Introduction
Benign prostatic hyperplasia (BPH) is a
hyper-proliferative disease that reduces the quality of life of
elderly men. The worldwide incidence of BPH is 20% in men at age
40, which rises to 70% by age 60 and 90% by age 90 (1,2). BPH is
characterized by a four-fold increase of stromal cells, which
results in prostate gland expansion; therefore, it is generally
considered a proliferative stromal disease (3,4). BPH
increases prostate size and tightens the urethra, producing
symptoms in the lower urinary tract, including urinary
intermittency, nocturia, frequency, dysuria, weak stream,
incomplete emptying and suprapubic pain (5).
The pressures of modern society have led to the
increased prevalence of mental health issues, which has prompted
the production and use of various antipsychotic drugs (6). Antipsychotic drugs may cause toxic
adverse effects, including menstrual disorder, amenorrhea, dysuria
and constipation in the human reproductive system, particularly in
the prostate (7), which has received
increasing attention from clinicians.
Sulpiride, a specific type 2 dopamine receptor
antagonist, produces various side effects, including insomnia,
fatigue, tachycardia, liver dysfunction and delayed dyskinesia when
the dose of sulpiride reaches 600 mg/day (8-11). PRL is associated
with the growth and development of BPH (9,12,13). PRL
levels increase, while testosterone (T) levels decrease with age,
which indicates that PRL serves a key role in BPH development in
the elderly (8,14). Ahonen et al (15) reported that PRL serves a primary role
in the differentiation and proliferation of the prostate in rats
and humans. The effects of PRL are mediated via signal transduction
pathways triggered by PRL receptors (16). Słuczanowska-Głąbowska et al
(9) revealed that PRL increases
while T decreases in experimental rats that received
metoclopramide. Morphological abnormalities were also observed in
columnar epithelial cells of the lateral, dorsal and ventral lobes;
however, prostate lobes did not exhibit morphological changes under
hyperprolactinemia (7). Previous
studies have indicated that PRL may promote prostate growth and
cell proliferation synergistically with androgens (8,17)
Conversely, it has also been reported that PRL acts independently
of T in prostate growth (18).
Additional studies have revealed that PRL stimulates the secretion
of prostate proteins and conversion of T to dihydrotestosterone
(19,20). The prostate is dependent on
androgens, which serve a role in BPH (21). Furthermore, testosterone regulation,
prostate structure and prostate function are influenced by tissue
growth and the hypothalamus-pituitary-gonadal axis (22). T secretion is regulated by
luteinizing hormone (LH) and follicle stimulating hormone (FSH)
(23-27).
Therefore, PRL may serve an important role in the regulation of
prostate cell growth and differentiation.
Proliferating cell nuclear antigen (PCNA) mediates
the proliferation of prostate cells in rats and, as such, is used
as a marker of proliferation (28).
In addition, B-cell lymphoma-2 (Bcl-2) is an anti-apoptotic protein
that promotes prostate hyperplasia (29). Shi et al (30) revealed that estrogens contributed to
the pathogenesis of BPH in elderly men. Estrogen receptor-α (ERα)
and estrogen receptor-β (ERβ) are expressed and are antagonistic;
ERα mediates cell proliferation while ERβ regulates apoptosis
(31,32). Furthermore, the androgen receptor
(AR) was demonstrated to promote prostate cells proliferation in
BPH development (33). Similar to
ERs, various co-regulators interact directly with AR, enhancing or
reducing its transcriptional activity (34).
In BPH, the relative ratio of stroma: Epithelium
increases with disease progression (35). Fibroblasts, myofibroblasts and smooth
muscle cells are the primary stromal components of prostate tissue
(36). It has also been revealed
that mesenchymal cell markers, including vimentin, fiber binding
proteins (fibronectin) and smooth muscle actin-α (α-SMA) serve
roles in BPH progression (37-39).
Studies that assess the mechanism of BPH primarily
utilize rodents, including Sprague-Dawley rats (40), Wistar rats (41), as well as non-rodent animal models,
including Beagle dogs (12).
However, Brown-Norway (BN) rats are rarely employed to model BPH.
Although in vitro/in vivo prostate models have been
developed to explore the mechanism of benign hyperplasia, to the
best of our knowledge, estrogen receptor subtypes, ARs and
mesenchymal cell biomarkers (including vimentin, fibronectin and
α-SMA) have not been assessed in the BN rat model. The aim of the
present study was to establish a useful model of BPH and explore
the mechanism of sulpiride-induced benign hyperplasia in male BN
rats.
Materials and methods
Animals and housing
A total of 36 male BN rats (10 weeks old; 280±20 g)
were obtained from Beijing Weitong Lihua Experimental Animal
Technology Co., Ltd., (Beijing, China) and housed in standard
polypropylene cages with sawdust bedding. Drinking water and a
pellet diet (Shanghai Shilin Biological Technology Co., Ltd.,
Shanghai, China) were available ad libitum. Rooms were maintained
at 20-26˚C with 40-70% humidity and a 12 h light/dark cycle. The
present study was approved by the Shanghai Institute of Planned
Parenthood Research Animal Care (Shanghai, China).
Animals were divided into three groups (n=12)
according to body weight following 5 days acclimatization as
follows: Vehicle group (290.0±49.0 g), 40 mg/kg sulpiride group
(292.3±55.4 g) and 120 mg/kg sulpiride group (294.3±50.5 g). All
animal procedures were approved by the Animal Care and Use
Committee of Shanghai Institute of Planned Parenthood Research
(Shanghai, China) and performed according to the Guide for the Care
and Use of Laboratory Animals.
Reagents
Sulpiride (cat. no. SLBG4648V; purity, 100%) was
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Sodium carboxymethyl cellulose (CMC-Na; cat. no. 20140520; purity,
100%) was purchased from Sinopharm Group Co., Ltd. (Beijing,
China). Sulpiride was dissolved in 0.5% CMC-Na. The characteristics
of primary antibodies are presented in Table I. UltraSensitive™ SP
(Mouse/Rabbit) IHC kit (cat. no. KIT-9710) and PBS were purchased
from Fuzhou Maixin Biotechnology Development Co., Ltd. (Fuzhou,
China).
 | Table ICharacteristics of primary
antibodies. |
Table I
Characteristics of primary
antibodies.
| Primary
antibodies | Supplier | Host species | Dilution | Retrieval | Incubation | Cat. no. |
|---|
| PCNA | Santa Cruz
Biotechnology, Inc. | Rabbit | 1:100 | 10 min x2 | 1 h; room
temperature | Sc-7907 |
| Bcl-2 | Santa Cruz
Biotechnology, Inc. | Rabbit | 1:100 | 10 min x2 | Overnight; 4˚C | Sc-492 |
| ERα | Santa Cruz
Biotechnology, Inc. | Rabbit | 1:75 | 10 min x2 | Overnight; 4˚C | Sc-7207 |
| ERβ | ProteinTech Group,
Inc. | Rabbit | 1:100 | 10 min x2 | Overnight; 4˚C | 14007-1-AP |
| AR | Santa Cruz
Biotechnology, Inc. | Mouse | 1:100 | 10 min x2 | Overnight; 4˚C | Sc-7305 |
| Vimentin | BD Biosciences | Mouse | 1:100 | 10 min x2 | Overnight; 4˚C | 550513 |
| Fibronectin | BD Biosciences | Mouse | 1:250 | 10 min x2 | Overnight; 4˚C | 610078 |
| α-SMA | Santa Cruz
Biotechnology, Inc. | Mouse | 1:100 | 10 min x2 | Overnight; 4˚C | Sc-53142 |
Experimental groups
Rats were randomly divided into three groups (n=12)
according to body weight following acclimatization, treated daily
with sulpiride (40 and 120 mg/kg, intragastrically) or vehicle
(0.5% CMC-Na) for 4 weeks and weighed once per week as previously
described (42). The 40 and 120
mg/kg dosages represent the therapeutic low and high dose used in
human treatment (43,44). On day 29 (24 h following final
treatment), 3% pentobarbital sodium (Sigma-Aldrich; Merck KGaA; cat
no. P3761; 39 mg/kg) anesthesia was administered via intravenous
injection and whole blood samples (~6-8 ml) were obtained from
aortaventralis. Following euthanasia, a median abdominal incision
was performed to expose the bladder and prostate. Prostates were
harvested and divided into three sections, including ventral,
dorsal and lateral lobes (VP, DP and LP, respectively) according to
their position relative to the urinary bladder. The lobes were
weighed and fixed in neutral 10% formalin for 24 h at room
temperature.
Histopathology
VP, DP and LP tissues were embedded in paraffin,
sectioned at 3 µm and submitted to routine hematoxylin for 5 min
and eosin for 1 min (H&E) staining at room temperature.
Histological changes were observed under an optical microscope at
magnification, x40 for the acinar luminal area and magnification,
x400 for the height of the prostatic epithelium (Nikon Eclipse 50i;
Nikon Corporation, Tokyo, Japan). The height of the prostatic
epithelium (HPE) and acinar luminal area were (ALA) assessed using
Nikon NIS-Elements BR 3.1 software (Nikon Corporation). A total of
20 epithelial samples per rat (240 per group) were randomly
selected for analysis by a blinded investigator.
