Introduction
Placenta accreta spectrum (PAS), which represents a
clinical challenge in obstetrics, is defined as myometrial
involvement by the fetal trophoblast (1,2). It may
lead to uncontrollable bleeding and threaten the lives of mother
and baby. Its prevalence has markedly increased in China over the
past 50 years, primarily due to the increasing number of pregnant
females undergoing primary and repeat cesarean sections (3). Obstetricians have implemented various
methods to improve the massive hemorrhage caused by placenta
implantation, including ascending uterine artery ligation (AUAL),
uterine artery embolization (UAE) and prophylactic abdominal aorta
balloon occlusion (ABO), but the therapeutic effects are varied
(4). Interventional radiology is
commonly applied and placement of prophylactic balloon catheters in
the common or internal iliac arteries are commonly used to help
control massive hemorrhage (5,6).
Development of an effective way to prenatally predict massive
hemorrhage may allow for appropriate pre-operative preparation,
including the arrangement for treatment by a skilled surgical
team.
There have been several reports on the association
between clinical information or therapeutic schedule and the risk
of massive hemorrhage (7,8). Wright et al (8) reported an association among PAS
(placenta accreta, increta or percreta), gestational age of <34
weeks at delivery and estimated blood loss (EBL)≥5,000 ml. It was
noted that patients with placenta previa, who delivered at an
earlier gestational age, were more likely to require ≥10 units of
blood. Shamshirsaz et al (7)
reported that a standardized approach for patients with morbidly
adherent placentation provided by a specific multidisciplinary team
was associated with improved maternal outcomes compared with a more
traditional non-multidisciplinary approach. There are several
reports on sonographic evaluation for predicting the risk of
massive bleeding. For instance, Hasegawa et al (9) reported that advanced maternal age,
previous cesarean section and presence of sponge-like tissue in the
cervix were risk factors of massive bleeding during cesarean
section in cases of placenta previa, regardless of whether
placental adherence was present. Baba et al (10) reported that anterior placentation was
a risk factor of massive hemorrhage during cesarean section for
placenta previa.
Chen et al (11) reported that low signal intensity
bands on T2-weighted imaging may be a predictor of poor maternal
outcome in patients with invasive placenta previa. However, studies
on the association between MRI features and hemorrhage of patients
with PAS are currently limited. The present study investigated
whether MRI features are able to predict massive hemorrhage of
patients with PAS. The results may be helpful for pre-operative
preparation.
Materials and methods
Patients
The present study was a retrospective study. A total
of 40 patients who underwent ultrasonography (US) and placenta MRI
examination from March 2015 to May 2018 were enrolled. The
inclusion criteria were as follows: (a) Patients with suspected PAS
or inconclusive results on US, (b) patients at high risk of PAS
with one or more of the following: Maternal age >35 years, grand
multiparity, previous uterine interventional procedures (e.g.
cesarean section, dilatation and curettage and myomectomy) and
placenta previa (12). The exclusion
criteria were as follows: (a) Medical records not available, (b)
only MRI data of post-partum placenta implantation available, (c)
early pregnancy, (d) patients who had induced labor rather than
cesarean section due to stillbirth in utero. The patients were
first diagnosed with suspected or inconclusive PAS using US, based
on the detection of any of the following: Loss/irregularity of the
echolucent area between uterus and placenta, thinning or
interruption of the hyperechoic interface between uterine serosa
and bladder wall, the presence of turbulent placental lacunae with
high-velocity flow (>15 cm/sec), hypervascularity of the uterine
serosa-bladder wall interface and irregular intraplacental
vascularization (13). All patients
received cesarean section and only one patient underwent subtotal
hysterectomy due to uncontrollable bleeding. The final diagnosis of
PAS was made based on intra-operative observation for 39 patients
and by histopathology for one patient who was treated by subtotal
hysterectomy. Finally, 29 patients (72.5%, 29/40) were confirmed as
having PAS (average age, 33.5±4.2 years), and 11 patients were
confirmed as non-PAS (average age, 32.8±3.2 years).
The retrospective study was performed in accordance
with the standards set out in the Code of Ethics of the World
Medical Association (Declaration of Helsinki) and the research
procedures were approved by the ethics review board of Shandong
Provincial Hospital (Jinan, China). Informed consent was obtained
from each patient.
MRI examination
All MRI examinations were performed with a 1.5-T
system (HDxt; GE Healthcare). An eight-channel pelvic phased-array
surface coil was used for signal reception. All patients were
imaged in the supine or left lateral position depending on
tolerability for each case. The MRI protocol included axial,
sagittal and coronal fast imaging employing steady-state
acquisition with the following settings: Repeat time (TR), 3.6
msec; echo time (TE), 1.6 msec; matrix size, 224×256; thickness,
5–7 mm; intersection gap, 1 mm; and field of view (FOV), 400×400
mm2, as well as single-shot fast spin-echo T2-weighted
imaging (TR, 1,800 msec; TE, 81 msec; matrix size, 288×192;
thickness, 5–6 mm; intersection gap, 1 mm; FOV, 380×380-400×400
mm2) and liver acquisition with volume acceleration (TR,
3.9 msec; TE, 1.8 msec; matrix size, 288×200; thickness, 2.5 mm;
intersection gap, 0.5 mm; and FOV, 400×400 mm2).
MRI data analysis
MRI data were analyzed independently by two
radiologists (JZ with five years and HX with ten years of
experience in evaluating the placenta using MRI) blinded to the
patients history, US examination results, presence of PAS and
intra-operative findings. MRI data were interpreted on a PACS view
station (Centricity RIS CE V2.0; GE Healthcare). A total of 11 MRI
features were evaluated, including placenta previa, focal defect of
the uteroplacental interface (UPI), myometrial thinning, disruption
of the inner layer of the UPI, intraplacental thick dark bands,
focal defect of the interval between the bladder and uterus,
increased placental vascularity, markedly heterogeneous placenta,
uterine bulge, increased uterine vascularity and increased
vascularity in the UPI (14). A
complete description of the MRI features is provided in
Supplemental Table SI. In case of
any disagreement, a third radiologist (QL) with 15 years of
experience in evaluating the placenta using MRI was consulted.
Clinical diagnosis of PAS
The reference standard for determining the actual
status of the placenta was established by one obstetrician (CZ with
20 years of experience in obstetrics) according to intra-operative
findings recorded in the electronic medical records of most
patients (n=39). The diagnostic criteria were as follows (15): Placenta accreta: i) No placental
tissue invading through the surface of the uterus. ii) Incomplete
separation with uterotonics and gentle cord traction and manual
removal of the placenta was required for the remaining placenta.
iii) Bleeding cannot be controlled autonomously. Placenta increta:
i) No placental tissue invading through the surface of the uterus.
ii) Placental tissue implanted in myometrium of uterus requiring to
be removed by forceps curettage. Placenta percreta:
Macroscopically, the whole layer of the uterus (including the
serosal surface), even the surrounding organs, was invaded by
placental tissues.
