Introduction
Cardio- and cerebrovascular disease incidence has
increased due to improved living standards (1,2).
Coronary heart disease (CHD) is one of the leading causes of death
in the world and adversely affects public health (3,4). An
increasing number of studies have indicated that vascular smooth
muscle cells (VSMCs) serve vital functions in the development of
CHD (5,6). However, the molecular mechanisms of
VSMCs in CHD have yet to be elucidated.
microRNAs (miRNAs/miRs) are a class of non-coding
RNAs of 20-22 nucleotides in length that are able to regulate
30-50% of all genes by binding to the 3'-untranslated regions
(3'-UTRs) (7). miRNAs regulate
various biological functions, including cell proliferation,
apoptosis and signal transduction (8). Previous studies have suggested that
miRNAs participate in the pathological processes of a number of
diseases, including cardiac hypertrophy (9), heart failure (10), myocardial ischemia (11) and reperfusion (12). Previous studies have demonstrated
that miR-24 is a vital molecule in mediating vascular endothelial
cells (13,14). miR-24-3p has been shown to play an
important role in regulating cell growth and metastasis in various
types of cancer (15-17).
In addition, miR-24-3p is involved in ischemia/reperfusion injury
in cardiomyocytes (18). However,
the function of miR-24-3p in VSMCs in coronary heart disease
remains to be elucidated.
The Bcl-2 and caspase protein families serve
different and vital roles in cell apoptosis (19). Bcl-2-like protein 11 (Bcl-2L11; Bim)
is a pro-apoptotic member of the Bcl-2 family, which induces
cytochrome c release from the mitochondria (20). Previous studies have reported that
Bcl-2L11 mediates the biological processes of cell growth and
apoptosis (21-23). Therefore, the
present study investigated the modulating effect of miR-24-3p in
VSMCs. Furthermore, the underlying mechanism by which miR-24-3p
regulated the apoptosis of VSMCs was clarified.
The purpose of the present study was to investigate
the expression of miR-24-3p in the blood samples of CHD patients,
examine the role of miR-24-3p in VSMCs, and further to explore the
molecular mechanism.
Materials and methods
Clinical specimen collection
Blood samples were collected from 30 patients with
coronary heart disease (22 male, 8 female; age range, 37-75 years)
and 30 healthy volunteers (22 male, 8 female; age range: 34-73
years) from Renmin Hospital (Shiyan, China) between February 2016
and June 2018. The specimens were rapidly frozen and stored at
-80˚C until use. All patients provided written informed consent and
approved the use of their samples in the present study. The study
procedures obtained approval from the Ethics Committee at Renmin
Hospital.
Cell culture
Human VSMCs were obtained from the American Type
Culture Collection (cat. no. ATCC® PCS-100-012). Cells
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA) at 37˚C in a 5%
CO2 incubator.
Cell transfection and reagents
In total, 100 nM miR-24-3p inhibitor (antagonist of
miR-24-3p; 5'-CUGUUCCUGCUGAACUGAGCCA-3'), 100 nM inhibitor control
(5'-CAGUACUUUUGUGUAGUACAA-3'), 100 nM miR-24-3p mimic (sense:
5'-UGGCUCAGUUCAGCAGGAACAG-3'; anti-sense:
5'-GUUCCUGCUGAACUGAGCCAUU-3), 100 nM mimic control (sense:
5'-UUCUCCGAACGUGUCACGUTT-3'; anti-sense:
5'-ACGUGACACGUUCGGAGAATT-3'; all from Shanghai GenePharma Co.,
Ltd.), 1 µM control small interfering (si)RNA (cat. no. sc-36869;
Santa Cruz Biotechnology, Inc.), 0.2 µM Bcl-2L11-siRNA (cat. no.
sc-29802; Santa Cruz Biotechnology, Inc.), 100 nM miR-24-3p
inhibitor + 1 µM control-siRNA, or 100 nM miR-24-3p inhibitor + 0.2
µM Bcl-2L11-siRNA were transfected into VSMCs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for in accordance with the manufacturer's
protocol. Reverse transcription-quantitative (RT-q)PCR was
performed to detect the efficiency of cell transfection 48 h after
incubation, at 37˚C.
CCK-8 assay
A Cell Counting Kit-8 (Beyotime Institute of
Biotechnology) was employed to detect the cell viability of VSMCs,
according to the manufacturer's protocol. Cells were seeded into
96-well culture plates (6x103 cells/well) and then
incubated in DMEM for 24 h at 37˚C. Then, the cells were
transfected with inhibitor control, miR-24-3p inhibitor, miR-24-3p
inhibitor + control-siRNA, or miR-24-3p inhibitor + Bcl-2L11-siRNA
for 48 h. Subsequently, CCK-8 reagent was added into each well and
the cells were incubated at 37˚C for another 2 h. The absorbance
(optical density) at a wavelength of 450 nm was measured using a
microplate reader (Eon; BioTek Instruments, Inc.).
Flow cytometry analysis
VSMCs were transfected with inhibitor control,
miR-24-3p inhibitor, miR-24-3p inhibitor + control-siRNA, or
miR-24-3p inhibitor + Bcl-2L11-siRNA for 48 h. Then, Annexin
V-FITC/propidium iodide (PI) dual staining was performed to
evaluate cell apoptosis, according to the manufacturer's protocol
(cat. no. KGA106; Nanjing KeyGen Biotech Co., Ltd.). Briefly, VSMCs
were digested with 0.2% trypsin, washed with PBS and fixed with 70%
ethanol overnight at 4˚C. Then, the cells were stained with 5 µl
Annexin V-FITC and 5 µl PI for 30 min at room temperature. Finally,
the stained cells were quantified using a FACSCalibur flow
cytometer (BD Biosciences) and the data were analyzed with FlowJo
7.6.1 software (FlowJo LLC).
