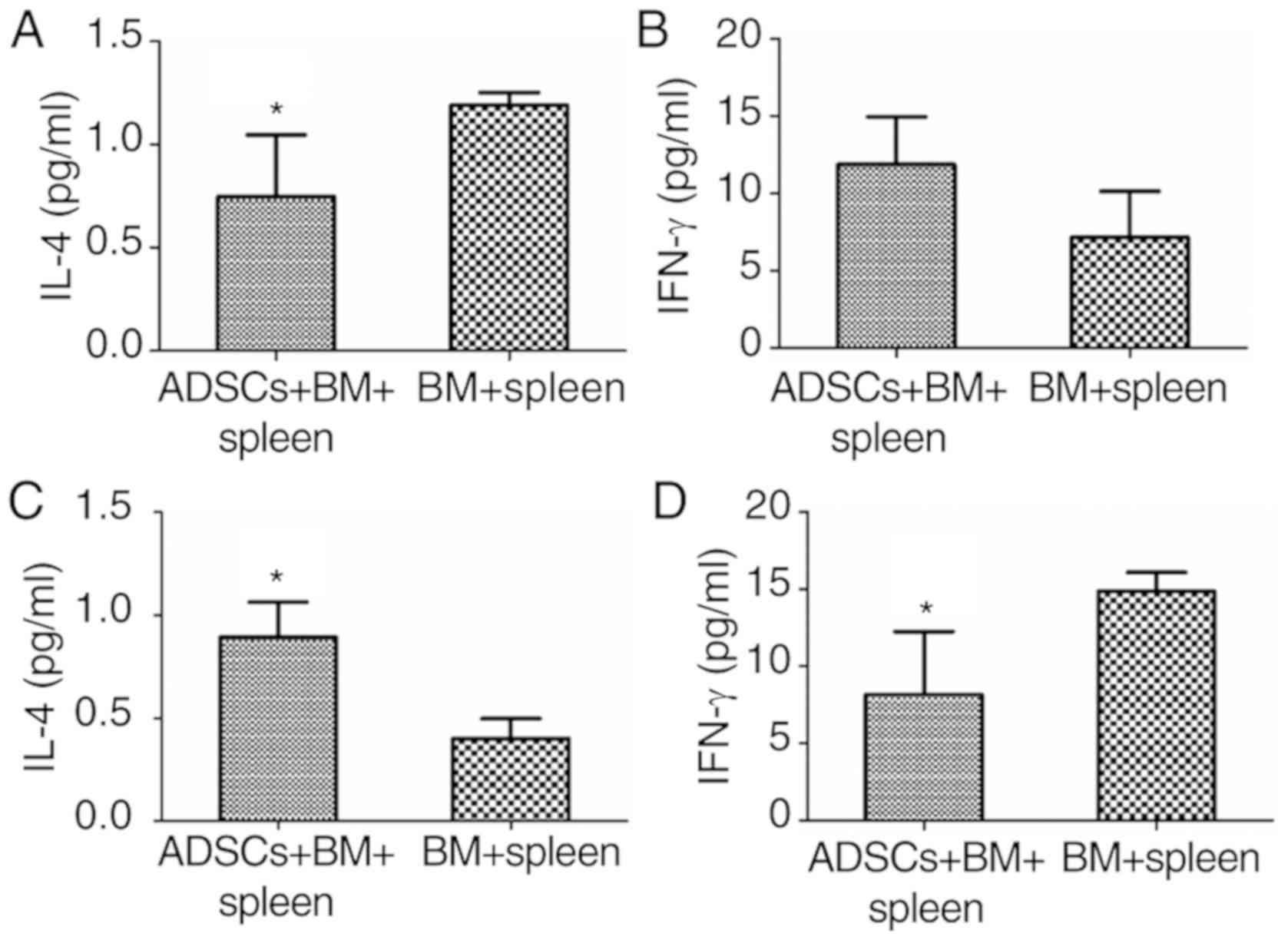 | Figure 5.Immune-related factor expression
during the restoration of aGVHD in rats. (A) IL-4 levels on day 7
(n=4, *P<0.05); (B) IFN-γ levels on day 7 (n=4, *P>0.05); (C)
IL-4 levels on day 14 (n=3, *P<0.05) and (D) IFN-γ levels on day
14 (n=3, *P<0.05). ADSCs, adipose-derived stem cells; aGVHD,
acute graft vs. host disease; IL-4, interleukin 4; INF-γ,
interferon-γ. Groups: BM, 2×108/kg bone marrow cells
were infused; BM+spleen, 2×108/kg bone marrow cells +
3×108/kg spleen cells were infused; ADSCs+BM+spleen,
2×108/kg bone marrow cells + 3×108/kg spleen
cells + 1×107/kg ADSCs were infused. |
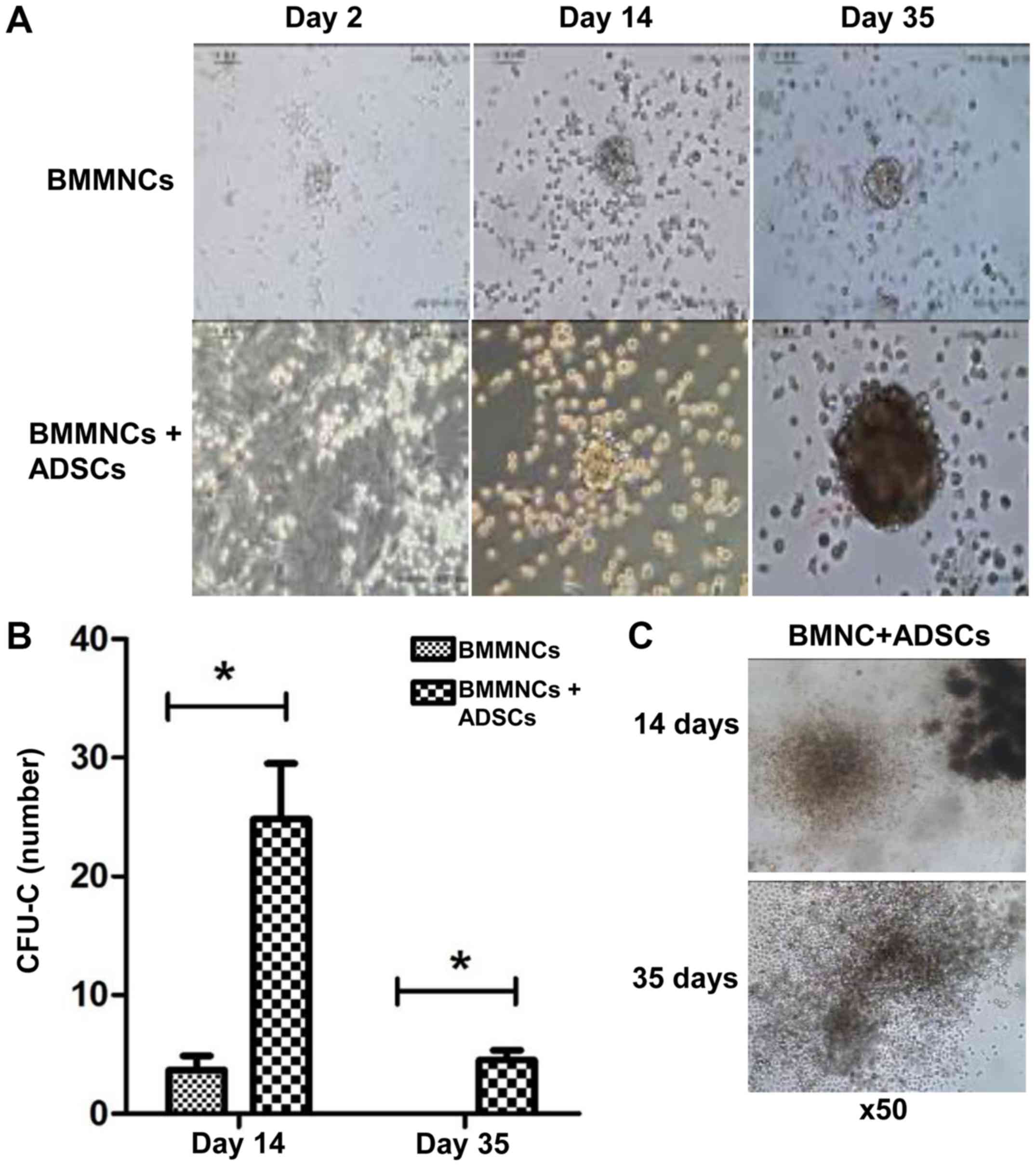
ADSCs support the proliferation of
hematopoietic stem/progenitor cells in vitro
Within the duration of coculture, CFUs of
hematopoietic stem/progenitor cells cultured with ADSCs were
readily identified, with their sizes gradually growing larger. By
contrast, few CFUs were observed when BMMNCs were cultured alone.
On day 14 and 35, the number of CFUs of cells supported by ADSCs
were significantly higher than that of cells cultured alone,
respectively (P<0.05) (Fig. 6A and
B). ADSCs were still able to promote the proliferation of
BMMNCs on day 35 (Fig. 6C). The
results indicate that ADSCs support the proliferation of
hematopoietic stem/progenitor cells in vitro.
