Introduction
Coronary heart disease (CHD) is correlated with
well-acknowledged cardiovascular risk factors (CVRFs), including
cigarette smoking, type 2 diabetes (T2D), hypertension,
hypercholesterolemia, family history and metabolic syndrome (MetS)
(1,2). Imaging studies have demonstrated an
association between plaque phenotypes and CVRFs in middle-aged and
elderly patients with CHD (3-5).
However, the pathophysiology of atherosclerosis in young patients
with CHD differs from that in older patients (6). To date, the association between CVRFs
and the characteristics of culprit coronary plaque in young
patients has remained to be fully elucidated. Furthermore, the
incidence of CHD has increased in young individuals. CHD may have
serious consequences, including premature death and long-term
disability (7).
Optical coherence tomography (OCT) has emerged as
the most accurate imaging modality for intracoronary evaluation,
with a resolution of 10-20 µm (8).
OCT has been widely used to investigate atherosclerotic plaque
microstructure, which may be a key factor in determining plaque
stability (9). OCT findings are
validated by histologic evaluation (10). In the present study, the association
between the phenotype of the culprit atherosclerotic plaque as
determined by OCT and CVRFs in young patients were assessed.
Patients and methods
Patients
The present study was a retrospective, single-center
study. Consecutive patients (age, 36±7 years; male 87%, female 13%)
who underwent OCT between April 2014 and March 2017 in the
Cardiology Department of Beijing Anzhen Hospital, including those
with stable CHD and acute coronary syndrome (ACS), were selected.
The exclusion criteria were a known history of severe hepatic or
renal dysfunction, an ongoing inflammatory condition, familial
hypercholesterolemia and arthritis. Patients with poor image
quality, incomplete pullback, or missing data were also excluded.
All of the patients provided informed consent and the study
protocol was approved by the Ethics Committee of the Beijing Anzhen
Hospital (Beijing, China).
Definition of CVRFs
The definition of MetS was based on the criteria
established in the Joint Scientific Statement (11). An adult with ≥3 of the following was
deemed to have MetS: Waist circumference, ≥90 cm for males or ≥80
cm for females; triglycerides, ≥150 mg/dl; high-density lipoprotein
cholesterol, ≤40 mg/dl; systolic blood pressure (SBP), ≥130 mmHg
and/or diastolic blood pressure (DBP), ≥85 mmHg, or treated
hypertension; and fasting blood glucose level, ≥100 mg/dl or
treated T2D. Smoking was defined as current cigarette smoking.
Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg, or
treated hypertension. T2D was defined as fasting blood glucose
>126 mg/dl or treated T2D (a diabetic diet or prescription of
oral hypoglycemic agent). Hypercholesterolemia was defined as total
cholesterol >200 mg/dl or treated hypercholesterolemia. A family
history of coronary artery disease (CAD) was defined as premature
CAD in a first-degree relative (a male aged <55 years or a
female aged <65 years).
Coronary angiography (CAG) and OCT
procedures
Diagnostic angiograms were recorded via radial
access using a 5.24-mm French (5-Fr) catheter and after
administering a 5,000-IU bolus of heparin. Culprit lesions were
identified via CAG and electrocardiographic ST-segment alterations.
The decision of whether to perform OCT was at the discretion of the
operator. A 0.014-inch guidewire was placed distally in the target
vessel and an intracoronary injection of 200 mg nitroglycerin was
administered via a 6-Fr guiding catheter. Frequency domain OCT
images were acquired using a C7-XR OCT Intravascular Imaging System
(St. Jude Medical), which was advanced to the culprit lesion.
During image acquisition, the coronary blood flow was replaced by
continuously flushing contrast media directly from the guiding
catheter at a rate of 3-4 ml/sec with a power injector, thus
creating a virtually blood-free environment with the integrated
automated pullback device at 20 mm/sec. In the OCT investigations,
5-10 ml of contrast media was used, the flouro time was 2-4 sec and
the radiation dose was 30.6-61.2 mGray.
