Introduction
Hashimoto's thyroiditis (HT) is a chronic autoimmune
thyroid disease characterized by autoimmune-mediated destruction of
the thyroid gland (1). Individuals
with HT may have no symptoms early on. However, over time, the
thyroid may enlarge, forming a painless goiter, and most patients
eventually develop hypothyroidism (2,3). The
diagnosis of HT relies on the demonstration of circulating
antibodies to thyroid peroxidase and thyroglobulin (4). Despite the large number of studies, HT
remains a complex and expanding disease that awaits further
understanding and novel forms of treatment.
Nicotinamide phosphoribosyltransferase (Nampt), also
known as pre-B cell colony-enhancing factor (PBEF) or visfatin, is
a member of the nicotinic acid phosphoribosyltransferase family
(5). It is the rate limiting
component in the mammalian NAD biosynthesis pathway. In addition,
Nampt acts as a cytokine with immunomodulating properties and an
adipokine with anti-diabetic properties (6-8). Recently, more and
more studies have revealed the association between Nampt and
autoimmune thyroid disease (AITD). Nampt is overexpressed in
leukocytes and thyroid tissues of patients with Graves' disease
(4,9,10). The
serum concentration of Nampt was also found to be increased in
Graves' disease (11). Clinical
studies and animal experiments have shown elevated plasma Nampt
concentrations in hyperthyroidism and hypothyroidism groups
(12,13). Li and Li reported that plasma Nampt
levels were higher in patients with HT than that noted in controls
(14). All of these studies revealed
the changes in Nampt expression in AITD; however, studies on the
exact role and the mechanisms of Nampt in AITD are rare.
Herein, a rat model of HT was constructed and it was
ascertained whether Nampt overexpression contributes to the
development of HT. Furthermore, the effect of Nampt overexpression
on Toll-like receptor 4 (TLR4) in HT was also investigated.
Materials and methods
Animal experiments
All the animal procedures were approved by the
Ethics Committee of The First Affiliated Hospital of Nanchang
University (Nanchang, Jiangxi, China). A total of 38 female SD rats
(8 weeks old) were obtained from Hunan Slac Jingda Laboratory
Animal Co., Ltd. (Changsha, Hunan, China). The rats were randomly
assigned into two groups: the control group (Control group, n=8)
and the Hashimoto's thyroiditis group (Model group, n=9). Rats in
the model group were injected with 0.2 ml porcine thyroglobulin (4
mg/ml; Huayang Zhenglong Biochemical Products) emulsified in
complete Freund's adjuvant (CFA; MP Biomedicals) at the footpad,
and with a booster injection of an equal dose of porcine
thyroglobulin in incomplete Freund's adjuvant (IFA) performed 2 and
4 weeks later. Rats in the control group were treated with
phosphate-buffered saline (PBS) instead of porcine thyroglobulin at
the same time. The rats in the normal and the model groups were
maintained under standard laboratory conditions, and allowed access
to deionized water or 0.05% Na I solution (Shanghai Zhanyun
Chemical Co., Ltd.). One week after the third immunization, the
thyroid tissues were collected for testing. Empty adenoviral
vector and adenoviral expression vector carrying the Nampt
gene were provided by Abmgoodchina Inc. Rats with HT were injected
with 109 vp of empty adenoviral vector (Model+NC group,
n=9) or adenoviral expression vector carrying the Nampt gene
(Model+Nampt group, n=9) through tail vein for 3 days. One week
later, thyroid tissues and serum samples were collected for
testing.
Hematoxylin and eosin (H&E)
staining
Thyroid tissues were fixed in formalin, and then
embedded in paraffin. After deparaffinization and rehydration, the
sections were stained with hematoxylin solution for 3 min followed
by differentiation in acid ethanol for 15 sec. Following rinsing in
distilled water, the sections were then stained with eosin solution
(Boster) for 3 min, dehydrated with graded alcohol, and cleared in
xylene. The slides were observed under an Olympus CX41 microscope
(magnification, x200; Olympus, Tokyo, Japan).
Immunohistochemistry (IHC)
Sections were baked at 65˚C for 2 h, followed by
incubation with xylene for 20 min and graded ethanol for 25 min.