Hormone level detection
Blood samples were harvested and centrifuged for 15
min (2,000 x g, 4˚C) to collect serum, which was immediately stored
at -80˚C. Serum PRL (cat. no. DEV9966), FSH (cat. no. LS-F6305), T
(cat. no. 582701) and LH (cat. no. 12281601A) levels were
determined using specific ELISA assay kits obtained from Demeditec
Diagnostics GmbH (Kiel, Germany), LifeSpan BioSciences, Inc.
(Seattle, WA, USA), Cayman Chemical Company (Ann Arbor, MI, USA)
and ENZO Life Sciences, Inc. (Farmingdale, NY, USA) according to
the manufacturers' protocol. The absorbance was read using a
microplate reader at 450 nm (Zenyth 200st; Biochrom Ltd.,
Cambridge, UK).
Immunohistochemical staining
(IHC)
Representative blocks of paraffin-embedded prostate
tissues were fixed as above and sliced to a 4 µm thickness, dewaxed
and rehydrated in a descending alcohol series. Sections were heated
to 95-100˚C in a microwave for 20 min for antigen retrieval and
washed with a 0.01 M sodium citrate buffer (pH 6.0). The
UltraSensitive™ SP (Mouse/Rabbit) IHC kit was used for
peroxidase staining. Endogenous peroxidase was quenched with
oxidase blocking solution (Reagent A) for 10 min at room
temperature. Following blocking with normal non-immune serum
(Reagent B) for 10 min at room temperature, sections were incubated
with primary antibodies as presented in Table I. Primary antibodies were replaced
with PBS in negative controls. The corresponding secondary
antibodies (Reagent C) and Streptomyces antibiotic peroxidase
solution (Reagent D) were added successively at room temperature
for 10 min, followed by staining with 3,3'-diaminobenzidine for
3-10 min at 25˚C). Sections were then treated with hematoxylin at
room temperature for 2 min, dehydrated in a descending series of
alcohol, washed with xylene, mounted and observed under an optical
microscope (magnification, x400; Nikon Eclipse 50i; Nikon
Corporation) by a blinded investigator. Finally, mean optical
densities were obtained using Image Pro-Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data were statistically analyzed using SPSS version
11.0 (SPSS, Inc., Chicago, IL, USA) and expressed as the mean ±
standard deviation. Statistical comparisons were performed using
one-way analysis of variance followed by Tukey's post hoc multiple
comparison test. If statistically significant, differences between
control and treatment groups were assessed using a least-squares
means test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Body and prostate lobe weights
During the 4 weeks of sulpiride administration,
animal body weights increased slightly compared with controls,
however no significant differences were observed (Table II). Following treatment with 40 and
120 mg/kg/day sulpiride, prostate weight and relative prostate
weight increased in a dose-dependent manner (Table II). In addition, treatment with
sulpiride resulted in a dose-dependent increase of DP and LP wet
and relative weights (Table III).
In particular, LP in sulpiride groups was significantly increased
(P<0.05 for sulpiride 40 mg/kg and P<0.01 for sulpiride 120
mg/kg). VP and relative weights in the 40 mg/kg sulpiride group
were reduced compared with the control values, while sulpiride 120