OCT image analysis
The operator who performed the pullback and an
independent investigator who was blinded to the clinical
presentation analyzed the OCT images offline. Any disagreements
were resolved by consensus. A thin-cap fibroatheroma (TCFA) was
defined as an OCT-delineated necrotic core subtending a >90̊ arc
and covered by a fibrous cap with a thickness of <65 µm
(5). Plaque erosion was defined by
the presence of preserved vascular integrity (intact fibrous cap),
a larger residual lumen and a platelet-rich thrombus (12). A vasa vasorum was defined as a small
black hole within a plaque, 50-300 µm in diameter, that was present
on at least 3 consecutive frames in pullback images (13). Cholesterol crystals were defined as
thin linear structures with high backscatter and without
attenuation within the plaque (14).
Plaque rupture, macrophage accumulation, calcified nodules and
percent area stenosis (AS%) were defined as per the International
Working Group for Intravascular Optical Coherence Tomography
consensus standards (15).
Statistical analysis
Categorical data are presented as counts and
proportions and were compared using a χ2 test. The
distributions of the continuous variables across the study groups
were tested with the Shapiro-Wilks test. Normally distributed data
are presented as the mean ± standard deviation and were compared
using an independent-samples t-test. Non-normally distributed data
are presented as the median (interquartile range) and were compared
using a non-parametric test. Univariate and multivariate logistic
regression analyses were performed to assess independent
predictors. All of the statistical calculations were performed
using SPSS software version 22 (IBM Corp.). P<0.05 was
considered to indicate statistical significance.
Results
Patient information
In the present study, 123 patients (age, 36±7 years;
male 87.0%, female 13%) who underwent CAG and OCT were analyzed.
Their baseline clinical characteristics and CAG data are presented
in Table I. Cigarette smoking,
hypertension, T2D, hypercholesterolemia and MetS were present in