High temperature and high pressure citrate buffer was used to
retrieve antigen. Endogenous peroxidase activity was quenched by
incubation with 3% H2O2 at room
temperature for 10 min. BSA (5%) was added to the sections to block
nonspecific staining. Antibodies against Nampt (cat. no. DF6059;
Affinity Biosciences) and TLR4 (cat. no. bs-20594R; Beijing
Biosynthesis Biotechnology Co., Ltd.) were diluted at 1:200, and
the sections were incubated with the primary antibodies at 4˚C
overnight. The secondary antibody (cat. no. ZB-2301; ZSBIO) was
used at a 1:100 dilution. The sections were incubated with the
secondary antibody at 37˚C for 30 min. After washing with PBS, the
sections were incubated with the DAB solution (CWBIO) and then
counterstained with hematoxylin (Boster).
Reverse transcriptionquantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (CWBIO)
and purified with an Ultrapure RNA Kit (CWBIO). The following
primers were used in the present study: Nampt,
5'-ATGCCGTGAAAAGAAGACAG-3' (forward) and 5'-TCCAGTTGGTGAGCCAGTAG-3'
(reverse); TLR4, 5'-AAGAGTCTAGCCGTCTTCAATC-3' (forward) and
5'-CAGCCAGCAATAAGTATCAGG-3' (reverse); GAPDH,
5'-TACCCACGGCAAGTTCAA-3' (forward) and 5-ACCAGCATCACCCCATTT-3'
(reverse). All primers were synthesized by Sangon Biotech Co., Ltd.
Total RNA was reverse transcribed into cDNA using a HiFiScript cDNA
Synthesis Kit (CWBIO) in accordance with the manufacturer's
instructions. qPCR was performed using UltraSYBR Mixture (CWBIO)
with an initial hold step (95˚C for 10 min) and 40 cycles at 95˚C
for 10 sec, 57˚C for 30 sec and 72˚C for 30 sec. Relative mRNA
expression was analyzed using the
2(-ΔΔCq) method (15).
Western blot analysis
Tissue samples were homogenized using liquid
nitrogen and lysed in RIPA lysis buffer (Applygen Technologies
Inc.) for 30 min. Lysates were then centrifuged at 4˚C for 10 min
at 12,000 x g, and the supernatants were collected. Total proteins
were separated by SDS-polyacrylamide gel electrophoresis, and
transferred onto PVDF membranes (Millipore). The membranes were
incubated with the appropriate primary antibodies, including rabbit
polyclonal anti-Nampt (cat. no. DF6059; Affinity Biosciences;
dilution, 1:500), rabbit polyclonal anti-TLR4 (cat. no. bs-20594R;
Beijing Biosynthesis Biotechnology Co., Ltd.; dilution, 1:1,000)
and mouse monoclonal anti-GAPDH (cat. no. TA-08; ZSBIO; dilution,
1:2,000) at 4˚C overnight, followed by incubation with
HRP-conjugated goat anti-rabbit IgG (H+L) (cat. no. ZB-2301; ZSBIO;
dilution, 1:2,000) or HRP-conjugated goat anti-mouse IgG (H+L)
(cat. no. ZB-2305; ZSBIO; dilution, 1:2,000) at 4˚C for 2 h.
Signals were visualized with the SuperSignal® West Pico
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.), and
the band density was quantified using Quantity One software
(version 4.6.9; Bio-Rad Laboratories, Inc.).
Measurement of anti-thyroglobulin and
anti-thyroid peroxidase antibodies
The thyroglobulin protein and thyroid peroxidase
were purchased from Beijing Biosynthesis Biotechnology Co., Ltd.
Anti-thyroglobulin antibodies (TGAb) and anti-thyroid peroxidase
antibodies (TPOAb) in serum were assessed by enzyme-linked
immunosorbent assay on an automatic chemiluminescence immunoassay
instrument (ADVIA Centaur CP, Siemens Medical Solutions
Diagnostics), in accordance with the manufacturer's guidelines.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp.), and the data are presented as means ± SD.