Discussion
Research concerning the mechanism of mesenchymal
stem cells (MSCs) on hematopoietic support and immune regulation
has mainly focused on bone marrow-derived mesenchymal stem cells
(BMSCs) and umbilical cord-derived mesenchymal stem cells (UMSCs).
Few studies have been conducted on the mechanism of adipose-derived
stem cell (ADSC) immune regulation. Moreover, some studies have
reported that BMSCs, ADSCs and UMSCs may have various differences
in regards to the mechanisms involved in tissue repair, immune
regulation and support of hematopoiesis (12–14). It
has been documented that acute graft vs. host disease (aGVHD), the
main cause of death after transplantation, can be relieved by
infusing BMSCs (15). Te Boome et
al (16) reported that an
increase in circulating immature myeloid dendritic cells is
associated with decreased mortality in patients with aGVHD treated
with BMSCs in vivo. An increase in serum level of IL-2 and a
lower IFN-γ to IL-4 ratio in BMSC-treated patients were observed by
Jitschin et al (17).
However, BMSCs have failed to significantly increase durable
complete remission rates in steroid-refractory aGVHD patients in a
phase III, multicenter, randomized controlled trial. Several
factors, including MSC sources and manufacturing process, may
account for the conflicting results (18). Compared to MSCs from bone marrow,
ADSCs are more accessible, with a higher yield at harvest and rapid
expansion in long-term expansion, being attractive in regenerative
medicine (19–21). The immunological regulatory
properties of ADSCs have been well documented. Previous studies
(22,23) demonstrated that ADSCs inhibited the
proliferation of T lymphocytes in a mixed lymphocyte reaction via
the production of PGE2. Yañez et al (24) demonstrated the potential effect of
ADSCs in alleviating aGVHD by the regulation of the levels of
IFN-γ, IL-12 and TNF-α in vitro. However, whether
transplantation of ADSCs reduces aGVHD in vivo remains
unclear. In the present study, it was demonstrated the prophylactic
effect of ADSCs on aGVHD with improved long-term hematopoiesis by
modulating the balance of IL-4 and INF-γ in a rat model.
The experiments in the present study revealed that
severe clinical manifestations of aGVHD are observed on day 14
after infusion of BM+spleen cells from male SD rats into
lethally irradiated female Wistar rats. With the mixture of
ADSCs to BM+spleen cells, it was discovered that aGVHD was
significantly alleviated, and the long-term survival rate on day 35
was apparently improved after allogeneic hematopoietic stem cell
transplantation. It has been well documented that disturbance in
the imbalance between Th1 and Th2 cytokines plays an important role
in the pathogenesis of aGVHD (25–27).
Furthermore, the present study showed that the serum level of Th1
cytokine IFN-γ was not significantly different, while the serum
level of Th2 cytokine IL-4 was lower at the early stage (day 7).
However, IFN-γ was decreased and IL-4 was increased significantly
at the late stage (day 14) after transplantation in the ADSC group
compared with the control group. As the current rat model exhibited
aGVHD on day 14, it was speculated that ADSCs participate in the
immune regulation and alleviate aGVHD symptoms after
transplantation.
The most innovative finding of the present study was
the long-term support of ADSCs on hematopoiesis in vivo and
in vitro. In particular, in vitro hematopoietic
support for 7 weeks was significantly better than that of BMSCs,
which has not been previously reported. In the present study, it
was demonstrated that the leukocyte number was reconstituted
faster, and Sry gene expression was only detected in the
ADSC group on day 21, suggesting that ADSCs strongly promote the
implantation of the donor hematopoietic stem cells in recipients
in vivo. de Barros et al (28) plated CD34+ cells onto
spheroid BMSCs and co-cultured them for up to 7 days, and found
that BMSCs self-assembled in a 3-dimensional spheroid and formed a
microenvironment that was informative for hematopoietic progenitor
cells. Arthur et al (29)
reported that Twist-1-overexpressing BMSCs exhibited an enhanced
capacity in maintaining human CD34+ hematopoietic stem
cells (HSCs) in long-term culture-initiating cell (LTC-IC) assays.
