Introduction
Endothelial progenitor cells (EPCs) are able to
mobilize into peripheral blood, home in to an injured area and
differentiate into endothelial cells (ECs), thereby contributing to
the improvement of endothelial function (1–3).
Previous studies have indicated that EPCs are able to exert a
protective effect in experimental disease models, including
hindlimb ischemia, myocardial infarction and kidney injury
(4–8).
Microvesicles (MVs) are small membrane particles
0.1–1 µm in size (9). In response to
different stimuli, MVs are released by various cell types,
including EPCs, and deliver proteins and genetic information,
including mRNA and microRNA (miRNA/miR), to recipient cells,
representing a novel method of cell-to-cell communication (10–12).
Recent studies have reported that MVs released from different
stimuli exert different functions at recipient cells (13,14).
Numerous mammalian organs are vulnerable to
hypoxic-ischemic (HI) insult. HI exposure induces a series of
changes in mammalian cells that remain incompletely understood. In
the short term, HI adaptations are important for improving survival
and function, and may be essential for optimal function.
However, to date, the effects of different
pathological stimuli on EPC-derived MVs have remained to be fully
determined. Oxygen-glucose deprivation (OGD) is a commonly used
model to mimic HI in vitro (15). The present study aimed to investigate
the potential effects of HI on EPC-derived MVs (EPC-MVs) by using
OGD culture.
Materials and methods
Culture of EPCs
EPC culture and characterization was performed as
previously described (16,17). In brief, mononuclear cells were
isolated from the spleens of Sprague Dawley (SD) rats (n=6; male;
age, 12 weeks; weight, 320–380 g; Third Military Medical
University, Chongqing, China). The cells were counted and seeded on
fibronectin-coated 24-well plates (BD Biosciences) at
1×106 cells/well and then grown in EC basal medium 2
(EBM-2; Gibco; Thermo Fisher Scientific, Inc.), supplemented with
5% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.) containing an EPC growth cytokine cocktail (Gibco; Thermo
Fisher Scientific, Inc.) in a 5% CO2 incubator at 37°C.
After 3 days, any non-adherent cells were removed by rinsing three
times with PBS. Thereafter, the culture medium was changed every
two days.
OGD
In vitro HI insult was simulated using OGD
methods. In brief, the cells were washed three times with
glucose-free medium (125 mM NaCl, 2.8 mM KCl, 1.5 mM
MgCl2, 0.05 mM MgSO4, 2 mM CaCl2,
0.83 mM NaH2PO4, 24 mM NaHCO3 and
2 mM HEPES) prior to oxygen removal and placed in an anaerobic
chamber perfused with a gas mixture of humidified 95% N2
and 5% CO2 for 3 h at 37°C. Control cells were subjected
to three washes to control for the mechanical stress associated
with medium changes, but otherwise remained in the control culture
medium at 37°C in a regular 5% CO2 and 95% air
incubator.
Preparation of MVs
MVs were generated from rat EPCs cultured in OGD
culture medium (OGD-MVs) or normoxic-condition culture medium
(n-MVs). In brief, EPCs were cultured in 100-mm cell culture
dishes. When the cells reached 80% confluence, they were washed
with PBS and then cultured in fresh culture growth medium (EBM-2)
or glucose-deprivation medium under oxygen-deprivation conditions
in the absence of serum. The cell medium, from 12 wells as an
aliquot, was collected and centrifuged at 300 × g for 5 min at 4°C,
followed by 2,000 × g for 15 min 4°C, to remove cells and cell
debris. The supernatants were ultracentrifuged at 100,000 × g
(Optima L-80 ultracentrifuge; Beckman Coulter) at 4°C for 2 h, and
then resuspended in PBS after two washes with PBS, each of which
was followed by ultracentrifugation at 100,000 × g for 1 h at 4°C.
The protein concentration of MVs was quantified via BCA Protein
Assay Kit (Beyotime Institute of Biotechnology).
Flow cytometry
Cultured cells were trypsinized and resuspended in
PBS containing 1% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.). Cell suspensions were incubated with different
antibodies, including CD133-APC (1:100; cat. no. 130-090-422;
Miltenyi Biotec), CD34-FITC (1:50; cat. no. sc-7324; Santa Cruz
Biotechnology, Inc.), von Willebrand factor (vWF)-FITC (1:200; cat.
no. ab8822; Abcam) and fetal liver kinase (Flk)-1-AF647 (1:50; cat.
no. sc-6251 AF647; Santa Cruz Biotechnology, Inc.) for 30 min at
room temperature in the dark. MVs were resuspended and incubated
for 30 min at 4°C in the dark with Annexin V-FITC (BD Biosciences).
Isotype-matched (IgG) non-specific antibodies served as negative
controls. After incubation, labeled cells or MVs were resuspended
with PBS and analyzed using flow cytometry (BD Biosciences).
Transmission electron microscopy
For scanning transmission electron microscopy, MVs
were fixed in Karnovsky fixative, dehydrated in alcohol, dried on a
glass surface and coated with gold by sputter. The specimens were
visualized using a Philips Tecnai-10 transmission electron
microscope (Philips).
Intracellular Ca2+
detection
The Ca2+-sensitive indicator Fluo-3/AM
(BD Biosciences) was used to determine intracellular
Ca2+. The dye-loading buffer contained Fluo-3-AM
(dissolved in DMSO and pluronic acid) at a final concentration of 4
mM in serum-free cell culture maintenance medium containing 20 mM
HEPES and 2.5 mM probenecid. The cells were incubated with the
dye-loading buffer for ~30 min at 37°C. Ca2+ levels were
determined using a FACScan flow cytometer (BD Biosciences) with an
excitation wavelength of 488 nm and an emission wavelength of 525
nm.
Co-culture assay of EPC-MVs and
EPCs
EPC-MVs were labeled with DiI (Sigma-Aldrich; Merck
KGaA) according to the manufacturer's protocol. In brief, EPC-MVs
were labeled with 10 µM DiI in PBS for 10 min at room temperature.
An equal volume of FBS was added to stop the staining reaction.
EPC-MVs were then ultracentrifuged and resuspended in culture
medium for co-culture experiments, wherein the labeled EPC-MVs were
added to EPCs, followed by culture for 24 h in an incubator (37°C,
5% CO2). Cell nuclei were stained with DAPI
(Sigma-Aldrich; Merck KGaA). The interaction between EPC-MVs and
EPCs was examined by fluorescence microscopy (Nikon Corp.).
Cell viability assay
Cell viability was assessed with a Cell Counting
Kit-8 (CCK-8; Dojindo). EPCs were seeded into 96-well plates
(50,000 cells in 100 µl/well). Following co-culture with n-MVs,
OGD-MVs and pretreated OGD-MVs by using RNase (Thermo Fisher
Scientific, Inc.), 10 µl CCK-8 solution was added to each well,
according to the manufacturer's protocol. After incubation at 37°C
for 3 h in a humidified CO2 incubator, the optical
density (OD) for each well was measured using a microplate reader
(Bio-Rad Laboratories, Inc.) at a wavelength of 450 nm. The OD
values were then used to calculate the cell viability by setting
the control as 100%.