Dual-luciferase reporter assay
A bioinformatics prediction program (TargetScan 7.2;
http://www.targetscan.org/vert_72/)
was used to predict the relationship between miR-24-3p and
Bcl-2L11, and binding sites between miR-24-3p and Bcl-2L11 were
observed. To confirm the prediction, the wild-type (WT-Bcl-2L11:
5'-CCCCUGCAGUGGAAACUGAGCCA-3') and mutant (MUT-Bcl-2L11:
5'-CCAAGCAAGUGGAAAAGCGCAAG-3') 3'UTR of Bcl-2L11, containing the
miR-24-3p-binding elements, were generated by RT-PCR using a
Transcriptor First Strand cDNA Synthesis Kit (Roche Molecular
Systems, Inc.) from total RNA preps extracted from VSMCs, using the
temperature protocol of 5 min at 25˚C followed by 60 min at 42˚C.
The sequences were then cloned into a pmiR-RB-Report™ dual
luciferase reporter gene plasmid vector (Guangzhou RiboBio Co.,
Ltd.). Then 100 ng Bcl-2L11-WT or 100 ng Bcl-2L11-MUT were
co-transfected with 100 nM miR-24-3p mimic or 100 nM mimic control
into VSMCs using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Dual-Luciferase® Reporter Assay kit (Promega
Corporation) was used to measure luciferase activity 48 h after
cell transfection, which were normalized to that of Renilla
luciferase.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to isolate the total RNA from
cells or blood samples, respectively, according to the
manufacturer's protocol. Then, 200 ng total RNA was reverse
transcribed into cDNA using the miScript RT kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. The temperature protocol for the reverse
transcription reaction was as follows: Initial annealing at 25˚C
for 5 min, followed by extension at 42˚C for 60 min and termination
at 80˚C for 2 min. The expression levels of miR-24-3p and Bcl-2L11
were quantified using a SYBR Green PCR Master Mix kit (Takara
Biotechnology Co., Ltd.). GAPDH and U6 were used to normalize mRNA
and miR-24-3p expression, respectively. The reaction conditions of
the qPCR were as follows: 95˚C for 5 min; 35 cycles of denaturation
at 94˚C (15 sec), annealing at 50˚C for 30 sec and chain extension
at 72˚C for 30 sec; and a final extension step at 72˚C for 10 min.
Primers were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China): U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; GAPDH forward,
5'-TGTTGCCATCAATGACCCCTT-3' and reverse, 5'-CTCCACGACGTACTCAGCG-3';
miR-24-3p forward, 5'-ACACTCCAGCTGGGTGGCTCAGTTCAGCAG-3' and
reverse, 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAG-3'; Bcl-2L11 forward,
5'-CACAAACCCCAAGTCCTCCT-3' and reverse, 5'-ACACCAGGCGGACAATGTAA-3';
caspase-3 forward, 5'-TGTCGATGCAGCAAACCTCA-3' and reverse,
5'-GACTTCTACAACGATCCCCTC-3'; Bax forward,
5'-CGTCCACCAAGAAGCTGAGCG-3' and reverse, 5'-CGTCCACCAAAGCTGAGCG-3';
and Bcl-2 forward, 5'-TTGGATCAGGGAGTTGGAAG-3' and reverse,
5'-TGTCCCTACCAACCAGAAGG-3'. The 2-ΔΔCq method (24) was used to calculate the relative
expression levels. The assay was repeated three times.
Western blot analysis
Proteins from VSMCs or blood samples were extracted
using RIPA buffer (Beyotime Institute of Biotechnology). The
protein concentration was detected by BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). Then, the extracted protein samples were
mixed with 5X loading buffer, boiled at 100˚C for 5 min,
centrifuged at 1,000 x g at 4˚C for 2 min. Protein samples (30 µg
protein/lane) were separated by 10% SDS-PAGE and subsequently
transferred onto a PVDF membrane. Then, the membranes were blocked
with 5% skimmed milk at room temperature for 1.5 h and subsequently
incubated with the primary antibodies at 4˚C overnight: Bcl-2L11
(cat. no. 2933; dilution 1:1,000), caspase-3 (cat. no. 14220;
dilution 1:1,000), Bcl-2 (cat. no. 4223; dilution 1:1,000), Bax
(cat. no. 5023; dilution 1:1,000) and GAPDH (cat. no. 5174;
dilution 1:1,000; all from Cell Signaling Technology Inc.). After
washing with TBS with Tween-20, the membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
secondary antibody (cat. no. 7074; dilution: 1:2,000; Cell
Signaling Technology, Inc.) for 2 h at room temperature. Finally,
the protein bands were visualized using ECL reagent (EMD
Millipore), according to the manufacturer's protocols.
Statistical analysis
All the aforementioned experiments were performed in
triplicate. Data are expressed as the mean ± SD. Statistical
analysis was performed using SPSS 19.0 software (IBM Corp.).
