Introduction
Atrial fibrillation (AF) is the most common
sustained cardiac arrhythmia that causes cardiac dysfunction and
strokes (1). Heart failure (HF) is
characterized by chamber dilatation, which may promote the
occurrence of AF through mechanoelectrical feedback and electrical
or structural remodeling (2-6).
Different 3',5'-cyclic nucleotide phosphodiesterase (PDE) isozymes
exert distinctive biological functions, therefore, pharmacological
inhibition of these PDEs might offer novel therapeutic strategies
through their abilities to modulate cardiovascular diseases,
including HF (7,8). In particular, PDE3-5 were reported to
be expressed in cardiomyocytes and might play a role in both HF and
AF (7,8). The PDE3 inhibitor, milrinone, exerts
positive inotropic, vasodilating and minimal chronotropic effects,
which are expected to improve HF (9). However, milrinone has potentially fatal
adverse effects, including ventricular arrhythmias, which limit its
use (10-14).
PDE4 is expressed by human atrial myocytes, and its D isoform
(PDE4D) has been linked to stroke risk in a number of genome-wide
association studies (15,16). In a human study, PDE4 activity
decreased by almost 50% in AF compared with patients with SR
(17). Furthermore, cardiac PDE4D
expression was also reduced in humans with atrial fibrillation, and
a clinical trial revealed a slight increase in incidence of atrial
arrhythmia in the PDE4 inhibitor (rofluminast)-treated group,
suggesting that a decrease in PDE4 activity may be linked to the
development of AF. The PDE5 inhibitor, sildenafil, blocks the
L-type calcium current (ICa-L) and the human
ether-a-go-go related gene potassium channel in ventricular
myocytes (18,19). Higher concentrations of sildenafil
(0.2 µg/ml), with a nitric oxide (NO) donor, increase ventricular
tachycardia or ventricular fibrillation, suggesting a potential
risk of atrial arrhythmogenesis with the use of PDE5 inhibitors
(20).
Pulmonary veins (PVs) form complex histological
components with vascular structures and cardiomyocytes (21). PVs serve critical roles in AF
initiation and maintenance through their distinctive electrical and
structural properties (22-25).
Furthermore, sinoatrial node (SAN) electrical activity can modulate
PV arrhythmogenesis by increasing PVs spontaneous activity, and
while PVs spontaneous activity exceeds SAN activity, atrial
arrhythmia may occur (26). In
addition to direct effects on cardiomyocytes, inhibitors of
different PDE subtypes significantly regulate vascular activity, as
well as cardiac contractility and pacemaker activity (7). Accordingly, it was hypothesized that
PDE3-5 inhibitors may differentially regulate PV and SAN electrical
activities, thereby contributing to the pathogenesis of AF. The
present study aimed to investigate the effects and mechanisms of
inhibitors of the three PDE subtypes on cardiac spontaneous
activity in SANs and PVs.
Materials and methods
Rabbit PV and atrial tissue
preparations
The present study was approved of the Institutional
Animal Care and Use Committee (approval no. IACUC-19-124) of the
National Defense Medical Center, Taipei, Taiwan and conformed to
the institutional Guide for the Care and Use of Laboratory Animals
and the ‘Guide for the Care and Use of Laboratory Animals’
published by the United States National Institutes of Health (8 ed.
Washington DC, 2011). Male New Zealand white rabbits (n=47; weight,
2.0-3.0 kg; age, 6-8 months) were used in the present study. All of
the rabbits had access to food and water ad libitum, and
were maintained in a temperature and humidity-controlled
environment (20-22˚C; 50-70% humidity) with a 12 h light/dark
cycle, and were raised in stainless steel cages. After rabbits were
euthanized using intramuscular injections of a mixture of Zoletil
50 (10 mg/kg) and xylazine (5 mg/kg) with an overdose of isoflurane
(5% in oxygen) from a precision vaporizer as previously described
(27,28). Hearts were rapidly removed and placed
in perfusion fluid after a midline thoracotomy. To dissect the PV,
the heart was opened by an incision along the mitral valve annulus,
extending from the coronary sinus to the septum, in Tyrode's
solution comprised of 137 mM sodium chloride, 4 mM potassium
chloride, 15 mM sodium bicarbonate, 0.5 mM monosodium phosphate,
0.5 mM magnesium chloride, 2.7 mM calcium chloride and 11 mM
dextrose. The PV was separated from the atrium at the level of the
left atrial-PV junction and separated from the lungs at the ending
of the PV myocardial sleeve. The SAN was isolated from the right
atrium. The adventitia or epicardial side of the preparation faced
upwards. One end of the preparation, consisting of the PV and
atrial-PV junction, was pinned with needles to the bottom of a
tissue bath. The distal-PV was connected to a FT03C force
transducer (Grass Instruments Co.) by silk threads. The PV tissue
strips were superfused at a constant rate (3 ml/min) with Tyrode's
solution and were saturated with a gas mixture of 97%
O2/3% CO2. The temperature was maintained at
37˚C and the preparations were allowed to equilibrate for 1 h
before the electrophysiological assessment.
Electrophysiological and
pharmacological studies
Transmembrane action potentials (APs) of the PVs
were recorded by machine-pulled glass capillary microelectrodes
filled with 3 mol/l KCl, which were connected to a Duo 773
electrometer (World Precision Instruments, Ltd.) under a tension of
1.47 mN (150 mg). Electrical and mechanical events (contractile
force and diastolic tension) were simultaneously displayed on a
4072 oscilloscope (Gould) and a TA11 recorder (Gould). Using a data
acquisition system, signals were recorded with direct coupling and
a filter with a 10-kHz low-pass cut-off frequency. Signals were
recorded digitally with a 16-bit accuracy, at a rate of 125 kHz.
Electrical stimulation was provided using a Grass S88 stimulator
through a SIU5B stimulus isolation unit (Grass Instruments Co.).