mg/kg demonstrated higher values (Table III).
 | Table IIEffects of Sulpiride on body and
prostate weight of benign hyperplasia prostate modeled Brown-Norway
rats. |
Table II
Effects of Sulpiride on body and
prostate weight of benign hyperplasia prostate modeled Brown-Norway
rats.
| | Body weight
(g) | Prostate |
|---|
| Group | Initial | Final | Weight gain | Weight (g) | Relative weight
(/100) |
|---|
| Control | 290.0±49.0 | 305.9±39.1 | 15.0±37.1 | 0.567±0.140 | 0.185±0.034 |
| Sulpiride (40
mg/kg) | 292.3±55.4 | 312.5±41.5 | 20.0±45.3 | 0.637±0.095 | 0.206±0.033 |
| Sulpiride (120
mg/kg) | 294.3±50.5 | 312.9±42.8 | 18.6±44.7 |
0.784±0.200a |
0.248±0.036a |
 | Table IIIEffects of sulpiride on prostate lobe
weight of benign hyperplasia prostate modeled Brown-Norway
rats. |
Table III
Effects of sulpiride on prostate lobe
weight of benign hyperplasia prostate modeled Brown-Norway
rats.
| | VP | DP | LP |
|---|
| Experimental
group | Weight (g) | Relative weight
(/1,000) | Weight (g) | Relative
weight(/1,000) | Weight (g) | Relative weight
(/1,000) |
|---|
| Control | 0.361±0.087 | 1.170±0.190 | 0.110±0.053 | 0.350±0.150 | 0.096±0.034 | 0.320±0.140 |
| Sulpiride (40
mg/kg) | 0.347±0.061 | 1.128±0.234 |
0.148±0.054a |
0.472±0.167a |
0.142±0.022a |
0.459±0.076a |
| Sulpiride (120
mg/kg) | 0.419±0.083 | 1.334±0.148 |
0.192±0.088b |
0.595±0.221b |
0.173±0.068b |
0.549±0.171b |
Histology
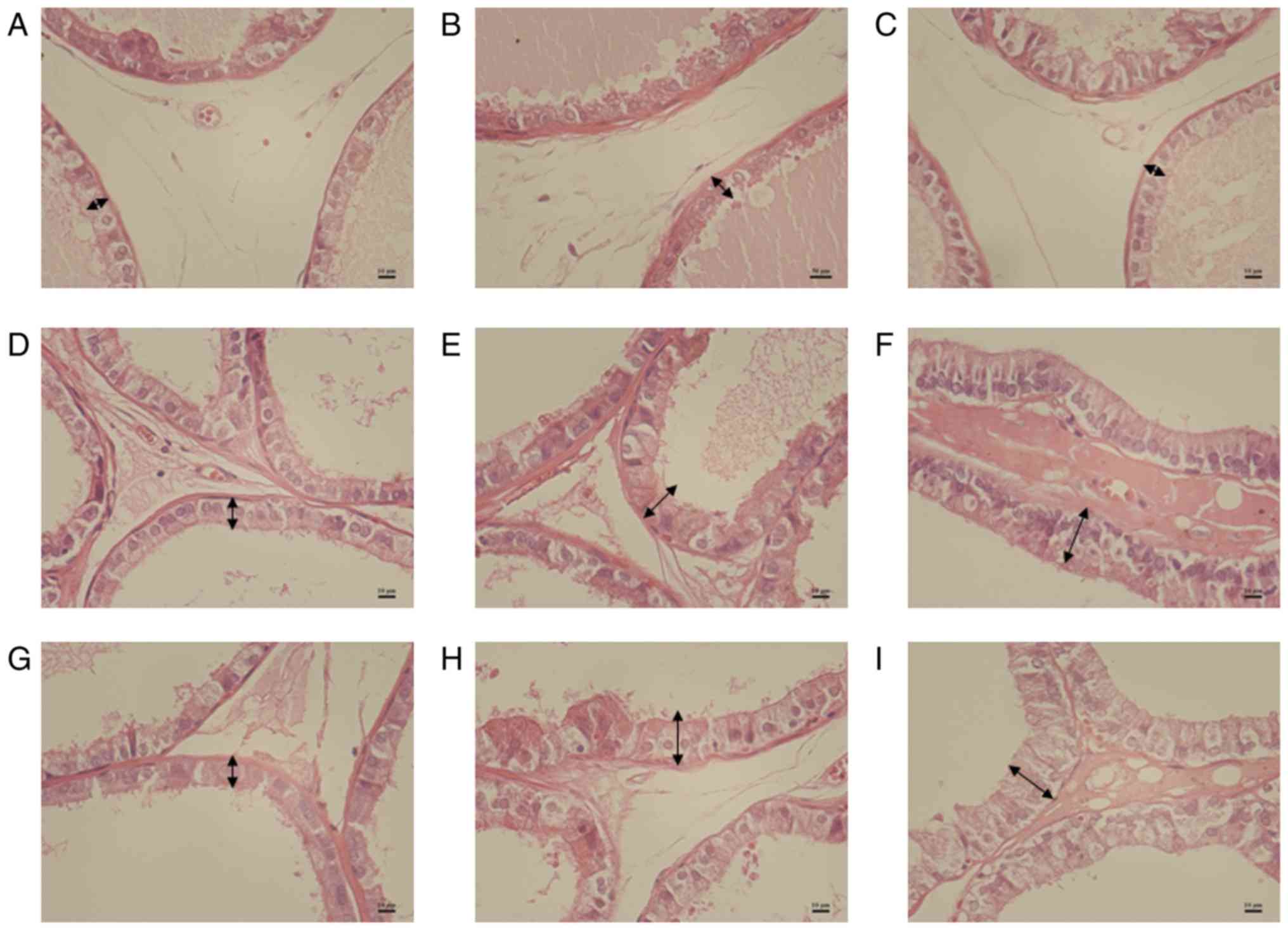
Histologically, proliferative features were more
prominent in the sulpiride groups compared with the control group
(Fig. 1). H&E staining revealed
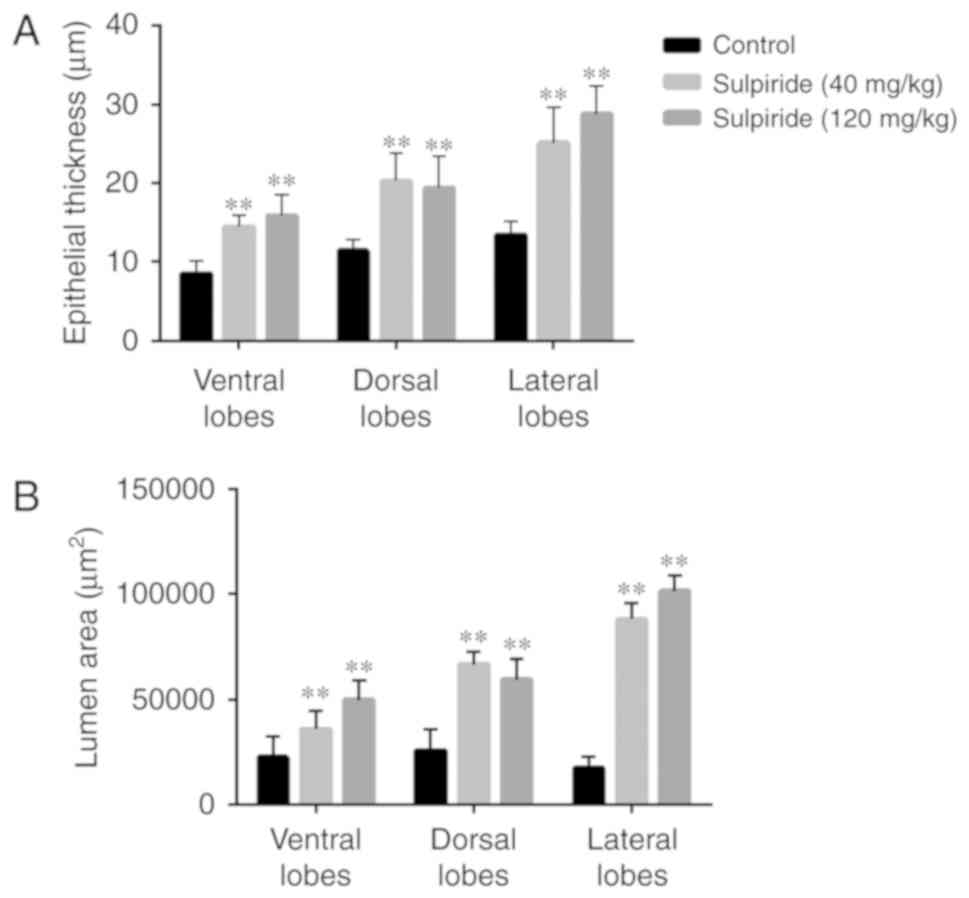
glandular prostatic hyperplasia with increased HPE and ALA in VP
and LP of 40 and 120 mg/kg sulpiride groups (Figs. 1 and 2). In the sulpiride groups, the HPE of LP
was significantly increased compared with control values
(P<0.01; Table IV). Following 4
weeks of treatment with sulpiride, HPE was significantly increased
in VP and LP tissues in the sulpiride groups compared with the
control group (P<0.01; Table
IV), particularly in the 120 mg/kg sulpiride group.
 | Table IVEffects of sulpiride on the height of
prostatic epithelium of benign hyperplasia prostate modeled
Brown-Norway rats. |
Table IV
Effects of sulpiride on the height of
prostatic epithelium of benign hyperplasia prostate modeled
Brown-Norway rats.
| Experimental
group | VP (µm) | DP (µm) | LP (µm) |
|---|
| Control | 8.54±1.61 | 11.41±1.41 | 13.41±1.71 |
| Sulpiride (40
mg/kg) |
14.46±1.49a |
20.27±3.54a |
25.14±4.50a |
| Sulpiride (120
mg/kg) |
15.92±2.60a |
19.41±4.05a |
28.77±3.62a |
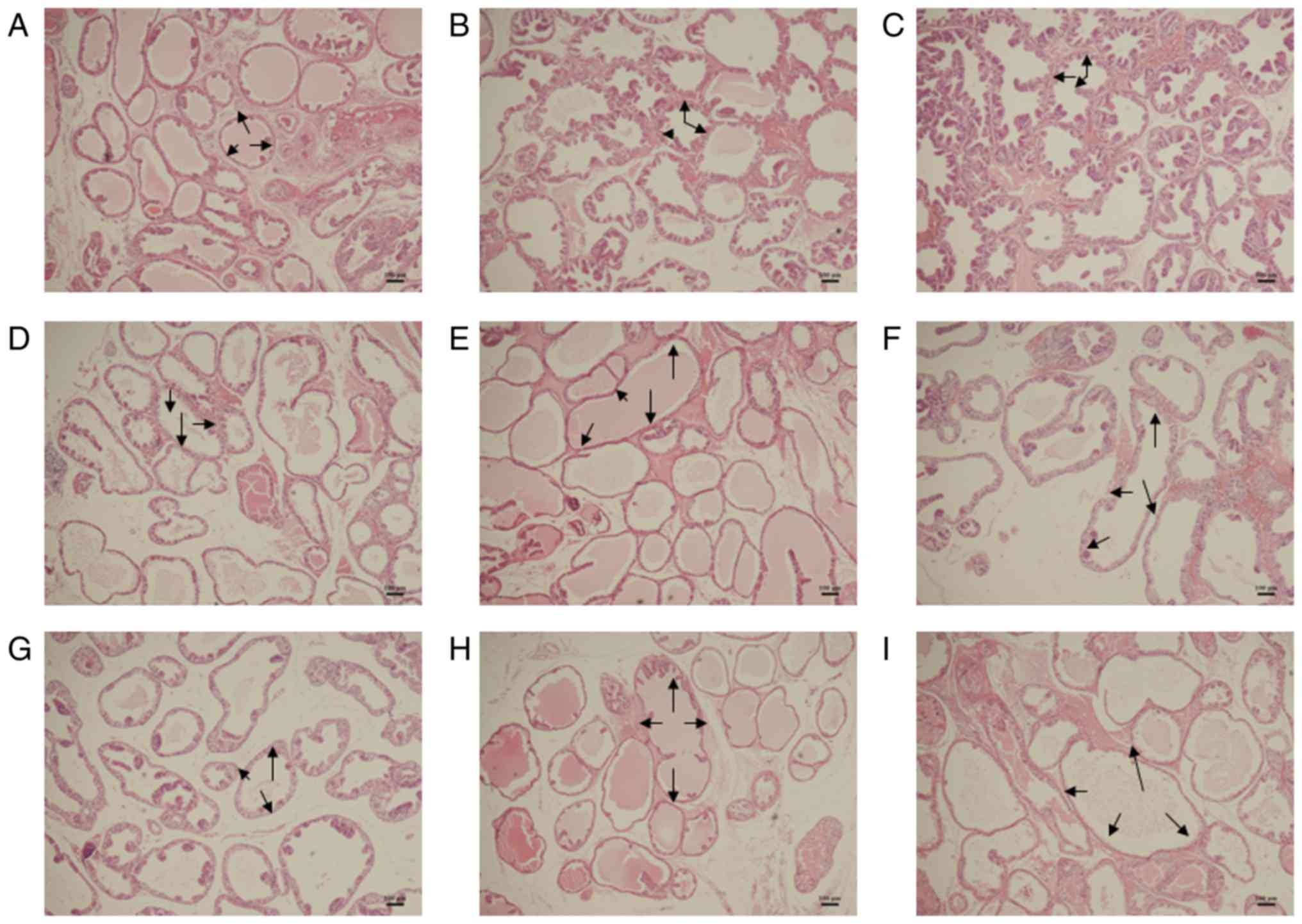
In the sulpiride groups, the ALAs of lobes were
significantly increased compared with the controls, particularly in
LP tissues (P<0.01; Table V;
Fig. 3). Following 4 weeks of
treatment with sulpiride, ALA in LP tissues was significantly
increased compared with control values (P<0.01; Table V), particularly in the 120 mg/kg
sulpiride (Table V; Fig. 3).
 | Table VEffects of sulpiride on prostate
acinar luminal areas of benign hyperplasia prostate modeled
Brown-Norway rats. |
Table V
Effects of sulpiride on prostate
acinar luminal areas of benign hyperplasia prostate modeled
Brown-Norway rats.