It was also confirmed that MSCs support the proliferation of
hematopoietic stem/progenitor cells in vitro by coculture of
human umbilical cord blood mononuclear cells and umbilical cord
mesenchymal stem cells for 14 days (30). Few studies have demonstrated that
ADSCs support the long-term proliferation of hematopoietic
stem/progenitor cells. A previous study (31) confirmed that ADSCs isolated from
inguen differentiate into osteoblast cells and adipose cells in
vitro. The present study found that ADSCs had similar effects
to BMSCs in the support of survival and proliferation of
hematopoietic stem/progenitor cells in short-term coculture (<14
days) in vitro. Moreover, the present study also showed that
ADSCs support the survival and proliferation of hematopoietic
stem/progenitor cells in long-term coculture (35 days), and the
partial hematopoietic CFU diameter in the ADSC group was greater
than that in the BMSC group, although the CFU counts were similar
in both groups. Therefore, the present study demonstrated that
ADSCs may also have long-term supportive function on hematopoiesis
in vitro. In conclusion, the present study demonstrated
preliminarily that rat ADSCs regulated immune responses and reduced
the occurrence of aGVHD in a rat allo-HSCT model, mainly through
Th1/Th2 cytokine immune adjustment mode. The present study verifies
that rat ADSCs can promote the long-term proliferation of
hematopoietic stem cells in vitro and prolong survival after
bone marrow transplantation in vivo. Further in-depth
research concerning these features of ADSCs will be carried out in
the future.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (no. 81460023) and Natural Science
Foundation of Xinjiang Uygur Autonomous Region (no. 200821113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MJ and LC designed and directed the experiment. XB,
HW and RZ performed the experiments. XD, NP and HY collected the
data and performed the statistical analysis. XB wrote the
manuscript. LC and XD reviewed and edited the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval for the study was granted from the
Ethics Committee of the First Affiliated Hospital of Xinjiang
Medical University (Urumqi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADSCs
|
adipose-derived stem cells
|
|
aGVHD
|
acute graft vs. host disease
|
|
BMSCs
|
bone marrow-derived stem cells
|
|
MSCs
|
mesenchymal stem cells
|
|
SD
|
Sprague-Dawley
|
|
BMMNCs
|
bone marrow mononuclear cells
|
|
HSCs
|
hematopoietic stem cells
|
|
IL-4
|
interleukin 4
|
|
INF-γ
|
interferon-γ
|
References
|
1
|
Piemontese S, Ciceri F, Labopin M,
Bacigalupo A, Huang H, Santarone S, Gorin NC, Koc Y, Wu D, Beelen
D, et al: A survey on unmanipulated haploidentical hematopoietic
stem cell transplantation in adults with acute leukemia. Leukemia.
29:1069–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray IR, West CC, Hardy WR, James AW,
Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C and Péault B:
Natural history of mesenchymal stem cells, from vessel walls to
culture vessels. Cell Mol Life Sci. 71:1353–1374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mirlashari MJ, Josefsen D, Landsverk K,
Hasvold G, Gullestad H and Kvalheim G: Culturing of human adipose
derived mesenchymal stem cells (ADSCS) under hypoxic conditions
affects production of wound healing cytokines and growth factors.
Cytotherapy. 16:S932014. View Article : Google Scholar
|
|
6
|
Institute of Laboratory Animal Resources
(US). Committee on Care and Use of Laboratory Animals. Guide for
the care and use of laboratory animals. US Department of Health and
Human Services, Public Health Service, National Institutes of
Health. 1986.
|
|
7
|
Frazier K, Williams S, Kothapalli D,
Klapper H and Grotendorst GR: Stimulation of fibroblast cell
growth, matrix production, and granulation tissue formation by
connective tissue growth factor. J Invest Dermatol. 107:404–411.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu J, Yan M, Jiang M, Shi W, Xu Y, Zhang D
and Li J: Establishment of a rat model of acute graft-versus-host
disease after allogeneic bone marrow transplantation. Chin J Com
Med. 17:581–584. 2007.
|
|
9
|
Vogelsang GB, Hess AD, Gordon G and Santos
GW: Treatment and prevention of acute graft-versus-host disease
with thalidomide in a rat model. Transplantation. 41:644–647. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renkonen R and Häyry P: Bone marrow
transplantation in the rat. I. Histologic correlations and
quantitation of cellular infiltrates in acute graft-versus-host
disease. Am J Pathol. 117:462–470. 1984.PubMed/NCBI
|
|
11
|
Ju XP, Xu B, Xiao ZP, Li JY, Chen L, Lu SQ
and Huang ZX: Cytokine expression during acute graft-versus-host
disease after allogeneic peripheral stem cell transplantation. Bone
Marrow Transplant. 35:1179–1186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong Y, Zhu T, Xia R, Li Y, Ge X, Zeng Q,
Ni J and Li Q: Mechanism of bone marrow mesenchymal stem cells for
treating model mice with aplastic anemia. J Clin Rehabil Tissue Eng
Res. 13:7138–7142. 2009.