Different concentrations of a PDE3 inhibitor (milrinone; 0.1, 1 and
10 µM; Sigma-Aldrich; Merck KGaA), PDE4 inhibitor (rolipram; 0.1, 1
and 10 µM; Sigma-Aldrich; Merck KGaA) or PDE5 inhibitor
(sildenafil; 0.1, 1 and 10 µM; Sigma-Aldrich; Merck KGaA) were
sequentially superfused to test for pharmacological responses. For
each concentration, PV and SAN preparations were treated for at
least 30 min. The electrical activity in isolated rabbit PVs was
recorded before and after the application of 10 µM milrinone with
or without isoproterenol (1 µM; Sigma-Aldrich; Merck KGaA), KT5823
(1 µM; Tocris Bioscience) [a potent selective inhibitor of cyclic
guanosine monophosphate (cGMP)-dependent protein kinase G (PKG)] or
H89 (a protein kinase A [PKA] inhibitor; 10 µM; Sigma-Aldrich;
Merck KGaA) (29).
Spontaneous activity was defined as the constant
occurrence of spontaneous APs in the absence of any electrical
stimuli. Early afterdepolarizations (EADs) were defined as the
interruption of the smooth contour of phase 2 or 3 of the APs.
Delayed afterdepolarizations (DADs) were defined as the presence of
a spontaneous hump-shaped depolarization of the impulse after full
repolarization had occurred. Burst firing was defined as the
occurrence of an accelerated spontaneous potential (faster than the
basal rate) with sudden onset and termination.
Statistical analysis
All continuous variables are expressed as the mean ±
