Introduction
Obesity is a metabolic disease that has emerged over
the past 3 decades as a major health drawback. The most recent data
estimate that more than 1.9 billion adults of over the age of 18
are overweight, with over 650 million of them being obese (1). In addition, while just under 1% of
children and adolescents aged 5-19 were obese in 1975, data in 2016
revealed that more than 124 million children and adolescents (6% of
girls and 8% of boys) were obese (2). Overweight and obesity increase the risk
of many health problems. It has a harmful metabolic consequence on
blood pressure, cholesterol, triglycerides, and insulin resistance,
and increases the risks of coronary heart disease, ischemic stroke
and type 2 diabetes mellitus (3). It
is also said to increase the risk of cancer of the breast, colon,
prostate, endometrium, kidney and gallbladder, and mortality rates
are increased by obesity (4).
Despite these complications of obesity, it is regarded as the most
preventable cause of death (5).
Therefore, effective management is essential.
Several prevention/treatment options are available
for the treatment of obesity. The most common treatment involves
losing weight through healthy eating and physical exercise. When
these methods are ineffective, weight loss medicines are
recommended to treat overweight and obesity. However, concerns have
been raised regarding the use of weight-loss medication whose side
effects may outweigh their benefits. Most anti-obesity medications
that were approved and marketed have now been withdrawn due to
serious adverse effects. For instance, fenfluramine and
dexfenfluramine, used to treat obesity, were recalled by the U.S.
Food and Drug Administration (FDA) because of concerns related to
heart valve problems (6).
Phentermine, diethylpropion, and mazindol were recalled by the
European Medicines Agency (EMA) due to their overwhelming side
effects (7). Rimonabant, a selective
CB1 blocker used to treat obesity that was available in 56
countries was never approved by the FDA and was recalled by the EMA
because of raised concerns of psychiatric adverse disorders
(8). Therefore, there is a need for
alternative therapy with little to no side effects on the
management of obesity and its related complications. Herbal
medicines have been used to control weight and treat obesity. For
instance, an increasing number of clinical trials have confirmed
the beneficial effects of green tea on obesity (9). The anti-obesity effect of green tea is
associated with its caffeine and catechins,
(-)-epigallocatechin-3-gallate contents (10).
Platycodon grandiflorum is a perennial plant
of the Campanulaceae family used as herbal medicine for the
treatment of respiratory disorders, asthma, and diabetes. It is
rich in bioactive compounds including flavonoids, polyphenols, and
the triterpenoid saponins, and possesses antioxidant, anticancer,
anti-inflammatory, and hepatoprotective properties (11-15).
Platycodins from P. grandiflorum showed anti-obesity effects
and lowered whole body cholesterol in a study (16). Apium graveolens or
celery, is a cultivated edible plant, commonly used as a vegetable
and has medicinal properties spanning anti-inflammatory,
antifungal, and antibacterial (17-19),
and is also used to treat a variety of diseases such as asthma,
hepatitis, bronchitis, and gastrointestinal infections (20). Celery has also been shown to reduce
blood glucose, blood lipids, and blood pressure (21). The main functional compounds found in
celery are chlorogenic acid, luteolin, apiin and apigenin (22). Green tea (Camellia
sinensis) is among one of the most consumed beverages in the
world. A large body of evidence points to the fact that green tea
and its isolated compounds have anti-inflammatory, antibacterial,
anti-viral and neuroprotective effects (9). Over the past years, the anti-obesity
effects of green tea in animals and humans have been closely
studied as a hot topic in functional food research. Indeed, there
are clinical shreds of evidence of the beneficial effects of green
tea on obesity (23-25).
The main constituents of green tea are catechins with some amount
of gallic acid, quercetin, kaempferol, myricetin, and chlorogenic
acid (26). The continuous search
for better therapies for the treatment of obesity and its
associated disorders has spark growing interest in the synergistic
effects of various phytochemicals and plant extracts for the
treatment of obesity. In this light, we sought to investigate the
synergistic effects of P. grandiflorum, A.
graveolens, and green tea in obesity and obesity-associated
disorders induced by a high-fat diet as part of our on-going
project to develop anti-obesity functional food products from a
combination of different extracts whose efficacy will be better
than other products in the market including green tea.
Materials and methods
Plant extraction
P. grandiflorum roots and A.
graveolens leaves were procured from a local market (Jeonbuk,
Korea) and a local farm (Busan, Korea), respectively. The plants
were washed in distilled water and dried at 40̊C for 16 h. The
P. grandiflorum roots and A. graveolens leaves (100 g
each) were extracted in 80% ethanol (2,000 ml), respectively for 72
h. The extracted samples were filtered using a 0.45 µm filter paper
(Advantec, Togo, Japan). The filtered samples were concentrated
under reduced pressure and lyophilized to obtain the dry powder of
P. grandiflorum roots extract (PGE) and A. graveolens
leaves extract (AGE), which were stored at -20̊C for subsequent
use. Green tea extract (total catechins ≥38%) was procured from
Mirae Biotech Co., Ltd (Gyeonggi-do, Korea) and stored at -20̊C for
direct use.
Animals and diet
C57BL/6N male mice (4 weeks of age) were procured
from Orient Bio Inc. (Gwangju, Korea). The mice were handled and
experiments were carried out based on Jeonju University
Institutional Animal Care and Use Committee guidelines with
permission to carry out the experiment obtained from Jeonju
University (JJU-IACUC-2018-8). Following the institution's
guidelines, the mice were maintained in standard environmental
conditions at a temperature of -22±2̊C, humidity of 50-60%, and
12/12 h light-dark cycle. The Mice were fed with commercial
standard laboratory diet and water ad libitum during an
acclimatization period of 1 week. After acclimatization, the 35
mice were grouped into 7 groups and fed either with a normal diet
(10% fat) or high-fat diet (HFD) (60% fat) for 16 weeks as follows:
Group 1: Non-HFD fed group (ND), n=5. Group 2: HFD fed group (HFD),
n=5. Group 3: HFD with 200 mg/kg PGE treatment, n=5. Group 4: HFD
with 200 mg/kg AGE treatment, n=5. Group 5: HFD with 200 mg/kg GTE
treatment, n=5. Group 6: HFD with 100 mg/kg PGE and 100 mg/kg AGE
mixture treatment, n=5. Group 7: HFD with 200 mg/kg PGE, AGE, and
GTE mixture (1:1:1 ratio) treatment, n=5. The extracts were orally
administered on a daily basis for 16 weeks. The ND and HFD group
received an equal amount of distilled water during extract
administration. All mice were euthanized at the end of the
experiment by cervical dislocation. Confirmation of death was done
by ensuring a firm toe pinch and lack of visible respiration. No
prior death was recorded for this experiment.
