Introduction
Vitiligo is a depigmentation disease of the skin and
mucous membranes caused by the selective destruction or malfunction
of melanocytes (1). Genetic and
environmental factors have been implicated in vitiligo onset
(2). The pathogenesis of vitiligo is
controversial; however, the main hypotheses indicate that
depigmentation occurs due to destruction of the melanocytes
mediated by autoimmunity (3), as
well as intrinsic anomalies of melanocytes (4). Currently, evidence suggests that both
hypotheses are linked through mechanisms of innate immunity
(5).
The melanocortin system is a regulatory module that
acts by coordinating and executing the local response to stress. It
consists of three melanocortin peptides: Alpha, beta and gamma
melanocyte stimulating hormone (alpha-MSH, beta-MSH and gamma-MSH),
and adrenocorticotropic hormone (ACTH), which are
post-translational products of proopiomelanocortin (POMC) (6). POMC is a protein synthesized in the
pituitary gland, and in other parts of the body, such as the skin,
where its products play important roles as regulators of
melanogenesis and melanocyte survival (7). Skin levels of the POMC and
POMC/corticotropin releasing hormone peptides are determined by
physiological changes associated with hair cycle (highest in anagen
phase), ultraviolet radiation exposure, immune cytokine release, or
the presence of cutaneous pathology (8).
The MSH and ACTH proteins bind to melanocortin
receptors of extracellular G proteins (MCR), of which five subtypes
have been described: Melanocortin receptor 1 (MCR1) to melanocortin
receptor 5 (MCR5) (9). The MC1R
receptor has shown high cutaneous expression, influencing both the
pigmentation and the immune system (10). It participates in the regulation of
skin color, in the protective effect of melanin and it has been
considered as a susceptibility gene for the development of melanoma
(11,12). MCIR receptor has become a new
therapeutic target of diseases that involve its participation.
Afamelanotide is a long-lasting synthetic analog of α-MSH, which
binds to the melanocortin-1 receptor and stimulates melanocyte
proliferation and melanogenesis (13,14).
In the pigmentation process, MC1R is a key signaling
molecule on melanocytes that responds to α-MSH by inducing
expression of enzymes responsible for eumelanin synthesis, through
a complex series of events, mainly mediated by the cAMP signaling
cascade, which leads to an increase in tyrosinase activity and
activation of biosynthesis of pigments (15). Kingo et al observed that the
expression levels of POMC is low and found that expression of MC1R
and MC4R was decreased in vitiligo-affected skin compared with
non-lesional skin from these patients (6). Additionally, the expression of these
receptors was elevated in the non-lesional skin of vitiligo
patients compared with the skin of healthy control subjects
(6). From these findings, they
concluded that the melanocortin system could be implicated in the
pathogenesis of vitiligo.
Currently, narrow-band ultraviolet type B light
(nb-UVB) is considered the gold standard for treating generalized
vitiligo (16,17). Its mechanism of action is through the
induction of local immunosuppression, stimulation of the
proliferation of melanocytes, induction of melanogenesis in the
skin (18), and the production of
the melanocyte stimulating hormone (MSH) (17).
Because the effect of nb-UVB phototherapy on gene
expression of the melanocortin system has not been studied to date,
the aim of this study is to analyse the expression profile of the
genes involved in the melanocortin system (POMC, MC1R and MC4R) in
the affected and non-affected skin of stable vitiligo patients
before and after nb-UVB phototherapy treatment, as well as to
analyse the clinical and biochemical variables that may be involved
in the treatment response.
Patients and methods
Subjects
The present study included 22 patients over 18 years
of age due to regulation of the Institutional Review Board, with
skin photo types II–V of the Fitzpatrick classification (19), with stable vitiligo (in which there
is no growth or appearance of new lesions within a period of 6
months), which affects an area greater than 10% and less than 80%
of the body surface, treated in the Dermatology Department of the
University Hospital ‘Dr. José E. González’, in Monterrey, Mexico,
between September 2013 and February 2016. The protocol and informed
consent form were approved by the Ethics and Research Committee of
the ‘Dr. Jose Eleuterio Gonzalez’ University Hospital of the School
of Medicine and registered the protocol and forms of informed
consent under the code DE12-006. Written informed consent was
obtained from all the participants. They underwent an interview and
clinical evaluation by dermatologists to confirm the diagnosis. The
patients didn't received any type of vitiligo treatment during the
previous 6 months. Patients with another vitiligo type, with
severe/poorly controlled systemic disease, for example type 2
Diabetes Mellitus (T2DM), or with skin cancer or a personal history
of skin cancer, and patients who were photosensitive, or
pregnant/lactating were excluded.
Demographic information, personal data, history of
the disease, age of onset, number and distribution of lesions,
height, weight and body mass index (BMI), family history of the
disease and association with other autoimmune diseases were
recorded.
Phototherapy treatment
Patients were treated with nb-UVB phototherapy
Spectra camera Model 311/350 nm (DAAVLIN) at 240 volts, 30 amps,
and 60 Hz in 2 sessions per week for a period of 6 months
(completing a total of 48 sessions), starting with a dose of 150
mJ/cm2 with incremental increases of 50
mJ/cm2 every third session (depending on the skin photo
type) until achieving the minimum dose of asymptomatic erythema.