SEM. Repeated-measures ANOVA followed by Duncan's post hoc test was
used to compare the difference before and after drug
administration. Electrophysiological and mechanical characteristics
were compared between different groups by a Wilcoxon rank-sum test
or an unpaired t-test, depending on the outcome of the normality
test. Nominal variables were compared by a χ2 analysis
with Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SigmaPlot software (version 12.0; Systat Software,
Inc.).
Results
Effects of the PDE3 inhibitor on the
electrical activities of isolated PVs and SANs
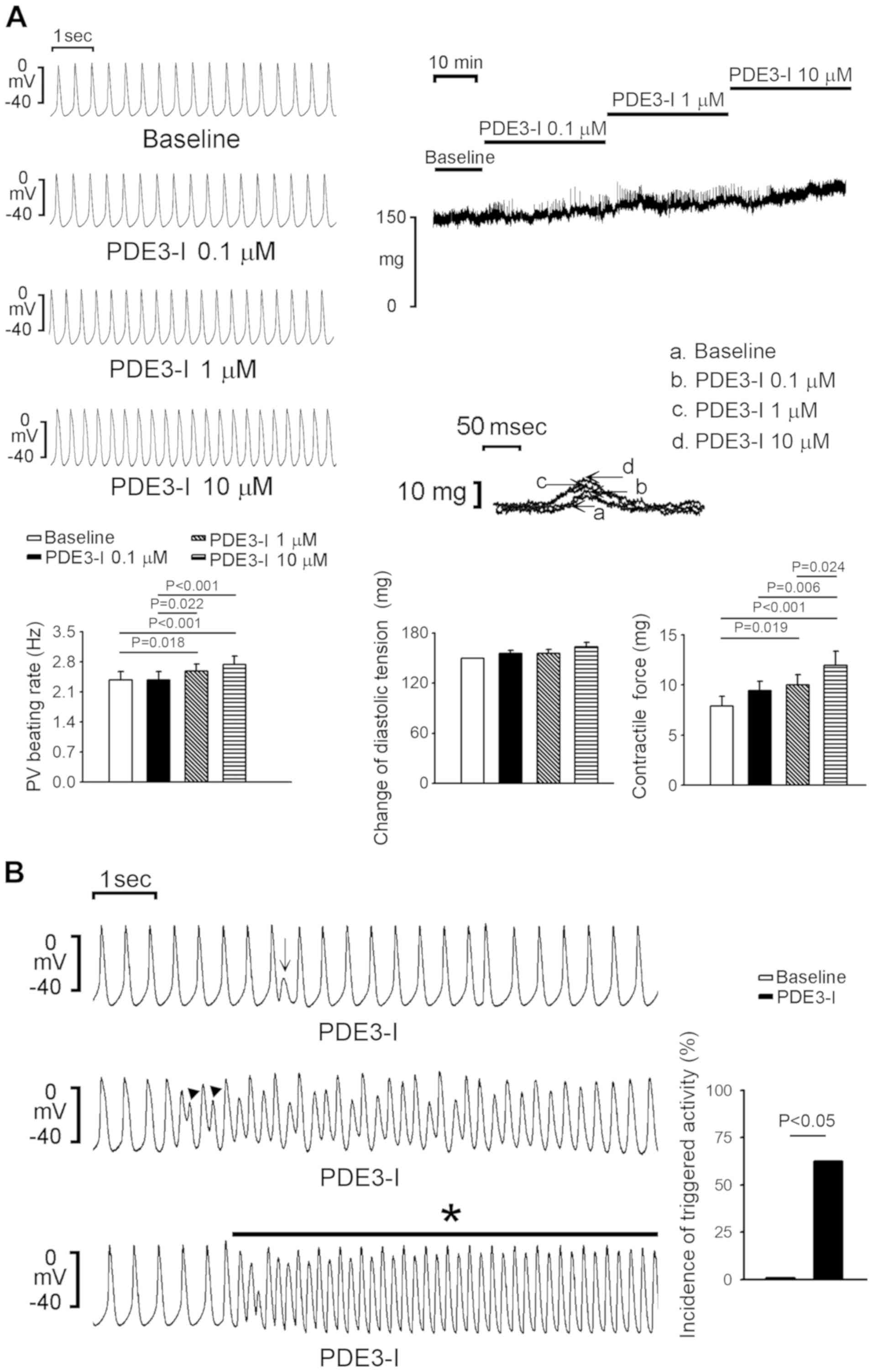
The PDE3 inhibitor (milrinone; 1 and 10 µM)
significantly increased PV spontaneous activity by 10.6±4.9%
(P=0.018) and 16.7±5.3% (P<0.001) and contractility by 31.7±9.8%
(P=0.019) and 58.8±19% (P<0.001) compared with the baseline,
respectively, but had no significant effect on the diastolic
tension of PVs (Fig. 1A). Milrinone
also significantly increased PV diastolic tension by 8.9±3.7% (P
=0.002) at 10 µM. Moreover, milrinone (≥1 µM) induced triggered
activity including EAD, or DAD or burst firing in PVs (0/8 vs. 5/8;
P<0.05; Fig. 1B). Similarly, the
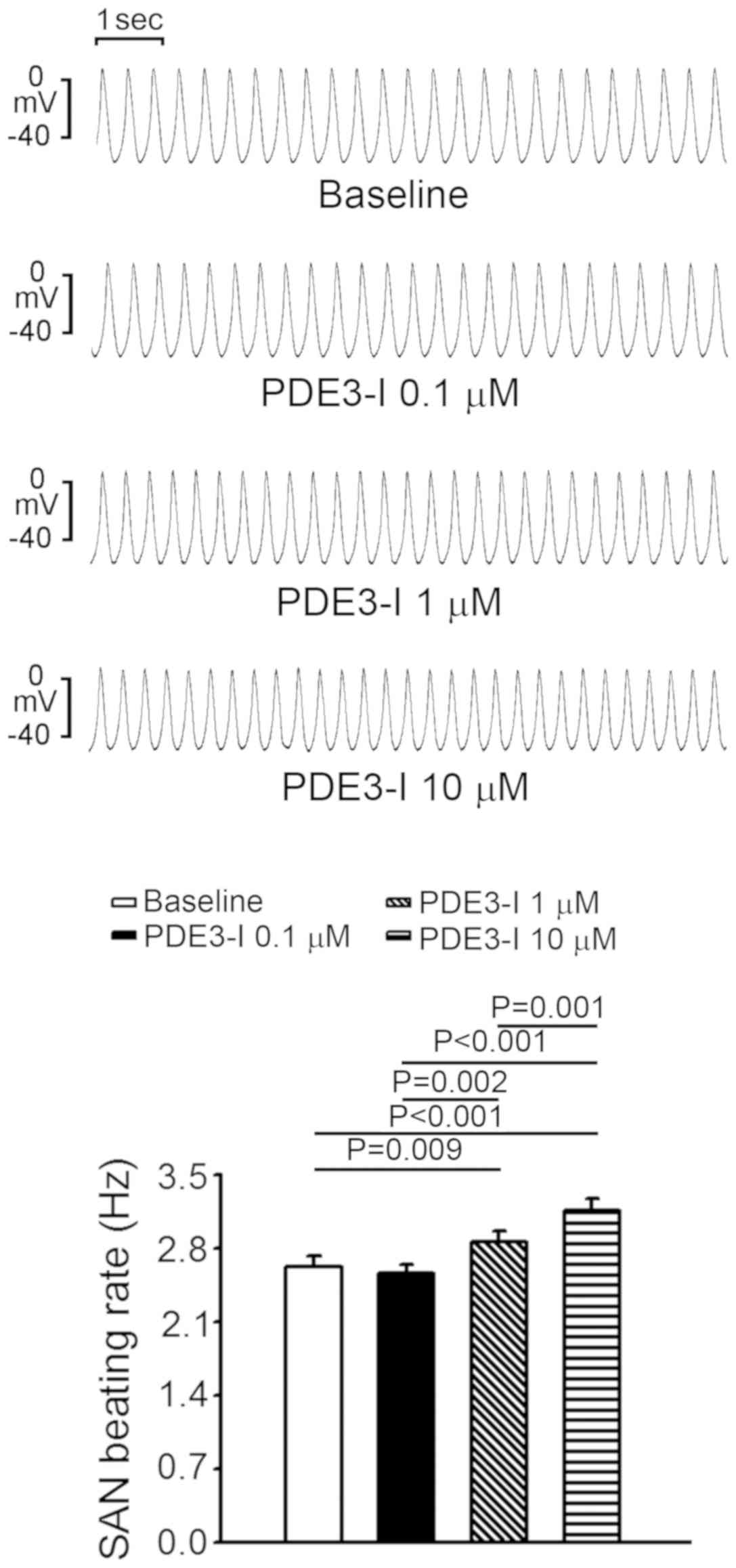
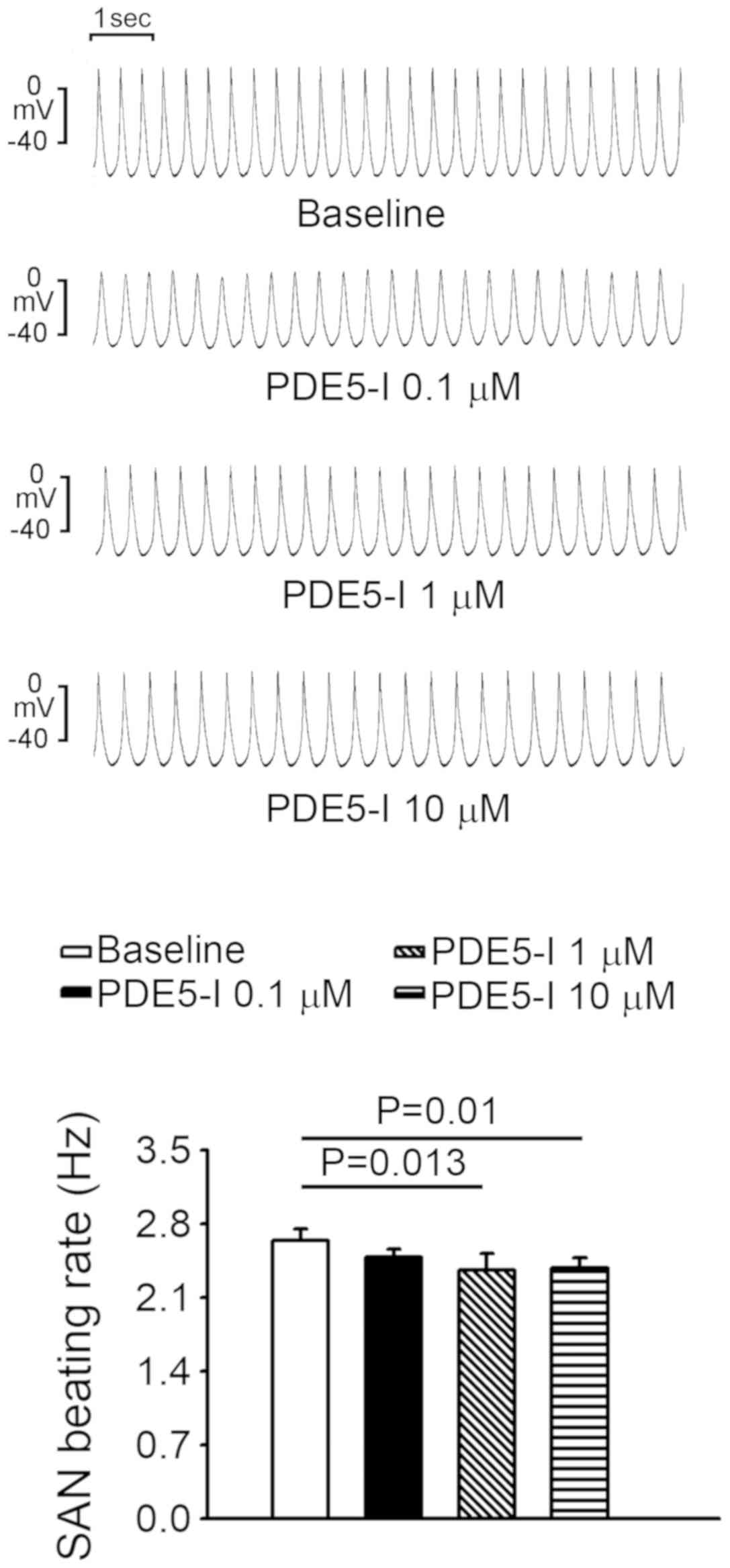
PDE3 inhibitor increased SAN activity by 9.3±4.3% and 20.7±4.6% at
1 and 10 µM compared with the baseline, respectively (Fig. 2). However, milrinone did not induce
burst firing in SANs.