Body weight gain and food intake
analysis
The weight of the mice in each group was recorded at
the start of the experimental period and at the end of the
experiment. The average difference between the initial and final
weight was taken to be the average weight gain in each group. The
amount of food intake was measured at the same time on the same day
of the week during the 16 weeks experimental period. The average
amount of daily food intake was calculated and recorded.
Serum biochemical analysis
At the end of the 16-weeks experimental period, the
mice were made to fast for 12 h and then were sacrificed. Blood was
immediately collected through the intraorbital vein. The blood
samples were centrifuged at 2,000 x g for 15 min at 4̊C, and the
serum was separated and stored at -70̊C for subsequent use. The
serum concentrations of total cholesterol (TC), high-density
lipoprotein cholesterol (HDL-C), triglycerides (TG) (Asan
Pharmaceutical Co., Ltd., Gyeonggi, Korea), leptin (R&D
Systems, Inc., Minneapolis, MN, USA), insulin (Alpco Diagnostics,
Windham, NH, USA), and glutamic oxaloacetic transaminase (GOT) and
glutamic-pyruvic transaminase (GPT) (Asan Pharmaceutical Co., Ltd.)
were analyzed by the use of a spectrophotometer (Tecan Group,
Männedorf, Switzerland). Low-density lipoprotein cholesterol
(LDL-C) was measured with the formula: LDL-C=TC-HDL-C-(TG/5). All
experiments were carried out following the manufacturer's
instructions. Blood glucose was measured with the aid of an
Accu-Check glucometer (Roche Diagnostics, Risch-Rotkreuz,
Switzerland).
Atherogenic index and cardiac risk
factor measurement
The atherogenic index was measured with the formula:
Atherogenic index=(TC-HDL)/HDL. The cardiac risk factor was
measured with the formula: Cardiac risk factor=TC/HDL.
Liver and adipose tissue analysis
After blood collection, liver and epididymal adipose
tissues were removed from the mice and weighed immediately. For
liver oxidative stress analysis, 0.3 g of liver tissues were
obtained and homogenized in 0.5 ml of phosphate-buffered saline and
then centrifuged at 2,000 x g for 15 min at 4˚C to obtain the
supernatant which was stored at -70˚C. Catalase (CAT) activity and
concentration of GSH (Cayman Chemical, Ann Arbor, MI, USA) were
measured using assay kits, following the manufacturer's
instructions using a Tecan spectrophotometer (Männedorf). For
histochemistry analysis, epididymal adipose and liver tissues were
fixed in 10% neutral formalin for 42 h. The tissues portions were
placed in cassettes and washed in three changes of phosphate
buffered saline (30 min for each wash); cleared in two changes of
xylene (30 min each) and embedded in 3 changes of paraffin (1 h
each). The tissues were blocked in paraffin and cut to a 5-µm
thickness. The section tissues were stained with hematoxylin and
eosin (H&E) and viewed under a light microscope (Leica,
Wetzlar, Germany). The epididymal adipocyte size was evaluated
using an ImageJ software program (developed by the National
Institutes of Health, Bethesda, MD, USA). In addition, an
independent who was blinded to the grouping of the mice expert
examined the stained liver sections.
Statistical analysis
Differences between groups were appraised by
analysis of variance (ANOVA) followed by Duncan's multiple-range
test. All data are given as the mean ± SD (standard deviation). A
P-value of <0.05 was set to indicate statistically significant
results.
Results
Synergistic effects of PAG mixture on
body weight, food intake, and food efficiency ratio
As shown in Fig. 1,
mice fed with HFD (HFD control group) significantly gained more
weight compared to the mice fed with normal laboratory diet (ND
group). When mice were fed with HFD and administered with either
PGE, AGE, GTE, PA mixture or PAG mixture, weight gain was
significantly decreased compared to the HFD group. Of note, mice
treated with the PAG mixture recorded the least amount of weight
gain as compared to the other treatment groups.
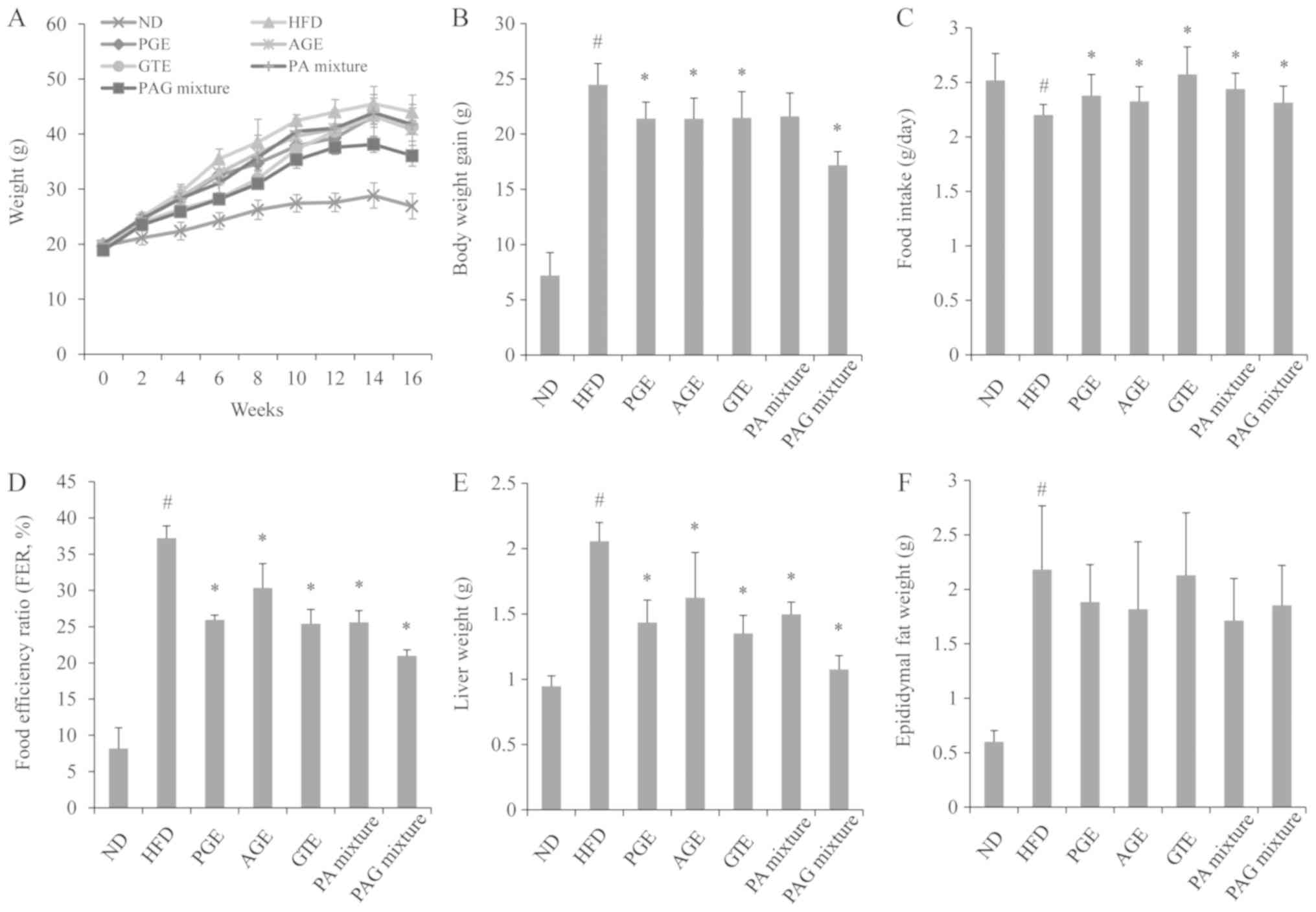 | Figure 1.Synergistic effect of PAG mixture on
(A) body weight, (B) weight gain, (C) food intake, (D) FER, (E)
liver and (F) epididymal fat weight in HFD-induced obese mice. Data
are presented as the mean ± standard deviation (n=5).