Comparisons between groups were estimated by Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-24-3p expression is upregulated in
blood samples from patients with CHD
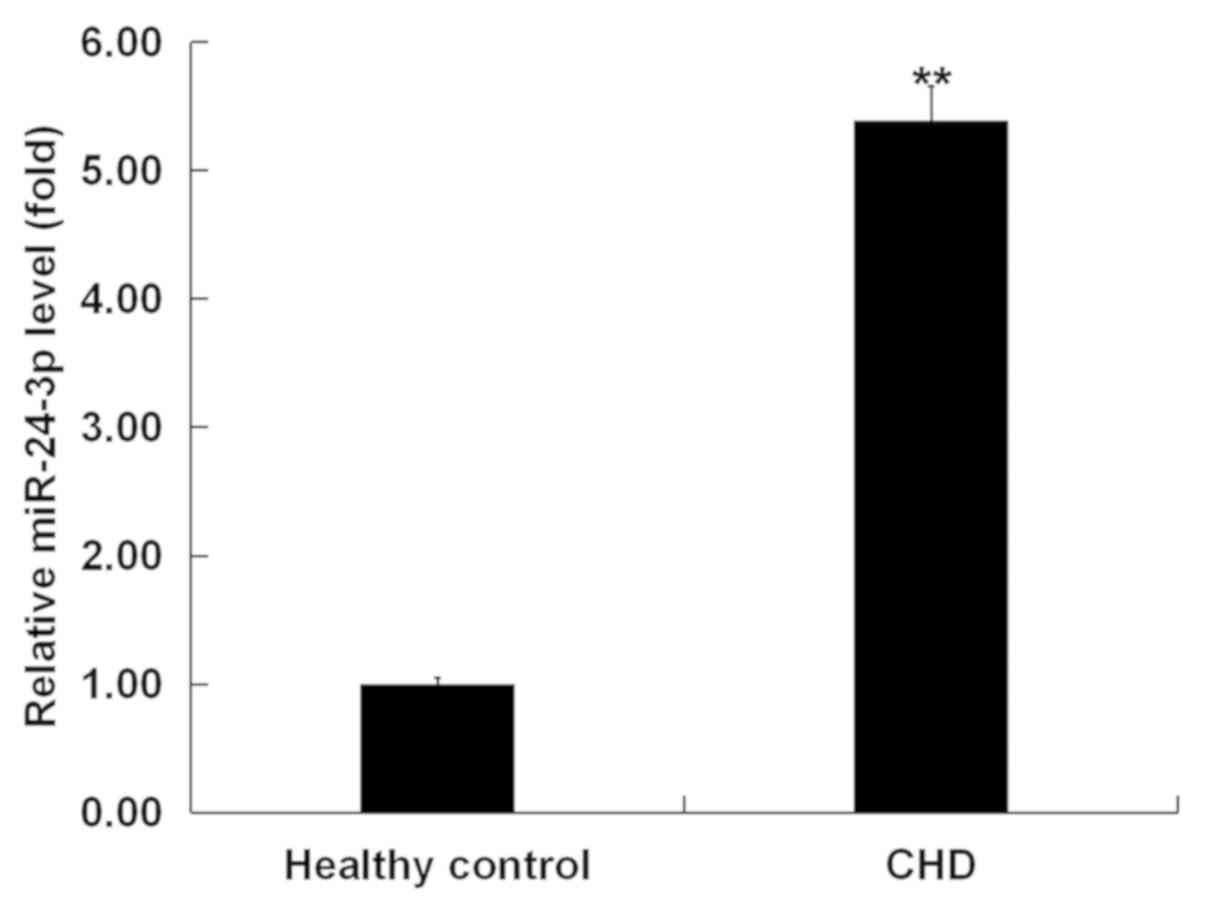
The present study first evaluated miR-24-3p
expression levels in peripheral blood samples from 30 patients with
CHD and normal controls. The results from RT-qPCR indicated that
the miR-24-3p expression was significantly higher in the peripheral
blood samples of patients with CHD than that in the healthy
volunteers (Fig. 1).
Bcl-2L11 is a direct target of
miR-24-3p
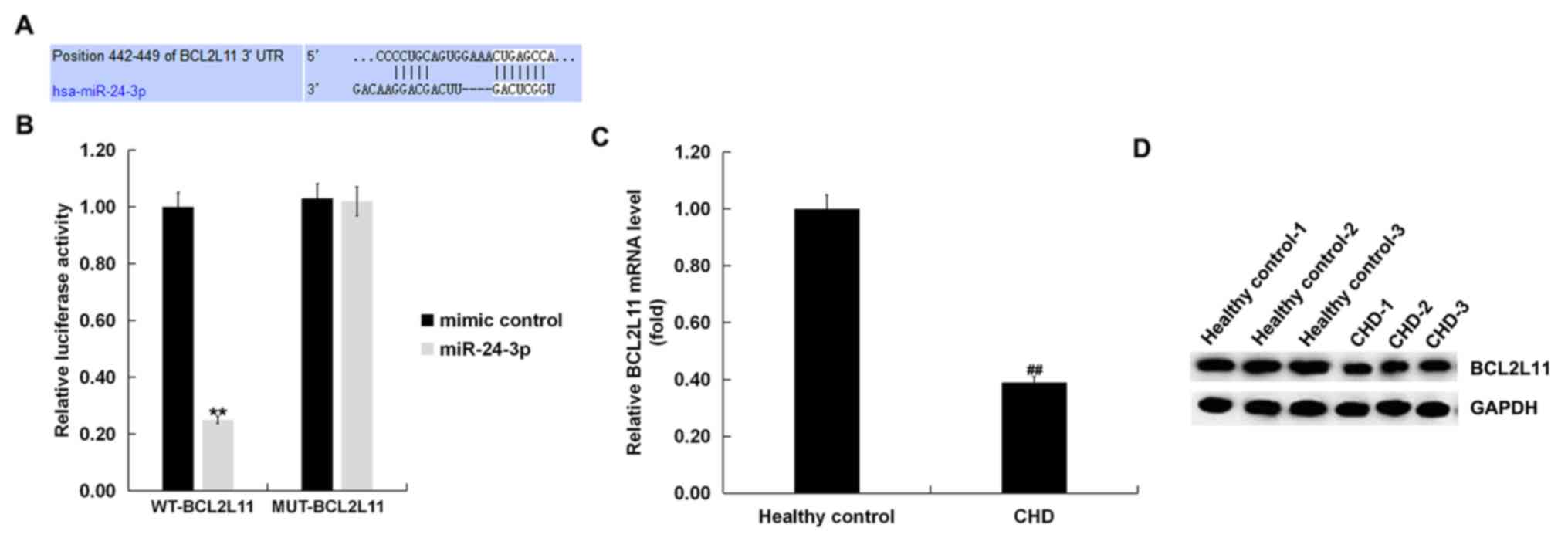
To investigate potential miR-24-3p target sites,
TargetScan was used to analyze the target genes of miR-24-3p. It
was found that Bcl-2L11 was a possible target of miR-24-3p and the
binding sites are shown in Fig. 2A.