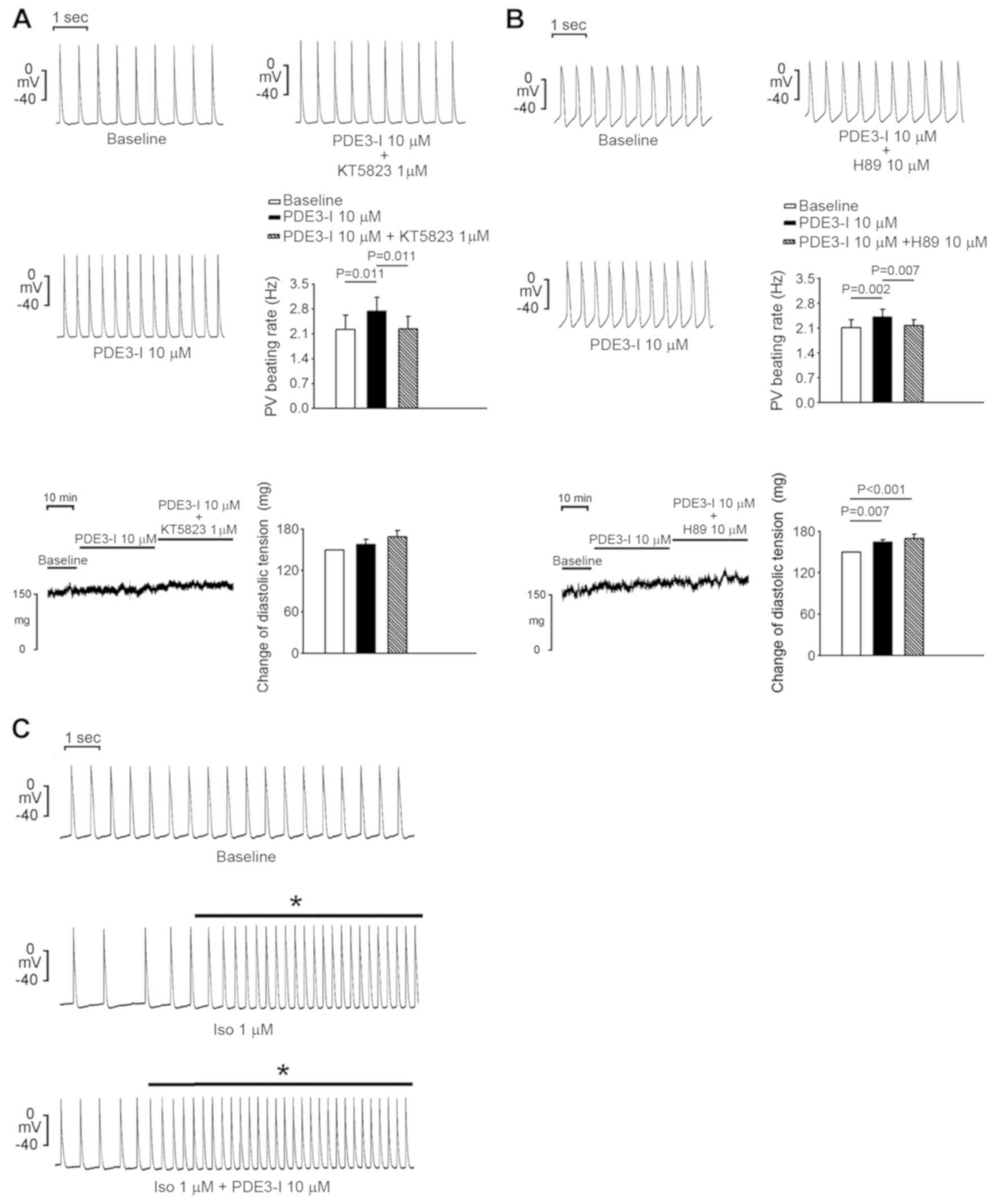
Application of KT5823 (1 µM), a selective PKG
inhibitor, abolished milrinone-accelerated PV electrical activity
(Fig. 3A). In addition, H89 (10 µM),
a PKA inhibitor, also suppressed milrinone-accelerated PV
electrical activity (Fig. 3B).
Isoproterenol increased PV spontaneous activity, diastolic tension
(Fig. 3B) and the occurrence of
burst firing compared with the baseline (4/5 vs. 0/5; P<0.05;
Fig. 3C). However, in the presence
of isoproterenol, milrinone (10 µM) did not significantly alter the
PV spontaneous activity or diastolic tension (P>0.05) compared
with the baseline. Taken together, PDE3 inhibition may regulate PV
electrical activity through adrenergic activity and activation of
the PKG and PKA signaling pathways.