#P<0.05 vs. ND; *P<0.05 vs. HFD group.
ND, mice fed the normal diet; HFD, mice fed the high-fat diet; PGE,
mice fed with HFD and administered with 200 mg/kg/day of PGE. AGE,
mice fed with HFD and administered with 200 mg/kg/day of AGE; GTE,
mice fed with HFD and administered with 200 mg/kg/day of GTE; PA
mixture, mice fed with HFD and administered with 100 mg/kg/day of
PGE + 100 mg/kg/day of AGE; PAG mixture, mice fed with HFD and
administered with 200 mg/kg/day of PGE + AGE + GTE mixture (1:1:1
ratio); FER, food efficiency ratio=body weight gain (g/day)/food
intake (g/day) x100. |
The daily food intake in mice fed with HFD was
significantly decreased compared to mice in the ND group. However,
HFD mice administered with either PGE, AGE, GTE, PA mixture or PAG
mixture, had a significant increase in the daily food intake
compared to the HFD control group. Food efficiency ratio was also
significantly increased in the HFD control group compared to the ND
group. The PGE, AGE, GTE, PA mixture or PAG mixture treatment
groups showed a significant reduction in food efficiency ratio
compared to the HFD control group. The PAG mixture treatment group
recorded the least food efficiency ratio.
Synergistic effects of PAG mixture on
serum lipid profile, atherogenic index, and cardiac risk
factor
As presented in Fig.
2, mice in the HFD control group showed a significant increase
in serum triglyceride, total cholesterol, LDL-cholesterol, and
HDL-cholesterol compared to the ND group. As compared to the mice
in the HFD control group, all of the PGE, AGE, GTE, PA mixture or
PAG mixture treatment groups showed a significant decrease in the
levels of triglycerides (Fig. 2A).
The total cholesterol (Fig. 2B), and
LDL-cholesterol (Fig. 2C) were also
decreased in all treatment groups except the AGE treatment group,
with the PAG treatment group recording the least levels. The
HDL-cholesterol level in serum was also decreased but their
differences were not significant among the treatment groups
(Fig. 2D).
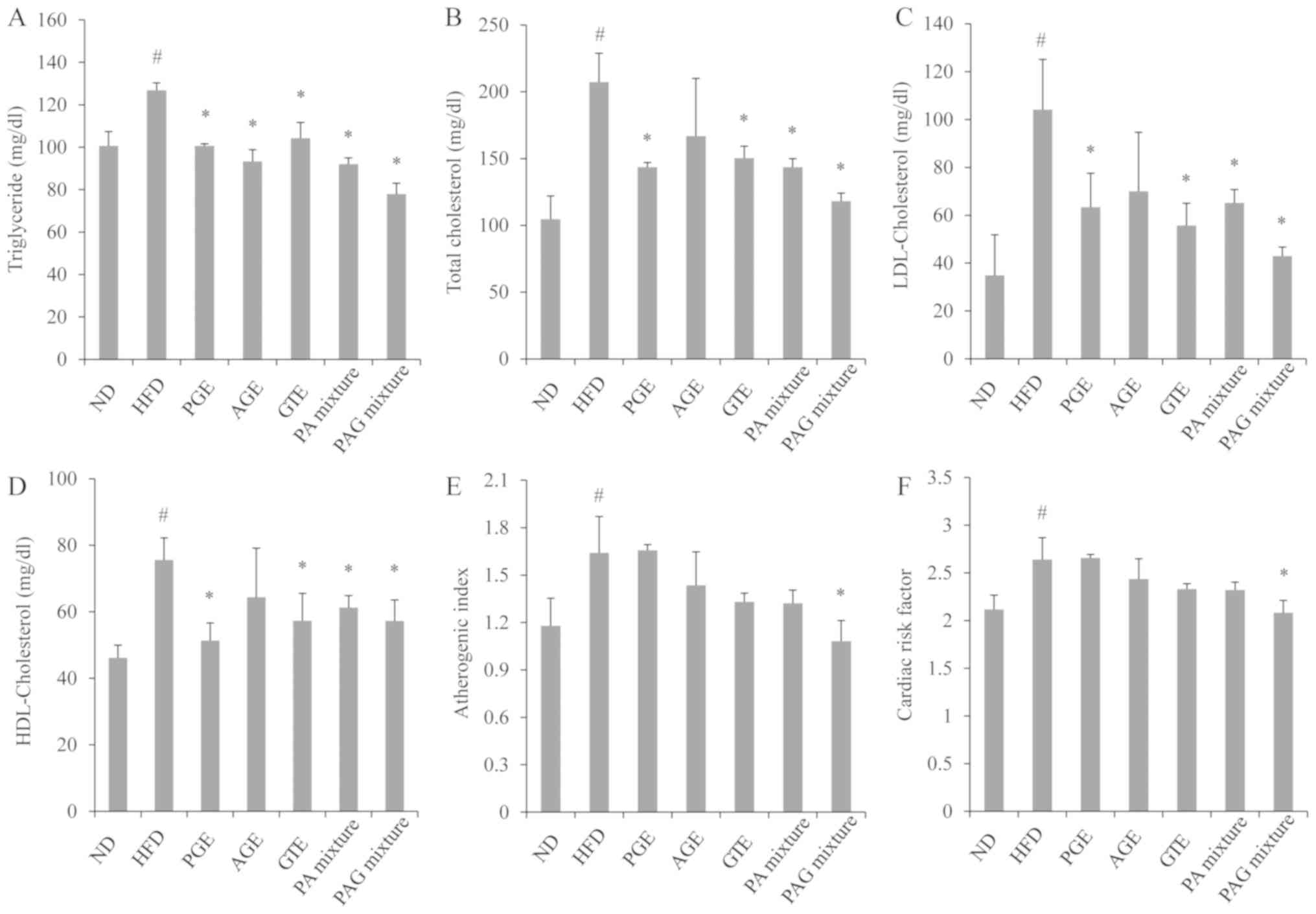 | Figure 2.Synergistic effects of (A) TG, (B)
TC, (C) LDL-C and (D) HDL-C PAG mixture on serum lipid profile, (E)