Hemorrhage analysis
The EBL was estimated by an experienced obstetrician
who participated in the operation based on the operative report,
which included fluid volume in the negative pressure aspirator,
dressing weight and other operative findings. The blood volume in
the mixture of blood and amniotic fluid was calculated according to
the total fluid volume, hematocrit of the mixture and prenatal
hematocrit. The EBL was estimated and documented during surgery.
Packed red blood cell (PRBC) transfusion and plasma transfusion
were also documented. Moderate hemorrhage was defined as
EBL<2,000 ml and PRBC transfusion <10 units. Massive
hemorrhage was defined as EBL≥2,000 ml or PRBC transfusion ≥10
units (7,8). The patients were divided into two
groups (moderate hemorrhage and massive hemorrhage) according to
EBL and PRBC transfusion.
Statistical analysis
Statistical analysis was performed with SPSS for
Windows, version 17.0 (SPSS, Inc.). Placenta previa is a
five-valued variable (without placenta previa, low-lying placenta,
marginal placenta previa, partial placenta previa or complete
placenta previa). The other 10 MRI features were binary variables
(with or without the MRI feature). Inter-observer agreement
regarding categorical data between the first two radiologists was
assessed using a Kappa test. Demographic profiles of numerical data
[including age, gestational age, EBL, PRBC transfusion, plasma
transfusion, interval time between MRI and operation, RBCs,
hemoglobin, hematocrit, prothrombin time, prothrombin time
international normalized ratio (INR) and activated partial
thromboplastin time (APTT)] of non-PAS and PAS patients, as well as
moderate hemorrhage and massive hemorrhage patients, were compared
using the independent-samples t-test. The numbers of cesarean
sections and abortions of the non-PAS and PAS patients, as well as
the number of patients with moderate and massive hemorrhage, were
compared using the Mann-Whitney U-test. The hemostatic methods for
the non-PAS and PAS patients, as well as the moderate hemorrhage
and massive hemorrhage patients, were compared using a
χ2 test.
The MRI features between the moderate and massive
hemorrhage patients were compared using the χ2 test. The
EBL, PRBC transfusion and plasma transfusion between patients with
and without each MRI feature were compared using the
independent-samples t-test. The EBL, PRBC transfusion and plasma
transfusion among different subtypes of placenta previa were
compared using one-way analysis of variance. For all statistical
analyses, P<0.05 was considered to indicate statistical
significance.
Results
Patients
As presented in Table
I, the clinical information of the patients was compared. The
EBL and plasma transfusion for the PAS patients were significantly
higher than those for the non-PAS patients (P<0.01). The
differences in the other demographic characteristics between the
non-PAS and the PAS patients were not significant (P>0.05).
There were 19 cases of moderate hemorrhage (including 6 cases of
placenta accreta, 11 cases of placenta increta and 2 cases of
placenta percreta), 10 cases of massive hemorrhage (including 5
cases of placenta increta and 5 cases of placenta percreta). The
EBL, PRBC transfusion and plasma transfusion for the patients in
the massive hemorrhage group were significantly higher than those
for the patients in the moderate hemorrhage group (P<0.001). The
mean APTT for the massive hemorrhage group was significantly longer
than that for the moderate hemorrhage group (P<0.05), but the
mean APTT of the two groups was in the normal range. A total of
three hemostatic methods have been applied for the patients
(Table I). One of the two PAS
patients treated with UAE underwent pre-operative UAE, while the
others underwent post-operative UAE. One of the 27 PAS patients
treated with AUAL underwent unilateral AUAL, while the other 26
patients underwent bilateral AUAL. The difference in hemostatic
methods between the moderate hemorrhage group and the massive
hemorrhage group was significant (P<0.05). The proportion of
patients who underwent ABO in the massive hemorrhage group was
larger than that in the moderate hemorrhage group. However, the
blood loss and transfusion in the massive hemorrhage group were
still higher than those in the moderate hemorrhage group. Other
hemostatic methods, including the use of oxytocin, tourniquet,
local suture ligation, uterine packing hemostasis and placement of
hemostatic gauze, were applied according to the intra-operative
conditions. Each patient was treated using multiple hemostasis
methods.
 | Table I.Demographic and clinical information
of all patients. |
Table I.
Demographic and clinical information
of all patients.
| Item | Non-PAS (n=11) | PAS (n=29) | P-value | Moderate hemorrhage
(n=19) | Massive hemorrhage
(n=10) | P-value |
|---|
| Maternal age
(years) | 32.8±3.2 (28–40) | 33.5±4.2 (27–40) | 0.635 | 34.5±4.4 (28–40) | 31.6±3.1 (27–36) | 0.076 |
| Gestational age
(days) | 239±26 (192–272) | 241±30
(126–280) | 0.864 | 242±23