To better understand the interaction between miR-24-3p and
Bcl-2L11, a luciferase reporter gene assay was performed. The
results suggested that miR-24-3p mimic markedly suppressed the
luciferase activity of cells co-transfected with miR-24-3p mimic
and Bcl-2L11-WT, whereas no significant differences were observed
in luciferase activity in cells co-transfected with miR-24-3p mimic
and Bcl-2L11-MUT (Fig. 2B).
Bcl-2L11 mRNA and protein levels were then detected
in the blood samples of patients with CHD using RT-qPCR and western
blotting. As shown in Fig. 2C, the
mRNA level of Bcl-2L11 was significantly reduced in the blood
samples of patients with CHD compared with healthy controls. In
addition, a relatively decreased protein expression level of
Bcl-2L11 was observed in the blood samples of CHD patients via
western blot analysis (Fig. 2D). In
summary, it was concluded that Bcl-2L11 was a direct target of
miR-24-3p. The expression levels of Bcl-2L11 in patients with CHD
were reduced.
Bcl-2L11-siRNA reverses the
incremental effects of miR-24-3p inhibitor on Bcl-2L11 expression
in VSMCs
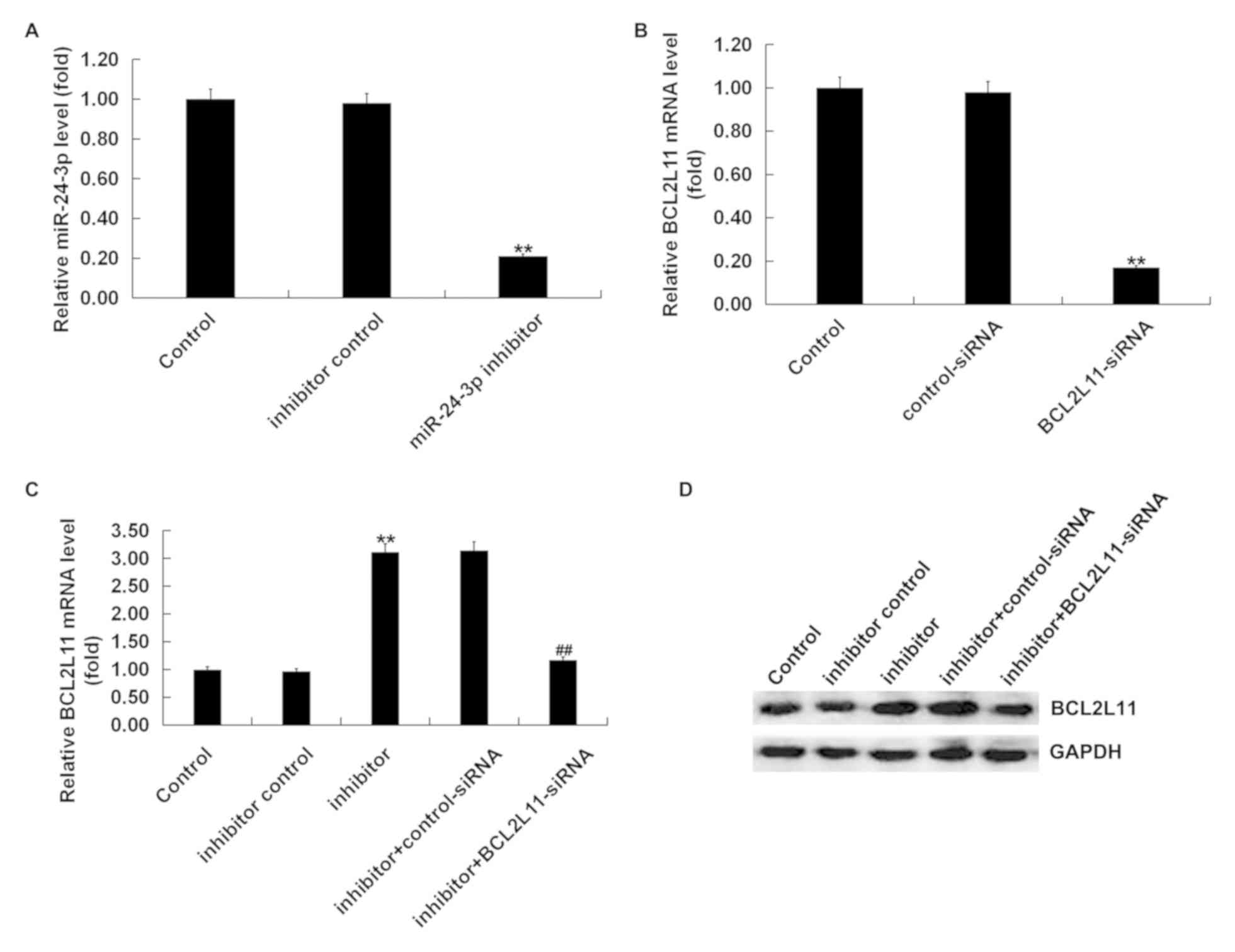
The functional relevance of Bcl-2L11 in
miR-24-3p-regulated effects in VSMCs was explored. Control-siRNA,
Bcl-2L11-siRNA, inhibitor control, miR-24-3p inhibitor, miR-24-3p
inhibitor + control-siRNA, or miR-24-3p inhibitor+Bcl-2L11-siRNA
were transfected into VSMCs for 48 h. RT-qPCR was performed to
evaluate the transfection efficiency. As presented in Fig. 3A, compared to the control group, the
levels of miR-24-3p were significantly decreased in VSMCs
transfected with miR-24-3p inhibitor. The Bcl-2L11 mRNA level was
significantly decreased in VSMCs transfected with Bcl-2L11-siRNA
compared with the control group (Fig.
3B). The results of the RT-qPCR and western blotting
demonstrated that the mRNA and protein levels of Bcl-2L11 increased
in the VSMCs transfected with miR-24-3p inhibitor compared with the
control group, and this increase was reversed by Bcl-2L11-siRNA
(Fig. 3C and D). Taken together, it was found that
Bcl-2L11 was negatively regulated by miR-24-3p in VSMCs.