atherogenic index and (F) cardiac risk factor in HFD-induced
obesity in mice. Data are presented as the mean ± standard
deviation (n=5). #P<0.05 vs. ND;
*P<0.05 vs. HFD group. ND, mice fed the normal diet;
HFD, mice fed the high-fat diet; PGE, mice fed with HFD and
administered with 200 mg/kg/day of PGE. AGE, mice fed with HFD and
administered with 200 mg/kg/day of AGE; GTE, mice fed with HFD and
administered with 200 mg/kg/day of GTE; PA mixture, mice fed with
HFD and administered with 100 mg/kg/day of PGE + 100 mg/kg/day of
AGE. PAG mixture, mice fed with HFD and administered with 200
mg/kg/day of PGE + AGE + GTE mixture (1:1:1 ratio); TG,
triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein
cholesterol; HDL-C, high-density lipoprotein cholesterol. |
The results of the atherogenic index and cardiac
risk factor demonstrated that mice in the HFD control group had a
significant increase in the atherogenic index and cardiac risk
factors. However, only mice fed with HFD and administered with
either GTE, PA mixture or PAG mixture had a significant reduction
in the atherogenic index and cardiac risk factors, with the PAG
mixture treatment group demonstrating better effects than the other
treatment groups (Fig. 2E and
F).
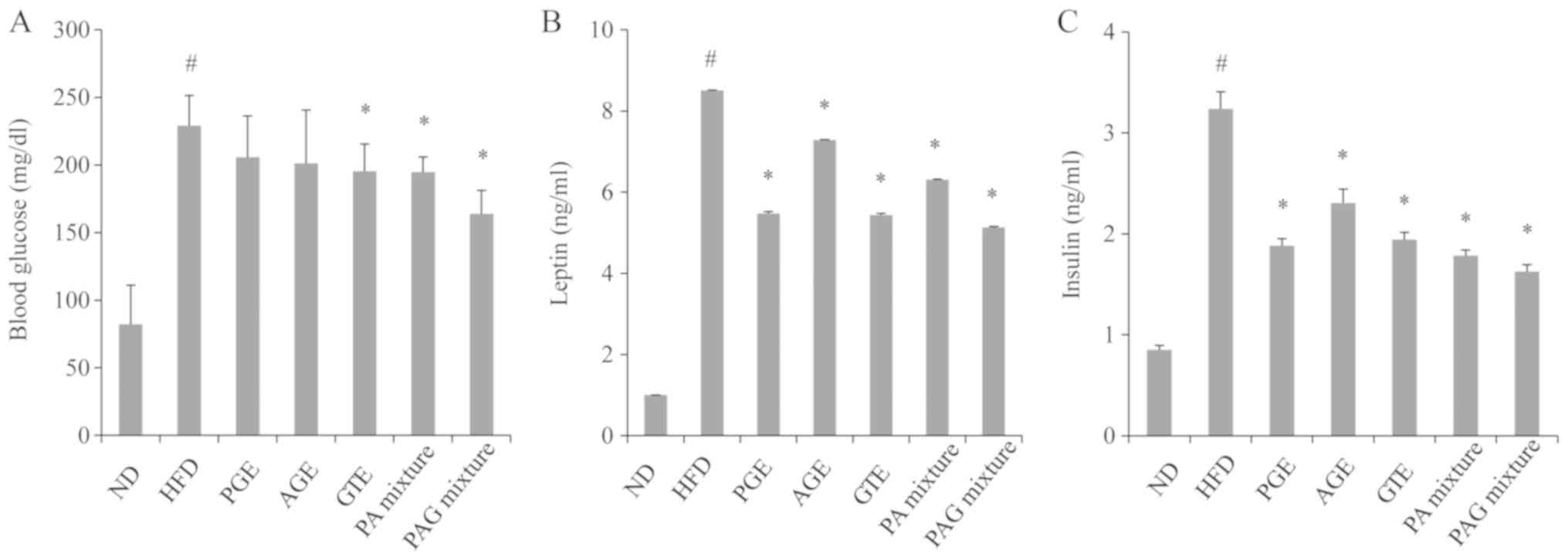
Synergistic effects of PAG mixture on
energy balancing metabolism
As shown in Fig. 3,
mice in the HFD control group recorded a significant increase in
blood glucose level, compared to mice in the ND group. Only the PA
mixture and PAG mixture treatment groups had a significant
reduction in serum glucose levels compared to the mice in the HFD
control group (Fig. 3A). Serum
leptin levels were also significantly increased in mice of the HFD
control group compared to the ND group. PGE, AGE, GTE, PA mixture
or PAG mixture treatment groups recorded a significant decrease in
leptin levels, compared to mice of the HFD control group (Fig. 3B). Similarly, insulin levels were
significantly increased in mice of the HFD control group. However,
the PGE, AGE, GTE, PA mixture or PAG mixture had the tendency to
downregulate serum insulin levels (Fig.