Effects of the PDE4 inhibitor on the
electrical activities of isolated PVs and SANs
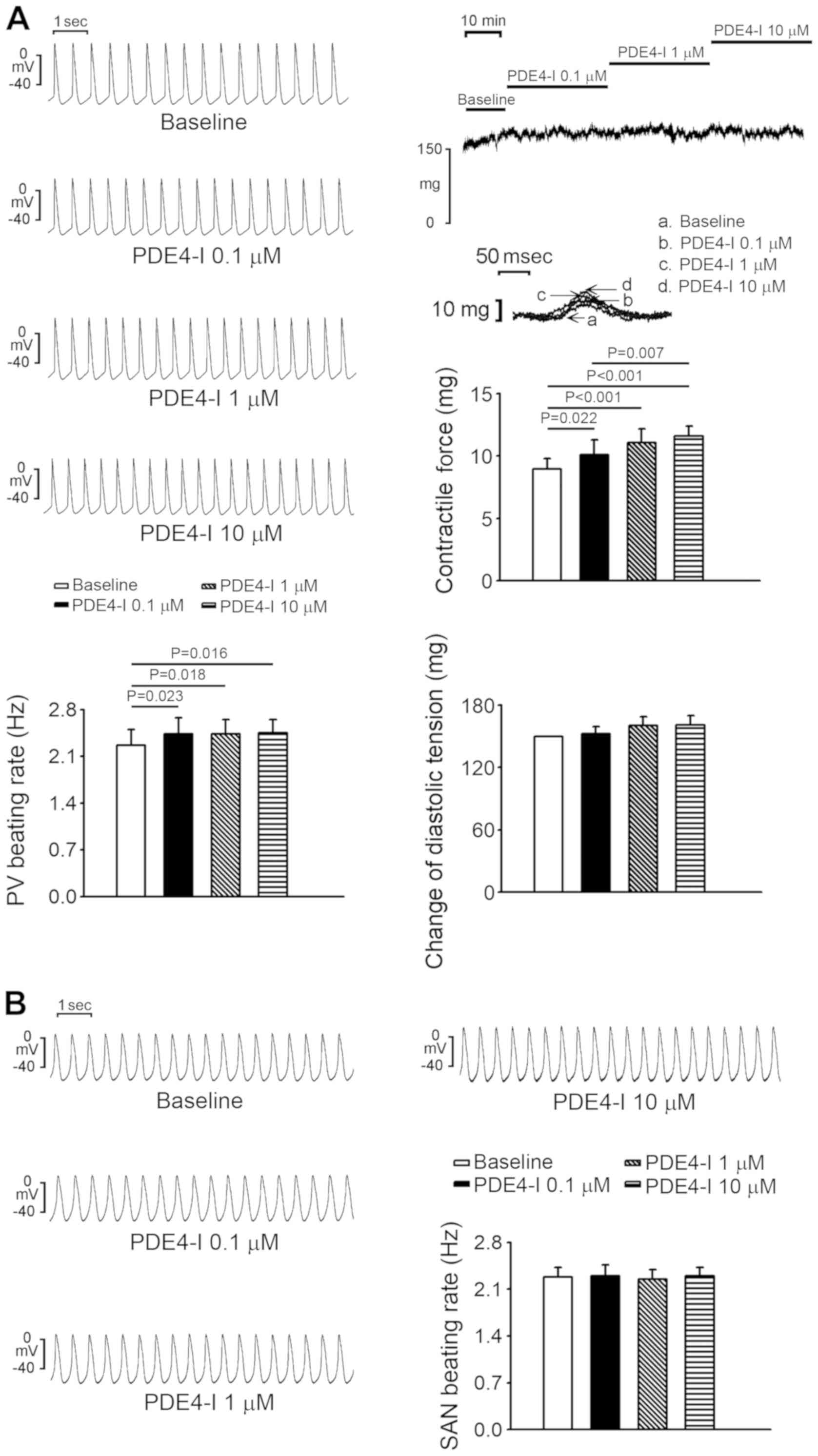
The PDE4 inhibitor (rolipram; 0.1, 1 and 10 µM)
increased PV spontaneous activity by 7.5±1.3, 8.2±3.1 and 9.5±4%,
PV diastolic tension by 1.6±4.7, 6.8±5.9 and 7.4±5.8%, and PV
contractility by 11.7±3.8, 23.8±3.1 and 32.3±7.5% compared with the
baseline, respectively (Fig. 4A).
Rolipram (0.1, 1 and 10 µM) did not significantly alter SAN
activity compared with the baseline (Fig. 4B), and there was only one triggered
beat in rolipram-treated PVs and none in rolipram-treated SANs (1/6
vs. 0/6; P>0.05).