The dose was maintained once the clinical response was observed. In
patients who presented irritation or intolerance, the dose was
reduced to the previously tolerated dose.
The follow-up of the treatment was documented
through baseline iconography and at 1, 3 and 6 months after the
initiation of treatment. Two independent investigators evaluated
clinical outcomes (repigmentation changes). The iconographies were
taken with a Sony Exmor DSC-HX1 9.1-megapixel camera
(Shinagawa-ku).
At the end of 48 nb-UVB phototherapy sessions, the
response to treatment observed in patients was classified into
three groups: Low (those subjects whose response to treatment was
less than 10%), medium (those subjects whose response to treatment
was between 10 and 29.9%), and high (those subjects whose response
to treatment was greater than 30%).
Biological samples
Venous blood samples were collected from each
subject to perform laboratory studies: Complete Blood Count (CBC),
Comprehensive Metabolic Panel (CMP), Antinuclear Antibodies (ANAs)
and Complete Thyroid Profile including levels of Triiodothyronine
(T3), L-thyroxine (T4) and thyroid stimulating hormone (TSH).
Four punch biopsies (4 mm) were performed in each
patient: 2 initial biopsies (one of a depigmented skin lesion and
one of non-affected-non-perilesional skin, to rule out degenerative
changes in melanocytes), and 2 biopsies at the end of nb-UVB
phototherapy treatment (one of the treated lesional skin that did
not repigment and one of the repigmented skin). The biopsies were
stored at −80°C immersed in RNA later (Ambion, Thermo Fisher
Scientific, Inc.) for preservation, stabilization and protection of
RNA, until they were processed for expression analysis. RNA
extraction was performed using RN easy Fibrous Tissue Mini kit
(Qiagen, Inc.) following the protocol established by the
manufacturer.
The total RNA amount was determined by
spectrophotometric quantification in microvolume with Nanodrop
equipment (Thermo Fisher Scientific, Inc.) and by quantification by
a fluorometric method with Qubit-RNA-BR Assay Kit for Qubit 2.0
(Thermo Fisher Scientific, Inc.). RNA quality was determined by
capillary electrophoresis using Experion Automated Electrophoresis
System (Bio-Rad Laboratories, Inc.), accessed by the determination
of 18S/28S ribosomal RNA ratio.
Expression analysis using TruSeq
Targeted RNA
Total RNA (100 µg) was extracted from the biopsies
of the patients and treated with DNase, and a reverse transcription
reaction was performed using the ProtoScript® reverse
transcriptase (New England Biolabs), following the indications
established for TruSeq Targeted RNA analysis (Illumina, Inc.).
The RNA expression analysis was performed by
next-generation sequencing (RNA-Seq) in an Illumina MiSeq sequencer
using MiSeq® Reagent kit v3 (150 cycles) and a
TruSeq® Targeted RNA Custom panel designed to detect the
expression profile of 3 genes involved in pigmentation: POMC, MC1R
and MC4R.
The data obtained by RNA-Seq were analysed within
Base Space (https://basespace.illumina.com) using the TruSeq
Targeted RNA tool. The count data for each sample were exported and
normalized by equalizing the total number of counts.
Statistical analysis
The sample size was calculated for comparing means,
considering the study of melanocortin expression previously
conducted by Kingo et al (6)
(alpha=0.05, standard deviation=0.002, mean=0.008 and delta=0.10),
and it was estimated that a minimum sample size of 19 subjects was
sufficient to carry out this study.
The statistical analysis was performed using the
statistical package IBM SPSS Statistics version 17.0 (IBM Corp.).
Descriptive statistics were used for the demographic data, as well
as for all defining variables. To contrast the normality, the
Kolmogorov-Smirnov and Shapiro-Wilk tests were carried out.
According to the type of distribution presented by the groups
(normal or non-normal) of continuous variable, comparisons were
made of 2 groups using Student's T-test and Mann-Whitney U-test,
respectively. The comparisons of more than two group means were
made using one-way analysis of variance (ANOVA) for the variables
with normal distribution, and the H-Kruskal-Wallis test for the
variables with non-normal distribution. Additionally, post hoc
tests were performed for comparison between groups (Tukey and
Bonferroni for equal variances, Tamhane and Dunnett T3 for
different variances). The association between variables was
determined using the chi-square test (χ2) and Fisher's
exact test for Antinuclear Antibodies and the degree of response to
treatment. To determine existing significant differences between
expression profiles, multiple comparison Kruskal-Wallis test was
used due to the non-normal distribution of the data, and
Mann-Whitney U test was used to confirm differences between two
independent groups of gene expression profiles. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient data
In this study, 22 patients with stable vitiligo were
included, 8 women (with an average age of 40±16.43, minimum of 19
years and a maximum of 70 years) and 14 men (with an average age of
39.79±12.18, minimum 24 years, maximum 69 years) with greater than
10% of their body surface affected by the disease, and skin photo
types III–IV (Fitzpatrick classification (19)).