54.5, 51.2, 17.9, 12.2 and 66.7% of the study population,
respectively. The percentages of patients using aspirin, statins,
beta-blockers, ACEIs or ARBs, and insulin were 22.8, 20.3, 25.2,
27.6 and 4.1%, respectively. The percentages of smokers in the
stable angina cohort vs. the ACS cohort were 39.1% vs. 63.6%,
respectively (P=0.013). Left-anterior descending lesions accounted
for 67.5% of all culprit lesions.
 | Table IBaseline characteristics of the
patients (n=123). |
Table I
Baseline characteristics of the
patients (n=123).
| Item | Value |
|---|
| Age (years) | 36±7 (20-45) |
| Male sex | 107 (87.0) |
| Family history of
CHD | 10 (8.1) |
| Smoking | 67 (54.5) |
| Hypertension | 63 (51.2) |
| Diabetes
mellitus | 22 (17.9) |
|
Hypercholesterolemia | 15 (12.2) |
| Metabolic
syndrome | 82 (66.7) |
| ACS | 77 (62.6) |
|
Smoking | 49 (63.6) |
| Stable CHD | 46 (37.4) |
|
Smoking | 18 (39.1) |
| Pharmacological
therapy | |
|
Aspirin | 28 (22.8) |
|
Statins | 25 (20.3) |
|
Beta
blockers | 31 (25.2) |
|
ACEI or
ARB | 34 (27.6) |
|
Insulin | 5 (4.1) |
| EF (%) | 63 (60-68) |
| Culprit vessel | |
|
Left
main | 4 (3.3) |
|
Left
anterior descending | 83 (67.5) |
|
Left
circumflex | 10 (8.1) |
|
Right
coronary artery | 26 (21.1) |
| CTNI (ng/ml) | 0.10 (0.02-7.33) |
| BNP (pg/ml) | 57 (35-340) |
Characteristics of OCT-derived plaques
and CVRFs
Distinct phenotypes of OCT-derived plaques and their
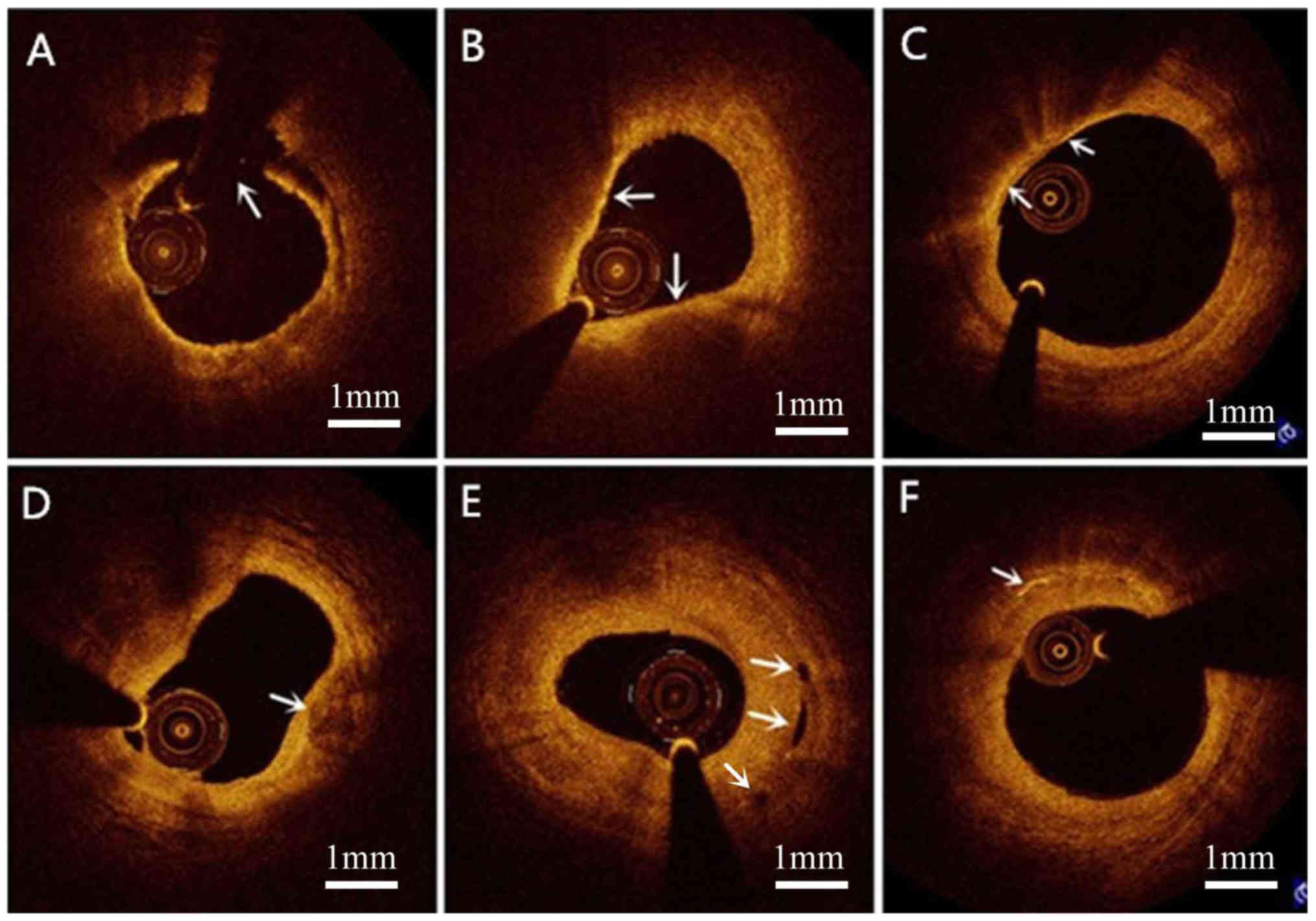
associations with CVRFs are presented in Table II. TCFAs and macrophage accumulation
were more prevalent in patients with than without MetS (P=0.020)
and hypertension (P<0.001), respectively. Cholesterol crystals
presented more frequently in patients with than without a family
history of CHD (P=0.004) and hypercholesterolemia (P=0.031). The
extent of plaque rupture was greater in smokers than in non-smokers
(P=0.002). Vasa vasorum was more common in the culprit lesions of
non-smokers than in those of smokers (P=0.003). By contrast, no
significant association was observed between erosions and CVRFs or
between calcified nodules and CVRFs in the present study.