| Experimental
group | VP
(µm2) | DP
(µm2) | LP
(µm2) |
|---|
| Control |
22,735.78±9,992.60 |
25,769.59±10,308.27 |
17,454.82±5,506.35 |
| Sulpiride (40
mg/kg) |
36,013.52±8,837.63a |
66,713.71±6,123.32a |
88,233.29±7,336.71a |
| Sulpiride (120
mg/kg) |
49,829.91±9,301.02a |
59,617.50±9,814.00a |
101,443.90±7,374.53a |
Serum PRL, FSH, T and LH levels
All groups treated with sulpiride exhibited higher
PRL levels compared with the control group, particularly following
treatment with 120 mg/kg sulpiride (P<0.001; Table VI). Furthermore, PRL levels
demonstrated a dose-dependent increase. All groups treated with
sulpiride had significantly increased T levels compared with the
control group (P<0.01 for sulpiride 120 mg/kg and P<0.001 for
sulpiride 40 mg/kg). Compared with the control group, the LH levels
were significantly decreased in the sulpiride groups (P<0.001;
Table VI). Additionally, the
results determined that LH levels decreased as the sulpiride dose
increased. FSH levels were increased in the sulpiride groups
compared with controls, particularly in the 40 mg/kg sulpiride
group (P<0.001; Table VI).
 | Table VIPlasma PRL, FSH, T and LH levels. |
Table VI
Plasma PRL, FSH, T and LH levels.
| Experimental
group | PRL (ng/ml) | FSH (ng/ml) | T (pg/ml) | LH (mIU/ml) |
|---|
| Control | 5.87±3.16 | 4.82±1.67 | 197.27±170.63 | 701.58±515.83 |
| Sulpiride (40
mg/kg) |
57.48±15.52b |
7.46±2.98b |
535.07±352.90b |
575.03±123.69b |
| Sulpiride (120
mg/kg) |
106.82±27.2b | 5.36±1.00 |
273.16±92.44a |
367.61±265.64b |
PCNA, Bcl-2, ERα, ERβ, AR, vimentin,
fibronectin and α-SMA expression levels in prostate lobes
The expression levels of PCNA, Bcl-2, ERα, ERβ, AR
and mesenchymal cell markers (including vimentin, fibronectin and
α-SMA) were immunohistochemically analyzed to further assess the
sulpiride-induced signaling pathway in BPH. The results
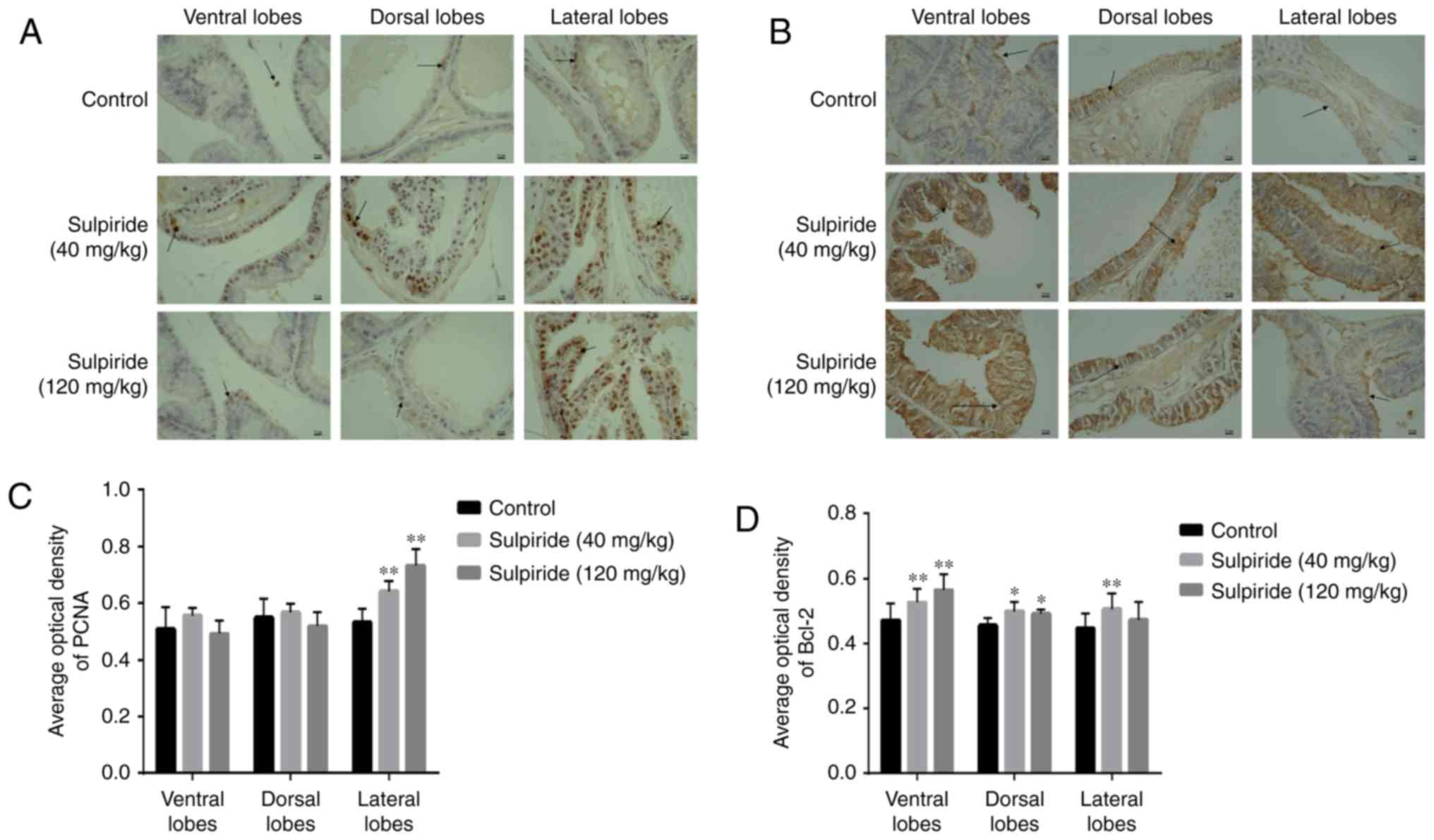
demonstrated that PCNA was primarily expressed in the nucleus of
epithelial cells (Fig. 4A). Compared
with the control group, sulpiride treatment resulted in increased
PCNA levels in LP (P<0.01; Fig.
4C); however, no significant differences were observed in VP
and DP. The Bcl-2 protein was primarily expressed in the cytoplasm
and was significantly increased in VP, DP and LP tissues in the
sulpiride groups compared with the controls (P<0.05 or
P<0.01; Fig. 4).
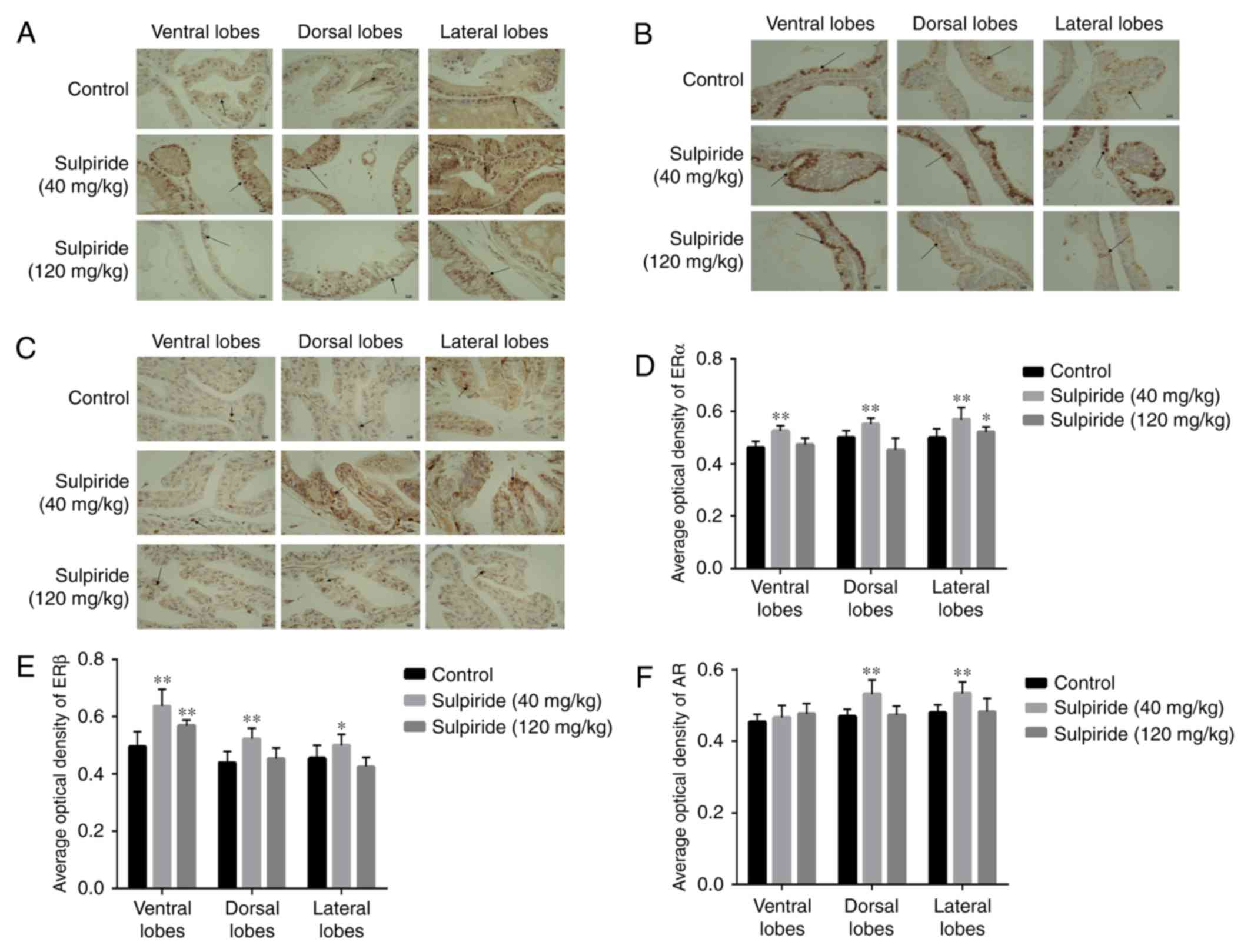
The results of IHC demonstrated that ERα was
primarily distributed in the epithelial cell nucleus (Fig. 5A) and ERβ was expressed abundantly in
the basal epithelial layer within cell nuclei (Fig. 5B). In the 120 mg/kg sulpiride group,
ERα levels in LP were slightly increased compared with control
values (P<0.05; Fig. 5D). Similar
results were observed in the 40 mg/kg sulpiride group (P<0.01;
Fig. 5D). AR was expressed in the
nucleus and cytoplasm of epithelial cells, but remained
predominantly nuclear. The expression of AR was similar to that of
ERα, unlike ERβ (Fig. 5D-F). Sulpiride 40 mg/kg significantly
upregulated ERα and AR levels in LP (P<0.01) whilst sulpiride
120 mg/kg downregulated ERβ, ERβ was significantly increased in LP
tissues treated with sulpiride 40 mg/kg (P<0.05; Fig. 5E). The effects of sulpiride treatment
at 40 mg/kg were markedly more pronounced than those exhibited at
120 mg/kg.