(189–269) | 237±42
(126–280) | 0.716 |
| EBL (ml) | 586±358
(300–1,400) | 1286±855
(300–3,500) | 0.001 | 816±347
(300–1,600) | 2180±824
(800–3,500) | <0.001 |
| PRBC transfusion
(units) | 1.95±2.76
(0–8) | 4.59±4.36
(0–14) | 0.07 | 2.11±2.05
(0–6) | 9.32±3.57
(4–14) | <0.001 |
| Plasma transfusion
(ml) | 127±205
(0–600) | 405±408
(0–1,200) | 0.008 | 166±197
(0–550) | 860±299
(400–1,200) | <0.001 |
| Interval time | 12.3±17.1
(0–47) | 9.6±13.2
(0–65) | 0.604 | 11.3±15.5
(1–65) | 6.5±6.9 (0–21) | 0.366 |
| RBC
(1012/l) (normal range 3.8–5.1) | 3.47±0.35
(2.94–4.03) | 3.7±0.42
(2.91–4.64) | 0.11 | 3.71±0.43
(3.14–4.64) | 3.69±0.41
(2.91–4.48) | 0.912 |
| Hemoglobin (g/l)
(normal range 115–150) | 99.7±13.7
(83–123) | 105.4±15.7
(74–137) | 0.295 | 105.5±16.6
(74–137) | 105.3±14.8
(81–127) | 0.971 |
| Hematocrit (%)
(normal range 35–45) | 30.4±3.6
(26.5–37.9) | 32.1±4.0
(24–39) | 0.215 | 31.9±4.0
(24–39) | 32.6±4.1
(27–38.2) | 0.636 |
| PT (sec) (normal
range 10.7–14.0) | 12.8±0.8
(11.4–14.1) | 12.6±1.7
(10.3–19) | 0.761 | 12.3±1.1
(10.3–14.5) | 13.3±2.5
(10.4–19) | 0.263 |
| INR (normal range
0.8–1.2) | 1.00±0.06
(0.94–1.10) | 1.02±0.14
(0.87–1.62) | 0.633 | 1.00±0.06
(0.87–1.11) | 1.06±0.22
(0.9–1.62) | 0.449 |
| APTT (sec) (normal
range 21–35) | 31.6±3.2
(25–34.3) | 30.1±4.7
(21.9–39) | 0.338 | 29.1±5.4
(21.9–39) | 32.0±2.1
(28.3–36.3) | 0.045 |
| Cesarean sections
per patient |
|
| 0.361 |
|
| 0.897 |
|
None | 0 | 3 |
| 2 | 1 |
|
|
Once | 10 | 24 |
| 16 | 8 |
|
|
Twice | 1 | 2 |
| 1 | 1 |
|
| Abortions per
patient |
|
| 0.126 |
|
| 0.677 |
|
None | 2 | 8 |
| 5 | 3 |
|
|
Once | 1 | 11 |
| 8 | 3 |
|
|
Twice | 6 | 9 |
| 5 | 4 |
|
| ≥Three
times | 2 | 1 |
| 1 | 0 |
|
| Hemostatic
methods |
|
| 0.437 |
|
| 0.035 |
|
ABO | 1 | 7 |
| 2 | 5 |
|
|
UAE | 0 | 2 |
| 0 | 2 |
|
|
AUAL | 9 | 27 |
| 18 | 9 |
|
All patients underwent planned cesarean section and
no emergency surgery was performed in the present study. One PAS
patient underwent subtotal hysterectomy. A total of 4 PAS patients
delivered with broken placentae. The other 35 patients delivered
with almost complete placentae. No maternal mortality occurred.
There was no case of bladder invasion. One stillborn fetus was
delivered.
Inter-observer agreement
Interobserver agreement was excellent for one of the
eleven MRI features (markedly heterogeneous placenta, κ>0.8),
good for three MRI features (intraplacental thick dark bands,
increased placental vascularity, increased uterine vascularity,
κ>0.6) and moderate for two MRI features (myometrial thinning,
focal defect of the interval between the bladder and uterus,
κ>0.4). For the remaining five MRI features (placenta previa,
focal defect of the UPI, disruption of the inner layer of the UPI,
uterine bulge and increased vascularity in UPI), the κ-values for
the 40 patients with suspected PAS (κ-all) and for the 29 patients
with confirmed PAS (κ-PAS) were not in the same interval, but the
inter-observer agreement was statistically significant. The
detailed κ-values of each MRI feature are provided in Table II. Inter-observer agreement was fair
(κ-all=0.375) only for the MRI feature of uterine bulge.
 | Table II.Inter-observer agreement for MRI
features. |
Table II.
Inter-observer agreement for MRI
features.
| MRI feature | κ-alla | P-value | κ-PASa | P-value |
|---|
| Placenta
previa | 0.787 | <0.001 | 0.886 | <0.001 |
| Focal defect of the
UPI | 0.615 | <0.001 | 0.557 | 0.002 |
| Myometrial
thinning | 0.593 | <0.001 | 0.551 | 0.003 |
| Disruption of the
inner layer of the UPI | 0.627 | <0.001 | 0.514 | 0.005 |
| Intraplacental
thick dark bands | 0.725 | <0.001 | 0.703 | <0.001 |
| Focal defect of the
IBU | 0.481 | <0.001 | 0.473 | 0.003 |
| Increased placental
vascularity | 0.654 | <0.001 | 0.623 | 0.001 |
| Markedly
heterogeneous placenta | 0.908 | <0.001 | 0.901 | <0.001 |
| Uterine bulge | 0.375 | 0.018 | 0.426 | 0.017 |
| Increased uterine
vascularity | 0.714 | <0.001 | 0.703 | <0.001 |
| Increased
vascularity in UPI | 0.644 | <0.001 | 0.519 | 0.005 |
Association between involvement depth
and hemorrhage
There were 6 cases of placenta accreta (20.7%), 16
cases of placenta increta (55.2%) and 7 cases of placenta percreta
(24.1%). EBL, PRBC transfusion and plasma transfusion of patients
with placenta accreta, increta and percreta are compared in
Table III. The EBL exhibited an
increasing trend along with the implant depth, but without
significant difference (P>0.05). The differences in PRBC
transfusion, as well as plasma transfusion, among the three groups
were significant (P<0.05). The differences in PRBC transfusion
and plasma transfusion between the placenta accreta and placenta
percreta groups, as well as that between the placenta increta and
placenta percreta groups, were significant (P<0.05). However,
there was no significant difference between the placenta accreta
and the placenta increta groups (P>0.05).
 | Table III.Association between involvement depth
and hemorrhage. |
Table III.
Association between involvement depth
and hemorrhage.