Representative OCT images are provided in Fig. 1.
 | Table IIOptical coherence tomography-derived
plaque characteristics according to cardiovascular risk
factors. |
Table II
Optical coherence tomography-derived
plaque characteristics according to cardiovascular risk
factors.
| | Family history of
CHD | Smoking | Hypertension | Diabetes
mellitus |
Hypercholesterolemia | Metabolic
syndrome |
|---|
| Item | Yes (n=10) | No (n=113) | P-value | Yes (n=67) | No (n=56) | P-value | Yes (n=63) | No (n=60) | P-value | Yes (n=22) | No (n=101) | P-value | Yes (n=21) | No (n=102) | P-value | Yes (n=82) | No (n=41) | P-value |
|---|
| TCFA | 5 | 82 | 0.155 | 51 | 36 | 0.168 | 49 | 38 | 0.112 | 14 | 73 | 0.444 | 15 | 72 | 1.000 | 64 | 23 | 0.020 |
| | (50.0) | (72.6) | | (76.1) | (64.3) | | (77.8) | (63.3) | | (63.6) | (72.3) | | (71.4) | (70.6) | | (78.0) | (56.1) | |
| Macrophage | 7 | 68 | 0.739 | 45 | 30 | 0.141 | 49 | 26 | <0.001 | 16 | 59 | 0.238 | 15 | 60 | 0.333 | 51 | 24 | 0.700 |
| accumulation | (70.0) | (60.2) | | (67.2) | (53.6) | | (77.8) | (43.3) | | (72.7) | (58.4) | | (71.4) | (58.5) | | (62.2) | (58.5) | |
| Calcified | 2 | 10 | 0.252 | 6 | 6 | 0.769 | 6 | 6 | 1.000 | 0 | 12 | 0.122 | 2 | 10 | 1.000 | 6 | 6 | 0.212 |
| nodule | (20.0) | (8.8) | | (9.0) | (10.7) | | (9.5) | (10.0) | | (0.0) | (11.9) | | (9.5) | (9.8) | | (7.3) | (14.6) | |
| Vasa | 3 | 29 | 0.719 | 10 | 22 | 0.003 | 18 | 14 | 0.543 | 5 | 27 | 0.794 | 8 | 24 | 0.180 | 22 | 10 | 0.830 |
| vasorum | (30.0) | (25.7) | | (14.9) | (39.3) | | (28.6) | (23.3) | | (22.7) | (26.7) | | (38.1) | (23.5) | | (26.8) | (24.4) | |
| Cholesterol | 6 | 18 | 0.004 | 12 | 12 | 0.654 | 16 | 8 | 0.113 | 7 | 17 | 0.137 | 8 | 16 | 0.031 | 16 | 8 | 1.000 |
| crystals | (60.0) | (15.9) | | (17.9) | (21.4) | | (25.4) | (13.3) | | (31.8) | (16.8) | | (38.1) | (15.7) | | (19.5) | (19.5) | |
| Erosion | 0 | 6 | 0.455 | 4 | 2 | 0.688 | 2 | 4 | 0.432 | 2 | 4 | 0.292 | 0 | 6 | 0.588 | 6 | 0 | 0.177 |
| | (0.0) | (5.3) | | (6.0) | (3.6) | | (3.2) | (6.7) | | (9.1) | (4.0) | | (0.0) | (5.9) | | (7.3) | (0.0) | |
| Plaque | 0 | 18 | 0.355 | 16 | 2 | 0.002 | 10 | 8 | 0.801 | 2 | 16 | 0.525 | 2 | 16 | 0.736 | 14 | 4 | 0.418 |
| rupture | (0.0) | (15.9) | | (23.9) | (3.6) | | (15.9) | (13.3) | | (9.1) | (15.8) | | (9.5) | (15.7) | | (17.1) | (9.8) | |
| %AS | 84.5± | 82.4± | 0.624 | 81.2± | 84.2± | 0.196 | 80.7± | 84.5± | 0.111 | 85.8± | 81.8± | 0.104 | 78.8± | 83.3± | 0.237 | 83.6± | 80.4± | 0.205 |
| | 14.8 | 12.9 | | 14.0 | 11.7 | | 14.5 | 11.1 | | 9.2 | 13.6 | | 16.4 | 12.2 | | 13.9 | 10.9 | |
Multivariate analysis
To assess the association between TFCAs and CVRFs or
between plaque rupture and CVRFs, multivariate regression analyses
were performed. Risk factors with P<0.100 from the univariate
analysis were included in the multivariate analyses. As presented
in Table III, after adjusting for
traditional confounding factors, MetS was independently associated
with TCFAs [risk ratio (RR), 2.421; 95% CI, 1.038-5.649; P=0.041].