Response to nb-UVB phototherapy
The clinical evaluation determined that all patients
had some degree of repigmentation (from 1 to 65%) after nb-UVB
phototherapy. In this regard, 8 (36.36%) subjects demonstrated a
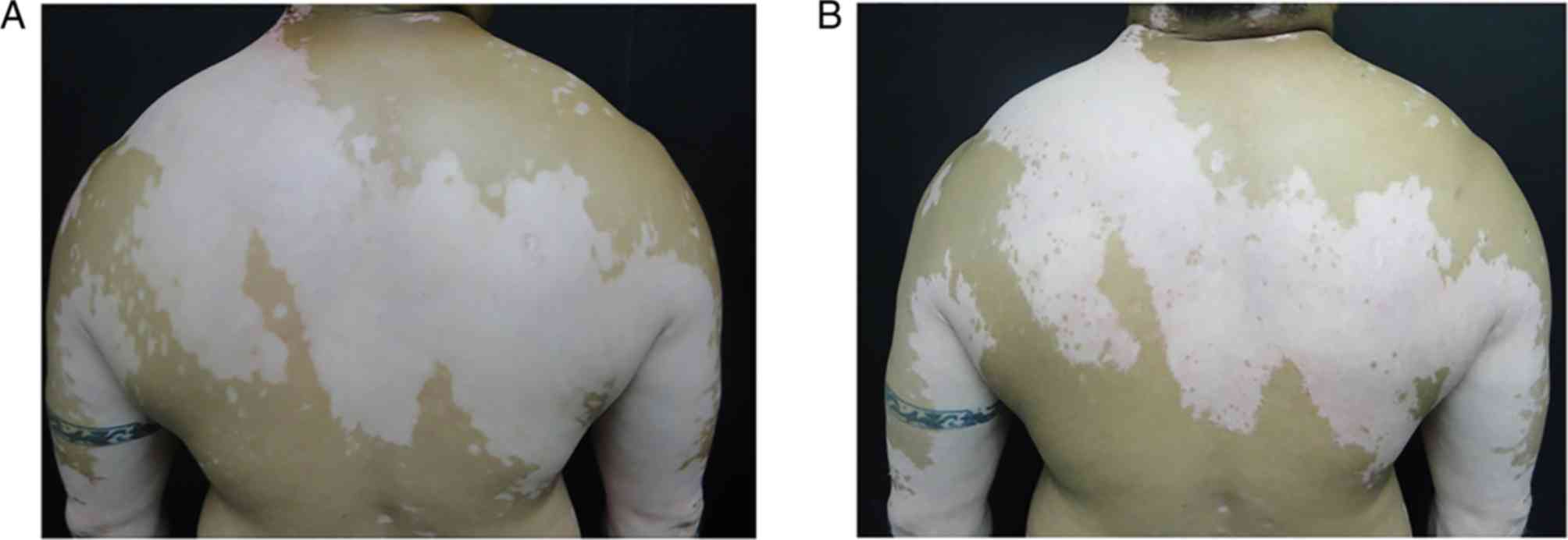
low response to treatment (Fig. 1),
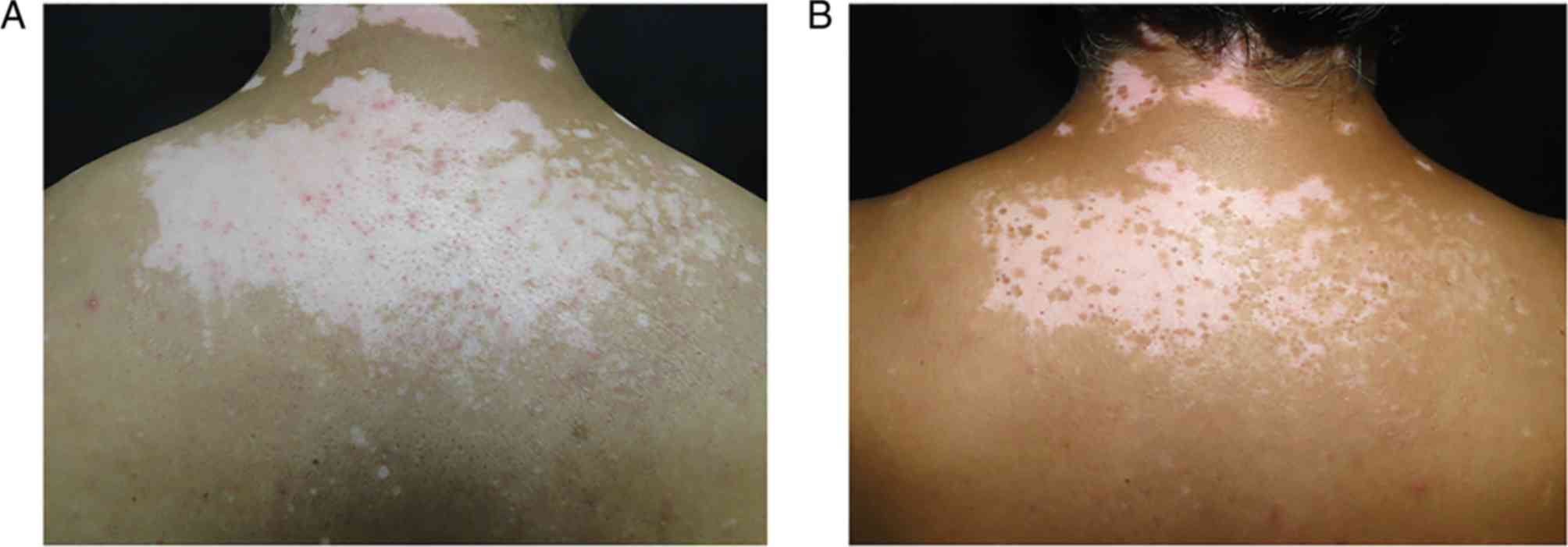
8 (36.36%) displayed a medium response (Fig. 2) and 6 (27.28%) exhibited a high
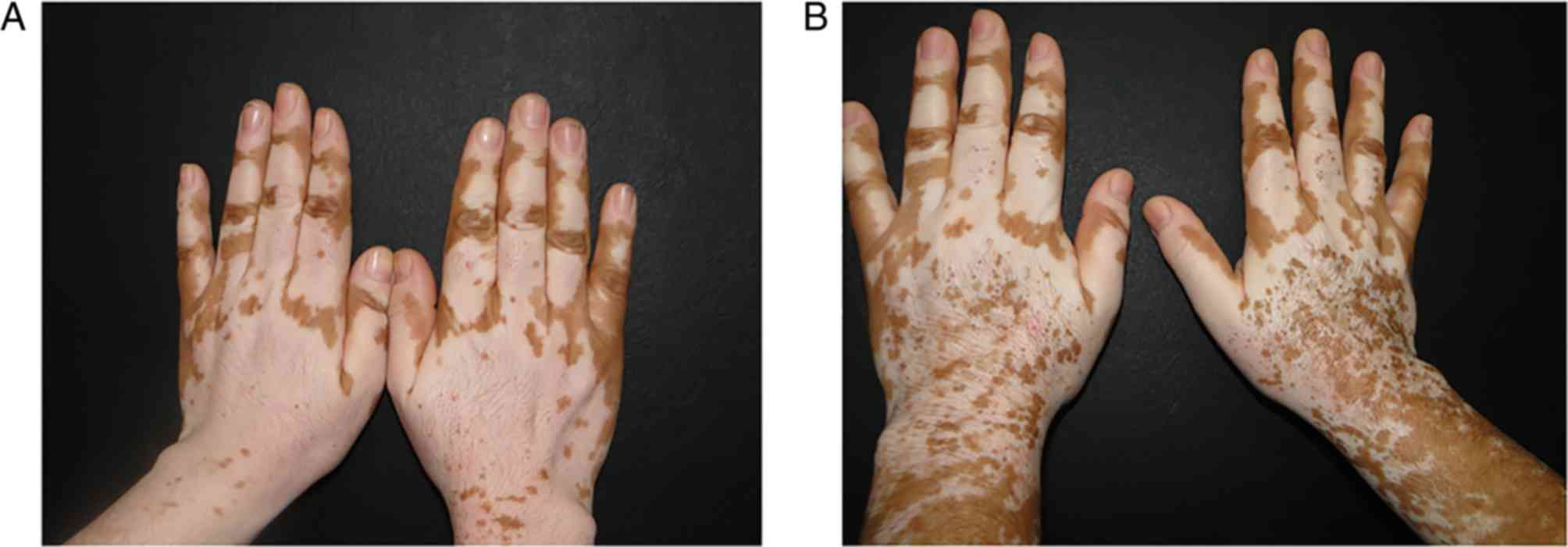
response (Fig. 3).
Effect of nb-UVB phototherapy on
various clinical and biochemical parameters
Regarding the biochemical and anthropometric
parameters analysed, it can be observed by ANOVA analysis that the
subjects who displayed a greater response to treatment have, on
average, higher levels of T4, and lower levels of T3, TSH, weight
and BMI (Table I). Concerning the
levels of triiodothyronine (T3), it could be observed that this
value is significantly lower in the subjects who presented a high
response to treatment, in comparison with those who presented a