| Involvement
depth | Patients (n) | EBL (ml) | PRBCs transfusion
(units) | Plasma transfusion
(ml) |
|---|
| Placenta
accreta | 6 |
717±299 | 1.33±2.07 |
67±103 |
| Placenta
increta | 16 | 1,275±801 | 4.09±3.72 | 350±322 |
| Placenta
percreta | 7 | 1,800±854 | 8.54±4.58 | 821±428 |
| P-value |
|
0.070 |
0.005 |
0.001 |
Association between MRI features and
hemorrhage
Differentiation between moderate and
massive hemorrhage group
To differentiate between the moderate and massive
hemorrhage groups, the MRI features of the two groups were
compared. Among the 29 PAS patients, the differences between the
two groups (moderate vs. massive hemorrhage group) were significant
in two MRI features (intraplacental thick dark bands, P=0.005;
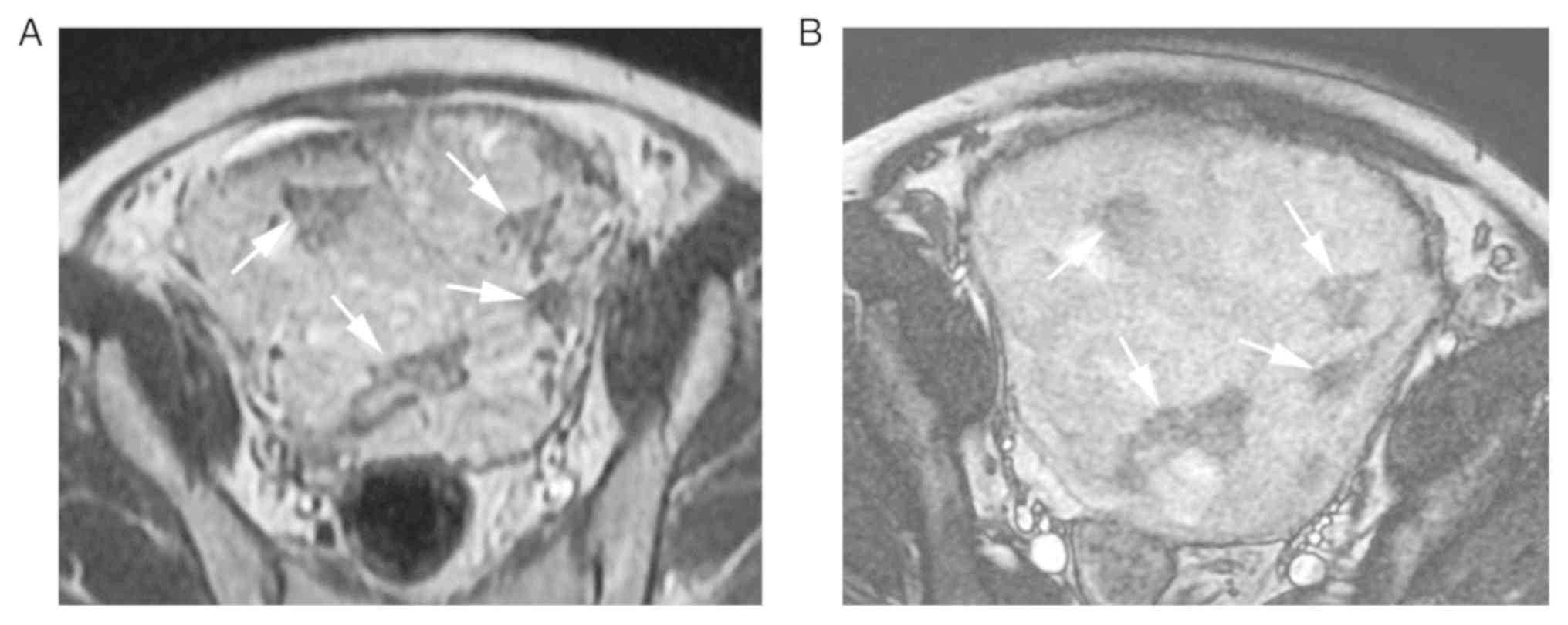
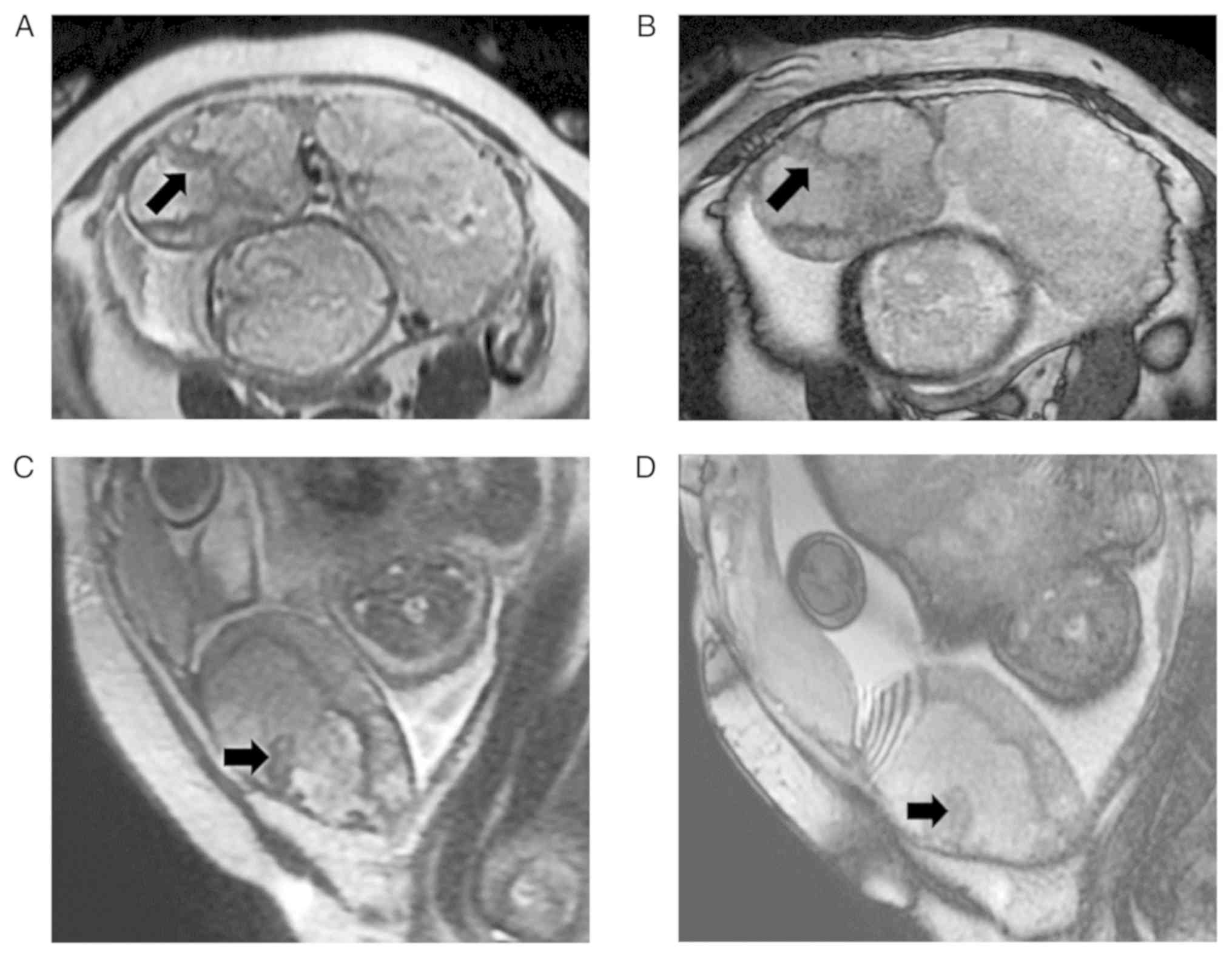
markedly heterogeneous placenta, P=0.020). The two MRI features are
provided in Figs. 1 and 2, respectively. The differences in the
other 9 MRI features between the two groups were not significant
(i.e. placenta previa, P=0.081; focal defect of the UPI, P=0.173;
myometrial thinning, P=0.059; disruption of the inner layer of the
UPI, P=0.054; focal defect of the interval between the bladder and
uterus, P=0.64; increased placental vascularity, P=0.198; uterine
bulge, P=0.777; increased uterine vascularity, P=0.596; increased
vascularity in UPI, P=0.206; Tables
IV and V). These results
indicated that the MRI features of intraplacental thick dark bands
and markedly heterogeneous placenta may be helpful in predicting
massive hemorrhage.
 | Table IV.EBL and blood transfusion in the
patients with PAS with different subtypes of placenta previa. |
Table IV.