Reverse transcription-quantitative
(RT-q)PCR analysis of miR-210
miR-210 expression was quantified by RT-qPCR, as
described previously (18). Total
RNA was extracted from MVs harvested from EPC culture medium using
a TRIzol isolation system, according to the instructions of the
manufacturer (Invitrogen; Thermo Fisher Scientific, Inc.).
Complementary DNA was synthesized using an miScript RT kit
(Qiagen). RT-qPCR was performed with specific primers for miR-210
or U6 and a miScript SYBR Green PCR Kit (Qiagen) on a real-time PCR
system (Bio-Rad Laboratories, Inc.). U6 was used as the internal
control. The primers used are listed in Table I. The thermocycling conditions were:
Initial denaturation at 94°C for 4 min, followed by a total of 35
cycles of 94°C for 20 sec, 60°C for 30 sec and 72°C for 30 sec. All
experiments were run in triplicate and the relative expression of
miR-210 was calculated using the 2−ΔΔCq method (19).
 | Table I.Primers used for quantitative PCR
analysis. |
Table I.
Primers used for quantitative PCR
analysis.
| Gene/primer
direction | Sequence |
|---|
| miR-210 |
|
|
Forward |
5′-GTGCAGGGTCCGAGGT-3′ |
|
Reverse |
5′-CTGTGCGTGTGACAGCGGCTGA-3′ |
| U6 |
|
|
Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
|
Reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
miR-210 overexpression in EPCs
EPCs were transfected with miR210 mimics (sense,
5′-CUGUGCGUGUGACAGCGGCUGA-3′ and antisense:
5′-UUGACACGCACACUGUCGCCGA; 20 nM; Western Biomedical Technology)
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) for 48 h. In the control group, EPCs were
transfected with scrambled miRNA (miRNA-NC; 20 nM; Western
Biomedical Technology). Overexpression was then verified by
RT-qPCR. The viability of the EPCs was detected using CCK-8 assay.
The control group were EPCs which were transfected with 20 nM
scrambled miRNA (miRNA-NC; sense, 5′-UUCUCCGAACGUGUGUCACGUTT-3′ and
antisense, 5′-ACGUGACACGUUCGGAGAATT-3′; Western Biomedical
Technology).
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed using SPSS v.18.0 software (SPSS, Inc.).
Statistical analyses were performed using one-way ANOVA with
Tukey's post hoc test for comparison among multiple groups or the
Student's t-test for comparison between two groups. P<0.05 was
considered to indicate statistical significance. All statistical
analyses were performed in a blinded manner.
Results
Isolation and identification of
EPCs
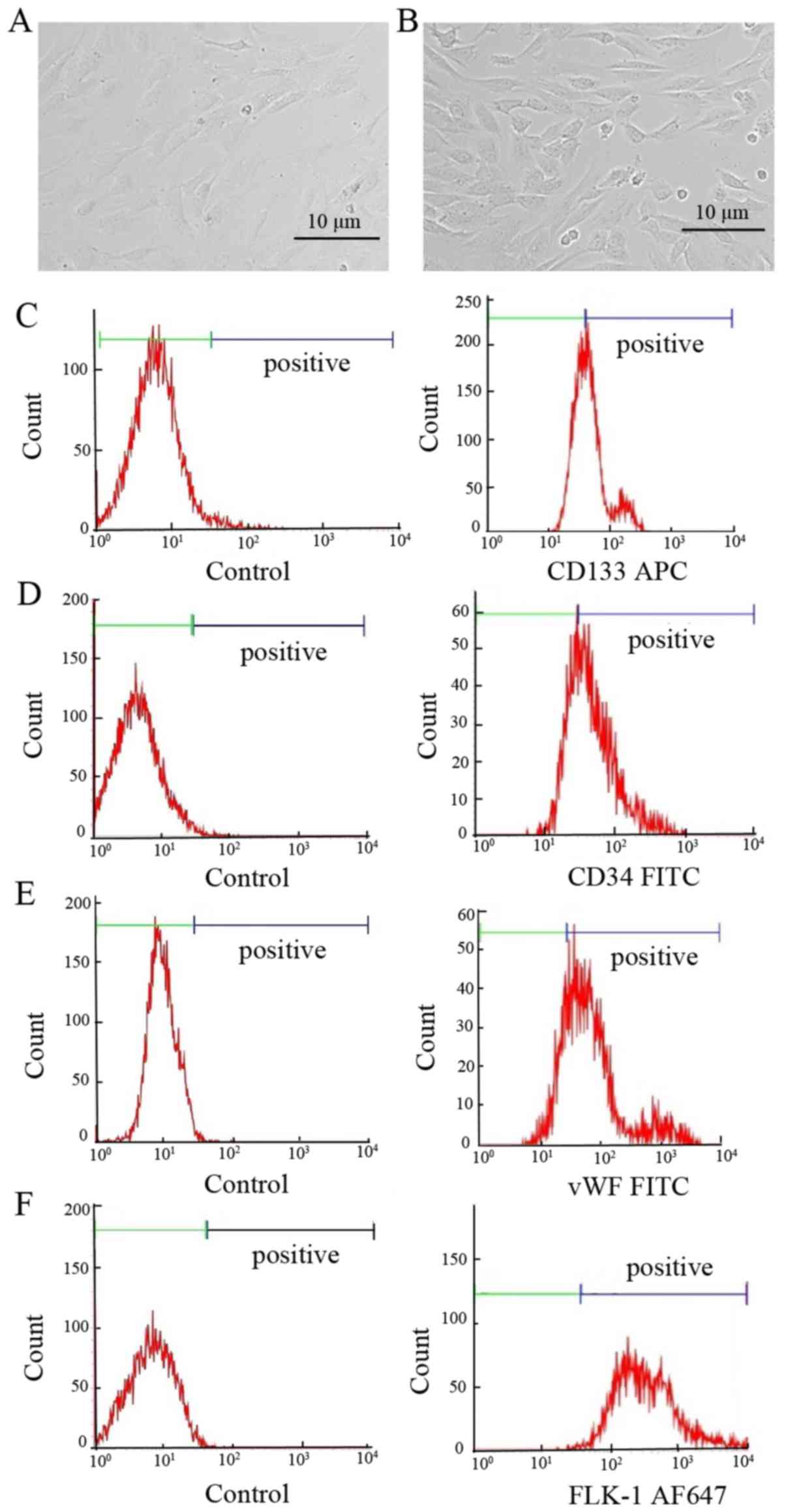
EPCs were isolated from 12-week-old SD rats and
successfully propagated in vitro. Isolated spleen-derived
EPCs exhibited a spindle-shaped morphology after 4–7 days of
culture (Fig. 1A and B). Although
there is currently no specific marker, it is generally agreed that
rat EPCs are positive for CD133, CD34, vWF and Flk-1 (20,21).