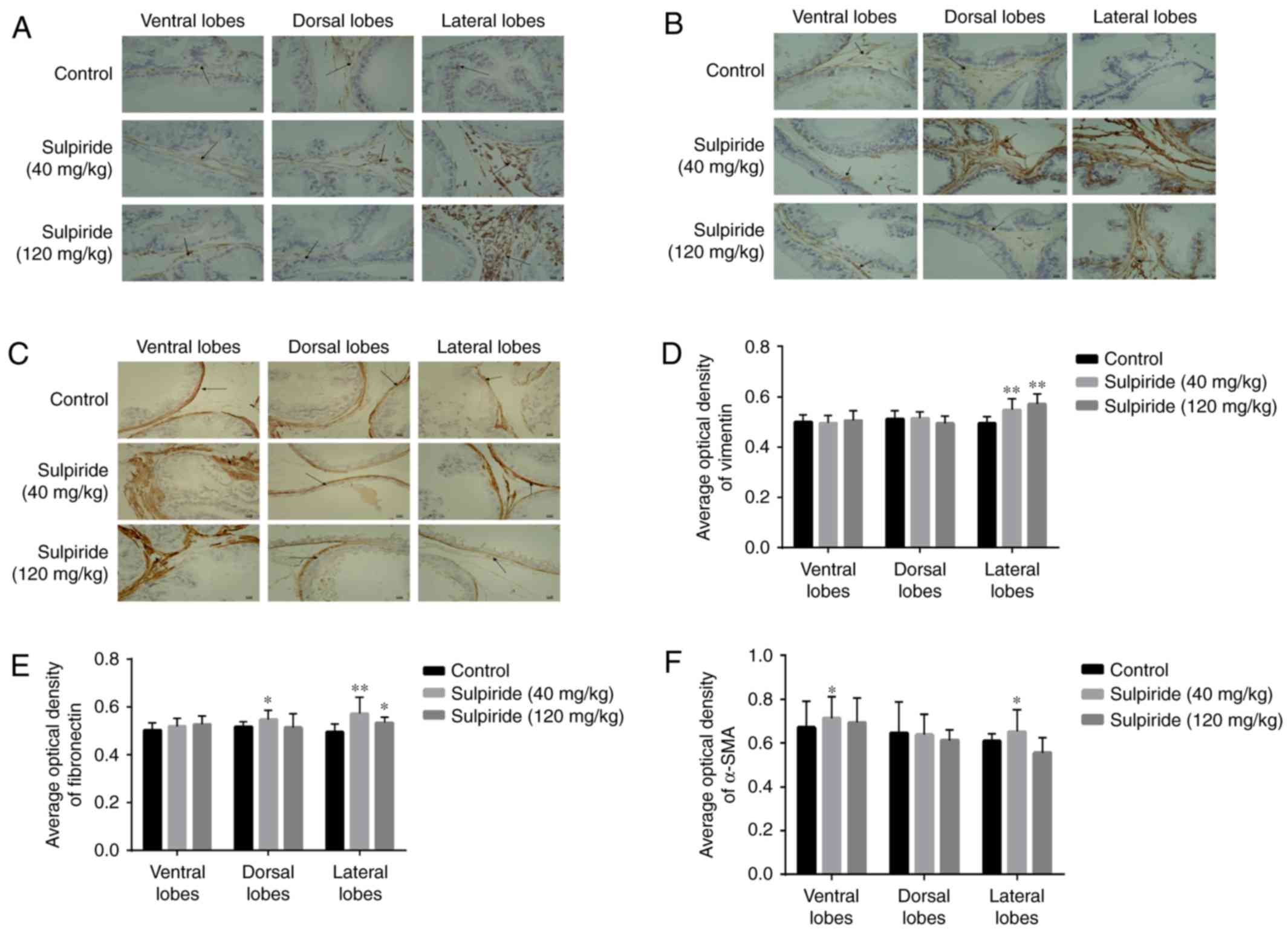
Vimentin constitutes a specific protein marker of
fibroblast cells (45) and was
primarily expressed in mesenchymal cells (Fig. 6). Compared with the control group,
treatment with 40 and 120 mg/kg sulpiride significantly upregulated
vimentin in LP tissues (P<0.01; Fig.
6D); however no significant differences in vimentin levels were
detected in VP and DP tissues. Fibronectin was primarily expressed
in mesenchymal cells (Fig. 6B).
Compared with controls, fibronectin expression in DP and LP
following treatment with 40 mg/kg sulpiride was significantly
increased (P<0.05 and P<0.01, respectively; Fig. 6E), as well as in LP tissues treated
with 120 mg/kg sulpiride (P<0.05, P<0.01). However,
fibronectin protein levels in VP from the 40 mg/kg treated group
and VP and DP tissues treated with 120 mg/kg exhibited no
significant differences. α-SMA, a specific marker of
myofibroblasts, was primarily expressed in the stromal cells of the
prostate (Fig. 6C). Compared with
controls, α-SMA levels were increased in VP and LP tissues
following treatment with 40 mg/kg sulpiride (P<0.05; Fig. 6F); however, levels decreased in LP
tissues following the administration of 120 mg/kg sulpiride.
Furthermore, α-SMA levels in DP tissues following treatment with 40
mg/kg sulpiride and in VP and DP tissues following 120 mg/kg
sulpiride treatment, demonstrated no significant differences
(Fig. 6F).
Discussion
The results of the present study revealed the
mechanism of sulpiride-induced BPH in male BN rats. Sulpiride
stimulates PRL secretion from the pituitary gland and causes
prostate toxicity (8). The present
study aimed to establish a useful model to assess the mechanism of
BPH development and the mechanism of sulpiride-induced BPH in male
BN rats.
BPH originates in the transitional and peripheral
zones of the humans prostate (46).
Murine dorsal and lateral prostate lobes (DLP) are homologous with
the peripheral zone in the human prostate and proliferation is more
evident in the DLP than in the VP in transgenic mice (47). The current histological findings
suggest that sulpiride significantly induces glandular hyperplasia
in LP as follows: Increases of ALA and HPE corroborated
experimental findings by Van Coppenolle et al (8). Ahonen et al (15) demonstrated that PRL stimulates
proliferation and acts as an androgen-independent suppressor of
apoptosis in prostate epithelial cells, leading to prostate
epithelial hyperplasia in DP and LP by using long-term organ
cultures of rat prostate tissue. In addition,
Słuczanowska-Głąbowska et al (9) demonstrated that the columnar and
cubical cells of the epithelium were tall and had irregular
disposition in sites of hyperplasia of the DP and LP in sexually
mature male Wistar rats treated with metoclopramide, while no
changes were observed in the VP. As previously mentioned, LP
hyperplasia in sulpiride-injected animals may be caused by
increased PRL levels (32).