Effects of the PDE5 inhibitor on the
electrical activities of isolated PVs and SANs
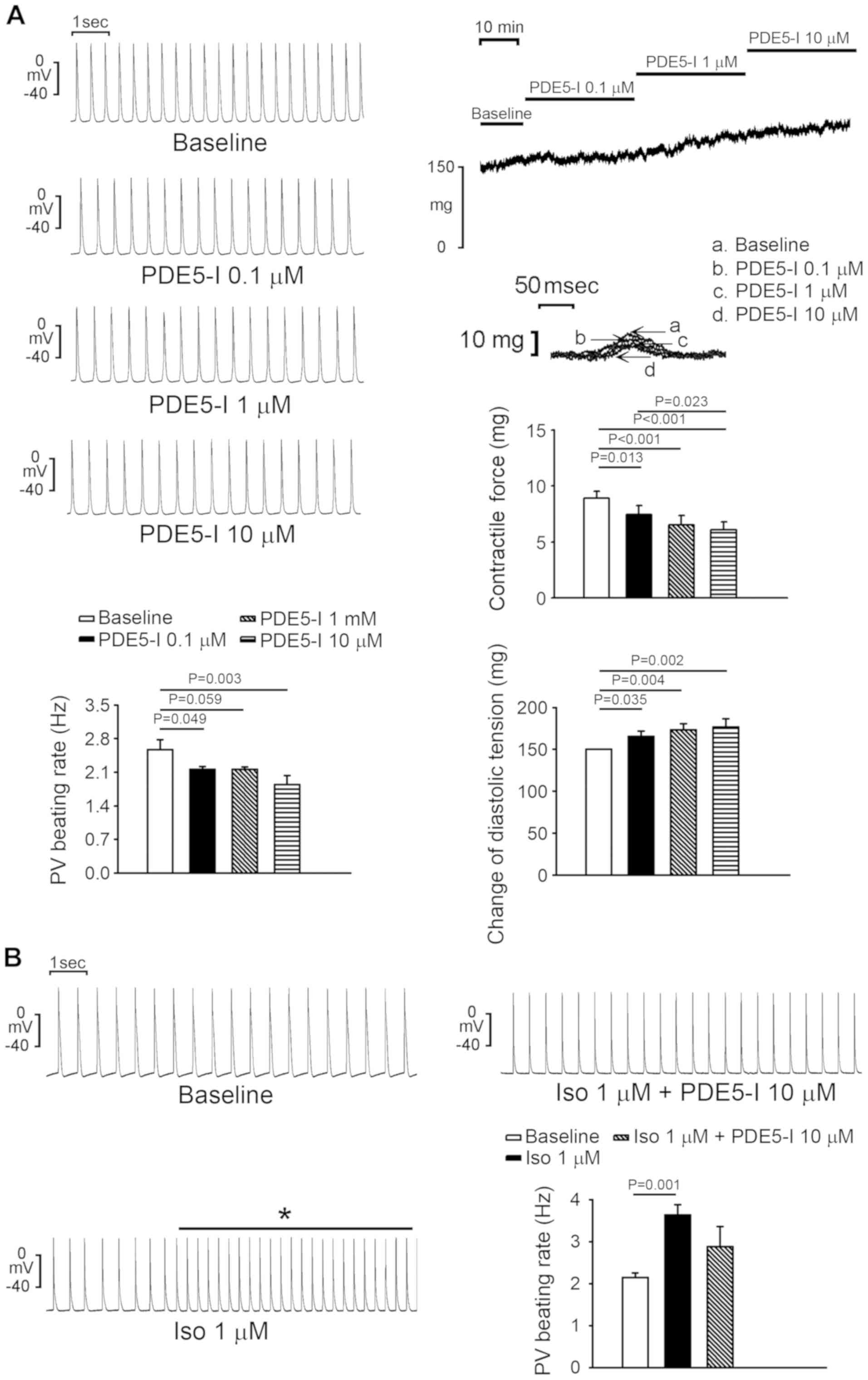
The PDE5 inhibitor, sildenafil, significantly
reduced PV spontaneous activity by 13.5±5.5% at 1 µM and 25.9±9% at
10 µM compared with the baseline (P<0.05; Fig. 5A). Sildenafil (0.1, 1 and 10 µM)
significantly reduced PV contractility by 16.6±4.6% (P =0.013),
27.5±7.0% (P<0.001) and 31.2±6.0% (P<0.001), respectively,
but increased PV diastolic tension by 10.3±4.1% (P=0.035),
15.5±4.9% (P=0.004) and 17.8±6.7% (P=0.002), respectively, compared
with the baseline, respectively. Moreover, the
isoproterenol-induced accelerated PV spontaneous activity was
attenuated by sildenafil (Fig. 5B).
In addition, sildenafil suppressed isoproterenol-induced PV burst
firing from 66.7 to 0% (P<0.001; n=9; Fig. 5B). Sildenafil (0.1, 1 and 10 µM)
reduced SAN activity compared with the baseline, however, at 10 µM
sildenafil reduced SAN activity by 9.7%, which was less than the
effect of sildenafil on PVs (Fig.
6).
Discussion
HF is a common risk factor for AF (2-4).
Milrinone potentiates the effect of cyclic adenosine monophosphate
(cAMP) and enhances the relaxation of the left ventricle by
increasing Ca2+-ATPase activity in the cardiac
sarcoplasmic reticulum, by increasing calcium ion uptake (7). Although the PDE3 inhibitor, milrinone,
improves HF through positive inotropic effects, milrinone also
increases the risk of AF in patients with HF (13). In the present study, milrinone
increased PV and SAN spontaneous activities to a similar extent.
The accelerating effect of milrinone on PVs was abolished by the
PKG inhibitor (KT5823; 1 µM) and the PKA inhibitor (H89; 10 µM),
suggesting that the electrophysiological effects of milrinone could
involve the PKG and PKA signaling pathways. Additionally, the
effect of milrinone on PV arrhythmogenesis in the presence of
isoproterenol indicated that milrinone and isoproterenol might
regulate PV electrical activity in a similar manner, via the
activation of cAMP.
Previous studies reported that milrinone might relax
the pulmonary arterial and venous vascular beds in guinea pig and
human lung tissues by activating the ATP-sensitive potassium
channel (30,31). However, the present study suggested
that milrinone increased diastolic tension in PVs. The discrepancy
may have been caused by the differences between distal, with simple
venous structures, and proximal PVs, since the atrial-PV junction
used in the present study contained the myocardial sleeve
surrounding the PV vascular components. Milrinone may increase PV
diastolic tension via positive inotropic effects on cardiomyocytes,
as shown by increased PV contractility.
PDE4 inhibition increased intracellular cAMP levels
and the ICa-L in atrial myocytes, as well as increasing
the frequency of spontaneous Ca2+ release (18), which may predispose individuals to
AF. However, in the present study, the PDE4 inhibitor, rolipram,
had no significant effect on PV and SAN spontaneous activities. The
results from the present study were consistent with that of a
previous study, which suggested that PDE4 inhibition did not
significantly alter SAN electrical activity (32). Therefore, clinical observations of an
increased risk of AF in patients receiving PDE4 inhibition therapy
might be explained by AF substrate modifications, rather than the
enhancement of the arrhythmogenesis that AF triggers (33).
PDE5 expression is strongly increased in HF
(34-36).
Previous studies reported that inhibition of PDE5 ameliorates
cardiac dysfunction and sildenafil blocks ICa-L in
ventricular myocytes in a dose-dependent manner (18). In the present study, sildenafil
significantly reduced PV and SAN spontaneous activities. The
stronger inhibitory effect of sildenafil on PV activity compared
with SAN activity might reduce the risk of AF. The rate reduction
effect of sildenafil on PVs may be partially attributed to the NO
synthase-mediated signaling pathways, since NO has been shown to
play an important role in PV arrhythmogenesis (37). Sildenafil increased the PV diastolic
tension, but decreased PV contractility, which may be accounted for
by the electrophysiological effects of sildenafil via
mechanoelectrical feedback. Contrastingly to PDE3 inhibitors, which
exhibit a high affinity for both cAMP and cGMP, sildenafil, as a
cGMP-specific PDE5 blocker (7),
abolished isoproterenol-induced increases in PV activity. This
result was in line with a previous report, which indicated that
sildenafil can attenuate β-receptor agonist-induced cAMP generation
and accelerate the beating rate of PVs (38). Similar to the present study, PDE5
inhibition was previously reported to have a negative chronotropic
effect on mice SANs (26).