3C). Among the treatment groups, the PAG mixture group appeared
to have better effects on energy balancing metabolisms compared to
the other groups.
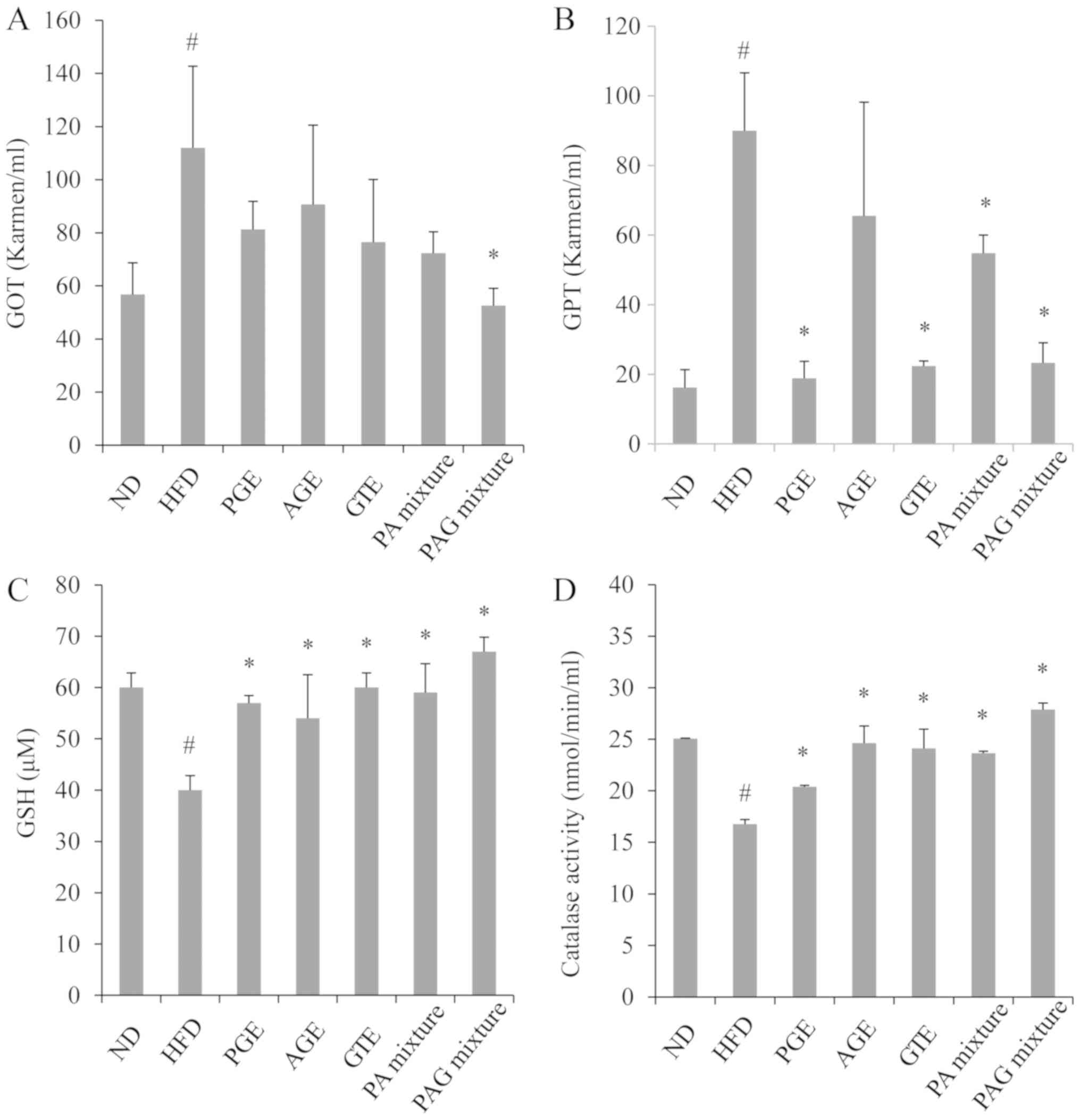
Synergistic effects of PAG mixture on
serum toxicity markers and liver oxidative stress
Mice fed with HFD resulted in a significant increase
in the liver weight (Fig. 1) and
serum levels of GOT and GPT compared to mice fed with a normal diet
(Fig. 4A and B). A significant decrease in endogenous GSH
antioxidant and catalase activities in the liver tissues were also
observed in mice fed with HFD compare to mice in the ND group
(Fig. 4C and D). Treatment with either the PGE, AGE, GTE,
PA mixture or PAG mixture significantly decreased liver weight,
with the PAG treatment group demonstrating better effects than the
other treatment groups (Fig. 1). The
serum GOT level was significantly decreased in the PA mixture and
PAG mixture treatment group (Fig.
4A), while serum GPT level was significantly decreased in the
PGE, GTE, PA mixture, and PAG mixture treatment groups (Fig. 4B). On the one hand, GSH antioxidant
and catalase activities were significantly upregulated in all
treated groups, with the PAG mixture treatment group demonstrating
the best results (Fig. 4C and
D). On the whole, the PGE, AGE, GTE,
PA mixture or PAG mixture treatment showed no detectable adverse
toxicity effects in the livers.