medium response to treatment (P=0.042) (Table I).
 | Table I.Biochemical and anthropometric
parameters according to the degree of response to nb-UVB
phototherapy. |
Table I.
Biochemical and anthropometric
parameters according to the degree of response to nb-UVB
phototherapy.
|
| Degree of response to
nb-UVB phototherapy |
|---|
|
|
|
|---|
| Parameters | Low (<10%) | Medium
(10–29.9%) | High (>30%) |
|---|
| T3 (ng/dl) | 147.94±51.60 | 129.11±18.24 |
104.37±14.27a |
| T4 (mcg/dl) | 7.53±2.52 | 7.51±1.00 | 7.90±1.10 |
| TSH (mlU/l) | 2.37±0.78 | 2.07±0.70 | 1.76±0.68 |
| Weight (kg) | 98.69±14.53 | 79.72±25.22 | 74.17±13.57 |
| BMI | 32.50±4.83 | 28.79±8.77 | 27.55±3.24 |
On the other hand, the chi square test analysis
(χ2 between the degree of response to treatment and the
presence of ANA's (considering as positive those subjects who
presented dilutions equal to or greater than 1:80), allowed to
determine that there is no association between the presence of
antinuclear antibodies and the degree of response to nb-UVB
phototherapy (P=0.097).