Accordingly, sildenafil may potentially protect individuals from AF
by reducing PV arrhythmogenesis.
Acute infusion of the kinase inhibitors, including
KT5823 and H89, was performed in the present study to investigate
the effect of milrinone on PVs. The suppression of the PDE3
accelerating effect on PVs following acute infusion of the kinase
inhibitors suggested a role for the cGMP and PKA signaling pathways
in PDE3 inhibitor mediated PV arrhythmogenesis. However, it is not
clear whether the PDE inhibitors may have further unknown effects
or whether the kinase inhibitors exert their effects at the
cellular level. Since only the acute effects of the kinase
inhibitors were evaluated in the present study, the protein
expression of components of the cGMP and PKA signaling pathways in
PVs at such a short exposure time is highly unlikely to be affected
or to be shown by western blot analysis. Therefore, this requires
further investigation.
In conclusion, different subtypes of PDE inhibitors
regulate PV and SAN electrical activities in distinct manners and
may contribute to susceptibility to atrial arrhythmogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of
Science and Technology (grant nos. MOST105-2314-B-016-035-MY3,
MOST105-2628-B-038-012-MY3, MOST105-2314-B-038-059-MY3,
MOST106-2314-B-038-060 and MOST107-2314-B-038-101-MY3), the Taipei
Medical University-Wan Fang Hospital (grant nos. 105-wf-eva-06,
105-swf-02, 105-wf-eva-08, 105-wf-eva-14, 106-eva-02, 106-eva-06,
106-swf-01, 107-wf-swf-02 and 107-wf-eva-13), the Cathay General
Hospital (grant no. 106CGH-TMU-04), the Chi-Mei Medical Center
(grant nos. 106CM-TMU-08 and CMNDMC10606) and the Ministry of
National Defense-Medical Affairs Bureau (grant no.
MAB-107-044).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YKL and CCC contributed to the experimental design,
performed the in vitro experiments, analyzed the
experimental results and wrote the paper. YKL and JHH and gave
various contributions in the statistical analysis, and
interpretation of the results and discussion. YAC contributed to
the in vitro experiments and provided technical assistance
in the study. SAC and YJC contributed to the experimental design,
analysis of the results and final revision of the paper for
publication. YYL and YCC conceived and designed the study, and
reviewed the paper prior to submission.
Ethics approval and consent to
participate
The present study was approved of the Institutional
Animal Care and Use Committee by the local review board (approval
no. IACUC-19-124) of the National Defense Medical Center, Taipei,
Taiwan and conformed to the institutional Guide for the Care and
Use of Laboratory Animals and the ‘Guide for the Care and Use of
Laboratory Animals’ published by the United States National
Institutes of Health (8 ed. Washington DC, 2011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsang TS and Gersh BJ: Atrial
fibrillation: An old disease, a new epidemic. Am J Med.
113:432–435. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schotten U, Neuberger HR and Allessie MA:
The role of atrial dilatation in the domestication of atrial
fibrillation. Prog Biophys Mol Biol. 82:151–162. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang SL, Chen YC, Chen YJ, Wangcharoen W,
Lee SH, Lin CI and Chen SA: Mechanoelectrical feedback regulates
the arrhythmogenic activity of pulmonary veins. Heart. 93:82–88.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanders P, Morton JB, Davidson NC, Spence
SJ, Vohra JK, Sparks PB and Kalman JM: Electrical remodeling of the
atria in congestive heart failure: Electrophysiological and
electroanatomic mapping in humans. Circulation. 108:1461–1468.
2003.PubMed/NCBIPubMed/NCBI
|
|
5
|
Li D, Melnyk P, Feng J, Wang Z, Petrecca
K, Shrier A and Nattel S: Effects of experimental heart failure on
atrial cellular and ionic electrophysiology. Circulation.
101:2631–2638. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yeh YH, Wakili R, Qi XY, Chartier D,
Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D and Nattel S:
Calcium-handling abnormalities underlying atrial arrhythmogenesis
and contractile dysfunction in dogs with congestive heart failure.
Circ Arrhythm Electrophysiol. 1:93–102. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Knight W and Yan C: Therapeutic potential
of PDE modulation in treating heart disease. Future Med Chem.
5:1607–1620. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miller CL and Yan C: Targeting cyclic
nucleotide phosphodiesterase in the heart: Therapeutic
implications. J Cardiovasc Transl Res. 3:507–515. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jaski BE, Fifer MA, Wright RF, Braunwald E
and Colucci WS: Positive inotropic and vasodilator actions of
milrinone in patients with severe congestive heart failure.
Dose-response relationships and comparison to nitroprusside. J Clin
Invest. 75:643–649. 1985.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Omori K and Kotera J: Overview of PDEs and
their regulation. Circ Res. 100:309–327. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Varma A, Shah KB and Hess ML:
Phosphodiesterase inhibitors, congestive heart failure, and sudden
death: Time for re-evaluation. Congest. Heart Fail. 18:229–233.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghosh R, Sawant O, Ganpathy P, Pitre S and
Kadam VJ: Phosphodiesterase inhibitors: Their role and
implications. Int J PharmTech Res. 1:1148–1160. 2009.
|
|
13
|
Cuffe MS, Califf RM, Adams KF Jr, Benza R,
Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, et
al: Outcomes of a prospective trial of intravenous milrinone for
exacerbations of chronic heart failure (OPTIME-CHF) Investigators.
Short term intravenous milrinone for acute exacerbation of chronic
heart failure: A randomized controlled trial. JAMA. 287:1541–1547.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wechsler J, Choi YH, Krall J, Ahmad F,
Manganiello VC and Movsesian MA: Isoforms of cyclic nucleotide
phosphodiesterase PDE3A in cardiac myocytes. J Biol Chem.