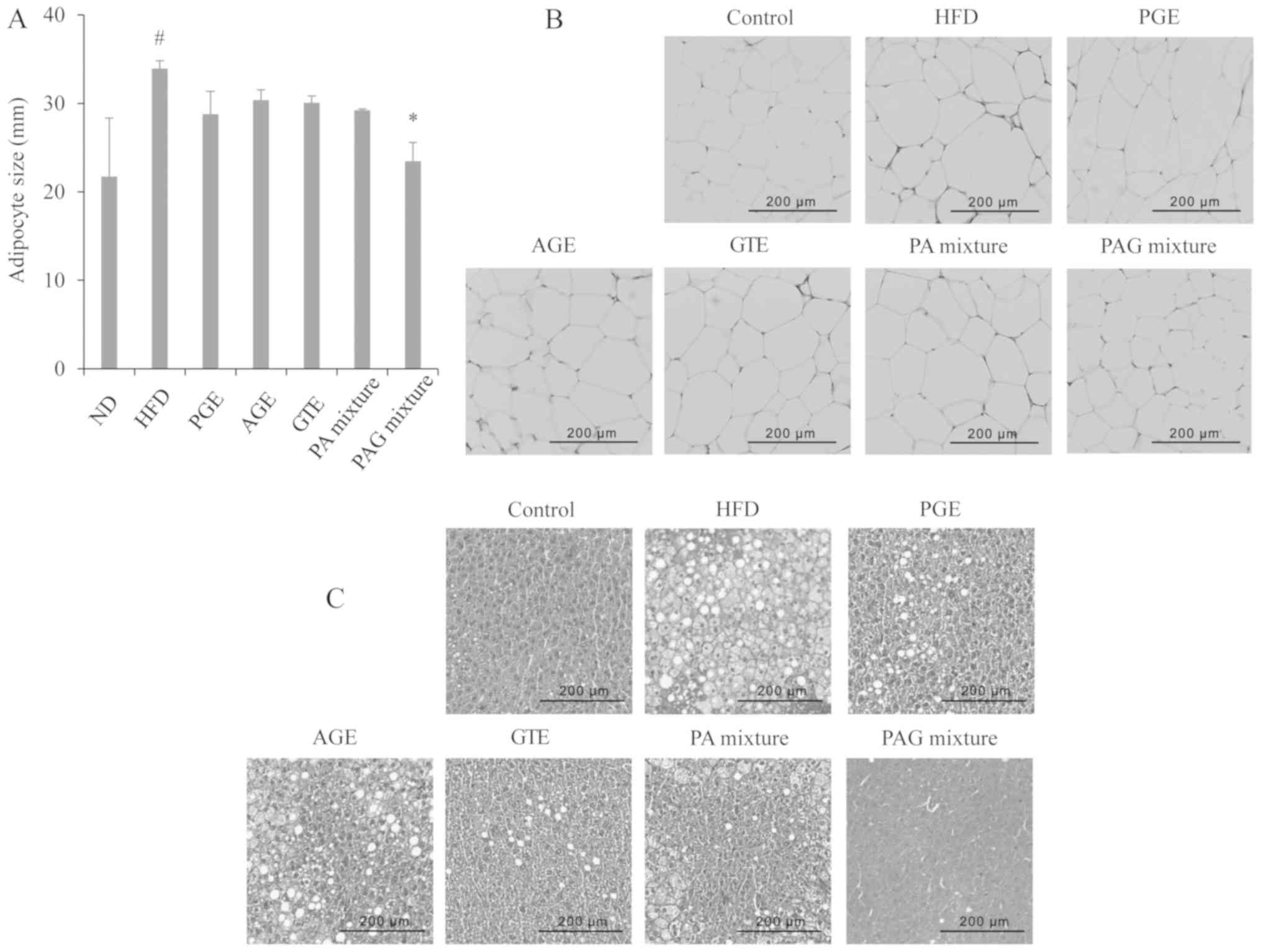
Synergistic effects of PAG mixture on
adipose tissue and fat deposition
Mice fed with HFD resulted in a significant increase
in the epididymal fat weight compared to mice that received the
normal diet (Fig. 1). However, mice
fed with HFD and treated with either the PGE, AGE, GTE, PA mixture,
or PAG mixture showed no significant decrease in the epididymal fat
weight compared to the HFD control group (Fig. 1). To estimate the adipocyte size,
using Image software, the diameter of adipocytes in H&E stained
sections were measured. The mice in the HFD control group showed a
significant increase in adipocyte diameter size compared to the ND
group. Of all the treatment groups, only the PAG group showed a
significant decrease in adipocyte diameter compared to the HFD
control group (Fig. 5A). The
adipocyte size can be seen clearly in the histological sections
presented in Fig. 5B.
Histological stain liver sections also showed that
the liver of mice in the HFD control group had extensive lipid
droplet accumulation compared to the ND group (Fig. 5C). Macrovesicular and micro lipid
droplets could be seen in the liver sections of mice in the HFD
control group thus demonstrating a typical fatty liver. All
treatment groups showed less lipid droplet accumulation compared to
the HFD control group. It is also of interest to note that the
liver conditions of the PAG treatment group appeared to be close to
those of the ND group (Fig. 5C).
Discussion
With the increasing global prevalence of obesity and
its associated disorders, coupled with the fact that therapies
designated for the prevention and treatment of obesity most often
render patients with severe consequences, alternative anti-obesity
therapies from natural products have taken the lead in modern day
research with the sole aim of developing better therapies for the
prevention and treatment of obesity and its associated disorders.
In the present study, we investigated the synergistic effects of
PGE, AGE, and GTE in obesity induced by HFD in mice. It was found
that the PGE, AGE, GTE, and PA mixture decreased the body weight of
mice that were fed with HFD. Although no significant difference was
found in the epididymal adipose tissue of the HFD control group and
the treatment groups, histological examinations of the epididymal
tissues, and measurement of the diameters of the adipocyte in
different microscopic fields revealed that the PAG mixture
treatment group had reduced adipose cell size. The results obtained
from the PAG mixture treatment appeared to be most effective in
preventing weight gain and inhibiting epididymal adipocyte size
expansion in the obese mice. Also, the results of the daily food
intake and food efficiency ratio further suggest that PAG was most
effective in preventing body weight gain among the treatment
groups. From these results, it can be inferred that PAG treatment
has the potential to be used as an anti-obesity agent for
preventing weight gain and preventing an increase in adipocyte size
in obesity and its associated disorders.
In animals as well as in humans, obesity or a
high-fat diet has been shown to influence lipid dynamics that
result in or increase the risk of cardiovascular diseases. In this
study, obesity led to an increment of serum lipid parameters such
as TG, total cholesterol, LDL, and HDL. These disturbances in the
lipid dynamics exposed the mice to the risk of developing
cardiovascular diseases as revealed by the atherogenic index and
cardiac risk factor data of this study. The study also showed that
PGE, AGE, GTE, PA mixture, and PAG mixtures decreased the serum
lipid parameters thereby decreasing the risk of cardiovascular
disease, with the PAG mixture demonstrating the overall best
effects. Previous studies have revealed the presence of
hypolipidaemic compounds in PGE, AGE, and GTE (16,21,27). The
effects of these extracts and their combinations could act by
directly preventing obesity and hence lipid serum levels or by
decreasing cholesterol absorption or better still interfering in
triglyceride or cholesterol synthesis. A study had earlier reported
that phenols in apples were responsible for hypolipidemic and
antiatherogenic in rats by suppressing cholesterol absorption while
promoting its catabolism (28).