Of the CVRFs, smoking retained an independent association with
plaque rupture (RR, 8.301; 95% CI, 1.813-38.015; P=0.006).
 | Table IIIUnivariate and multivariate analysis
for TCFA and plaque rupture predictors. |
Table III
Univariate and multivariate analysis
for TCFA and plaque rupture predictors.
| A, Predictors of
TCFA |
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factor | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Gender | 1.54 | 0.514-4.610 | 0.440 | | | |
| Smoking | 1.771 | 0.809-3.877 | 0.153 | | | |
| Hypertension | 2.026 | 0.917-4.477 | 0.081 | 1.574 | 0.679-3.650 | 0.291 |
| Diabetes
mellitus | 0.671 | 0.254-1.774 | 0.421 | | | |
|
Hypercholesterolemia | 1.042 | 0.369-2.942 | 0.939 | | | |
| Metabolic
syndrome | 2.783 | 1.240-6.246 | 0.013 | 2.421 | 1.038-5.649 | 0.041 |
| Aspirin | 0.678 | 0.277-1.660 | 0.395 | | | |
| Statins | 0.542 | 0.216-1.355 | 0.190 | | | |
| Beta blocker | 0.827 | 0.343-1.993 | 0.673 | | | |
| ACEI or ARB | 1.210 | 0.499-2.934 | 0.674 | | | |
| Insulin | 0.607 | 0.097-3.796 | 0.594 | | | |
| B, Predictors of
plaque rupture |
| | Univariate
analysis | Multivariate
analysis |
| Factor | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Smoking | 8.471 | 1.855-38.690 | 0.006 | 8.301 | 1.813-38.015 | 0.006 |
| Hypertension | 1.226 | 0.449-3.352 | 0.691 | | | |
| Diabetes
mellitus | 0.531 | 0.113-2.499 | 0.423 | | | |
|
Hypercholesterolemia | 0.566 | 0.120-2.670 | 0.472 | | | |
| Metabolic
syndrome | 1.904 | 0.585-6.205 | 0.285 | | | |
| Aspirin | 0.380 | 0.082-1.763 | 0.217 | | | |
| Statins | 0.446 | 0.095-2.081 | 0.304 | 2.064 | 0.422-10.093 | 0.371 |
| Beta blocker | 0.328 | 0.071-1.514 | 0.153 | | | |
| ACEI or ARB | 0.285 | 0.062-1.314 | 0.107 | | | |
| Insulin | 4.250 | 0.658-27.443 | 0.128 | | | |
Discussion
The major results of the present study were as
follows: i) MetS was independently associated with TCFAs; and ii)
smoking was independently associated with plaque rupture. To the
best of our knowledge, the present study was the first OCT study
investigating the association between culprit plaque phenotype and
CVRFs in young patients.
Young individuals with premature CHD may have fewer
risk factors of CHD, but MetS is frequently present in this group
of patients and puts them at a high risk of early-onset clinical
CHD (16). In the present study,
66.7% of the patients had MetS. Kalantzi et al (7) reported that MetS is highly associated
with ACS in patients <45 years of age and is more predictive
than other cardiovascular risk factors. TCFAs are known as
important predictors of cardiovascular events (CVEs) (17). Using virtual-histology intravascular
ultrasound (VH-IVUS), Zheng et al (3) analyzed the volumetric plaque
composition of the coronary arterial tree and its association with
other CVRFs and MetS in patients diagnosed with ischemic heart
disease (age, 59±9 years) and indicated that MetS patients had more
frequent VH-IVUS-derived TCFAs within the tree than non-MetS
patients. Similarly, the present study suggested that patients with
MetS had more frequent TCFAs than patients without MetS. Zheng
et al (3) also demonstrated
that T2D is independently associated with TCFAs.
Previous studies investigating features of coronary
plaques in patients with MetS have provided conflicting results.