Expression analysis
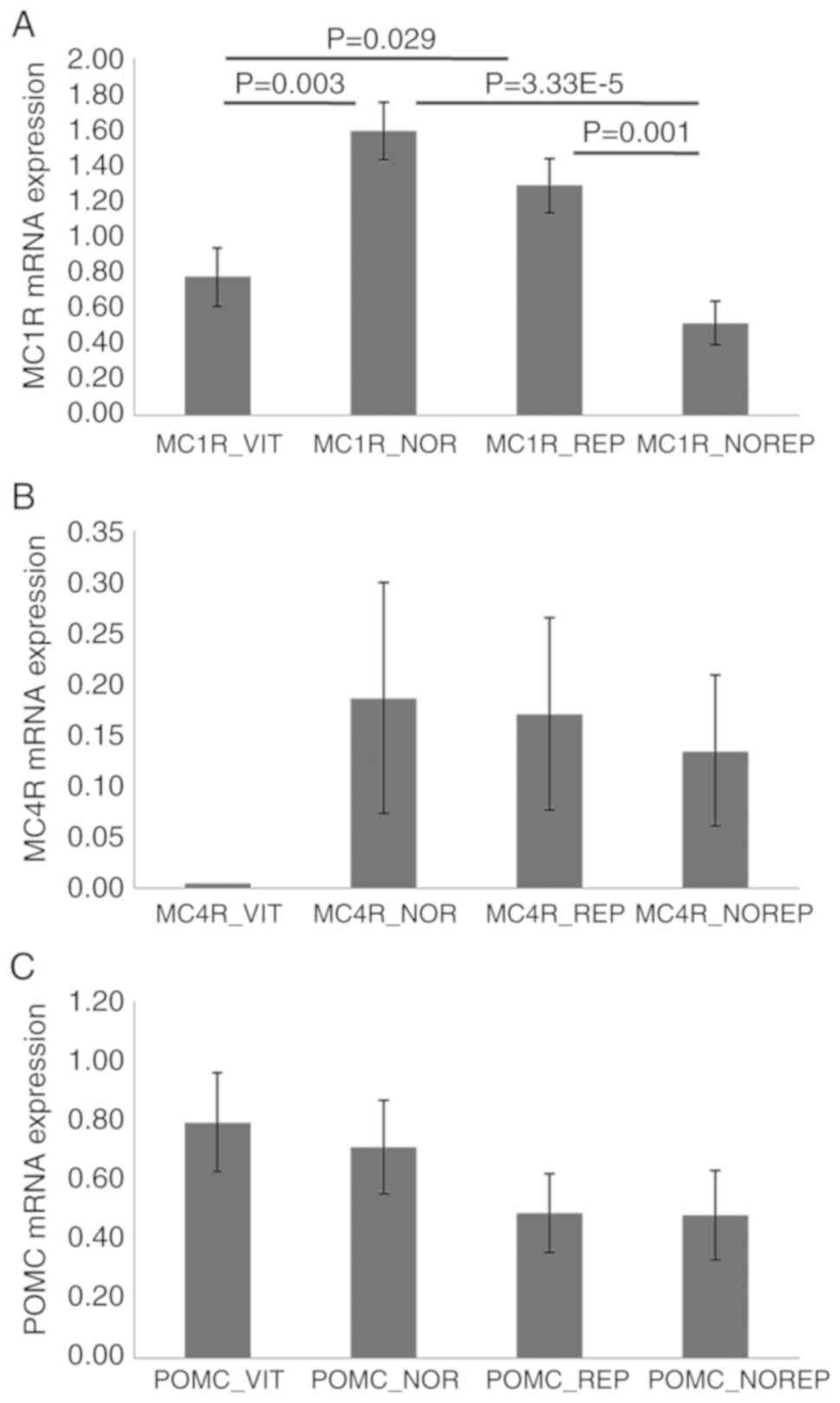
For the expression profile analysis of the POMC,
MC1R and MC4R genes, the 4 skin biopsies obtained from the 22
patients included in this study were used. The results by
Kruskal-Wallis and Mann-Whitney U test analysis showed: POMC
(vitiligo skin biopsy expression 0.79±0.17, normal skin biopsy
expression 0.71±0.16, repigmented skin biopsy expression 0.49±0.13,
and expression of skin biopsy that did not repigment following
treatment 0.48±0.15); MC1R (vitiligo skin biopsy expression
0.78±0.16, normal skin biopsy expression 1.59±0.16, repigmented
skin biopsy expression 1.29±0.15, and expression of skin biopsy
that did not repigment following treatment 0.52±0.12); and MC4R
(vitiligo skin biopsy expression no detected, normal skin biopsy
expression 0.19±0.11, repigmented skin biopsy expression 0.17±0.09,
and expression of skin biopsy that did not repigment following
treatment 0.13±0.07) (Fig. 4), and a
statistically significant difference was obtained in the expression
for MC1R gene (P≤0.05). Nevertheless, the expression profiles
obtained demonstrated no statistically significant relationship
with the degree of response to treatment experienced by the
patients (P>0.05).