EPCs at passage 5 were identified by flow cytometry using these
cell makers. In the present study, 55–95% of the cells were tested
positive for CD133-, CD34-, vWF- or Flk-1 (Fig. 1C-F; Table
II). The data confirm that the cells used in the present study
were indeed EPCs.
 | Table II.Ratio of positively labeled cells
identified by flow cytometry. |
Table II.
Ratio of positively labeled cells
identified by flow cytometry.
| Marker | Positive | Negative |
|---|
| CD133 | 58.98±1.98 | 43.72±2.05 |
| CD34 | 71.80±1.66 | 30.79±1.69 |
| vWF | 82.40±1.07 | 19.22±1.16 |
| Flk-1 | 95.61±0.65 | 2.1±1.21 |
OGD promotes the release of MVs
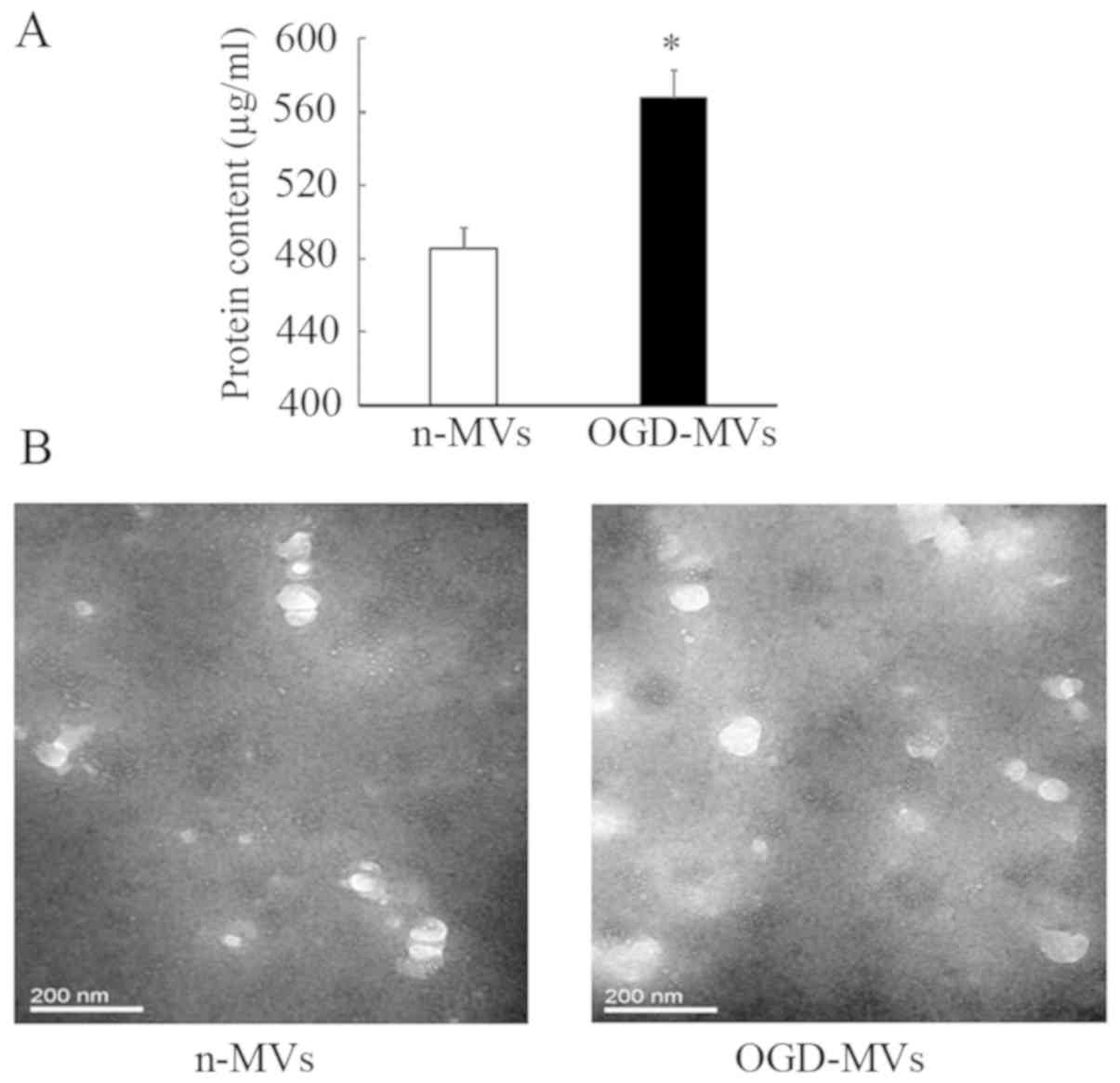
Protein quantification indicated that OGD treatment
increased the total protein in MVs harvested from EPC culture
medium. A clear increase in the total protein content of MVs was
observed after OGD (485.42±11.01 vs. 567.79±14.89 µg/ml with n-MVs
and OGD-MVs, respectively; P<0.05; Fig. 2A). In addition, in the images
captured via transmission electron microscopy, more MVs were
observed in the OGD group compared with those in the normoxic group
(Fig. 2B). These results implied
that increased numbers of MVs were released by EPCs after OGD
treatment.