Aside from androgens, the effects of serum PRL on
prostate cell differentiation are important (32). It remains unclear whether PRL acts
synergistically with or independently of T and current data remains
controversial (6,12,48). The
results of the present study revealed increased PRL, FSH and T
levels in sulpiride-treated groups compared with controls, while LH
levels decreased. The present study also demonstrated that PRL and
T levels increased, which is in disagreement with the results of
the study by Van Coppenolle et al (8). This discrepancy may result from the use
of different animal species in each study. The results of the
present study may also be explained by the reduced activity of
5α-reductase, which can cause increased serum T levels (49). However, this requires further study
to confirm. Furthermore, Kindblom et al (50) demonstrated that PRL stimulates BPH in
mice independently of T. This suggests that PRL affects the
prostate and increases T levels, thereby promoting BPH in BN rats,
which is similar to the results obtained from Wennbo et al
(51). Rubin et al (52,53)
confirmed the prior hypothesis that PRL stimulates T secretion,
which reported that PRL accentuates the effects of androgens in the
stimulation of prostate growth and function. Additionally, Van
Coppenolle et al (8)
demonstrated that T levels are higher in controls compared with
animals treated with sulpiride, which is not congruent with the
aforementioned results. Previous studies have indicated that an
increase in PRL inhibits gonadotrop-releasing hormone (GnRH)
release from the hypothalamus and reduces LH release via a negative
feedback regulatory mechanism (54,55). The
structure and function of the prostate are influenced by the
hypothalamus-pituitary-gonadal axis (56), while tissue growth and hormone
secretion are primarily affected by T regulation (57). In turn, T secretion is controlled by
LH and FSH (58,59). Sulpiride has an effect on the
hypothalamic tuberoinfundibular dopaminergic neurons at the
pituitary and increases PRL release (60). Furthermore, PRL stimulates the
release of endogenous opioids, which strongly inhibits GnRH release
(61). LH was downregulated and FSH
was upregulated via the feedback regulation mechanism utilized by
the hypothalamus-pituitary-gonadal axis; elevated FSH stimulates
the release of T, which in turn inhibits GnRH release.
The results of the present study indicate that
sulpiride promotes prostate growth in BN rats by increasing PRL and
T levels. In addition, synergistic effects between PRL and T were
demonstrated. PCNA is used as a proliferation marker in the rat
prostate (28). IHC results in the
present study revealed that PCNA was primarily expressed in the
nucleus of epithelial cells. In addition, PCNA expression in LP
samples was significantly increased following sulpiride treatment
compared with the control group. However, PCNA levels were
unchanged in VP and DP samples. A previous study has indicated that
the VP and DP in BN rats are insensitive to PRL, no PCNA
overexpression was detected in the present study (47). It has been demonstrated that Bcl-2
reduces cell apoptosis in prostate tissues (62). As revealed by IHC in the present
study, the Bcl-2 protein was primarily expressed in the cytoplasm
of epithelial cells and its levels increased significantly in rats
treated with sulpiride with the exception of LP at sulpiride 120
mg/kg. Van Coppenolle et al (8) demonstrated that Bcl-2 expression is
increased in the LP of sulpiride treated rats. In the present
study, LP hyperplasia may be explained by the PRL-induced
proliferation as mediated by PCNA overexpression and apoptosis
inhibition via Bcl-2 overexpression. These experimental results
suggest that sulpiride treatment promotes proliferation and
inhibits epithelial cell apoptosis, promoting prostate epithelial
hyperplasia.
Estrogen receptors belong to the nuclear receptor
family of proteins that includes ERα and ERβ; the former serves a
role in the promotion of prostate cell proliferation and the latter
exerts beneficial effects by repressing prostate growth (63). Sulpiride competes with estradiol for
the ERα binding due to its structural similarity and binds specific
DNA sequences to induce target genes following nuclear entry
(64). As demonstrated in the
present study, ERα and ERβ are primarily expressed in the nucleus
of epithelial cells. In sulpiride treatment groups, ERα levels in
LP tissues were significantly increased compared with control
values. The 40 mg/kg sulpiride group significantly increased ERα
levels in different lobes. The prostate is an androgen-dependent
organ and AR serves a pivotal role in the regulation of its
function, growth and differentiation (65). The transcription factor AR is
activated by its ligand, binding a specific androgen response
element to promote transcription (33). In the present study, AR was expressed
in the nucleus and cytoplasm of epithelial cells, with levels
increasing in VP with the dosage of 120 mg/kg sulpiride. However,
40 mg/kg sulpiride group significantly increased AR in DP and LP
tissues, more so than the 120 mg/kg dosage. The expression trend of
AR was similar to that of ERα, but opposite to that of ERβ. Higher
AR expression in the lateral lobes of BN rats may be explained by
the following mechanisms. Banerjee et al (66) reported that AR expression is not
dependent on T, as AR levels remain unchanged in castrated rats. In
addition, Sluczanowska-Glabowska et al (9) demonstrated that AR is elevated in LP
tissues of the experimental group, but lower in the VP and DP
tissues. Prins et al (67)
revealed that increased PRL leads to AR upregulation in the LP of
experimental animals. Furthermore, Holland and Lee (68) demonstrated that increased serum PRL
and decreased T results in the increased sensitivity of the LP to
PRL. As demonstrated in the present study, ERα and AR levels were
increased following sulpiride treatment, indicating that sulpiride
causes LP hyperplasia of the prostate via the ERα and AR signaling
pathways.
The present study evaluated the expression of
stromal cell biomarkers following sulpiride administration. The
stromal compartment contains fibroblasts, vasculature, nerves and
immune components (69,70). Mesenchymal cells consist of
fibroblasts, myofibroblasts and smooth muscle cells (71). Mesenchymal cell markers, including
vimentin, α-SMA and fibronectin, are associated with the
development of prostate tissues (72-76). As demonstrated
by IHC in the present study, vimentin, fibronectin and α-SMA were
primarily expressed in mesenchymal cells. In addition, vimentin
expression in LP was significantly increased following the
administration of 40 and 120 mg/kg sulpiride. These findings
suggest that sulpiride also causes hyperplasia in the stromal
component of LP, indicating that BPH is a proliferative stromal
disease, similar to spontaneous prostatic hyperplasia (4).
PCNA as a proliferation marker and Bcl-2 as an
anti-apoptotic marker mediate cell proliferation and apoptosis,
respectively. The present study demonstrated that the expression of
PCNA and Bcl-2 was upregulated in LP of 40 mg/kg sulpiride group.
Van Coppenolle et al (8)
demonstrated that PRL stimulates Bcl-2 expression in the prostate
gland of sulpiride-treated Wistar rats and inhibits prostate cell
apoptosis. The results of the present study revealed that BN rats
treated with sulpiride exhibited higher PRL levels compared with
control rats. It was hypothesized that higher Bcl-2 levels caused
by higher PRL inhibits prostate cell apoptosis except for the LP
tissues at sulpiride 120 mg/kg.
A previous study has determined that vimentin, an
important intermediate fibrin protein in cells, functions as a
general intermediate fiber and participates in the process of cell
apoptosis (77). Morishima et
al (78) revealed that apoptosis
was accompanied by vimentin fragmentation. Fibronectin serves an
important role in cell migration, adherence and proliferation. Wu
et al (79) demonstrated that
a lack of fibronectin triggers mesangial apoptosis via the
intrinsic pathway. In the present study it was determined that
sulpiride promotes the expression of stromal cell biomarkers
vimentin and fibronectin in BN rats. Based on these results, it was
hypothesized that sulpiride may inhibit the apoptosis of prostate
cells by upregulating vimentin and fibronectin.
The aforementioned results of the current study
indicate that sulpiride promotes proliferation and inhibits
prostate cell apoptosis by upregulating PCNA, Bcl-2 and stromal
cell biomarker (vimentin and fibronectin) expression.
The present study established a prostate hyperplasia
model in BN rats treated with sulpiride (40 mg/kg/day), the results
of which indicate that sulpiride causes LP hyperplasia in BN rats
by promoting proliferation and inhibiting apoptosis in prostate
cells, likely via ERα and AR signaling. However, further study is
required to fully elucidate the possible mechanisms by which
sulpiride activates ERα and AR functions and the pathways
involved.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Experimental Animal Scientific and Technological Innovative Action
Plan (grant no. 17140900801) and the Shanghai Professional Service
Platform of Non-clinical Evaluation of Drugs Against Women's and
Children's Diseases (grant no. 17DZ2293600).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and YL designed the study. CZ performed the
experiments and analyzed the experimental data. DC, CS, DH and JZ
participated in the animal experiments. YC performed the ELISA
experiments. XM and LL performed the tissue specimen preparation.
All authors have read and approved this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Shanghai
Institute of Planned Parenthood Research Animal Care (Shanghai,
China). All animal procedures were approved by the Animal Care and
Use Committee of Shanghai Institute of Planned Parenthood Research
(Shanghai, China) and performed according to the Guide for the Care
and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sampson N, Untergasser G, Plas E and
Berger P: The ageing male reproductive tract. J Pathol.
211:206–218. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Afriyie DK, Asare GA, Bugyei K, Adjei S,
Lin JM, Peng J and Hong ZF: Treatment of benign prostatic
hyperplasia with Croton membranaceus in an experimental animal
model. J Ethnopharmacol. 157:90–98. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gratzke C, Schlenker B, Weidlich P, Seitz
M, Reich O and Stief CG: Benign prostatic hyperplasia: Background
and diagnosis. MMW Fortschr Med. 149:25–28. 2007.(In German).