Discussion
Nb-UVB phototherapy has been considered the first
choice treatment in vitiligo vulgaris as it is effective and
well-tolerated (20). In this study,
all patients treated for stable vitiligo displayed some degree of
repigmentation, reaffirming the usefulness of nb-UVB phototherapy
as a therapeutic alternative in cases of stable vitiligo. The UV
radiation can upregulate α-MSH receptor (MC1R) expression and
activity, POMC expression, and POMC peptide production; include
α-MSH, β-endorphin, and ACTH molecules that participate in the
regulation of skin pigmentation, to protect skin from UV induced
injury, and to modulate skin immune response (21). It's well known that UV light
stimulates the production of endothelin-1 (ET-1) and POMC by
keratinocytes, factors that can act in a paracrine manner by
stimulating the function of melanocytes (22).
Nb-UVB phototherapy acts through the induction of
local immunosuppression and the stimulation of the proliferation of
melanocytes in the skin and in the outer sheath of the hair
follicle root; there is also a stimulating effect on melanogenesis
and on the production of melanocyte-stimulating hormone (MSH)
(17).
Currently, compared with other technologies such as
RTq-PCR and microarrays, RNA sequencing (RNA-Seq) using
next-generation sequencing (NGS) allows determination of gene
expression levels with higher efficiency, lower cost and shorter
times (23). In vitiligo, this
technology has already been used to determine the expression
profiles in skin biopsies of subjects affected by this disease
(24).
Skin pigmentation is due to the pigment produced by
melanocytes, eumelanin and pheomelanin, and their accumulation on
melanin granules in the keratinocytes. These pigments produced by
melanocytes are responsible for skin and hair color. Skin
pigmentation is an important protective mechanism against the DNA
damaging and mutagenic effects of solar UV radiation (25).
The melanocortin receptor family is a member of the
class A rhodopsin-like family of G-protein coupled receptors and
consists of five members: MC1R to MC5R; MC1R is found on both
melanocytes and leukocytes and its activation promotes UV
resistance and anti-inflammatory signaling (26). Melanocortin 1 receptor (MC1R) is a
key signaling molecule on melanocytes that responds to α-MSH by
inducing expression of enzymes responsible for eumelanin synthesis.
Moreover α-MSH/MC1R signaling is a potent anti-inflammatory pathway
and has been shown to promote anti-melanoma immunity (27). Human MC1R genetics variants are
associated with sunlight sensitivity (28), and altered expression of MC1R have
been associated with red hair and skin cancer risk (29).
The melanocortin system can play an important role
in the pathogenesis of vitiligo, and can be affected by this
disease, as was reported in the studies of Kingo et al
(6) and Nagui et al (30). In our study, we analysed the
expression profiles of POMC, MC1R and MC4R before and after nb-UVB
phototherapy treatment of patients with stable vitiligo.
Considering the skin samples obtained from the patients before
starting the treatment, we observed that the expression profile for
the MC1R and MC4R genes were higher in normal skin biopsies, and
these results are consistent with those described by Kingo et
al and Nagui et al (6,30).
However, for the POMC gene, we found that there is no statistically
significant difference between normal skin and skin with vitiligo
prior to treatment with nb-UVB phototherapy, unlike what was
described by both of these authors. In Fig. 2 we can observe a slight increase in
the level of POMC expression in the skin with vitiligo within the
analysed subjects. This slight increase could be due to the need of
POMC to stimulate pigmentation in the areas affected by
vitiligo.
Regarding the expression profiles for the MC1R gene
observed after treatment levels of expression in the skin that
repigmented after treatment and the levels in the normal skin of
the patient before treatment were statistically similar. This
allowed us to suppose that this increase in expression accounts for
the repigmentation experimented by the skin (Fig. 4). Otherwise, the skin that did not
repigment, presented statistically lower levels than normal skin
and skin that was repigmented and even lower than the skin with
vitiligo onset (Fig. 4).
Concerning the MC4R gene, we observed that the
expression levels after treatment were increased in both biopsies.
In addition, the expression profiles of skin biopsies that showed
repigmentation and normal tissue before treatment were similar.
This increase in expression levels of repigmented tissue is related
to the response to treatment. In the non-repigmented skin, the low
expression profile for this gene would have been expected, as was
observed in the case of MC1R. However, the result obtained leads us
to consider two possibilities: (a) that this skin will repigment
and there was not enough treatment time to see a positive clinical
response; or (b) that this gene is not fundamental in pigmentation,
and its expression levels are very low. As already described in the
study of Millington (2006) the melanocortin 4 receptor (MC4R) is
expressed mainly in the brain, spinal cord, muscle and adipose
tissue, with its main function being the regulation of energy
metabolism (10,31).