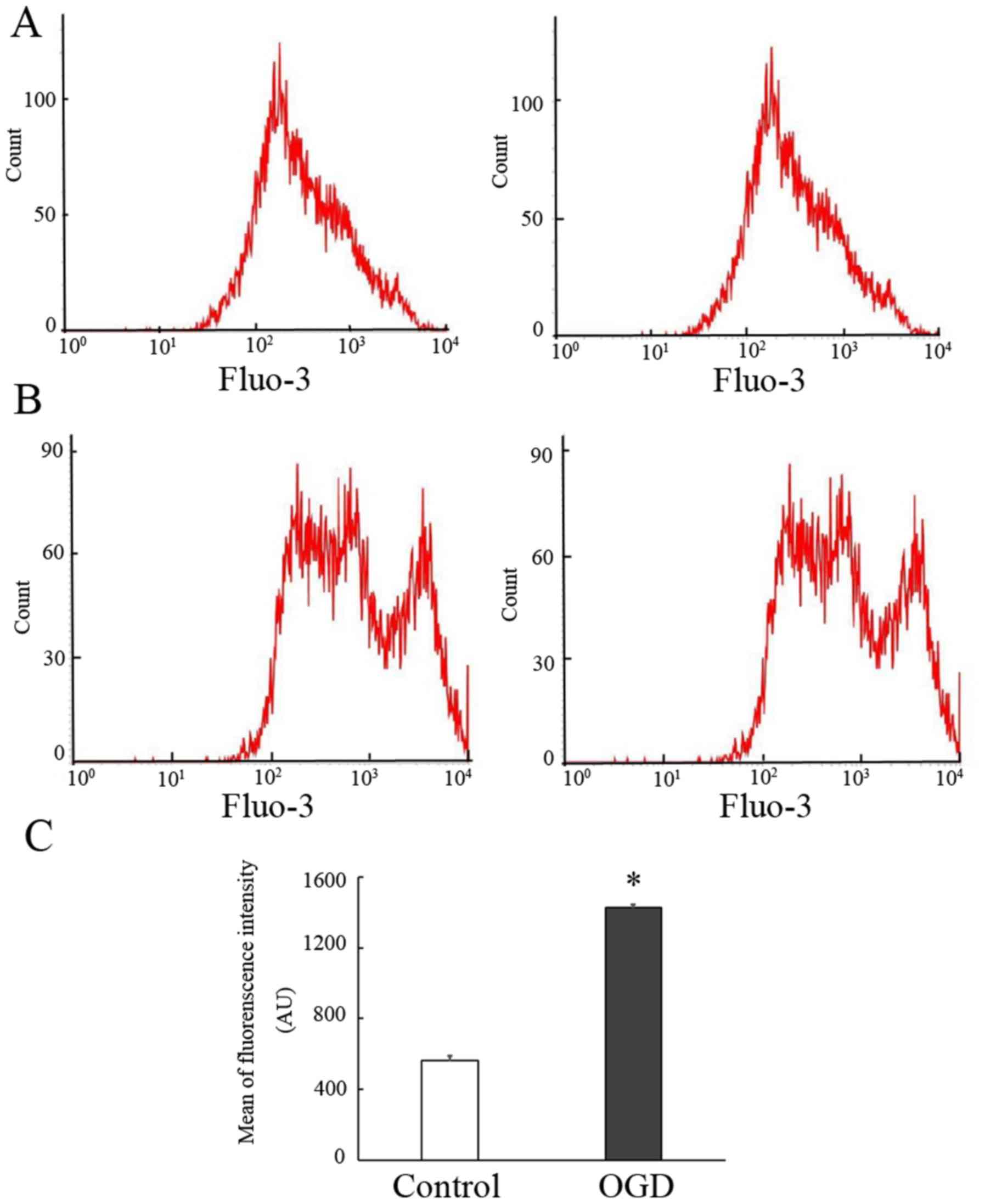
Previous studies have suggested that Ca2+
influx has a vital role in the process of MV release and that HI
may induce cellular Ca2+ influx (22). To determine whether the release of
MVs after OGD pre-treatment is associated with Ca2+
influx, intracellular Ca2+ levels were detected using
the fluorescent Ca2+ indicator Fluo-3/AM. In the present
study, it was indicated that treatment of EPCs with OGD resulted in
an apparent Ca2+ fluctuation. As presented in Fig. 3, a clear increase in the fluorescence
intensity was observed in the OGD group as compared with that in
the normoxic group (562.75±27.40 vs. 1,426.50±18.34% in the
normoxic and OGD groups, respectively; P<0.05). The results
suggested that the release of MVs from EPCs following OGD treatment
was also associated with Ca2+ influx.
OGD treatment induces increased
expression of miR210 in MVs
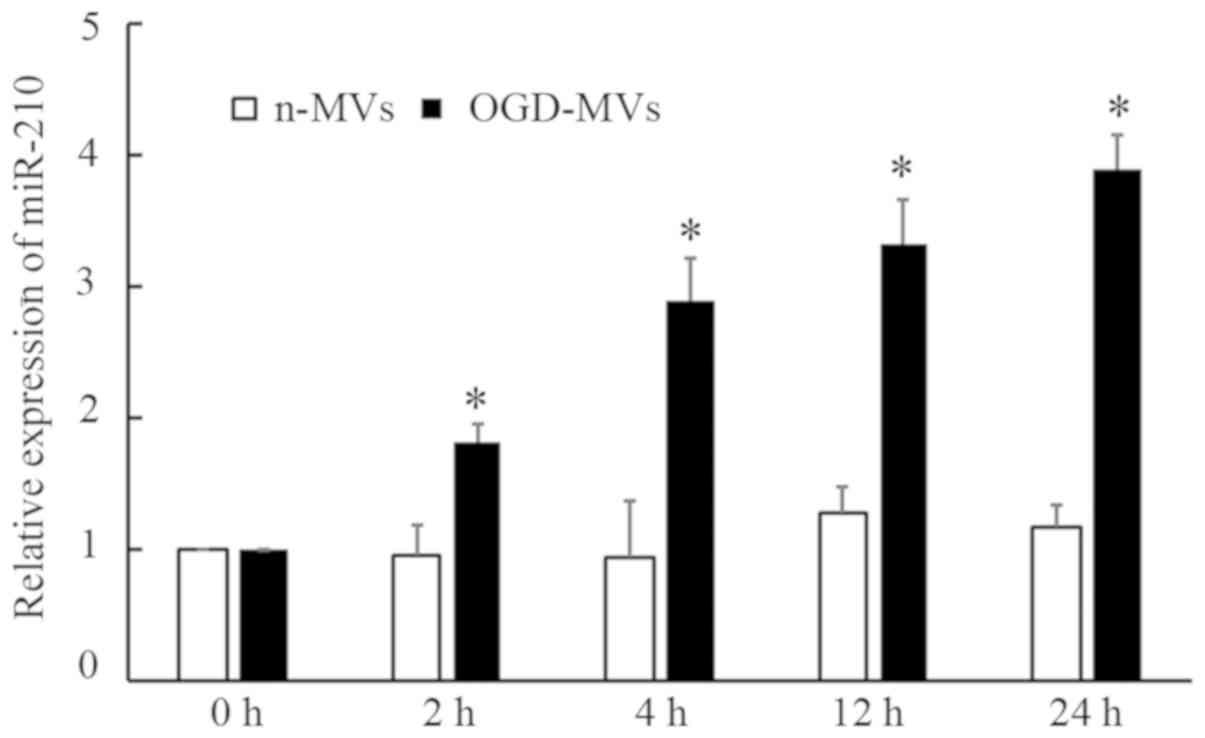
Considering miR-210 is sensitive to hypoxia and is
involved in certain pathogeneses associated with hypoxia, miR-210
levels in MVs were examined. As presented in Fig. 4, RT-qPCR analysis revealed that the
levels of miR-210 were markedly increased in OGD-MVs as compared
with those in the normoxic group. In addition, a time-dependent
increase was also observed in miR-210 expression in the OGD group.
These results demonstrated that miR-210 may be produced and
secreted by EPCs, and packaged further into MVs in response to OGD
(HI insult).