PubMed/NCBI
|
|
4
|
Schauer IG and Rowley DR: The functional
role of reactive stroma in benign prostatic hyperplasia.
Differentiation. 82:200–210. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Akanni OO, Abiola OJ and Adaramoye OA:
Methyl Jasmonate ameliorates testosterone propionate-induced
prostatic hyperplasia in castrated wistar rats. Phytother Res.
31:647–656. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Kristiansen JE, Dastidar SG, Palchoudhuri
S, Roy DS, Das S, Hendricks O and Christensen JB: Phenothiazines as
a solution for multidrug resistant tuberculosis: From the origin to
present. Int Microbiol. 18:1–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martynova NA and Gorokhova LG: Toxicity
assessment of sulpiride as the basis of its hygienic
standardization. Gig Sanit. 94:114–117. 2015.PubMed/NCBI
|
|
8
|
Van Coppenolle F, Slomianny C, Carpentier
F, Le Bourhis X, Ahidouch A, Croix D, Legrand G, Dewailly E,
Fournier S, Cousse H, et al: Effects of hyperprolactinemia on rat
prostate growth: Evidence of androgeno-dependence. Am J Physiol
Endocrinol Metab. 280:E120–E129. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Słuczanowska-Głąbowska S, Laszczyńska M,
Wylot M, Głąbowski W, Piasecka M and Gącarzewicz D: Morphological
and immunohistochemical comparison of three rat prostate lobes
(lateral, dorsal and ventral) in experimental hyperprolactinemia.
Folia Histochem Cytobiol. 48:447–454. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Payne MR, Howie PW, McNeilly AS, Cooper W,
Marnie M and Kidd L: Sulpiride and the potentiation of progestogen
only contraception. Br Med J (Clin Res Ed). 291:559–561.
1985.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Farnsworth WE: Prolactin effect on the
permeability of human benign hyperplastic prostate to testosterone.
Prostate. 12:221–229. 1988.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wolf K, Kayacelebi H, Urhausen C,
Piechotta M, Mischke R, Kramer S, Einspanier A, Oei CH and
Gunzel-Apel A: Testicular steroids, prolactin, relaxin and prostate
gland markers in peripheral blood and seminal plasma of normal dogs
and dogs with prostatic hyperplasia. Reprod Domest Anim. 47:(Suppl
6). 243–246. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Drobnis EZ and Nangia AK: Psychotropics
and male reproduction. Adv Exp Med Biol. 1034:63–101.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zeng QS, Xu CL, Liu ZY, Wang HQ, Yang B,
Xu WD, Jin TL, Wu CY, Huang G, Li Z, et al: Relationship between
serum sex hormones levels and degree of benign prostate hyperplasia
in Chinese aging men. Asian J Androl. 14:773–777. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ahonen TJ, Härkönen PL, Rui H and
Nevalainen MT: PRL signal transduction in the epithelial
compartment of rat prostate maintained as long-term organ cultures
in vitro. Endocrinology. 143:228–238. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Thomas LN, Merrimen J, Bell DG, Rendon R
and Too CK: Prolactin- and testosterone-induced carboxypeptidase-D
correlates with increased nitrotyrosines and Ki67 in prostate
cancer. Prostate. 75:1726–1736. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pechenino AS and Brown TR: Superoxide
dismutase in the prostate lobes of aging brown Norway rats.
Prostate. 66:522–535. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reiter E, Lardinois S, Klug M, Sente B,
Hennuy B, Bruyninx M, Closset J and Hennen G: Androgen-independent
effects of prolactin on the different lobes of the immature rat
prostate. Mol Cell Endocrinol. 112:113–122. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bole-Feysot C, Goffin V, Edery M, Binart N
and Kelly PA: Prolactin (PRL) and its receptor: Actions, signal
transduction pathways and phenotypes observed in PRL receptor
knockout mice. Endocr Rev. 19:225–268. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Costello LC and Franklin RB: Effect of
prolactin on the prostate. Prostate. 24:162–166. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Roehrborn CG: Pathology of benign
prostatic hyperplasia. Int J Impot Res. 20 (Suppl 3):S11–S18.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Porcaro AB, Migliorini F, Petrozziello A,
Sava T, Romano M, Caruso B, Cocco C, Ghimenton C, Zecchinini AS,
Lacola V, et al: Follicle-stimulating hormone and the
pituitary-testicular-prostate axis at the time of initial diagnosis
of prostate cancer and subsequent cluster selection of the patient
population undergoing standard radical prostatectomy. Urol Int.
90:45–55. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chowen-Breed J, Fraser HM, Vician L,
Damassa DA, Clifton DK and Steiner RA: Testosterone regulation of
proopiomelanocortin messenger ribonucleic acid in the arcuate
nucleus of the male rat. Endocrinology. 124:1697–1702.
1989.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rasmussen DD, Sarkar DK, Roberts JL and
Gore AC: Chronic daily ethanol and withdrawal: 4. Long-term changes
in plasma testosterone regulation, but no effect on GnRH gene
expression or plasma LH concentrations. Endocrine. 22:143–150.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ottenweller JE, Li MT, Giglio W, Anesetti
R, Pogach LM and Huang HF: Alteration of follicle-stimulating
hormone and testosterone regulation of messenger ribonucleic acid
for Sertoli cell proteins in the rat during the acute phase of
spinal cord injury. Biol Reprod. 63:730–735. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mattsson P and Medvedev A: Modeling of
testosterone regulation by pulse-modulated feedback. Adv Exp Med
Boil. 823:23–40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schanbacher BD: Testosterone regulation of
luteinizing hormone and follicle stimulating hormone secretion in
young male lambs. J Anim Sci. 51:679–684. 1980.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alonso-Magdalena P, Brössner C, Reiner A,
Cheng G, Sugiyama N, Warner M and Gustafsson JA: A role for
epithelial-mesenchymal transition in the etiology of benign
prostatic hyperplasia. Proc Natl Acad Sci USA. 106:2859–2863.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shoieb SM, Esmat A, Khalifa AE and
Abdel-Naim AB: Chrysin attenuates testosterone-induced benign
prostate hyperplasia in rats. Food Chem Toxicol. 111:650–659.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shi X, Peng Y, Du X, Liu H, Klocker H, Lin
Q, Shi J and Zhang J: Estradiol promotes epithelial-to-mesenchymal
transition in human benign prostatic epithelial cells. Prostate.
77:1424–1437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tsurusaki T, Aoki D, Kanetake H, Inoue S,
Muramatsu M, Hishikawa Y and Koji T: Zone-dependent expression of
estrogen receptors alpha and beta in human benign prostatic
hyperplasia. J Clin Endocrinol Metab. 88:1333–1340. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nicholson TM and Ricke WA: Androgens and
estrogens in benign prostatic hyperplasia: Past, present and
future. Differentiation. 82:184–199. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song L, Shen W, Zhang H, Wang Q, Wang Y
and Zhou Z: Differential expression of androgen, estrogen, and
progesterone receptors in benign prostatic hyperplasia. Bosn J
Basic Med Sci. 16:201–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Choi HM, Jung Y, Park J, Kim HL, Youn DH,
Kang J, Jeong MY, Lee JH, Yang WM, Lee SG, et al: Cinnamomi Cortex
(Cinnamomum verum) suppresses testosterone-induced benign prostatic
hyperplasia by regulating 5α-reductase. Sci Rep.
6(31906)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roper WG: The prevention of benign
prostatic hyperplasia (bph). Med Hypotheses. 100:4–9.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Luo Y, Waladali W, Li S, Zheng X, Hu L,
Zheng H, Hu W and Chen C: 17beta-estradiol affects proliferation
and apoptosis of rat prostatic smooth muscle cells by modulating
cell cycle transition and related proteins. Cell Biol Int.
32:899–905. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saito M, Tsounapi P, Oikawa R, Shimizu S,
Honda M, Sejima T, Kinoshita Y and Tomita S: Prostatic ischemia
induces ventral prostatic hyperplasia in the SHR; possible
mechanism of development of BPH. Sci Rep. 4(3822)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Byun SS, Jeong H, Jo MK and Lee E:
Relative proportions of tissue components in the prostate: Are they
related to the development of symptomatic BPH in Korean men?
Urology. 66:593–596. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lean FZ, Kontos S and Palmieri C:
Expression of β-catenin and mesenchymal markers in canine prostatic
hyperplasia and carcinoma. J Comp Pathol. 150:373–381.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu JH, Jiang XR, Liu GM, Liu XY, He GL and
Sun ZY: Oral exposure to low-dose bisphenol A aggravates
testosterone-induced benign hyperplasia prostate in rats. Toxicol
Ind Health. 27:810–819. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shao R, Shi J, Liu H, Shi X, Du X, Klocker
H, Lee C, Zhu Y and Zhang J: Epithelial-to-mesenchymal transition
and estrogen receptor α mediated epithelial dedifferentiation mark
the development of benign prostatic hyperplasia. Prostate.
74:970–982. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sanchez P, Torres JM, Vilchez P, Del Moral
RG and Ortega E: Effects of sulpiride on mRNA levels of steroid
5alpha-reductase isozymes in prostate of adult rats. Iubmb Life.