Leptin and insulin among other hormones are key
obesity markers that correlate positively with adiposity and an
increase in fat mass and are involved in the regulation of appetite
and satiety, thus affecting energy metabolism (29). Also, leptin and insulin resistance is
seen in obesity and its leads to hyperinsulinemia and
hyperleptinemia (30,31). Thus, the normalization of these
obesity-related parameters will lead to fat and glucose metabolism
thus providing useful methods for treating obesity and its related
complications. In the present study, serum insulin and leptin were
increased with HFD treatment. HFD has been reported to increase
insulin and leptin levels, causing hyperinsulinemia and
hyperleptinemia (32). It was also
revealed in this study that treatment with PGE, AGE, GTE, PA
mixture, and PAG mixtures suppressed the increase in insulin and
leptin levels with PAG mixture demonstrating the overall best
results. The effects of these extracts in decreasing insulin and
leptin levels might also be responsible for the decrease in glucose
levels seen with the extract treatment, especially with the PAG
mixture treatment group. Several reports have shown a reduction in
body weight gain with HFD treatment of mice through leptin
modulation (33-35).
It is also of interest to note that insulin determines leptin
levels (32). Therefore, it can be
concluded that a significant decrease in insulin level may have
resulted in the suppression of leptin and hence glucose levels with
the extracts treatment.
A high-fat diet is also well known to increase liver
weight probably through fatty acid deposition, causes liver injury
and also induces oxidative burden in mice liver. Consistently,
feeding mice with HFD led to higher liver weight, while
administration of the PGE, AGE, GTE, PA mixture, and PAG mixtures
led to a decrease in liver weight. Histological findings using
H&E staining revealed severe hepatic steatosis in HFD-fed mice.
The presence of macrovesicular and micro lipid droplets could be
seen in stained sections. In addition, HFD resulted in liver
oxidative stress and injury as evidenced by enhanced GOT, GPT, and
diminished catalase activity and GSH levels. Administration of PGE,
AGE, GTE, PA mixture, and in particular PAG mixtures to mice fed
with HFD reduced fatty acid deposition in the liver; reduced GOT
and GPT; and up-regulated catalase activity and GSH levels in the
mice. These results further suggest that PGE, AGE, and GTE
combinations possess liver-protective actions.
The observed effects of PAG mixtures on weight gain,
adipocyte size, lipid profiles, obesity-related hormones, and liver
protection in our study were in compliance with reports from other
studies. Varying combinations of Phyllostachys
pubescens leaf extract and Scutellaria
baicalensis root extract significantly reduced weight gain,
adipose tissue weight, size of adipocytes, and the levels of
glucose, leptin, and lipid profile in serum, and fat accumulation
in the liver (36). Treatment with
mixtures of Salacia reticulate extract and
cyclodextrin also significantly suppresses body weight gain,
visceral fat mass plasma leptin, and TG levels of rats fed with HFD
(37). Here, we hypothesized that
the potential mechanism of action of these extracts could involve
increasing energy expenditure, enhancing lipolysis, preventing
energy intake, and elevating fatty acid oxidation-related genes
especially as one of the extracts used in this study (Green tea)
had previously been shown to regulated these parameters in previous
studies (9,38). The synergistic effects that were seen
in this study could have arisen from an increase in the number or
amount of antiobesity constituents as a result of combining the
three extracts. Also, an important observation in this study was
the fact that the combination of the three extracts did not
demonstrate any adverse effects (evidenced by no mouse death
recorded) as seen with drug-drug interaction in combinational
therapies.
In conclusion, oral administration of PAG mixture
significantly reduced body weight, adipocyte size, serum
triglyceride, total cholesterol, LDL cholesterol and leptin,
insulin and glucose levels in high-fat-diet-induced obese mice. The
mixed extract also decreased the serum levels of GOT and GPT,
upregulated catalase activity and GSH level, thus preventing liver
injury. Therefore, it can be assumed that a combination of
Platycodon grandiflorum, Apium
graveolens, and green tea exert anti-obesity effects. The
overall findings of this study indicate that the PAG mixture is
worthy of further investigation as a potential anti-obesity agent.
As a limitation to the present study, multiple ratios of the
extracts were not tested to determine the optimal ratio for better
efficacy.
Acknowledgements
Not applicable.
Funding
This research was supported by a grant (grant no.
20170914-C1-014) from the Jeonbuk Research & Development
Program funded by Jeonbuk Province.
Availability of data and materials
The datasets used and/or analyzed data during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
BOC and SIJ designed the research. HJK, DNC, JYS,
JSK and SJK performed the research. BOC, JC and SIJ analyzed the
research data. BOC, JC, SIJ and DNC wrote the manuscript draft.
BOC, SIJ and DNC reviewed and edited the final manuscript. BOC
managed the research project. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The mice were handled and experiments were carried
out based on Jeonju University Institutional Animal Care and Use
Committee guidelines with permission to carry out the experiment
obtained from Jeonju University (approval no.
JJU-IACUC-2018-8).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jane L, Atkinson G, Jaime V, Hamilton S,
Waller G and Harrison S: Intermittent fasting interventions for the
treatment of overweight and obesity in adults aged 18 years and
over: A systematic review protocol. JBI Database System Rev
Implement Rep. 13:60–68. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
WHO: Obesity and overweight. February 16,
2018.
|
|
3
|
Bloomgarden ZT: Obesity, hypertension, and
insulin resistance. Diabetes Care. 25:2088–2097. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gallagher EJ and LeRoith D: Obesity and
Diabetes: The increased risk of cancer and cancer-related
mortality. Physiol Rev. 95:727–748. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kushner RF: Medical management of obesity.
Semin Gastrointest Dis. 13:123–132. 2002.PubMed/NCBI
|
|
6
|
Connolly HM, Crary JL, McGoon MD, Hensrud
DD, Edwards BS, Edwards WD and Schaff HV: Valvular heart disease
associated with fenfluramine-phentermine. N Engl J Med.
337:581–588. 1997.PubMed/NCBI
|
|
7
|
Glazer G: Long-term pharmacotherapy of
obesity 2000: A review of efficacy and safety. Arch Intern Med.
161:1814–1824. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Powell AG, Apovian CM and Aronne LJ: New
drug targets for the treatment of obesity. Clin Pharmacol Ther.
90:40–51. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y
and Wan X: The anti-obesity effects of green tea in human
intervention and basic molecular studies. Eur J Clin Nutr.