Specifically, a previous IVUS study demonstrated no significant
association between the presence of TCFAs and MetS in patients with
stable angina pectoris (age, 64.7±9.5 years) (18). Another study using OCT indicated that
coronary plaques in patients with MetS (age, 60±11 years) and T2D
(age, 59±11 years) contain larger amounts of lipids, but neither
MetS nor T2D was significantly associated with TCFAs (19). These conflicting results may have
several reasons. First, the population of the present study was
significantly younger than that in the aforementioned studies. The
pathophysiology underlying atherosclerosis and plaque
characteristics differ between young and old patients with CHD
(20). Furthermore, in the study by
Yonetsu et al (19), selected
198 patients who had nonculprit or nontarget coronary plaques with
area stenosis >50% as measured by OCT; however, whether they are
culprit or non-culprit may affect the characteristics of plaques
(21).
The present study indicated that cigarette smoking
is independently associated with plaque rupture. Cigarette smoking
is associated with a high incidence of CVEs, including ACS
(22). It is also associated with a
higher burden of necrotic cores in coronary atherosclerotic
plaques, which may be one of the mechanisms underlying the
increased risk it poses for plaque rupture and CVEs (23,24). By
far, the most common risk factor for early-onset CHD is cigarette
smoking (6), which increases the
risk of plaque rupture. Cigarette smokers accounted for 54.5%
(n=67) of the patients of the present study. Cigarette smoking is
thought to increase the burden of cardiovascular disease by
inducing endothelial dysfunction, increasing the burden of coronary
atherosclerosis and increasing the risk of plaque rupture and CVEs
(25). T2D was not significantly
associated with TCFAs or rupture in the present study. This may be
due to the larger number of patients with T2D than without (63.6%
vs. 10.9%) using statins, which may reduce TCFAs and plaque rupture
(26).
The universally acknowledged features of vulnerable
plaques currently include TCFAs, macrophage accumulation, calcified
nodules, vasa vasorum and cholesterol crystals (27-31).
The association between TCFAs and CVRFs was described above. In the
present study, macrophage accumulation was more common in patients
with hypertension and cholesterol crystals were present more often
in patients with a family history of CHD and hypercholesterolemia.
By contrast, no significant correlation was observed between
calcified nodules or vasa vasorum and CVRFs in the patients of the
present study. The sample examined was not extracted from the
general population but was rather composed of relatively young
patients. Thus, the results may not be extrapolatable to the
general population. In addition, the results may be affected by
lifestyle factors, including diet and physical exercise.
In conclusion, by using OCT evaluation, the present
study demonstrated that young patients with MetS had more extensive
TCFAs and that young cigarette smokers were at increased risk for
culprit plaque rupture. Young patients with CHD should therefore
actively control their body weight, blood lipids, blood pressure
and blood sugar levels, as well as quit smoking, so as to reduce
the occurrence/risk of TCFAs and ruptures.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and JG conceived the study. FH, WL, YD and YL
collected and analyzed the patients' general information. FH and WL
wrote the manuscript. SY, XM and ZW analyzed the OCT images. All of
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Beijing Anzhen Hospital
(Beijing, China) approved the study protocol and all of the
participants provided written informed consent.
Patient consent for publication
All of the participants provided written informed
consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Authors/Task Force Members. Piepoli MF,
Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M,
Corrà U, Cosyns B, Deaton C, et al: 2016 European Guidelines on
cardiovascular disease prevention in clinical practice: The Sixth
Joint Task Force of the European Society of Cardiology and Other
Societies on Cardiovascular Disease Prevention in Clinical Practice
(constituted by representatives of 10 societies and by invited
experts) Developed with the special contribution of the European
Association for Cardiovascular Prevention & Rehabilitation
(EACPR). Atherosclerosis. 252:207–274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilson PW, D'Agostino RB, Parise H,
Sullivan L and Meigs JB: Metabolic syndrome as a precursor of
cardiovascular disease and type 2 diabetes mellitus. Circulation.