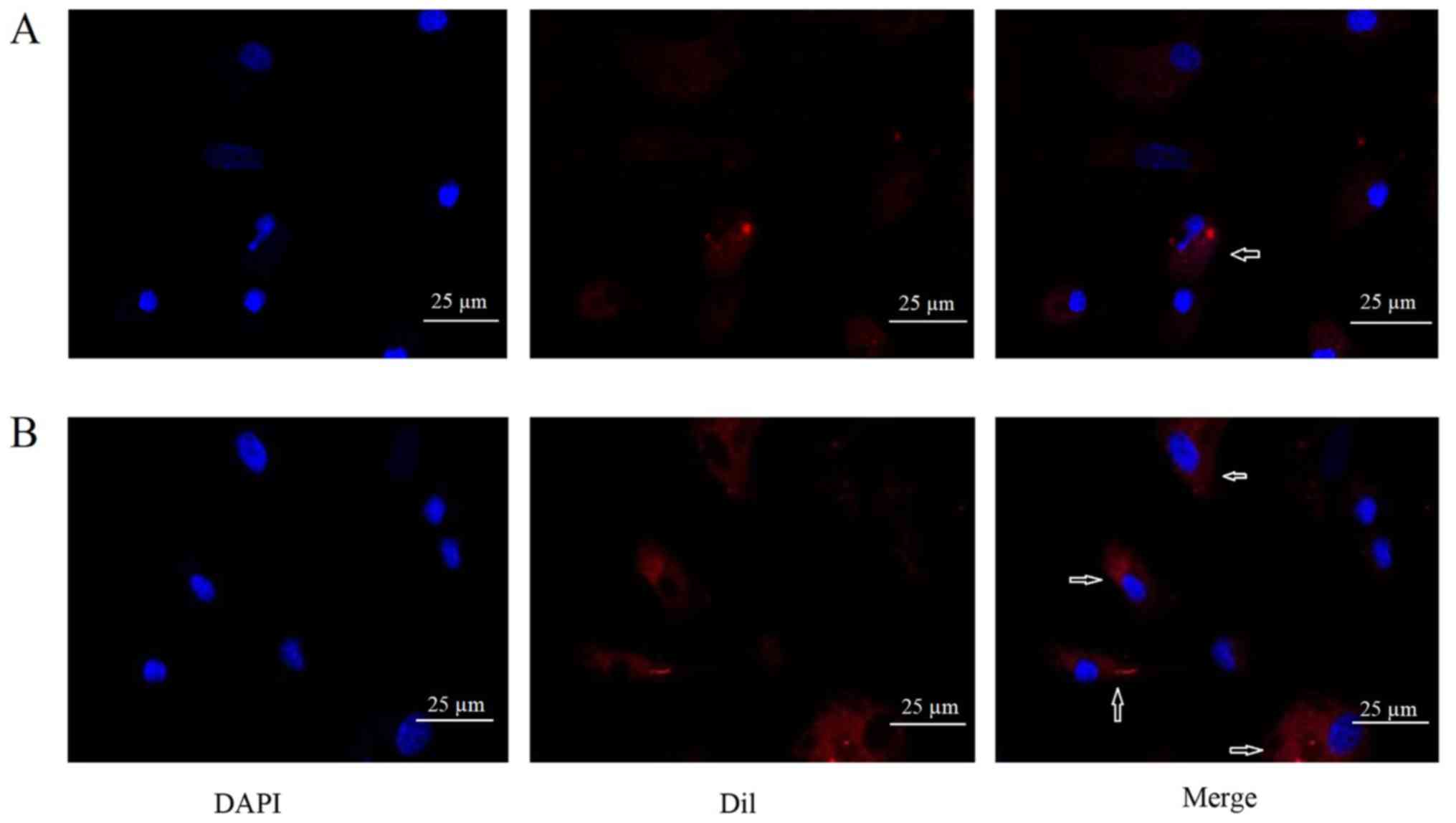
MVs incorporated into EPCs after in
vitro co-incubation
To observe whether MVs could be incorporated into
EPCs, DiI-labeled MVs were added into EPCs culture. After
co-incubation of DiI-labeled MVs with EPCs for 24 h, DiI
fluorescence was detected in EPCs. Immunofluorescence analysis
suggested that the MVs were incorporated into the EPCs (Fig. 5).
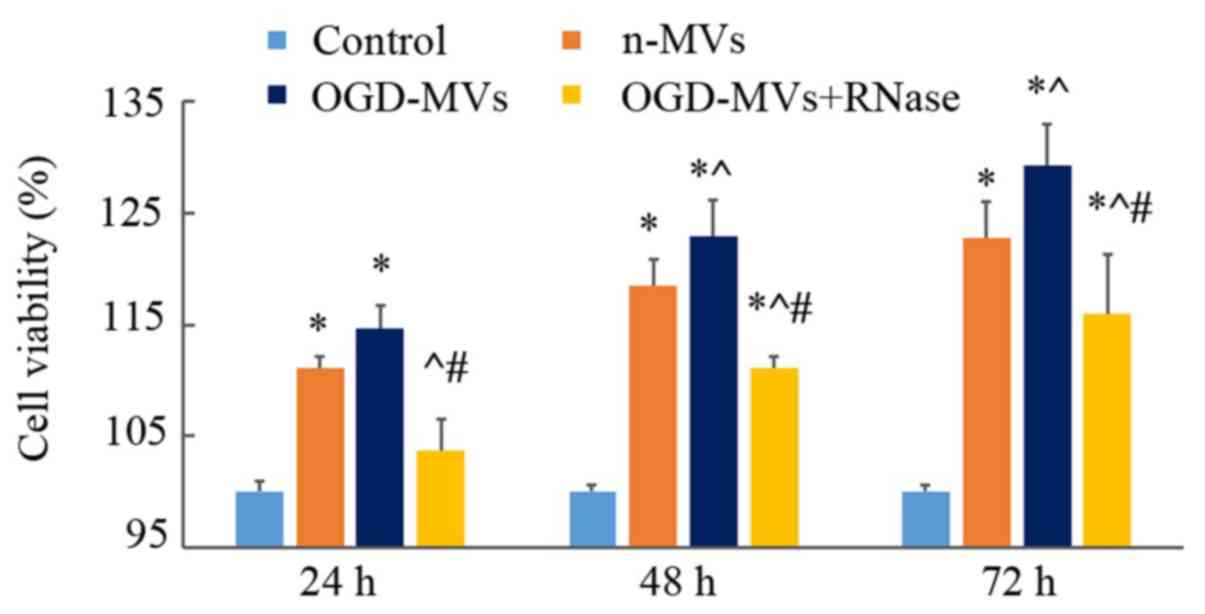
MVs promote the proliferation of
EPCs
As OGD led to upregulated miR-210 expression in MVs
and miR-210 is associated with cell proliferation, it was presumed
that MVs treated with OGD may be involved in modulating the
proliferation of EPCs. For this purpose, EPCs were cultured with
MVs collected from OGD and normoxic culture media, and it was
revealed that co-culture with MVs increased EPC proliferation; the
increase in the OGD-MV group was the most significant (Fig. 6).