Regarding the biochemical parameters and the
response to treatment experimented by patients, it was observed
that there is a relationship between the levels of T3 and the
response to treatment (Table I). In
particular, in the subjects who presented a high response to
treatment (greater than 30% repigmentation), the levels of this
hormone were found to be decreased (104.37±14.27 ng/dl) compared to
the groups of patients who displayed a low or medium response to
treatment, which exhibited substantially higher levels of this
hormone. In a retrospective study conducted by Subba et al,
alterations in the levels of T3, T4 and TSH in subjects affected by
vitiligo were described (32). In a
study conducted in a Turkish population, high levels of T3 and T4
were described in subjects with different types of vitiligo; and
progression of this disease was seen in comparison with control
subjects (33).
van Beek et al (2008) described that T4
up-regulates the proliferation of hair matrix keratinocytes and
prolonged the duration of the hair growth phase (anagen) in
vitro with human hair follicles, whereas their apoptosis is
down-regulated by T3 and T4; on the other hand, both T3 and T4,
significantly stimulated intrafollicular melanin synthesis
(34). The association between
thyroid problems and vitiligo is known, mainly in people with
hypothyroidism (35), supporting the
theory of a thyroid dysfunction and the participation of autoimmune
processes in the development of this pathology (36).
In conclusion, Nb-UVB phototherapy is effective in
the treatment of patients with stable vitiligo, favouring
repigmentation and producing changes in expression profiles of
three genes involved in the melanocortin system, POMC, MC1R and
MC4R, related to the repigmentation process in stable vitiligo
patients, mainly MC1R (P<0.05). Although we reaffirm that the
melanocortin system intervenes in the pathogenesis of stable
vitiligo, additional studies are required to determine the
performance of these genes in other variants of this disease. It is
also necessary to evaluate the biochemical parameters of vitiligo
patients, mainly T3 levels, since elevated levels of this hormone
may lead to a poor response to phototherapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Department of
Dermatology, ‘Dr. Jose Eleuterio Gonzalez’ University Hospital of
the School of Medicine, Monterrey, Nuevo Leon, Mexico.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JOG, JOC and OW conceived the current study. JOG,
JOC, OW and MSS designed the present study. JOG and MHR selected
the patients. JOG, MHR and MSS analyzed the data. JOG, MSS
interpreted the data. JOG, JOC, OW, MHR and MSS drafted and
critically revised the manuscript. JOG, JOC, OW and MSS improved
the manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
The University Hospital-UANL Institutional Review
Board approved and registered the study under the trial number,
DE12-006.
Patient consent for publication
All patients consented to the publication of their
data and associated images.
Competing interests
The authors declare that they have no competing
interesets.
References
|
1
|
Picardo M, Dell'Anna ML, Ezzedine K,
Hamzavi I, Harris JE, Parsad D and Taieb A: Vitiligo. Nat Rev Dis
Primers. 1:150112015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen C, Gao J, Sheng Y, Dou J, Zhou F,
Zheng X, Ko R, Tang X, Zhu C, Yin X, et al: Genetic susceptibility
to vitiligo: GWAS approaches for identifying vitiligo
susceptibility genes and loci. Front Genet. 7:32016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandoval-Cruz M, García-Carrasco M,
Sánchez-Porras R, Mendoza-Pinto C, Jiménez-Hernández M,
Munguía-Realpozo P and Ruiz-Argüelles A: Immunopathogenesis of
vitiligo. Autoimmun Rev. 10:762–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schallreuter KU, Bahadoran P, Picardo M,
Slominski A, Elassiuty YE, Kemp EH, Giachino C, Liu JB, Luiten RM,
Lambe T, et al: Vitiligo pathogenesis: Autoimmune disease, genetic
defect, excessive reactive oxygen species, calcium imbalance, or
what else? Exp Dermatol. 17:139–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richmond JM, Frisoli ML and Harris JE:
Innate immune mechanisms in vitiligo: Danger from within. Curr Opin
Immunol. 25:676–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kingo K, Aunin E, Karelson M, Philips MA,
Rätsep R, Silm H, Vasar E, Soomets U and Kõks S: Gene expression
analysis of melanocortin system in vitiligo. J Dermatol Sci.
48:113–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rousseau K, Kauser S, Pritchard LE,
Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ and
White A: Proopiomelanocortin (POMC), the ACTH/melanocortin
precursor, is secreted by human epidermal keratinocytes and
melanocytes and stimulates melanogenesis. FASEB J. 21:1844–1856.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slominski A, Wortsman J, Luger T, Paus R
and Solomon S: Corticotropin releasing hormone and
proopiomelanocortin involvement in the cutaneous response to
stress. Physiol Rev. 80:979–1020. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y: Structure, function and regulation
of the melanocortin receptors. Eur J Pharmacol. 660:125–130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Millington GW: Proopiomelanocortin (POMC):
The cutaneous roles of its melanocortin products and receptors.
Clin Exp Dermatol. 31:407–412. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi Y, Brenner M and Hearing VJ: The
regulation of skin pigmentation. J Biol Chem. 282:27557–27561.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wolf Horrell EM, Boulanger MC and D'Orazio
JA: Melanocortin 1 receptor: Structure, function, and regulation.