Bcl-2L11-siRNA reverses the effect of
miR-24-3p inhibitor on cell proliferation and apoptosis in
VSMCs
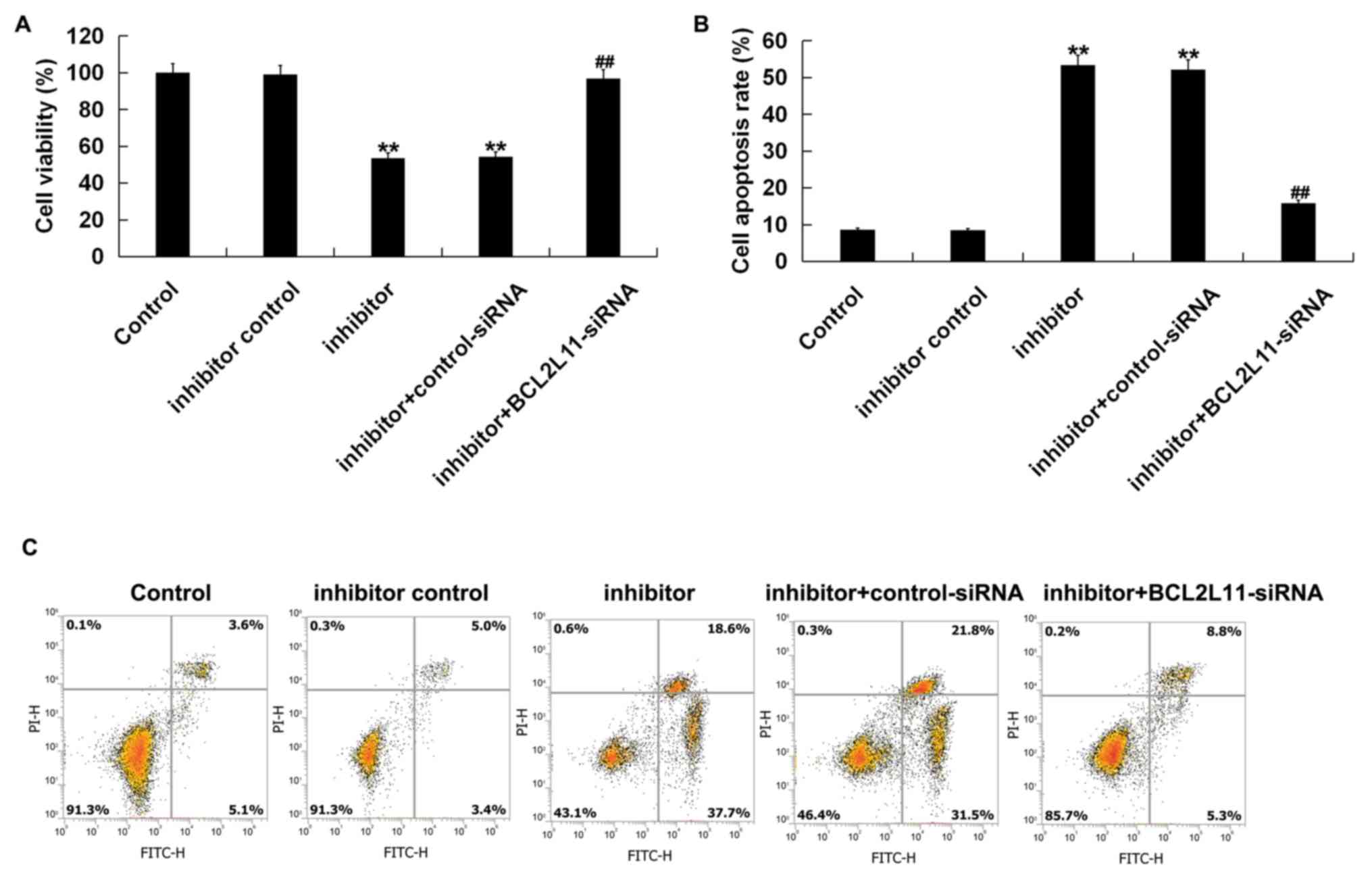
In order to further investigate the effect of
miR-24-3p on VSMCs, a CCK-8 assay and flow cytometry were performed
to assess VSMC viability and apoptosis. VSMCs were transfected with
an inhibitor control, miR-24-3p inhibitor, miR-24-3p inhibitor +
control-siRNA, or miR-24-3p inhibitor + Bcl-2L11-siRNA for 48 h.
The results of the CCK-8 analysis indicated that cell viability was
significantly decreased in the miR-24-3p inhibitor group compared
with the control group, whereas this decrease was reversed by
Bcl-2L11-siRNA (Fig. 4A). Flow
cytometry analysis demonstrated that compared with the control
group, miR-24-3p inhibitor significantly induced apoptosis in
VSMCs, while Bcl-2L11-siRNA clearly reversed these effects
(Fig. 4B and C). These data demonstrated that miR-24-3p
inhibitor could inhibit VSMC viability and induce VSMC apoptosis by
targeting Bcl-2L11.