60:68–72. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Lee BH, Kang SG, Kim TW, Lee HJ, Yoon HK
and Park YM: Hyperprolactinemia induced by low-dosage amisulpride
in Korean psychiatric patients. Psychiatry Clin Neurosci. 66:69–73.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Martinot JL, Paillère-Martinot ML, Poirier
MF, Dao-Castellana MH, Loc'H C and Mazière B: In vivo
characteristics of dopamine D2 receptor occupancy by amisulpride in
schizophrenia. Psychopharmacology (Berl). 124:154–158.
1996.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Abe Y, Yonemura K, Nishida K and Takagi K:
Giant cell tumor of bone: Analysis of proliferative cells by
double-labeling immunohistochemistry with anti-proliferating cell
nuclear antigen antibody and culture procedure. Nihon Seikeigeka
Gakkai Zasshi. 68:407–414. 1994.PubMed/NCBI
|
|
46
|
Sun HB, Xia SJ and Tang XD: Expression of
different genes in transitional zone and peripheral zone of human
normal prostate. Zhonghua Yi Xue Za Zhi. 85:610–613. 2005.(In
Chinese). PubMed/NCBI
|
|
47
|
Liu YN, Abou-Kheir W, Yin JJ, Fang L,
Hynes P, Casey O, Hu D, Wan Y, Seng V, Sheppard-Tillman H, et al:
Critical and reciprocal regulation of KLF4 and SLUG in transforming
growth factor β-initiated prostate cancerepithelial-mesenchymal
transition. Mol Cell Biol. 32:941–953. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shao R, Shi J, Liu H, Shi X, Du X, Klocker
H, Lee C, Zhu Y and Zhang J: Epithelial-to-mesenchymal transition
and estrogen receptor α mediated epithelial dedifferentiation mark
the development of benign prostatic hyperplasi. Prostate.
74:970–982. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sánchez P, Torres JM, Castro B, Frias JF
and Ortega E: Effects of metoclopramide on mRNA levels of steroid
5α-reductase isozymes in prostate of adult rats. J Physiol Biochem.
69:133–140. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kindblom J, Dillner K, Ling C, Törnell J
and Wennbo H: Progressive prostate hyperplasia in adult prolactin
transgenic mice is not dependent on elevated serum androgen levels.
Prostate. 53:24–33. 2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wennbo H, Kindblom J, Isaksson OG and
Törnell J: Transgenic mice overexpressing the prolactin gene
develop dramatic enlargement of the prostate gland. Endocrinology.
138:4410–4415. 1997.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rubin RT, Poland RE and Tower BB:
Prolactin-related testosterone secretion in normal adult men. J
Clin Endocrinol Metab. 42:112–116. 1976.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rubin RT, Gouin PR, Lubin A, Poland RE and
Pirke KM: Nocturnal increase of plasma testosterone in men:
Relation to gonadotropins and prolactin. J Clin Endocrinol Metab.
40:1027–1033. 1975.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shen YC, Kang CH and Chiang PH: Efficacy
of switching therapy of luteinizing hormone-releasing hormone
analogue for advanced prostate cancer. Kaohsiung J Med Sci.
32:567–571. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Miki K, Sasaki H, Kido M, Takahashi H,
Aoki M and Egawa S: A comparative study on the efficacies of
gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist
in neoadjuvant androgen deprivation therapy combined with
transperineal prostate brachytherapy for localized prostate cancer.
BMC Cancer. 16(708)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yamanaka H, Kosaku N, Makino T and Shida
K: Fundamental and clinical study of the anti-prostatic effect of
allylestrenol. Hinyokika Kiyo. 29:1133–1145. 1983.(In Japanese).
PubMed/NCBI
|
|
57
|
Liu RF, Fu G, Li J, Yang YF, Wang XG, Bai
PD and Chen YD: Roles of autophagy in androgen-induced benign
prostatic hyperplasia in castrated. rats. Exp Ther Med.
15:2703–2710. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hammond GL, Kontturi M, Määttälä P, Puukka
M and Vihko R: Serum FSH, LH and prolactin in normal males and
patients with prostatic diseases. Clin Endocrinol (Oxf). 7:129–135.
1977.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Porcaro AB, Petrozziello A, Ghimenton C,
Migliorini F, Sava T, Caruso B, Cocco C, Romano M and Artibani W:
Along the pituitary-testis-prostate axis, serum total testosterone
is a significant preoperative variable independently contributing
to separating the prostate cancer population into prostatectomy
Gleason score groups. Anticancer Res. 32:5015–5022. 2012.PubMed/NCBI
|
|
60
|
Yuan ZF, Yang SC and Pan JT: Effects of
prolactin-releasing peptide on tuberoinfundibular dopaminergic
neuronal activity and prolactin secretion in estrogen-treated
female rats. J Biomed Sci. 9:112–118. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Calogero AE, Weber RF, Raiti F, Burrello
N, Moncada ML, Mongioi A and D'Agata R: Involvement of
corticotropin-releasing hormone and endogenous opioid peptides in
prolactin-suppressed gonadotropin-releasing hormone release in
vitro. Neuroendocrinology. 60:291–296. 1994.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ogura T, Tanaka Y, Tamaki H and Harada M:
Docetaxel induces Bcl-2- and pro-apoptotic caspase-independent
death of human prostate cancer DU145 cells. Int J Oncol.
48:2330–2338. 2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ellem SJ and Risbridger GP: The dual,
opposing roles of estrogen in the prostate. Ann N Y Acad Sci.
1155:174–186. 2009.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Weng YI, Hsu PY, Liyanarachchi S, Liu J,
Deatherage DE, Huang YW, Zuo T, Rodriguez B, Lin CH, Cheng AL and
Huang TH: Epigenetic influences of low-dose bisphenol A in primary
human breast epithelial cells. Toxicol Appl Pharmacol. 248:111–121.
2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huang DY, Zheng CC, Pan Q, Wu SS, Su X, Li
L, Wu JH and Sun ZY: Oral exposure of low-dose bisphenol A promotes
proliferation of dorsolateral prostate and induces
epithelial-mesenchymal transition in aged rats. Sci Rep.
8(490)2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Banerjee PP, Banerjee S and Brown TR:
Increased androgen receptor expression correlates with development
of age-dependent, lobe-specific spontaneous hyperplasia of the
brown Norway rat prostate. Endocrinology. 142:4066–4075.
2001.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Prins GS: Prolactin influence on cytosol
and nuclear androgen receptors in the ventral, dorsal, and lateral
lobes of the rat prostate. Endocrinology. 120:1457–1464.
1987.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Holland JM and Lee C: Effects of pituitary
grafts on testosterone stimulated growth of rat prostate. Biol
Reprod. 22:351–355. 1980.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Da J, Lu M and Wang Z: Estrogen receptor
alpha (ERα)-associated fibroblasts promote cell growth in prostate
cancer. Cell Biochem Biophys. 73:793–798. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Corradi LS, Jesus MM, Fochi RA, Vilamaior
PS, Justulin LJ Jr, Góes RM, Felisbino SL and Taboga SR: Structural
and ultrastructural evidence for telocytes in prostate stroma. J
Cell Mol Med. 17:398–406. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang S, Wu H and Liu C: Inhibition of
lymphocyte proliferation: An ability shared by murine mesenchymal
stem cells, dermal fibroblasts and chondrocytes. Transpl Immunol
Feb. 20:2018.(Epub ahead of print). PubMed/NCBI View Article : Google Scholar
|
|
72
|
Van de Voorde WM, Elgamal AA, Van Poppel
HP, Verbeken EK, Baert LV and Lauweryns JM: Morphologic and
immunohistochemical changes in prostate cancer after preoperative
hormonal therapy. A comparative study of radical prostatectomies.
Cancer-Am Cancer Soc. 74:3164–3175. 1994.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hetzl AC, Montico F, Kido LA and Cagnon
VH: Prolactin, EGFR, vimentin and α-actin profiles in elderly rat
prostate subjected to steroid hormonal imbalance. Tissue Cell.
48:189–196. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lindsay CR, Le Moulec S, Billiot F, Loriot
Y, Ngo-Camus M, Vielh P, Fizazi K, Massard C and Farace F: Vimentin
and Ki67 expression in circulating tumour cells derived from
castrate-resistant prostate cancer. BMC Cancer.
16(168)2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Burch TC, Watson MT and Nyalwidhe JO:
Variable metastatic potentials correlate with differential plectin
and vimentin expression in syngeneic androgen independent prostate
cancer cells. PLoS One. 8(e65005)2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Haixuan Qiao, Qingming Wang and Huipeng
Chen: Changes in vimentin during apoptosis. Bull Acad Military Med
Sci. 275(277)2005.
|
|
78
|
Morishima N: Changes in nuclear morphology
during apoptosis correlate with vimentin cleavage by different
caspases located either upstream or downstream of Bcl-2 action.
Genes Cells. 4:401–414. 1999.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wu D: Mechanism of apoptosis induced by
knockdown of fbronectin in mesangial cells. (unpublished PhD
thesis). General Hospital of PLA.
|