Previous studies have indicated that treatment of
MVs with high concentrations of RNase inactivated the RNAs carried
by MVs (10,23). In the present study, the involvement
of MVs in EPC proliferation was further confirmed by using RNase to
pre-treat MVs. With the digestion of RNase, the proliferation of
EPCs was visibly decreased as compared with that in the other two
co-culture groups lacking pre-treatment with RNase, but it remained
slightly higher than that in the control group (Fig. 6).
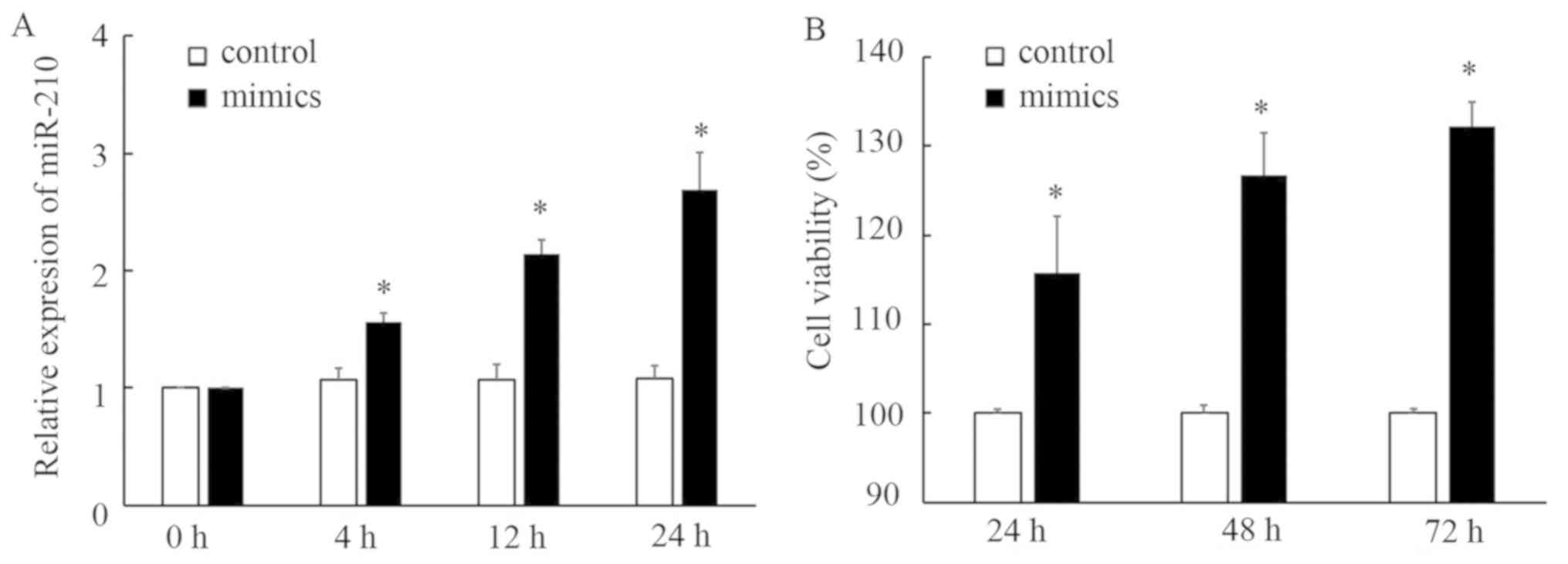
miR-210 mimics promote the
proliferation of EPCs
To further confirm whether miR-210 is involved in
EPC proliferation, EPCs were transfected with miR-210 mimics. As
presented in Fig. 7, transfection of
miR-210 mimics led to overexpression of miR-210 and a significant
increase in the proliferation of EPCs, indicating an association
between miR-210 and EPC proliferation.
Discussion
In the present study, MVs derived from EPCs that had
been subjected to OGD were obtained. There were three major
results: First, it was demonstrated that OGD induced
Ca2+ influx in EPCs. Furthermore, it was revealed that
OGD increased the release of EPC-MVs compared with that under
normoxic conditions. Finally, it was demonstrated that OGD
increased the level of miR-210, and transfection of miR-210 mimics
into EPC-MVs led to stimulation of EPC proliferation under normal
culture conditions.
Therapeutic angiogenesis and vasculogenesis have
emerged as promising therapies to treat ischemic diseases (24,25).
Ischemic injury is multifactorial in its etiology. One of the
factors involves the disruption of vascular integrity, causing
vessel vulnerability. It is well known that the endothelium serves
as a barrier between vessel walls and the blood, and has an
essential role in regulating vascular homeostasis (26). It has been demonstrated that EPCs are
able to mobilize in the peripheral blood, home in to an injured
area, differentiate into ECs and promote vascular repair.
Furthermore, evidence indicates that transplantation of EPCs
promotes new vessel formation and alleviates injury to ischemic
tissues, suggesting that EPCs actively contribute to angiogenesis
and vasculogenesis (27–29).
Transplantation of EPCs is a potential therapy for
ischemic diseases (5–8). However, similar to other types of stem
cells, certain limitations still exist, including the mode of
action, immunogenicity, tumorigenicity, proliferation capacity and
overall feasibility of use (30).
Further studies on the optimization of the safety and efficacy of
stem cell transplantation are required, while recent studies have
provided novel insight, including information on MVs.
MVs shed from the surface of viable cells and act as
paracrine mediators due to their ability to incorporate into target
cells in order to exert their functions. In particular, the
therapeutic potential of MVs has been taken into consideration when
designing studies. By using a model of kidney injury induced by
ischemia-reperfusion, it has been demonstrated that the injection
of MSC-derived MVs has a significant protective effect on renal
function (31,32). Another study suggested that MVs
derived from ECs modulated astrocyte function, blood-brain barrier
integrity and cerebral blood flow, indicating MVs may serve as a
novel therapeutic target for ischemic stroke (33). The biological functions of MVs are
tightly associated with their contents. Contents of MVs are not
randomly packaged, but influenced by various factors, including
external stimuli. Hence, the package of MVs is a precisely
regulated process. RNA inactivation in MVs, achieved by treatment
with high concentrations of RNase, is able to reduce the protective
effects of MVs (32,34), indicating that genetic information
transferred by MVs has a pivotal role in their biological
functions.
Endogenous miRNAs have been identified to have
essential roles in gene regulation to modulate physiological and
pathological processes. miRNAs are short, non-coding RNA molecules
of ~19–23 nucleotides in length, which function as inhibitors of
target gene expression by inducing mRNA degradation or
translational repression (35,36).