miR-24-3p affects VSMC apoptosis by
regulating Bcl-2L11/Bcl-2/Bax/caspase-3 expression
To further explore the underlying mechanism of
miR-24-3p inhibitor-induced cell apoptosis, the expression levels
of Bcl-2L11, caspase-3, Bcl-2 and Bax were determined by western
blot analysis and RT-qPCR. VSMCs were transfected with inhibitor
control, miR-24-3p inhibitor, miR-24-3p inhibitor + control-siRNA,
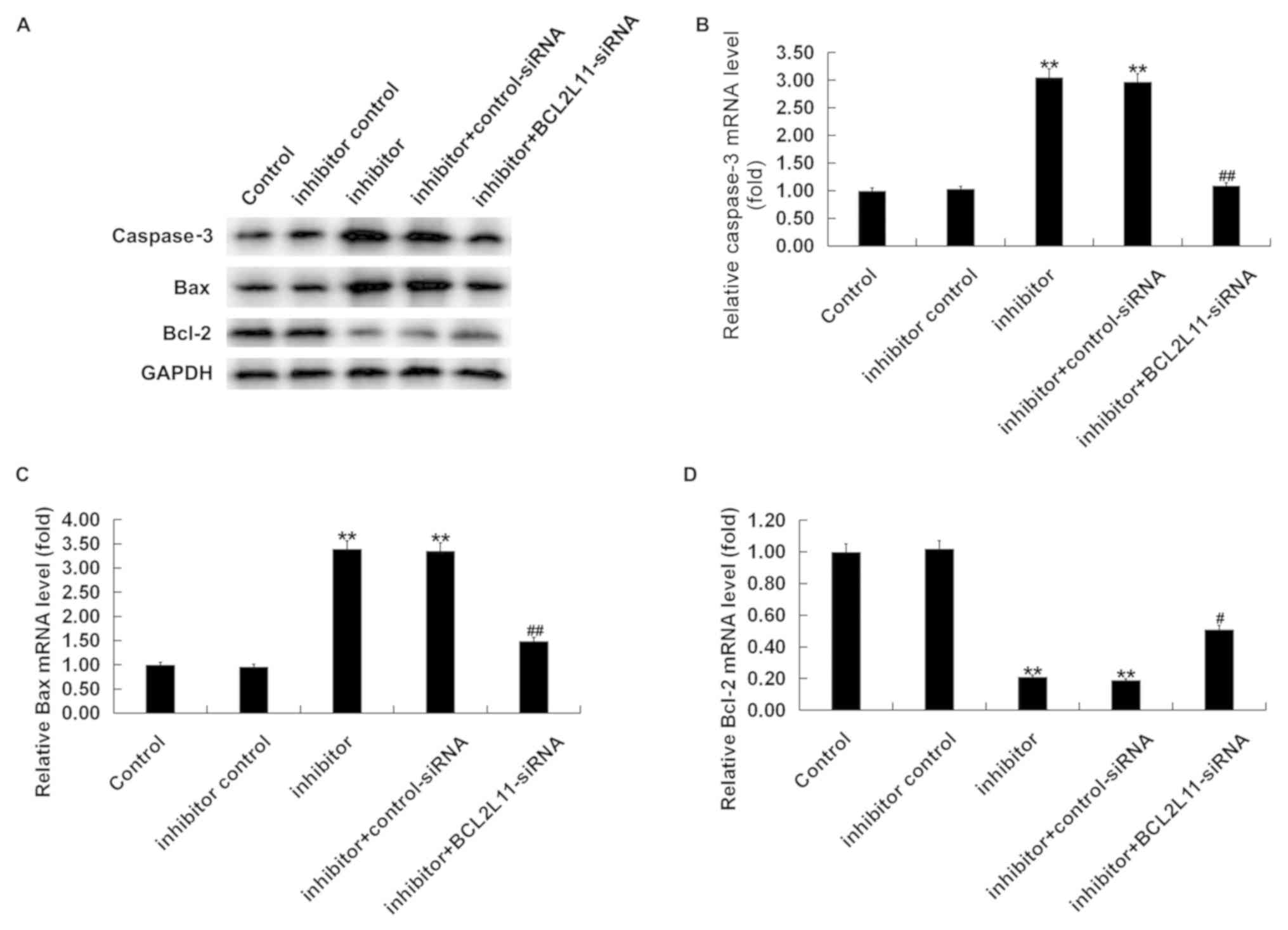
or miR-24-3p inhibitor + Bcl-2L11-siRNA for 48 h. As shown in
Fig. 5A, miR-24-3p inhibitor
increased the protein levels of Bcl-2L11, caspase-3 and Bax, while
it decreased the protein levels of Bcl-2 in VSMCs, and these
changes were reversed by Bcl-2L11-siRNA. RT-qPCR assay indicated
that miR-24-3p inhibitor significantly increased the mRNA levels of
Bcl-2L11, caspase-3 and Bax, while it decreased the mRNA levels of
Bcl-2 (Fig. 5). All these changes
were reversed by Bcl-2L11-siRNA.
Discussion
VSMCs are the main cell type in blood vessels and,
abnormal proliferation and apoptosis of VSMCs may result in the
rapid development of disease (25,26).
Recently, growing evidence has identified miRNAs as new biomarkers
for a number of cardiovascular diseases (27,28). It
is necessary to identify disease-specific miRNAs and their targets
to understand their roles in disease (29-31).
It has been reported that various miRNAs participate in mediating
the functions of VSMCs, including miR-21, miR-214 and miR-146a
(32-34).
In the present study, the expression level of miR-24-3p in the
blood samples of 30 patients with CHD was explored using RT-qPCR
and it was found that the miRNA-24-3p level was higher in the blood
samples of patients with CHD compared with the normal controls. The
results demonstrated that miRNA-24-3p might play a vital role in
modulating CHD. Luciferase reporter analysis identified that
Bcl-2L11 was a direct target of miRNA-24-3p in VSMCs. The
measurements of the mRNA and protein Bcl-2L11 expression in
peripheral blood samples of CHD patients and normal volunteers
showed that Bcl-2L11 was downregulated in the blood samples of CHD
patients. However, the relationship between miRNA-24-3p expression
and Bcl-2L11 expression in patients with CHD was not analyzed. This
might be a limitation of the present study, and is something to
explore in the future.
Then, the role and mechanism of miRNA-24-3p in
regulating VSMCs was investigated. It has been reported that
Bcl-2L11 is a pro-apoptotic Bcl-2 family member and it is activated
in a number of activities, including mediating excitotoxic
apoptosis, mitochondrial depolarization and factor translocation
(35-37).