EBL and blood transfusion in the
patients with PAS with different subtypes of placenta previa.
| Subtype of placenta
previa | Moderate
hemorrhage | Massive
hemorrhage | Total | EBL (ml) | Transfused PRBC
(units) | Transfusion of
plasma (ml) |
|---|
| Without | 1 | 0 | 1 | 600±0
(600–600) | 2.00±0.00
(2.00–2.00) | 0±0 (0–0) |
| Low-lying | 4 | 0 | 4 |
1,050±412 (600–1,600) | 3.00±2.58
(0.00–6.00) | 238±281
(0–550) |
| Marginal | 4 | 0 | 3 | 633±321
(400–1,000) | 0.00±0.00
(0.00–0.00) | 0±0 (0–0) |
| Partial | 1 | 1 | 2 | 1,250±1,061
(500–2,000) | 3.88±5.48
(0.00–7.75) | 500±707
(0–1,000) |
| Complete | 10 | 9 | 19 |
1,479±941 (300–3,500) |
5.87±4.53 (0.00–14.00) | 516±402
(0–1,200) |
| Total | 19 | 10 | 29 | P=0.477 | P=0.206 | P=0.187 |
 | Table V.Differentiation between moderate and
massive hemorrhage group of PAS patients with MRI features. |
Table V.
Differentiation between moderate and
massive hemorrhage group of PAS patients with MRI features.
| MRI feature | Moderate hemorrhage
(n=19) | Massive hemorrhage
(n=10) | P-value |
|---|
| Focal defect of the
UPI | 13 | 9 | 0.173 |
| Myometrial
thinning | 11 | 9 | 0.059 |
| Disruption of the
inner layer of the UPI | 15 | 10 | 0.054 |
| Intraplacental
thick dark bands | 1 | 5 | 0.005 |
| Focal defect of
IBU | 1 | 1 | 0.640 |
| Increased placental
vascularity | 2 | 3 | 0.198 |
| Markedly
heterogeneous placenta | 2 | 5 | 0.020 |
| Uterine bulge | 3 | 2 | 0.777 |
| Increased uterine
vascularity | 4 | 3 | 0.596 |
| Increased
vascularity in UPI | 5 | 5 | 0.206 |
MRI features as grouping
variables
The differences in EBL, PRBC transfusion and plasma
transfusion were compared between the patients with and without
each MRI feature (Table VI). There
were no significant differences in EBL and blood transfusion among
the different subtypes of placenta previa (Table IV). The differences in EBL between
the patients with and without the three MRI features were
significant (i.e., focal defect of the UPI, intraplacental thick
dark bands, markedly heterogeneous placenta; P<0.05). The three
MRI features are presented in Fig.
3. The differences in PRBC transfusion and plasma transfusion
between the patients with and without the six MRI features were
significant (P<0.05). The six MRI features included myometrial
thinning, disruption of the inner layer of the UPI, intraplacental
thick dark bands, increased placental vascularity, markedly
heterogeneous placenta and increased vascularity in UPI (Table VI). Representative images of the six
MRI features are displayed in Figs.
1–3.
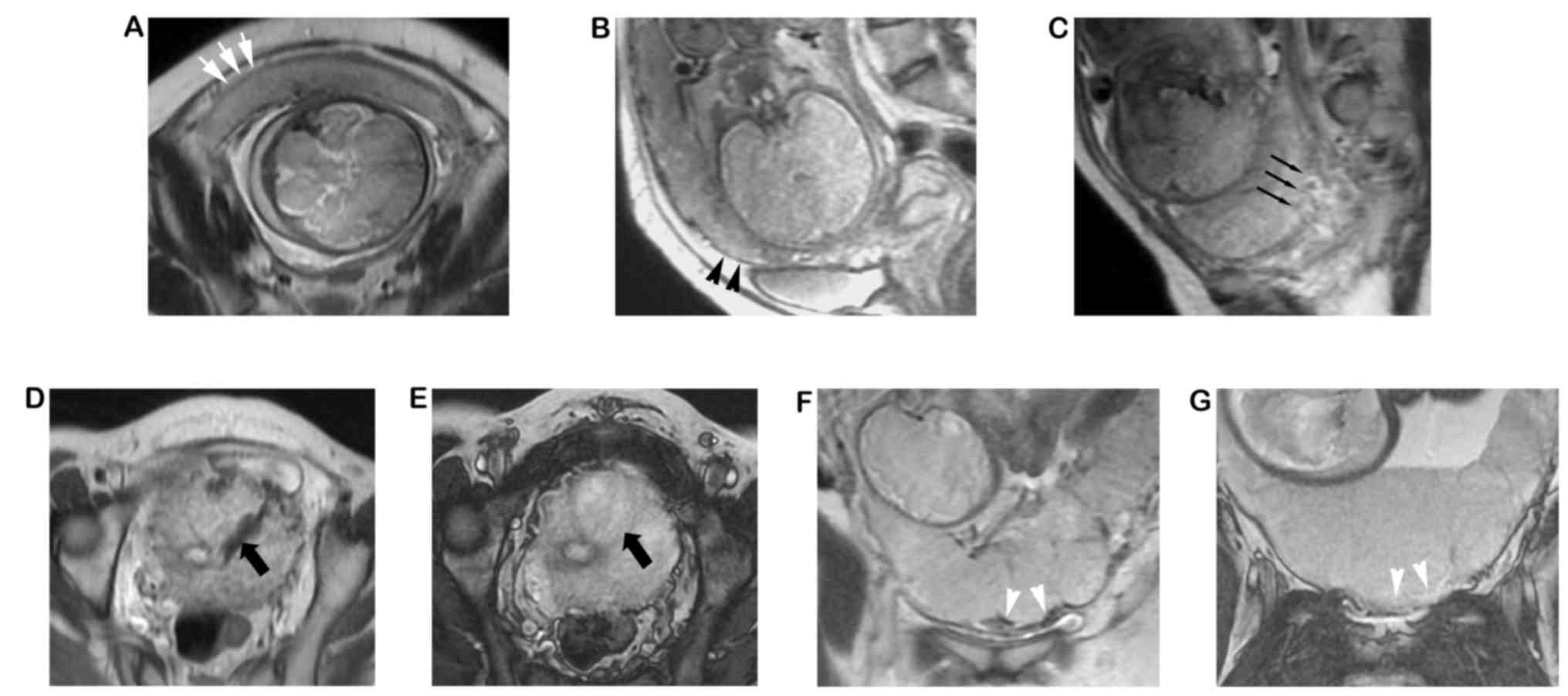 | Figure 3.MRI images from five different
patients. (A) Image obtained from patient 1, where focal defects of
the UPI were indicated by white arrows. Thickness, 2.2 mm. The
three layers of the myometrium could not be displayed clearly. (B)
Patient 2, displaying myometrial thinning (black arrowheads;
thickness, 1.7 mm) and focal defect of the UPI; (C) Patient 3,
displaying disruption on the inner layer of the UPI (black thin
arrows), where the inner layer of the UPI was interrupted; (D and
E) Patient 4, exhibiting increased placental vascularity (black
thick arrows). (D) The vessel exhibited a lack of flow on the
single-shot fast spin echo T2-weighted image and was isointense on
the (E) fast imaging employing steady-state acquisition image. (F
and G) Patient 5, where increased vascularity was observed at the
UPI. (F) A lack of flow on the SSFSE T2-weighted image and (G)
isointense on the FIESTA image (white arrowheads). SSFSE,
single-shot fast spin echo; FIESTA, fast imaging employing
steady-state acquisition; UPI, uteroplacental interface. |
 | Table VI.Differences in EBL, PRBC transfusion
and plasma transfusion between the patients with and without each
MRI feature. |
Table VI.