Serval studies have demonstrated that expression of a certain set
of miRNAs is upregulated under hypoxia, including miR-210 (37–40).
miR-210 is evolutionarily conserved and widely expressed in various
cell and tissue types. In specific cell types, including primary
vascular ECs, hypoxia induces an augmented expression of miR-210
(38,41). Data on the genetic structure indicate
that the promoter region of miR-210 carries a functioning hypoxia
response element (HRE), which may be recognized by
hypoxia-inducible factor-1α for the induction of miRNA
transcription upon exposure to hypoxia (42,43).
Thus, miR-210 is sensitive to hypoxic stimuli and has an important
role in modulating hypoxia-induced pathogeneses. A diverse range of
genes have been demonstrated to be direct targets of miR-210,
including iron-sulfur cluster assembly protein ISCU1/2, fibroblast
growth factor receptor-like 1, FLASH/caspase-8 associated
protein-2, HOXA3, RAD52 homolog, DNA repair protein and tyrosine
kinase ligand ephrin-A3 (41,44–48).
Through the suppression of direct target transcripts, miR-210 is
involved in modulating the processes of cellular survival,
metabolism, proliferation, DNA repair and endothelial angiogenesis
(38).
Thus, together with the in vitro results of
the present study, it is indicated that HI pre-treatment may
activate stronger protective effects of EPC-MVs through increasing
the release and elevating the expression of miR-210 in the EPC-MVs.
It is possible that the beneficial effects of EPC-MVs induced by HI
insult may contribute to injury repair. However, further studies
focusing on the detailed effects and mechanisms of the release and
contents of EPC-MVs induced by HI insult are required.
In conclusion, the present study demonstrated that
treatment with EPC-MVs produced under HI conditions was able to
promote the proliferation of EPCs, which was associated with
elevated expression of miR-210. The effects of HI insult on EPC-MVs
may offer novel therapeutic strategies for tissue injury.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81401247), Sichuan Science
and Technology Program (grant nos. 2016JY0126 and 2019YJ0648) and
Science and Technology Project of Chengdu, Sichuan Province, China
(grant no. 2014-HM01-00348-SF).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
WZ designed the study, performed the experiments and
the data analysis and wrote the manuscript. QL performed cell
culture, preparation of MVs and transmission electron microscopy.
JM performed the flow cytometry and RT-qPCR experiments. RJ
collected and analyzed the data and revised the manuscript.
Ethics approval and consent to
participate
All procedures conformed to the local principles of
laboratory animal care and were approved by the Institutional
Animal Care and Use Committee of Chengdu Women's & Children's
Central Hospital (Sichuan, China; approval no. 2019-31).
Precautions were taken to minimize suffering and the number of
animals used in each experiment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EPC
|
endothelial progenitor cell
|
|
MV
|
microvesicle
|
|
EPC-MV
|
EPC-derived microvesicle
|
|
miRNA
|
microRNA
|
|
HI
|
hypoxia-ischemia
|
|
OGD
|
oxygen-glucose deprivation
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Szmitko PE, Fedak PW, Weisel RD, Stewart
DJ, Kutryk MJ and Verma S: Endothelial progenitor cells: New hope
for a broken heart. Circulation. 107:3093–3100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sekiguchi H, Ii M, Jujo K, Yokoyama A,
Hagiwara N and Asahara T: Improved culture-based isolation of
differentiating endothelial progenitor cells from mouse bone marrow
mononuclear cells. PLoS One. 6:e286392011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hristov M and Weber C: Endothelial
progenitor cells: Characterization, pathophysiology, and possible
clinical relevance. J Cell Mol Med. 8:498–508. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li SC, Acevedo J, Wang L, Jiang H, Luo J,
Pestell RG, Loudon WG and Chang AC: Mechanisms for progenitor
cell-mediated repair for ischemic heart injury. Curr Stem Cell Res
Ther. 7:2–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jujo K, Ii M and Losordo DW: Endothelial
progenitor cells in neovascularization of infarcted myocardium. J
Mol Cell Cardiol. 45:530–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwon O, Miller S, Li N, Khan A, Kadry Z
and Uemura T: Bone marrow-derived endothelial progenitor cells and
endothelial cells may contribute to endothelial repair in the
kidney immediately after ischemia-reperfusion. J Histochem
Cytochem. 58:687–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu P, Li Q, Liu Y, Zhang J, Seldeen K and
Pang M: Pro-angiogenic efficacy of transplanting endothelial
progenitor cells for treating hindlimb ischemia in hyperglycemic
rabbits. J Diabetes Complications. 29:13–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teraa M, Sprengers RW, Schutgens RE,
Slaper-Cortenbach IC, van der Graaf Y, Algra A, van der Tweel I,
Doevendans PA, Mali WP, Moll FL and Verhaar MC: Effect of
repetitive intra-arterial infusion of bone marrow mononuclear cells
in patients with no-option limb ischemia: The randomized,
double-blind, placebo-controlled rejuvenating endothelial
progenitor cells via transcutaneous intra-arterial supplementation
(JUVENTAS) trial. Circulation. 131:851–860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rautou PE and Mackman N: Microvesicles as
risk markers for venous thrombosis. Expert Rev Hematol. 6:91–101.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deregibus MC, Cantaluppi V, Calogero R, Lo
Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B and Camussi G:
Endothelial progenitor cell derived microvesicles activate an
angiogenic program in endothelial cells by a horizontal transfer of
mRNA. Blood. 110:2440–2448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mause SF and Weber C: Microparticles:
Protagonists of a novel communication network for intercellular
information exchange. Circ Res. 107:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Nedawi K, Meehan B and Rak J:
Microvesicles: Messengers and mediators of tumor progression. Cell
Cycle. 8:2014–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Breakefield XO, Frederickson RM and
Simpson RJ: Gesicles: Microvesicle ‘cookies’ for transient
information transfer between cells. Mol Ther. 19:1574–1576. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antonyak MA, Li B, Boroughs LK, Johnson
JL, Druso JE, Bryant KL, Holowka DA and Cerione RA: Cancer
cell-derived microvesicles induce transformation by transferring
tissue transglutaminase and fibronectin to recipient cells. Proc
Natl Acad Sci USA. 108:4852–4857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tasca CI, Dal-Cim T and Cimarosti H: In
vitro oxygen-glucose deprivation to study ischemic cell death.