112:3066–3072. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng M, Choi SY, Tahk SJ, Lim HS, Yang
HM, Choi BJ, Yoon MH, Park JS, Hwang GS and Shin JH: The
relationship between volumetric plaque components and classical
cardiovascular risk factors and the metabolic syndrome a 3-vessel
coronary artery virtual histology-intravascular ultrasound
analysis. JACC Cardiovasc Interv. 4:503–510. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rivera JJ, Nasir K, Cox PR, Choi E, Yoon
Y, Cho I, Chun EJ, Choi S, Blumenthal RS and Chang HJ: Association
of traditional cardiovascular risk factors with coronary plaque
sub-types assessed by 64-slice computed tomography angiography in a
large cohort of asymptomatic subjects. Atherosclerosis.
206:451–457. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De Rosa R, Vasa-Nicotera M, Leistner DM,
Reis SM, Thome CE, Boeckel J, Fichtlscherer S and Zeiher AM:
Coronary atherosclerotic plaque characteristics and cardiovascular
risk factors-insights from an optical coherence tomography study.
Circ J. 81:1165–1173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aggarwal A, Srivastava S and Velmurugan M:
Newer perspectives of coronary artery disease in young. World J
Cardiol. 8:728–734. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kalantzi K, Korantzopoulos P, Tzimas P,
Katsouras CS, Goudevenos JA and Milionis HJ: The relative value of
metabolic syndrome and cardiovascular risk score estimates in
premature acute coronary syndromes. Am Heart J. 155:534–540.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rathore S, Terashima M, Matsuo H,
Kinoshita Y, Kimura M, Tsuchikane E, Nasu K, Ehara M, Asakura Y,
Katoh O and Suzuki T: Association of coronary plaque composition
and arterial remodelling: A optical coherence tomography study.
Atherosclerosis. 221:405–415. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Finn AV, Nakano M, Narula J, Kolodgie FD
and Virmani R: Concept of vulnerable/unstable plaque. Arterioscler
Thromb Vasc Biol. 30:1282–1292. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jang IK, Bouma BE, Kang DH, Park SJ, Park
SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, et
al: Visualization of coronary atherosclerotic plaques in patients
using optical coherence tomography: Comparison with intravascular
ultrasound. J Am Coll Cardiol. 39:604–609. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith
SC Jr, et al: Harmonizing the Metabolic Syndrome: A Joint Interim
Statement of the International Diabetes Federation Task Force on
Epidemiology and Prevention; National Heart, Lung, and Blood
Institute; American Heart Association; World Heart Federation;
International Atherosclerosis Society; and International
Association for the Study of Obesity. Circulation. 120:1640–1645.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jia H, Dai J, Hou J, Xing L, Ma L, Liu H,
Xu M, Yao Y, Hu S, Yamamoto E, et al: Effective anti-thrombotic
therapy without stenting: Intravascular optical coherence
tomography-based management in plaque erosion (the EROSION study).
Eur Heart J. 38:792–800. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tian J, Hou J, Xing L, Kim SJ, Yonetsu T,
Kato K, Lee H, Zhang S, Yu B and Jang IK: Significance of
intraplaque neovascularisation for vulnerability: Optical coherence
tomography study. Heart. 98:1504–1509. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishimura S, Ehara S, Hasegawa T,
Matsumoto K, Yoshikawa J and Shimada K: Cholesterol crystal as a
new feature of coronary vulnerable plaques: An optical coherence
tomography study. J Cardiol. 69:253–259. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tearney GJ, Regar E, Akasaka T,
Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM,
Chowdhary S, et al: Consensus standards for acquisition,
measurement, and reporting of intravascular optical coherence
tomography studies: A report from the International Working Group
for Intravascular Optical Coherence Tomography Standardization and
Validation. J Am Coll Cardiol. 59:1058–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Iribarren C, Go AS, Husson G, Sidney S,
Fair JM, Quertermous T, Hlatky MA and Fortmann SP: Metabolic
syndrome and early-onset coronary artery disease: Is the whole
greater than its parts? J Am Coll Cardiol. 48:1800–1807.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Iannaccone M, Quadri G, Taha S, D'Ascenzo