68:1075–1087. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suzuki T, Pervin M, Goto S, Isemura M and
Nakamura Y: Beneficial effects of tea and the green tea catechin
epigallocatechin-3-gallate on obesity. Molecules. 21(pii:
E1305)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma G, Guo W, Zhao L, Zheng Q, Sun Z, Wei
J, Yang J and Xu X: Two new triterpenoid saponins from the root of
Platycodon grandiflorum. Chem Pharm Bull (Tokyo).
61:101–104. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee JY, Hwang WI and Lim ST: Antioxidant
and anticancer activities of organic extracts from Platycodon
grandiflorum A. De Candolle roots. J Ethnopharmacol.
93:409–415. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee KJ, You HJ, Park SJ, Kim YS, Chung YC,
Jeong TC and Jeong HG: Hepatoprotective effects of Platycodon
grandiflorum on acetaminophen-induced liver damage in mice.
Cancer Lett. 174:73–81. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jeong CH, Choi GN, Kim JH, Kwak JH, Kim
DO, Kim YJ and Heo HJ: Antioxidant activities from the aerial parts
of Platycodon grandiflorum. Food Chem. 118:278–282. 2010.
View Article : Google Scholar
|
|
15
|
Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS
and Kim YS: Inhibition of inducible nitric oxide synthase and
cyclooxygenase II by Platycodon grandiflorum saponins via
suppression of nuclear factor-kappaB activation in RAW 264.7 cells.
Life Sci. 76:2315–2328. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao HL, Harding SV, Marinangeli CP, Kim
YS and Jones PJ: Hypocholesterolemic and anti-obesity effects of
saponins from Platycodon grandiflorum in hamsters fed
atherogenic diets. J Food Sci. 73:H195–H200. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mencherini T, Cau A, Bianco G, Della
Loggia R, Aquino RP and Autore G: An extract of Apium
graveolens var. dulce leaves: Structure of the major
constituent, apiin, and its anti-inflammatory properties. J Pharm
Pharmacol. 59:891–897. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Momin RA and Nair MG: Mosquitocidal,
nematicidal, and antifungal compounds from Apium graveolens
L. seeds. J Agric Food Chem. 49:142–145. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baananou S, Bouftira I, Mahmoud A, Boukef
K, Marongiu B and Boughattas NA: Antiulcerogenic and antibacterial
activities of Apium graveolens essential oil and extract.
Nat Prod Res. 27:1075–1083. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kooti W and Daraei N: A review of the
antioxidant activity of celery (Apium graveolens L). J Evid
Based Complementary Altern Med. 22:1029–1034. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jung UJ, Cho YY and Choi MS: Apigenin
ameliorates dyslipidemia, hepatic steatosis and insulin resistance
by modulating metabolic and transcriptional profiles in the liver
of high-fat diet-induced obese mice. Nutrients.
8(E305)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu G, Zhuang L, Song D, Lu C and Xu X:
Isolation, purification, and identification of the main phenolic
compounds from leaves of celery (Apium graveolens L. var.
dulce Mill./Pers.). J Sep Sci. 40:472–479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chantre P and Lairon D: Recent findings of
green tea extract AR25 (Exolise) and its activity for the treatment
of obesity. Phytomedicine. 9:3–8. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brown AL, Lane J, Holyoak C, Nicol B,
Mayes AE and Dadd T: Health effects of green tea catechins in
overweight and obese men: A randomised controlled cross-over trial.
Br J Nutr. 106:1880–1889. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cardoso GA, Salgado JM, Cesar Md C and
Donado-Pestana CM: The effects of green tea consumption and
resistance training on body composition and resting metabolic rate
in overweight or obese Women. J Med Food. 16:120–127.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Singhal K, Raj N, Gupta K and Singh S:
Probable benefits of green tea with genetic implications. J Oral
Maxillofac Pathol. 21:107–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmad RS, Butt MS, Sultan MT, Mushtaq Z,
Ahmad S, Dewanjee S, De Feo V and Zia-Ul-Haq M: Preventive role of
green tea catechins from obesity and related disorders especially
hypercholesterolemia and hyperglycemia. J Transl Med.
13(79)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Osada K, Suzuki T, Kawakami Y, Senda M,
Kasai A, Sami M, Ohta Y, Kanda T and Ikeda M: Dose-dependent
hypocholesterolemic actions of dietary apple polyphenol in rats fed
cholesterol. Lipids. 41:133–139. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Staiger H and Haring HU: Adipocytokines:
Fat-derived humoral mediators of metabolic homeostasis. Exp Clin
Endocrinol Diabetes. 113:67–79. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: A review. Obes Rev. 8:21–34. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Könner AC, Hess S, Tovar S, Mesaros A,
Sánchez-Lasheras C, Evers N, Verhagen LA, Brönneke HS, Kleinridders
A, Hampel B, et al: Role for insulin signaling in catecholaminergic
neurons in control of energy homeostasis. Cell Metab. 13:720–728.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Harte RA, Kirk EA, Rosenfeld ME and
LeBoeuf RC: Initiation of hyperinsulinemia and hyperleptinemia is
diet dependent in C57BL/6 mice. Horm Metab Res. 31:570–575.
1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sasaki T: Age-associated weight gain,
leptin, and SIRT1: A possible role for hypothalamic SIRT1 in the
prevention of weight gain and aging through modulation of leptin
sensitivity. Front Endocrinol (Lausanne). 6(109)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Myers MG Jr, Leibel RL, Seeley RJ and
Schwartz MW: Obesity and leptin resistance: Distinguishing cause
from effect. Trends Endocrinol Metab. 21:643–651. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Carter S, Caron A, Richard D and Picard F:
Role of leptin resistance in the development of obesity in older
patients. Clin Interv Aging. 8:829–844. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim DS, Kim SH and Cha J: Antiobesity
effects of the combined plant extracts varying the combination
ratio of phyllostachys pubescens leaf extract and scutellaria
baicalensis root extract. Evid Based Complement Alternat Med.
2016(9735276)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kishino E, Ito T, Fujita K and Kiuchi Y: A
mixture of the Salacia reticulata (Kotala himbutu) aqueous
extract and cyclodextrin reduces the accumulation of visceral fat
mass in mice and rats with high-fat diet-induced obesity. J Nutr.
136:433–439. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rains TM, Agarwal S and Maki KC:
Antiobesity effects of green tea catechins: A mechanistic review. J
Nutr Biochem. 22:1–7. 2011.PubMed/NCBI View Article : Google Scholar
|