Methods Mol Biol. 1254:197–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunt KR, Hall SR, Ward CA and Melo LG:
Endothelial progenitor cell and mesenchymal stem cell isolation,
characterization, viral transduction. Methods Mol Med. 139:197–210.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosell A, Arai K, Lok J, He T, Guo S,
Navarro M, Montaner J, Katusic ZS and Lo EH: Interleukin-1beta
augments angiogenic responses of murine endothelial progenitor
cells in vitro. J Cereb Blood Flow Metab. 29:933–943. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samaan S, Khella HW, Girgis A, Scorilas A,
Lianidou E, Gabril M, Krylov SN, Jewett M, Bjarnason GA, El-said H
and Yousef GM: miR-210 is a prognostic marker in clear cell renal
cell carcinoma. J Mol Diagn. 17:136–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang N, Li D, Jiao P, Chen B, Yao S, Sang
H, Yang M, Han J, Zhang Y and Qin S: The characteristics of
endothelial progenitor cells derived from mononuclear cells of rat
bone marrow in different culture conditions. Cytotechnology.
63:217–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yao W, Firth AL, Sacks RS, Ogawa A, Aguer
WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA,
et al: Identification of putative endothelial progenitor cells
(CD34+ CD133+ Flk-1+) in endarterectomized tissue of patients with
chronic thromboembolic pulmonary hypertension. Am J Physiol Lung
Cell Mol Physiol. 296:L870–L878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morel O, Jesel L, Freyssinet JM and Toti
F: Cellular mechanisms underlying the formation of circulating
microparticles. Arterioscler Thromb Vasc Biol. 31:15–26. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bruno S, Grange C, Deregibus MC, Calogero
RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati
B, et al: Mesenchymal stem cell-derived microvesicles protect
against acute tubular injury. J Am Soc Nephrol. 20:1053–1067. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Losordo DW and Dimmeler S: Therapeutic
angiogenesis and vasculogenesis for ischemic disease: Part II:
Cell-based therapies. Circulation. 109:2692–2697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choksy SA and Chan P: Therapeutic
angiogenesis. Br J Surg. 93:261–263. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller MM and Griesmacher A: Markers of
endothelial dysfunction. Clin Chem Lab Med. 38:77–85. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zampetaki A, Kirton JP and Xu Q: Vascular
repair by endothelial progenitor cells. Cardiovasc Res. 78:413–421.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawamoto A, Asahara T and Losordo DW:
Transplantation of endothelial progenitor cells for therapeutic
neovascularization. Cardiovasc Radiat Med. 3:221–225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takizawa S, Nagata E, Nakayama T, Masuda H
and Asahara T: Recent progress in endothelial progenitor cell
culture systems: Potential for stroke therapy. Neurol Med Chir
(Tokyo). 56:302–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaneko Y, Tajiri N, Shinozuka K, Glover
LE, Weinbren NL, Cortes L and Borlongan CV: Cell therapy for
stroke: Emphasis on optimizing safety and efficacy profile of
endothelial progenitor cells. Curr Pharm Des. 18:3731–3734. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cantaluppi V, Gatti S, Medica D,
Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C
and Camussi G: Microvesicles derived from endothelial progenitor
cells protect the kidney from ischemia-reperfusion injury by
microRNA-dependent reprogramming of resident renal cells. Kidney
Int. 82:412–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aghajani Nargesi A, Lerman LO and Eirin A:
Mesenchymal stem cell-derived extracellular vesicles for kidney
repair: Current status and looming challenges. Stem Cell Res Ther.
8:2732017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pan Q, He C, Liu H, Liao X, Dai B, Chen Y,
Yang Y, Zhao B, Bihl J and Ma X: Microvascular endothelial
cells-derived microvesicles imply in ischemic stroke by modulating
astrocyte and blood brain barrier function and cerebral blood flow.
Mol Brain. 9:632016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aliotta JM, Pereira M, Johnson KW, de Paz
N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, Lee D, et al:
Microvesicle entry into marrow cells mediates tissue-specific
changes in mRNA by direct delivery of mRNA and induction of
transcription. Exp Hematol. 38:233–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chekulaeva M and Filipowicz W: Mechanisms
of miRNA-mediated post-transcriptional regulation in animal cells.
Curr Opin Cell Biol. 21:452–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Q, Yu P, Zeng Q, Luo B, Cai S, Hui K,
Yu G, Zhu C, Chen X, Duan M and Sun X: Neuroprotective effect of
hydrogen-Rich saline in global cerebral ischemia/reperfusion rats:
Up-regulated tregs and down-regulated miR-21, miR-210 and NF-κB
expression. Neurochem Res. 41:2655–2665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: MicroRNA-210 is upregulated by
hypoxia-inducible factor-1α in the stromal cells of giant cell
tumors of bone. Mol Med Rep. 12:6185–6192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pulkkinen K, Malm T, Turunen M, Koistinaho
J and Ylä-Herttuala S: Hypoxia induces microRNA miR-210 in vitro
and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially
regulated by miR-210. FEBS Lett. 582:2397–2401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Z, Hu Z, Lu Z, Cai S, Gu X, Zhuang H,
Ruan Z, Xia Z, Irwin MG, Feng D and Zhang L: Differential microRNA
profiling in a cellular hypoxia reoxygenation model upon
posthypoxic propofol treatment reveals alterations in autophagy
signaling network. Oxid Med Cell Longev. 2013:3784842013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan SY and Loscalzo J: MicroRNA-210: A
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu LL, Li D, He YL, Zhou YZ, Gong SH, Wu
LY, Zhao YQ, Huang X, Zhao T, Xu L, et al: miR-210 protects renal
cell against hypoxia-induced apoptosis by targeting HIF-1 alpha.
Mol Med. 23:258–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cicchillitti L, Di Stefano V, Isaia E,
Crimaldi L, Fasanaro P, Ambrosino V, Antonini A, Capogrossi MC,
Gaetano C, Piaggio G and Martelli F: Hypoxia-inducible factor 1-α
induces miR-210 in normoxic differentiating myoblasts. J Biol Chem.
287:44761–44771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang Z, Sun H, Dai H, Walsh RM, Imakura
M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, et al:
MicroRNA miR-210 modulates cellular response to hypoxia through the
MYC antagonist MNT. Cell Cycle. 8:2756–2768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fasanaro P, D'Alessandra Y, Di Stefano V,
Melchionna R, Romani S, Pompilio G, Capogrossi MC and Martelli F:
MicroRNA-210 modulates endothelial cell response to hypoxia and
inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol
Chem. 283:15878–15883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan SY, Zhang YY, Hemann C, Mahoney CE,
Zweier JL and Loscalzo J: MicroRNA-210 controls mitochondrial
metabolism during hypoxia by repressing the iron-sulfur cluster
assembly proteins ISCU1/2. Cell Metab. 10:273–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang X, Ding L, Bennewith KL, Tong RT,
Welford SM, Ang KK, Story M, Le QT and Giaccia AJ:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HW, Haider HK, Jiang S and Ashraf M:
Ischemic preconditioning augments survival of stem cells via
miR-210 expression by targeting caspase-8-associated protein 2. J
Biol Chem. 284:33161–33168. 2009. View Article : Google Scholar : PubMed/NCBI
|