F, Montefusco A, Omede P, Jang IK, Niccoli G, Souteyrand G, Yundai
C, et al: Prevalence and predictors of culprit plaque rupture at
OCT in patients with coronary artery disease: A meta-analysis. Eur
Heart J Cardiovasc Imaging. 17:1128–1137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee MG, Jeong MH, Kim DH, Lee KH, Park KH,
Sim DS, Yoon NS, Yoon HJ, Kim KH, Park HW, et al: Can metabolic
syndrome predict the vulnerable plaque in patients with stable
angina pectoris? Virtual histology-intravascular ultrasound
analysis. J Cardiol. 59:266–274. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yonetsu T, Kato K, Uemura S, Kim BK, Jang
Y, Kang SJ, Park SJ, Lee S, Kim SJ, Jia H, et al: Features of
coronary plaque in patients with metabolic syndrome and diabetes
mellitus assessed by 3-vessel optical coherence tomography. Circ
Cardiovasc Imaging. 6:665–673. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barbero U, Scacciatella P, Iannaccone M,
D'Ascenzo F, Niccoli G, Colombo F, Ugo F, Colangelo S, Mancone M,
Calcagno S, et al: Culprit plaque characteristics in younger versus
older patients with acute coronary syndromes: An optical coherence
tomography study from the FORMIDABLE registry. Catheter Cardiovasc
Interv. 92:E1–E8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trusinskis K, Juhnevica D, Strenge K and
Erglis A: iMap intravascular ultrasound evaluation of culprit and
non-culprit lesions in patients with ST-elevation myocardial
infarction. Cardiovasc Revasc Med. 14:71–75. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Erhardt L: Cigarette smoking: An
undertreated risk factor for cardiovascular disease.
Atherosclerosis. 205:23–32. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abtahian F, Yonetsu T, Kato K, Jia H,
Vergallo R, Tian J, Hu S, McNulty I, Lee H, Yu B and Jang IK:
Comparison by optical coherence tomography of the frequency of
lipid coronary plaques in current smokers, former smokers, and
nonsmokers. Am J Cardiol. 114:674–680. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bolorunduro O, Cushman C, Kapoor D,
Alexander K, Cuellar-Silva J, Giri S, Robinson V and Ibebuogu UN:
Comparison of coronary atherosclerotic plaque burden and
composition of culprit lesions between cigarette smokers and
non-smokers by in vivo virtual histology intravascular ultrasound.
J Invasive Cardiol. 27:354–358. 2015.PubMed/NCBI
|
|
25
|
Csordas A and Bernhard D: The biology
behind the atherothrombotic effects of cigarette smoke. Nat Rev
Cardiol. 10:219–230. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gili S, Iannaccone M, Colombo F,
Montefusco A, Amabile N, Calcagno S, Capodanno D, Scalone G,
Rognoni A, Omedè P, et al: Effects of statins on plaque rupture
assessed by optical coherence tomography in patients presenting
with acute coronary syndromes: Insights from the optical coherence
tomography (OCT)-FORMIDABLE registry. Eur Heart J Cardiovasc
Imaging. 19:524–531. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kume T and Uemura S: Current clinical
applications of coronary optical coherence tomography. Cardiovasc
Interv Ther. 33:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee T, Mintz GS, Matsumura M, Zhang W, Cao
Y, Usui E, Kanaji Y, Murai T, Yonetsu T, Kakuta T and Maehara A:
Prevalence, predictors, and clinical presentation of a calcified
nodule as assessed by optical coherence tomography. JACC Cardiovasc
Imaging. 10:883–891. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gonzalez L and Trigatti BL: Macrophage
apoptosis and necrotic core development in atherosclerosis: A
rapidly advancing field with clinical relevance to imaging and
therapy. Can J Cardiol. 33:303–312. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Janoudi A, Shamoun FE, Kalavakunta JK and
Abela GS: Cholesterol crystal induced arterial inflammation and
destabilization of atherosclerotic plaque. Eur Heart J.
37:1959–1967. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Taruya A, Tanaka A, Nishiguchi T, Matsuo
Y, Ozaki Y, Kashiwagi M, Shiono Y, Orii M, Yamano T, Ino Y, et al:
Vasa vasorum restructuring in human atherosclerotic plaque
vulnerability: A clinical optical coherence tomography study. J Am
Coll Cardiol. 65:2469–2477. 2015.PubMed/NCBI View Article : Google Scholar
|