As Bcl-2L11 was found to be a direct target of miRNA-24-3p in
VSMCs, it was hypothesized that altering the expression of
miRNA-24-3p in the VSMCs of CHD patients could change the Bcl-2L11
expression and the growth of VSMCs. To test this hypothesis,
inhibitor control, miRNA-24-3p inhibitor, miRNA-24-3p inhibitor +
control-siRNA, or miRNA-24-3p inhibitor + Bcl-2L11-siRNA were
transfected into VSMCs. The results showed that miRNA-24-3p
inhibitor significantly increased Bcl-2L11 expression in VSMCs,
while this increase was eliminated by Bcl-2L11-siRNA. miRNA-24-3p
inhibitor significantly suppressed cell viability and induced
apoptosis in VSMCs. However, Bcl-2L11-siRNA significantly reversed
the effects of miRNA-24-3p inhibitor on cell viability and cell
apoptosis in VSMCs. These data indicated that miRNA-24-3p regulated
the apoptosis of VSMCs in patients with CHD. This was in accordance
with the observations of previous studies (38,39).
However, the effect of miR-24-3p upregulation on VSMCs was not
investigated and this might be a limitation of the present study,
to be addressed in the future.
Cell apoptosis occurs through two pathways: The
death receptor-regulated external signaling pathway and the
mitochondria-regulated internal signaling pathway (40). The present study investigated the
signaling pathway in which miRNA-24-3p regulated apoptosis in the
VSMCs of patients with CHD. It was observed that miRNA-24-3p
inhibitor significantly upregulated the expression of Bcl-2L11,
caspase-3 and Bax in VSMCs, and the expression of Bcl-2 was
suppressed. The effects of miR-24-3p inhibitor on the expression of
these genes were reversed by Bcl-2L11-siRNA.
In conclusion, the present study suggested that
miRNA-24-3p exhibited a vital role in regulating the viability and
apoptosis of VSMCs by targeting Bcl-2L11. This may provide
potential therapeutic targets for the interference and treatment of
CHD. However, the current study is only a preliminary study of the
expression of miRNA-24-3p in CHD patients and its role in VSMCs. To
substantiate the role of miRNA-24-3p in CHD, more detailed research
is needed. For example, other targets of miR-24-3p in CHD should be
investigated to fully demonstrate the function of miRNA-24-3p in
VSMCs. The effect of Bcl-2L11-siRNA and miR-24-3p mimic on VSMCs
should be investigated. In addition, the role of miRNA-24-3p in CHD
in vivo needs further study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
HXZ and SZX contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. YF contributed to data collection,
statistical analysis and manuscript preparation. JS and JXZ
contributed to data collection and statistical analysis. All
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent and
approved the use of their samples in the present study. The study
procedures obtained approval from the Ethics Committee at Renmin
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morera LP, Marchiori GN, Medrano LA and
Defagó MD: Stress, dietary patterns and cardiovascular disease: A
mini-review. Front Neurosci. 13(1226)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Portegies ML, Koudstaal PJ and Ikram MA:
Cerebrovascular disease. Handb Clin Neurol. 138:239–261.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xiao X, Liu HX, Shen K, Cao W and Li XQ:
Canonical transient receptor potential channels and their link with
cardio/cerebro-vascular diseases. Biomol Ther (Seoul). 25:471–481.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xing F, Dong Y, Tao J, Gao X, Zhou J, Chen
S, Ji C, Yao T and Wu S: Impact of isolated diastolic hypertension
on new-onset cardiovascular and cerebro-vascular diseases. Zhonghua
Liu Xing Bing Xue Za Zhi. 35:956–960. 2014.(In Chinese). PubMed/NCBI
|
|
5
|
Liu L, Cheng Z and Yang J: miR-23
regulates cell proliferation and apoptosis of vascular smooth
muscle cells in coronary heart disease. Pathol Res Pract.
214:1873–1878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoshioka S, Tsukamoto T and Chihara K:
Vascular smooth muscle cells in coronary heart disease. Nihon
Rinsho. 61 (Suppl 4)(S80-S85)2003.(In Japanese). PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qiu Z, He Y, Zhang Y, Guo J and Wang L:
Characterization of miRNAs and their target genes in He-Ne laser
pretreated wheat seedlings exposed to drought stress. Ecotoxicol
Environ Saf. 164:611–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nolan J, Stallings RL and Piskareva O:
Assessment of basic biological functions exerted by miRNAs. Methods
Mol Biol. 1509:11–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ooi JY, Bernardo BC and McMullen JR: The
therapeutic potential of miRNAs regulated in settings of
physiological cardiac hypertrophy. Future Med Chem. 6:205–222.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Katz MG, Fargnoli AS, Williams RD, Kendle
AP, Steuerwald NM and Bridges CR: MiRNAs as potential molecular
targets in heart failure. Future Cardiol. 10:789–800.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang H, Lu J, Wu S, Yang S, Wang L, Zhou
H, Fu Y and Liu J: Effects of electroacupuncture at different
acupoints on apoptosis and the expression of miRNAs in myocardial
cells in rats model of myocardial ischemia. Zhongguo Zhen Jiu.