Differences in EBL, PRBC transfusion
and plasma transfusion between the patients with and without each
MRI feature.
|
| EBL (ml) | PRBC transfusion
(Units) | Plasma transfusion
(ml) |
|---|
|
|
|
|
|
|---|
| MRI feature | No | Yes | P-value | No | Yes | P-value | No | Yes | P-value |
|---|
| Placenta
previa | – | – | 0.477 | – | – | 0.206 | – | – | 0.187 |
| Focal defect of the
UPI | 843±326 | 1,427±926 | 0.018 | 2.86±3.44 | 5.15±4.54 | 0.232 | 200±365 | 470±407 | 0.129 |
|
| (600–1,500) | (300–3,500) |
| (0.00–10.00) | (0.00–14.00) |
| (0–1000) | (0–1,200) |
|
| Myometrial
thinning | 922±689 | 1,450±887 | 0.126 | 2.17±2.60 | 5.69±4.59 | 0.015 | 156±219 | 518±427 | 0.006 |
|
| (300–2,500) | (400–3,500) |
| (0.00–7.50) | (0.00–14.00) |
| (0–600) | (0–1,200) |
|
| Disruption of the
inner | 750±520 | 1,372±874 | 0.181 | 1.00±1.15 | 5.17±4.42 | 0.001 | 50±100 | 462±411 | 0.000 |
| layer of the
UPI | (300–1,500) | (400–3,500) |
| (0.00–2.00) | (0.00–14.00) |
| (0–200) | (0–1,200) |
|
| Intraplacental
thick | 965±552 | 2517±679 | 0.001 | 3.47±3.81 | 8.92±3.77 | 0.004 | 304±366 | 792±341 | 0.007 |
| dark bands | (300–2,500) | (1,600–3,500) |
| (0.00–14.00) | (4.00–14.00) |
| (0–1000) | (400–1,200) |
|
| Focal defect of the
IBU | 1,248±873 | 1,800±283 | 0.388 | 4.19±4.10 | 10.00±5.66 | 0.068 | 370±391 | 875±460 | 0.092 |
|
| (300–3,500) | (1,600–2,000) |
| (0.00–14.00) | (6.00–14.00) |
| (0–1,200) | (550–1,200) |
|
| Increased
placental | 1,154±845 | 1,920±638 | 0.067 | 3.55±3.60 | 9.60±4.56 | 0.003 | 325±381 | 790±325 | 0.017 |
| vascularity | (300–3,500) | (1,000–2,500) |
| (0.00–12.00) | (4.00–12.00) |
| (0–1,200) | (400–1,200) |
|
| Heterogeneous
placenta | 964±565 | 2,300±845 | 0.000 | 3.44±3.90 | 8.21±3.91 | 0.009 | 300±374 | 736±345 | 0.011 |
|
| (300–2,500) | (1,000–3,500) |
| (0.00–14.00) | (4.00–14.00) |
| (0–1,000) | (400–1,200) |
|
| Uterine bulge | 1,254±864 | 1,440±891 | 0.667 | 4.13±4.10 | 6.80±5.40 | 0.220 | 375±397 | 550±477 | 0.393 |
|
| (400–3,500) | (300–2,500) |
| (0.00–14.00) | (0.00–14.00) |
| (0–1,200) | (0–1,200) |
|
| Increased
uterine | 1,172±770 | 1,643±1,069 | 0.211 | 4.42±4.20 | 5.14±5.15 | 0.710 | 389±411 | 457±428 | 0.706 |
| vascularity | (300–3,000) | (500–3,500) |
| (0.00–14.00) | (0.00–14.00) |
| (0–1,200) | (0–1,200) |
|
| Increased
vascularity | 1,142±820 | 1,560±896 | 0.217 | 3.33±2.85 | 7.00±5.75 | 0.028 | 295±315 | 615±494 | 0.042 |
| in UPI | (300–3,500) | (500–3,000) |
| (0.00–10.00) | (0.00–14.00) |
| (0–1,000) | (0–1,200) |
|
Discussion
In the present study, not only all of the PAS
patients but also all the non-PAS patients had placenta previa
and/or a history of at least one prior cesarean section and/or one
abortion. There was a large proportion of PAS (72.5%, 29/40) in the
present study. This may be due to inclusion of patients with
suspicious PAS or inconclusive findings on US who are at high risk
of PAS.
There is an emphasis in China on uterine-sparing
management. Therefore, the diagnosis of PAS for most patients was
confirmed according to intra-operative findings. There is a recent
International Federation of Gynecology and Obstetrics (FIGO) system
of clinical classification based on clinical findings (16). The diagnosis of placenta accreta and
percreta in the present study was basically consistent with that of
the FIGO system. In the present study, the diagnosis of placenta
increta was based on the placenta separation method of forceps
curettage. Although the diagnosis of placenta increate was
described differently, the meaning was consistent with that of the
FIGO system.