|
|
13
|
Wu KH, Tsai C, Wu HP, Sieber M, Peng CT
and Chao YH: Human application of ex vivo expanded umbilical
cord-derived mesenchymal stem cells: Enhance hematopoiesis after
cord blood transplantation. Cell Transplant. 22:2041–2051. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Te Boome LC, Mansilla C, van der Wagen LE,
Lindemans CA, Petersen EJ, Spierings E, Thus KA, Westinga K,
Plantinga M, Bierings M, et al: Biomarker profiling of
steroid-resistant acute GVHD in patients after infusion of
mesenchymal stromal cells. Leukemia. 29:1839–1846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jitschin R, Mougiakakos D, Von Bahr L,
Völkl S, Moll G, Ringden O, Kiessling R, Linder S and Le Blanc K:
Alterations in the cellular immune compartment of patients treated
with third-party mesenchymal stromal cells following allogeneic
hematopoietic stem cell transplantation. Stem Cells. 31:1715–1725.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galipeau J: The mesenchymal stromal cells
dilemma-does a negative phase III trial of random donor mesenchymal
stromal cells in steroid-resistant graft-versus-host disease
represent a death knell or a bump in the road? Cytotherapy. 15:2–8.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bora P and Majumdar AS: Adipose
tissue-derived stromal vascular fraction in regenerative medicine:
A brief review on biology and translation. Stem Cell Res Ther.
8:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Zhu Y, Li Y, Cao J, Zhang H, Chen
M, Wang L and Zhang C: Long-term engraftment of myogenic
progenitors from adipose-derived stem cells and muscle regeneration
in dystrophic mice. Hum Mol Genet. 24:6029–6040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai W, Jiang M, Cui L and Yi S: An
experimental study for the immunological properties of human
adipose-derived stem cells after expansion. J Tissue Eng Reconstr
Sur. 4:312–314. 2008.
|
|
23
|
Cui L, Yin S, Liu W, Li N, Zhang W and Cao
Y: Expanded adipose-derived stem cells suppress mixed lymphocyte
reaction by secretion of prostaglandin E2. Tissue Eng.
13:1185–1195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yañez R, Lamana ML, García-Castro J,
Colmenero I, Ramírez M and Bueren JA: Adipose tissue-derived
mesenchymal stem cells have in vivo immunosuppressive properties
applicable for the control of the graft-versus-host disease. Stem
Cells. 24:2582–2591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao Y, Song X, Cheng H, Tang G, Hu X, Zhou
H and Wang J: Dysfunction of bone marrow vascular niche in acute
graft-versus-host disease after MHC-haploidentical bone marrow
transplantation. PLoS One. 9:e1046072014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nasef A, Chapel A, Mazurier C, Bouchet S,
Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D and
Fouillard L: Identification of IL-10 and TGF-beta transcripts
involved in the inhibition of T-lymphocyte proliferation during
cell contact with human mesenchymal stem cells. Gene Expr.
13:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Najar M, Rouas R, Raicevic G, Boufker HI,
Lewalle P, Meuleman N, Bron D, Toungouz M, Martiat P and Lagneaux
L: Mesenchymal stromal cells promote or suppress the proliferation
of T lymphocytes from cord blood and peripheral blood: The
importance of low cell ratio and role of interleukin-6.
Cytotherapy. 11:570–583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Barros AP, Takiya CM, Garzoni LR,
Leal-Ferreira ML, Dutra HS, Chiarini LB, Meirelles MN, Borojevic R
and Rossi MI: Osteoblasts and bone marrow mesenchymal stromal cells
control hematopoietic stem cell migration and proliferation in 3D
in vitro model. PLoS One. 5:e90932010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arthur A, Cakouros D, Cooper L, Nguyen T,
Isenmann S, Zannettino AC, Glackin CA and Gronthos S: Twist-1
enhances bone marrow mesenchymal stromal cell support of
hematopoiesis by modulating CXCL12 expression. Stem Cells.
34:504–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun HP, Zhang X, Chen XH, Zhang C and Gao
L, Feng YM, Peng XG and Gao L: Human umbilical cord blood-derived
stromal cells are superior to human umbilical cord blood-derived
mesenchymal stem cells in inducing myeloid lineage differentiation
in vitro. Stem Cells Dev. 21:1429–1440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang R, Bi X, Ma Y, Duan X and Jiang M:
Biological characterization of C57 mouse bone marrow mesenchymal
stem cells using a whole bone marrow adherent culture technique.
Chin J Tissue Eng Res. 18:45–50. 2014.
|