36:281–286. 2016.(In Chinese). PubMed/NCBI
|
|
12
|
Gottlieb RA and Pourpirali S: Lost in
translation: MiRNAs and mRNAs in ischemic preconditioning and
ischemia/reperfusion injury. J Mol Cell Cardiol. 95:70–77.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: miR-24 limits aortic vascular inflammation and murine abdominal
aneurysm development. Nat Commun. 5(5214)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zheng Y, Li Y, Liu G, Qi X and Cao X:
MicroRNA-24 inhibits the proliferation and migration of endothelial
cells in patients with atherosclerosis by targeting importin-α3 and
regulating inflammatory responses. Exp Ther Med. 15:338–344.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu D, Zhang X, Lin Y, Liang S, Song Z and
Dong C: MT1JP inhibits tumorigenesis and enhances cisplatin
sensitivity of breast cancer cells through competitively binding to
miR-24-3p. Am J Transl Res. 11:245–256. 2019.PubMed/NCBI
|
|
16
|
Wang J, Yin K, Lv X, Yang Q, Shao M, Liu X
and Sun H: MicroRNA-24-3p regulates Hodgkin's lymphoma cell
proliferation, migration and invasion by targeting DEDD. Oncol
Lett. 17:365–371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu G, Jia Z and Dou Z: miR-24-3p regulates
bladder cancer cell proliferation, migration, invasion and
autophagy by targeting DEDD. Oncol Rep. 37:1123–1131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei W, Peng J and Shen T: Rosuvastatin
alleviates ischemia/reperfusion injury in cardiomyocytes by
downregulating Hsa-miR-24-3p to target upregulated uncoupling
protein 2. Cell Reprogram. 21:99–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Li HM, Zhang XN, Xiong CM and Ruan
JL: Dioscin-induced apoptosis of human LNCaP prostate carcinoma
cells through activation of caspase-3 and modulation of Bcl-2
protein family. J Huazhong Univ Sci Technolog Med Sci. 34:125–130.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khawaja NR, Carre M, Kovacic H, Estève MA
and Braguer D: Patupilone-induced apoptosis is mediated by
mitochondrial reactive oxygen species through Bim relocalization to
mitochondria. Mol Pharmacol. 74:1072–1083. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang H, Duan J, Qu Y, Deng T, Liu R,
Zhang L, Bai M, Li J, Ning T, Ge S, et al: Onco-miR-24 regulates
cell growth and apoptosis by targeting Bcl-2L11 in gastric cancer.
Protein Cell. 7:141–151. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Tan M, Li H, Li H and Sun Y:
Inactivation of SAG or ROC1 E3 ligase inhibits growth and survival
of renal cellcarcinoma cells: Effect of BIM. Transl Oncol.
12:810–818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim JH, Lee DK, Kim J, Choi S, Park W, Ha
KS, Kim TH, Choe J, Won MH, Kwon YG and Kim YM: A miRNA-101-3p/Bim
axis as a determinant of serum deprivation-induced endothelial cell
apoptosis. Cell Death Dis. 8(e2808)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schwartz M, Bockmann S and Hinz B:
Up-regulation of heme oxygenase-1 expression and inhibition of
disease-associated features by cannabidiol in vascular smooth
muscle cells. Oncotarget. 9:34595–34616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Torremade N, Bozic M, Panizo S,
Barrio-Vazquez S, Fernandez-Martín JL, Encinas M, Goltzman D,
Arcidiacono MV, Fernandez E and Valdivielso JM: Vascular
calcification induced by chronic kidney disease is mediated by an
increase of 1α-hydroxylase expression in vascular smooth muscle
cells. J Bone Miner Res. 31:1865–1876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou SS, Jin JP, Wang JQ, Zhang ZG,
Freedman JH, Zheng Y and Cai L: miRNAS in cardiovascular diseases:
Potential biomarkers, therapeutic targets and challenges. Acta
Pharmacol Sin. 39:1073–1084. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kaneto CM, Nascimento JS, Prado MSJG and
Mendonça LSO: Circulating miRNAs as biomarkers in cardiovascular
diseases. Eur Rev Med Pharmacol Sci. 23:2234–2243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen WX, Ren LH and Shi RH: Implication of
miRNAs for inflammatory bowel disease treatment: Systematic review.
World J Gastrointest Pathophysiol. 5:63–70. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang SS, Wu LJ, Li JJ, Xiao HB, He Y and
Yan YX: A meta-analysis of dysregulated miRNAs in coronary heart
disease. Life Sci. 215:170–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ding L, Wang M, Sun D and Li A: A novel
method for identifying potential disease-related miRNAs via a
disease-miRNA-target heterogeneous network. Mol Biosyst.
13:2328–2337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li FP, Lin DQ and Gao LY: LncRNA TUG1
promotes proliferation of vascular smooth muscle cell and
atherosclerosis through regulating miRNA-21/PTEN axis. Eur Rev Med
Pharmacol Sci. 22:7439–7447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Afzal TA, Luong LA, Chen D, Zhang C, Yang
F, Chen Q, An W, Wilkes E, Yashiro K, Cutillas PR, et al: NCK
associated protein 1 modulated by miRNA-214 determines vascular
smooth muscle cell migration, proliferation, and neointima
hyperplasia. J Am Heart Assoc. 5(e004629)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu ZW, Liu YF, Wang S and Li B:
Corrigendum miRNA-146a induces vascular smooth muscle cell
apoptosis in a rat model of coronary heart disease via NF-kB
pathway. Genet Mol Res. 14:18703–18712. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guo C, Li Y, Zhang R, Zhang Y, Zhao J, Yao
J, Sun J, Dong J and Liao L: Protective effect of salidroside
against diabetic kidney disease through inhibiting BIM-mediated
apoptosis of proximal renal tubular cells in rats. Front Pharmacol.
9(1433)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kumar A, Ghosh S and Chandna S: Evidence
for microRNA-31 dependent Bim-Bax interaction preceding
mitochondrial Bax translocation during radiation-induced apoptosis.
Sci Rep. 5(15923)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Choi YB and Nicholas J: Bim nuclear
translocation and inactivation by viral interferon regulatory
factor. PLoS Pathog. 6(e1001031)2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pang J, Zhang Z, Zheng T, Yang YJ, Li N,
Bai M, Peng Y, Zhang J, Li Q and Zhang B: Association of green tea
consumption with risk of coronary heart disease in Chinese
population. Int J Cardiol. 179:275–278. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Deng X, Liu Y, Luo M and Wu J, Ma R, Wan Q
and Wu J: Circulating miRNA-24 and its target YKL-40 as potential
biomarkers in patients with coronary heart disease and type 2
diabetes mellitus. Oncotarget. 8:63038–63046. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen J, Benlahrech A, Kelleher P and
Patterson S: Increased activity of extrinsic and intrinsic
apoptosis pathways in different mononuclear cell types in HIV type
1-infected patients regardless of whether they are depleted in
disease. AIDS Res Hum Retroviruses. 29:709–717. 2013.PubMed/NCBI View Article : Google Scholar
|