The EBL is notoriously subjective and subject to
error, particularly when the volume is very low or very high
(17). EBL was estimated by an
experienced obstetrician in this study. Different obstetricians may
have different estimates. Therefore, the association between PRBC
transfusion and plasma transfusion, as well as MRI features, was
evaluated in the present study. There were no significant
differences in red blood cells, hemoglobin, hematocrit, prothrombin
time and INR between the massive hemorrhage group and the moderate
hemorrhage group. There was a significant difference in APTT
between the two groups, but the APTT of each group was in the
normal range. They were therefore unlikely to have influenced the
PRBC and plasma transfusion.
The κ-all and κ-PAS values exhibited certain
differences, which may be due to the small number of patients,
resulting in the low robustness of the κ-values. The inter-observer
agreement for most MRI features was equal to or superior to
moderate. The inter-observer agreement for only uterine bulge
(κ-all) was fair. The inter-observer reliability may be influenced
in part by the differences in the experience of the radiologists
interpreting the images. The eleven MRI features in the present
study were proved to have a role in differentiating PAS from normal
placentae or determining implant depth (14,18–22).
The EBL exhibited a trend to increase along with the
placental implant depth in the present study. However, there was no
significant difference among the three groups (P=0.070). Of note,
the EBL does not always increase with the increase of the implant
depth; however, studies on the association between MRI features and
hemorrhage of PAS patients prior to delivery are limited. Chen
et al (11) defined blood
loss of >1,000 ml during surgery as significant hemorrhage. Poor
maternal outcome was defined as parturient with significant
hemorrhage or emergency hysterectomy. They reported that low signal
intensity bands on T2-weighted imaging may be a predictor of poor
maternal outcome after UAE-assisted cesarean section in patients
with invasive placenta previa. The intraplacental thick dark bands
and markedly heterogeneous placenta were reported to be important
MRI features not only in predicting massive hemorrhage but also the
differentiating factors for EBL, PRBC transfusion and plasma
transfusion. Intra-placental thick dark bands were the result of
fibrin deposition (14). In certain
previous studies, intra-placental thick dark bands can
differentiate between PAS and non-PAS (14,18,22). In
the present study, intra-placental thick dark bands were observed
more frequently in the massive hemorrhage group than in the
moderate hemorrhage group. Fibrin deposition may result in narrowed
intervillous space (18,23). The maternal vessels (spiral arteries
and draining veins) may be dilated or increased to enhance blood
flow to the placenta. Increased and/or dilated vessels may result
in more hemorrhage when the placenta is manually removed.
A markedly heterogeneous placenta is associated with
invasive placentation (18,19). Lax et al (19) and Ueno et al (22) indicated that a markedly heterogeneous
placenta was more frequently observed in cases of PAS than in
normal placentae. Bour et al (14) reported that a markedly heterogeneous
placenta was not significantly associated with the diagnosis of
PAS, but more frequently observed in patients with placenta
percreta than in those with placenta accreta. In the present study,
it was observed that a markedly heterogeneous placenta was more
frequent in the massive hemorrhage group than in the moderate
hemorrhage group. It was indicated that the characterization of
markedly heterogeneous placenta partly depended on the presence of
intraplacental thick dark bands and increased placental
vascularity.
Bour et al (14) reported that thinning or focal defect
of the UPI was significantly associated with the diagnosis of
invasive placenta and was the single independent predictor of
invasive placenta. This study suggested that the EBL of patients
with focal defect of the UPI was more than that of the patients
without this MRI feature. However, the blood transfusion difference
between the patients with and without the MRI feature was not
significant.
Most PAS patients have the MRI feature of myometrial
thinning, but this feature is not unique, as numerous maternal
patients with a normal placenta also have such a feature (22). The disruption of the inner layer of
the UPI had 81% sensitivity for the diagnosis of PAS (14). Increased placental vascularity was
significantly associated with PAS (18,22,24).
Increased vascularity in UPI was identified as a novel MRI feature
in the present study. The maternal spiral arteries at the
myometrium-placenta interface run parallel to the villous branches
of the chorionic arteries and perpendicular to the decidua surface.
The vessels at the UPI may be prone to rupture when the placenta is
manually removed. Although the difference in EBL between the
patients with and without the four MRI features was not
significant, the difference in blood transfusion was significant,
which may reflect blood loss to a certain extent.
The present study had several limitations. First, it
was a retrospective study. The hemostasis methods were not arranged
in advance. The proportion of patients who underwent ABO in the
massive hemorrhage group was greater than that in the moderate
hemorrhage group, but the ABO was more effective (25). Therefore, the hemostasis method of
ABO should not influence the results. In addition, patients who
underwent MRI had already been screened by US, and there was
already suspicion for PAS, particularly suspicion for placenta
increta and percreta. The diagnostic accuracy was therefore biased
prior to interpretation. However, the radiologists were blinded to
the EBL, PRBC transfusion and plasma transfusion. Therefore, any
bias made during PAS diagnosis were unlikely to have affected the
results. A further limitation is the inaccurate estimation of blood
loss, particularly in those patients with massive hemorrhage
(8,17). To minimize this bias, EBL and blood
transfusion were analyzed and the hemostatic methods that may
affect blood loss were recorded. In addition, the size of the PAS
cohort was small, which may influence the results of the
statistical analysis. Finally, the present study was a
retrospective single-center study and selection bias may have been
present. Further multiple-center studies with larger samples are
warranted.
In conclusion, two MRI features, intraplacental
thick dark bands and markedly heterogeneous placenta, are helpful
in predicting massive hemorrhage in patients with PAS. Focal defect
of the UPI, myometrial thinning, disruption of the inner layer of
the UPI, increased placental vascularity and increased vascularity
at the UPI may also contribute to predicting hemorrhage to a
certain extent. Patients with these MRI features may have a higher
risk of massive hemorrhage and pre-operative preparations should be
arranged for them in advance.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Primary
Research and Development Plan of Shandong Province (grant no.
2016GSF201095).
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
All authors of this manuscript have made substantial
contributions to this work. All authors have read and approved the
final manuscript. The specific contribution of each author is
listed as follows: Study design: YL, ZH;Study conception: CZ, YL,
JZ. Case collection: JZ, ZH, HX, QL. Statistical analysis: JZ.
Image capture: YX, ZL; US operation: XH; Clinical supervision: CZ,
YL; Manuscript preparation: JZ, YL, ZH; Manuscript revision: QL,
ZH, XH.