Front Genet. 7:952016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tafreshi N, Tichacek CJ, Pandya DN,
Doligalski ML, Budzevich MM, Kil H, Bhatt NB, Kock ND, Messina JL,
Ruiz EE, et al: Melanocortin 1 receptor targeted α-particle therapy
for metastatic Uveal melanoma. J Nucl Med. 60:1124–1133. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zubair R, Lyons AB, Vellaichamy G, Peacock
A and Hamzavi I: What's new in pigmentary disorders. Dermatol Clin.
37:175–181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
García-Borrón J, Abdel-Malek Z and
Jiménez-Cervantes C: MC1R, the cAMP pathway, and the response to
solar UV: Extending the horizon beyond pigmentation. Pigment Cell
Melanoma Res. 27:699–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vázquez-Martínez OT, Velásquez-Arenas L,
Méndez-Olvera N and Ocampo-Candiani J: Vitiligo. Overview and
current therapeutics. DCMQ. 3:2006.
|
|
17
|
Majid I: Vitiligo management: An update.
BJMP. 3:62010.
|
|
18
|
Carsberg CJ, Warenius HM and Friedmann PS:
Ultraviolet radiation-induced melanogenesis in human melanocytes.
Effects of modulating protein kinase C. J Cell Sci. 107:2591–2597.
1994.PubMed/NCBI
|
|
19
|
Fitzpatrick TB: The validity and
practicality of sun-reactive skin types I through VI. Arch
Dermatol. 124:869–871. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stinco G, Trevisan G, Buligan C, Gregoraci
G, De Marchi S, di Meo N and Patrone P: Narrow band-ultraviolet B
versus clobetasol propionate foam in the treatment of vitiligo: A
retrospective study. Dermatol Ther (Heidelb). 3:95–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV light touches the brain and
endocrine system through skin, and why. Endocrinology.
159:1992–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Costin GE and Hearing VJ: Human skin
pigmentation: Melanocytes modulate skin color in response to
stress. FASEB J. 21:976–994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Gerstein M and Snyder M: RNA-Seq:
A revolutionary tool for transcriptomics. Nat Rev Genet. 10:57–63.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salinas-Santander M, Trevino V, De la
Rosa-Moreno E, Verduzco-Garza B, Sanchez-Dominguez CN,
Cantu-Salinas C, Ocampo-Garza J, Lagos-Rodríguez A, Ocampo-Candiani
J and Ortiz-López R: CAPN3, DCT, MLANA and TYRP1 are overexpressed
in skin of vitiligo vulgaris Mexican patients. Exp Ther Med.
15:2804–2811. 2018.PubMed/NCBI
|
|
25
|
Swope VB and Abdel-Malek ZA: MC1R: Front
and center in the bright side of dark eumelanin and DNA repair. Int
J Mol Sci. 19(pii): E26672018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolf Horrell EM, Boulanger MC and D'Orazio
JA: Melanocortin 1 receptor: Structure, function and regulation.
Front Genet. 7:952016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nasti T and Timares L: MC1R, eumelanin and
pheomelanin: Their role in determining the susceptibility to skin
cancer. Photochem Photobiol. 91:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernando B, Sanz-Page E, Pitarch G,
Mahiques L, Valcuende-Cavero F and Martinez-Cadenas C: Genetic
variants associated with skin photosensitivity in a southern
European population from Spain. Photodermatol Photoimmunol
Photomed. 34:415–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beaumont KA, Newton RA, Smit DJ, Leonard
JH, Stow JL and Sturm RA: Altered cell surface expression of human
MC1R variant receptor alleles associated with red hair and skin
cancer risk. Hum Mol Genet. 14:2145–2154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagui NA, Mahmoud SB, Abdel Hay RM,
Hassieb MM and Rashed LA: Assessment of gene expression levels of
proopiomelanocortin (POMC) and melanocortin-1 receptor (MC1R) in
vitiligo. Australas J Dermatol. 58:e36–e39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You P, Hu H, Chen Y, Zhao Y, Yang Y, Wang
T, Xing R, Shao Y, Zhang W, Li D, et al: Effects of melanocortin 3
and 4 receptor deficiency on energy homeostasis in rats. Sci Rep.
6:349382016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Subba K, Karn D and Khatri R:
Triiodothyronin, thyroxine and thyrotropin in vitiligo. Kathmandu
Univ Med J (KUMJ). 9:7–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arıcan Ö, Şaşmaz S and Çetinkaya A: Role
of thyroid hormones in vitiligo type and progression. Turkderm.
37:269–273. 2003.
|
|
34
|
van Beek N, Bodó E, Kromminga A, Gáspár E,
Meyer K, Zmijewski MA, Slominski A, Wenzel BE and Paus R: Thyroid
hormones directly alter human hair follicle functions: Anagen
prolongation and stimulation of both hair matrix keratinocyte
proliferation and hair pigmentation. J Clin Endocrinol Metab.
93:4381–4388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta Y and Ammini AC: Vitiligo,
hypothyroidism and cardiomyopathy. Indian J Endocrinol Metab.
16:463–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kasumagic-Halilovic E, Prohic A, Begovic B
and Ovcina-Kurtovic N: Association between vitiligo and thyroid
autoimmunity. J Thyroid Res. 2011:9382572011. View Article : Google Scholar : PubMed/NCBI
|