277:38072–38078. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bevan S, Porteous L, Sitzer M and Markus
HS: Phosphodiesterase 4D gene, ischemic stroke, and asymptomatic
carotid atherosclerosis. Stroke. 36:949–953. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Milton AG, Aykanat VM, Hamilton-Bruce MA,
Nezic M, Jannes J and Koblar SA: Association of the
phosphodiesterase 4D (PDE4D) gene and cardioembolic stroke in an
Australian cohort. Int J Stroke. 6:480–486. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Calverley PM, Rabe KF, Goehring UM,
Kristiansen S, Fabbri LM and Martinez FJ: Roflumilast in
symptomatic chronic obstructive pulmonary disease: Two randomised
clinical trials. Lancet. 374:685–694. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiang CE, Luk HN, Wang TM and Ding PY:
Effects of sildenafil on cardiac repolarization. Cardiovasc Res.
55:290–299. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dustan Sarazan R, Crumb WJ Jr, Beasley CM
Jr, Emmick JT, Ferguson KM, Strnat CA and Sausen PJ: Absence of
clinically important HERG channel blockade by three compounds that
inhibit phosphodiesterase-5-sildenafil, tadalafil, and vardenafil.
Eur J Pharmacol. 502:163–167. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Swissa M, Ohara T, Lee MH, Kaul S, Shah
PK, Hayashi H, Chen PS and Karagueuzian HS: Sildenafil-nitric oxide
donor combination promotes ventricular tachyarrhythmias in the
swine right ventricle. Am J Physiol Heart Circ Physiol.
282:H1787–H1792. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen YJ and Chen SA: Electrophysiology of
pulmonary veins. J Cardiovasc Electrophysiol. 17:220–224.
2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Honjo H, Boyett MR, Niwa R, Inada S,
Yamamoto M, Mitsui K, Horiuchi T, Shibata N, Kamiya K and Kodama I:
Pacing-induced spontaneous activity in myocardial sleeves of
pulmonary veins after treatment with ryanodine. Circulation.
107:1937–1943. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patterson E, Lazzara R, Szabo B, Liu H,
Tang D, Li YH, Scherlag BJ and Po SS: Sodium-calcium exchange
initiated by the Ca2+ transient: An arrhythmia trigger within
pulmonary veins. J Am Coll Cardiol. 47:1196–1206. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen SA, Hsieh MH, Tai CT, Tsai CF,
Prakash VS, Yu WC, Hsu TL, Ding YA and Chang MS: Initiation of
atrial fibrillation by ectopic beats originating from the pulmonary
veins: Electrophysiological characteristics, pharmacological
responses, and effects of radiofrequency ablation. Circulation.
100:1879–1886. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P,
Chang MS and Lin CI: Effects of rapid atrial pacing on the
arrhythmogenic activity of single cardiomyocytes from pulmonary
veins: Implication in initiation of atrial fibrillation.
Circulation. 104:2849–2854. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lu YY, Lin YK, Wen ZH, Chen YC, Chen SA
and Chen YJ: Latrunculin B modulates electrophysiological
characteristics and arrhythmogenesis in pulmonary vein
cardiomyocytes. Clin Sci. 130:721–732. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chang SL, Hsiao YW, Tsai YN, Lin SF, Liu
SH, Lin YJ, Lo LW, Chung FP, Chao TF, Hu YF, et al: Interleukin-17
enhances cardiac ventricular remodeling via activating MAPK pathway
in ischemic heart failure. J Mol Cell Cardiol. 122:69–79.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pelizzo G, Avanzini MA, Icaro Cornaglia A,
Osti M, Romano P, Avolio L, Maccario R, Dominici M, De Silvestri A,
Andreatta E, et al: Mesenchymal stromal cells for cutaneous wound
healing in a rabbit model: Pre-clinical study applicable in the
pediatric surgical setting. J Transl Med. 13(219)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu YY, Cheng CC, Wu HJ, Lin YK, Chen YC,
Chen SA and Chen YJ: Effects of ANP on pulmonary vein
electrophysiology, Ca2+ homeostasis and adrenergic arrhythmogenesis
via PKA. Clin Exp Pharmacol Physiol. 47:247–254. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rieg AD, Suleiman S, Perez-Bouza A,
Braunschweig T, Spillner JW, Schröder T, Verjans E, Schälte G,
Rossaint R, Uhlig S and Martin C: Milrinone relaxes pulmonary veins
in guinea pigs and humans. PLoS One. 9(e87685)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kato R, Sato J and Nishino T: Milrinone
decreases both pulmonary arterial and venous resistances in the
hypoxic dog. Br J Anaesth. 81:920–924. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vinogradova TM, Sirenko S, Lukyanenko YO,
Yang D, Tarasov KV, Lyashkov AE, Varghese NJ, Li Y, Chakir K, Ziman
B and Lakatta EG: Basal spontaneous firing of rabbit sinoatrial
node cells is regulated by dual activation of PDEs
(Phosphodiesterases) 3 and 4. Circ Arrhythm Electrophysiol.
11(e005896)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Van Wagoner DR and Lindsay BD:
Phosphodiesterase-4 activity: A critical modulator of atrial
contractility and arrhythmogenesis. J Am Coll Cardiol.
59:2191–2192. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu
G, Fassett J, Tao Y, Zhang P, dos Remedios C, et al: Oxidative
stress regulates left ventricular PDE5 expression in the failing
heart. Circulation. 121:1474–1483. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nagendran J, Archer SL, Soliman D, Gurtu
V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB,
et al: Phosphodiesterase type 5 is highly expressed in the
hypertrophied human right ventricle, and acute inhibition of
phosphodiesterase type 5 improves contractility. Circulation.
116:238–248. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hwang IC, Kim YJ, Park JB, Yoon YE, Lee
SP, Kim HK, Cho GY and Sohn DW: Pulmonary hemodynamics and effects
of phosphodiesterase type 5 inhibition in heart failure: A
meta-analysis of randomized trials. BMC Cardiovasc Disord.
17(150)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin YK, Lu YY, Chen YC, Chen YJ and Chen
SA: Nitroprusside modulates pulmonary vein arrhythmogenic activity.
J Biomed Sci. 17(20)2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Isidori AM, Cornacchione M, Barbagallo F,
et al: Inhibition of type 5 phosphodiesterase counteracts
β2-adrenergic signalling in beating cardiomyocytes. Cardiovasc Res.
106:408–420. 2015.PubMed/NCBI View Article : Google Scholar
|