Ethics approval and consent to
participate
The study was performed in accordance with the
standards set out in the Code of Ethics of the World Medical
Association (Declaration of Helsinki) and the research procedures
were approved by the ethics review board of Shandong Provincial
Hospital (Jinan, China). Informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
PAS
|
placenta accrete spectrum
|
|
EBL
|
estimated blood loss
|
|
UPI
|
uteroplacental interface
|
|
AUAL
|
ascending uterine artery ligation
|
|
UAE
|
uterine artery embolization
|
|
ABO
|
aorta balloon occlusion
|
|
US
|
ultrasonography
|
|
FOV
|
field of view
|
|
PRBCs
|
packed red blood cells
|
References
|
1
|
Chattopadhyay SK, Kharif H and Sherbeeni
MM: Placenta praevia and accreta after previous caesarean section.
Eur J Obstet Gynecol Reprod Biol. 52:151–156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fox KA, Shamshirsaz AA, Carusi D, Secord
AA, Lee P, Turan OM, Huls C, Abuhamad A, Simhan H, Barton J, et al:
Conservative management of morbidly adherent placenta: Expert
review. Am J Obstet Gynecol. 213:755–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi H, Quan X, Sun X, Center R, Hospital Z
and University SM: Antenatal MRI findings of placental accreta.
Chin J Med Imaging. 23:474–477. 2015.
|
|
4
|
Huang G, Zhou R and Hu Y: A new suture
technique for cesarean delivery complicated by hemorrhage in cases
of placenta previa accreta. Int J Gynaecol Obstet. 124:262–263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minas V, Gul N, Shaw E and Mwenenchanya S:
Prophylactic balloon occlusion of the common iliac arteries for the
management of suspected placenta accreta/percreta: Conclusions from
a short case series. Arch Gynecol Obstet. 291:461–465. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salim R, Chulski A, Romano S, Garmi G,
Rudin M and Shalev E: Precesarean prophylactic balloon catheters
for suspected placenta accreta: A randomized controlled trial.
Obstet Gynecol. 126:1022–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shamshirsaz AA, Fox KA, Salmanian B,
Diaz-Arrastia CR, Lee W, Baker BW, Ballas J, Chen Q, Van Veen TR,
Javadian P, et al: Maternal morbidity in patients with morbidly
adherent placenta treated with and without a standardized
multidisciplinary approach. Am J Obstet Gynecol. 212:218.e1–e9.
2015. View Article : Google Scholar
|
|
8
|
Wright JD, Pri-Paz S, Herzog TJ, Shah M,
Bonanno C, Lewin SN, Simpson LL, Gaddipati S, Sun X, DAlton ME and
Devine P: Predictors of massive blood loss in women with placenta
accreta. Am J Obstet Gynecol. 205:38.e1–e6. 2011. View Article : Google Scholar
|
|
9
|
Hasegawa J, Matsuoka R, Ichizuka K, Mimura
T, Sekizawa A, Farina A and Okai T: Predisposing factors for
massive hemorrhage during Cesarean section in patients with
placenta previa. Ultrasound Obstet Gynecol. 34:80–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baba Y, Matsubara S, Ohkuchi A, Usui R,
Kuwata T, Suzuki H, Takahashi H and Suzuki M: Anterior placentation
as a risk factor for massive hemorrhage during cesarean section in
patients with placenta previa. J Obstet Gynaecol Res. 40:1243–1248.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen T, Xu XQ, Shi HB, Yang ZQ, Zhou X and
Pan Y: Conventional MRI features for predicting the clinical
outcome of patients with invasive placenta. Diagn Interv Radiol.
23:173–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elhawary TM, Dabees NL and Youssef MA:
Diagnostic value of ultrasonography and magnetic resonance imaging
in pregnant women at risk for placenta accreta. J Matern Fetal
Neonatal Med. 26:1443–1449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cali G, Giambanco L, Puccio G and Forlani
F: Morbidly adherent placenta: Evaluation of ultrasound diagnostic
criteria and differentiation of placenta accreta from percreta.
Ultrasound Obstet Gynecol. 41:406–412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bour L, Place V, Bendavid S, Fargeaudou Y,
Portal JJ, Ricbourg A, Sebbag D, Dohan A, Vicaut E and Soyer P:
Suspected invasive placenta: Evaluation with magnetic resonance
imaging. Eur Radiol. 24:3150–3160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YL, Song T, Liu Y, Huang J and He Y:
Diagnosis of prenatal MRI in placenta implantation abnormality.
Chin J Med Imaging. 23:470–473, 477. 2015.
|
|
16
|
Jauniaux E, Ayres-de-Campos D,
Langhoff-Roos J, Fox KA and Collins S; FIGO Placenta Accreta
Diagnosis and Management Expert Consensus Panel, : FIGO
classification for the clinical diagnosis of placenta accreta
spectrum disorders. Int J Gynaecol Obstet. 146:20–24. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dildy GA III, Paine AR, George NC and
Velasco C: Estimating blood loss: Can teaching significantly
improve visual estimation? Obstet Gynecol. 104:601–606. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derman AY, Nikac V, Haberman S, Zelenko N,
Opsha O and Flyer M: MRI of placenta accreta: A new imaging
perspective. AJR Am J Roentgenol. 197:1514–1521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lax A, Prince MR, Mennitt KW, Schwebach JR
and Budorick NE: The value of specific MRI features in the
evaluation of suspected placental invasion. Magn Reson Imaging.
25:87–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahaim NS and Whitby EH: The MRI features
of placental adhesion disorder and their diagnostic significance:
Systematic review. Clin Radiol. 70:917–925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teo TH, Law YM, Tay KH, Tan BS and Cheah
FK: Use of magnetic resonance imaging in evaluation of placental
invasion. Clin Radiol. 64:511–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ueno Y, Kitajima K, Kawakami F, Maeda T,
Suenaga Y, Takahashi S, Matsuoka S, Tanimura K, Yamada H, Ohno Y
and Sugimura K: Novel MRI finding for diagnosis of invasive
placenta praevia: Evaluation of findings for 65 patients using
clinical and histopathological correlations. Eur Radiol.
24:881–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huppertz B: The anatomy of the normal
placenta. J Clin Pathol. 61:1296–1302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Familiari A, Liberati M, Lim P, Pagani G,
Cali G, Buca D, Manzoli L, Flacco ME, Scambia G and Dantonio F:
Diagnostic accuracy of magnetic resonance imaging in detecting the
severity of abnormal invasive placenta: A systematic review and
meta-analysis. Acta Obstet Gynecol Scand. 97:507–520. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li K, Zou Y, Sun J and Wen H: Prophylactic
balloon occlusion of internal iliac arteries, common iliac arteries
and infrarenal abdominal aorta in pregnancies complicated by
placenta accreta: A retrospective cohort study. Eur Radiol.
28:4959–4967. 2018. View Article : Google Scholar : PubMed/NCBI
|