Comparisons of data between groups were made using one-way analysis
of variance (ANOVA) followed by Bonferroni's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Construction of an HT model in
rats
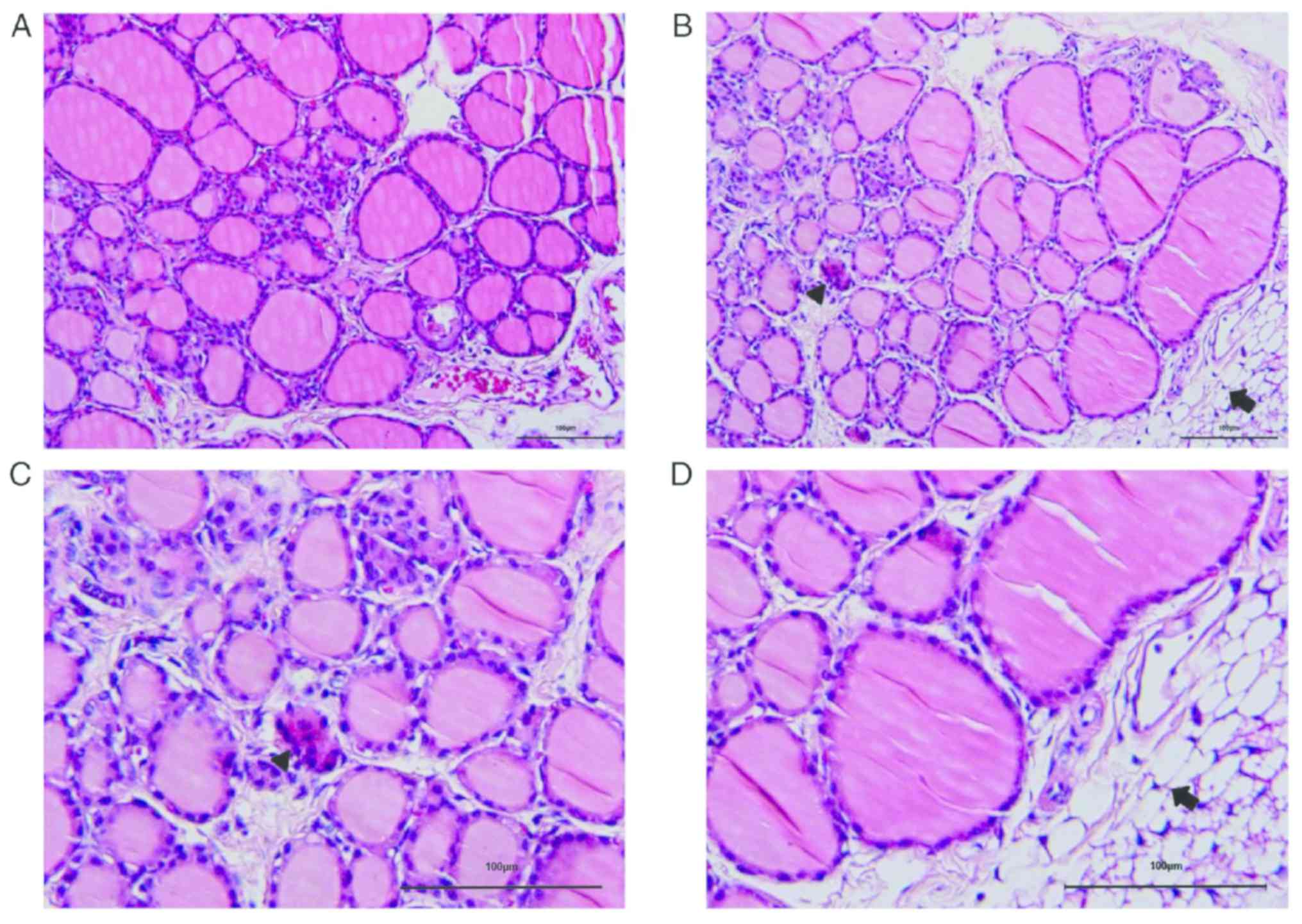
H&E staining of thyroid tissues are shown in
Fig. 1. Rats in the control group
(Fig. 1A) displayed intact and
even-distributed thyroid follicles, and lymphocyte infiltration was
barely presented in the thyroid tissues. The pathological grading
of the sections in the model group was grade I. It was shown that
the thyroid follicles in the HT rats were disordered and destroyed,
and lymphocyte infiltration was observed around the thyroid
follicles (Fig. 1B-D).
Expression of Nampt in the thyroid
tissues of the HT rats
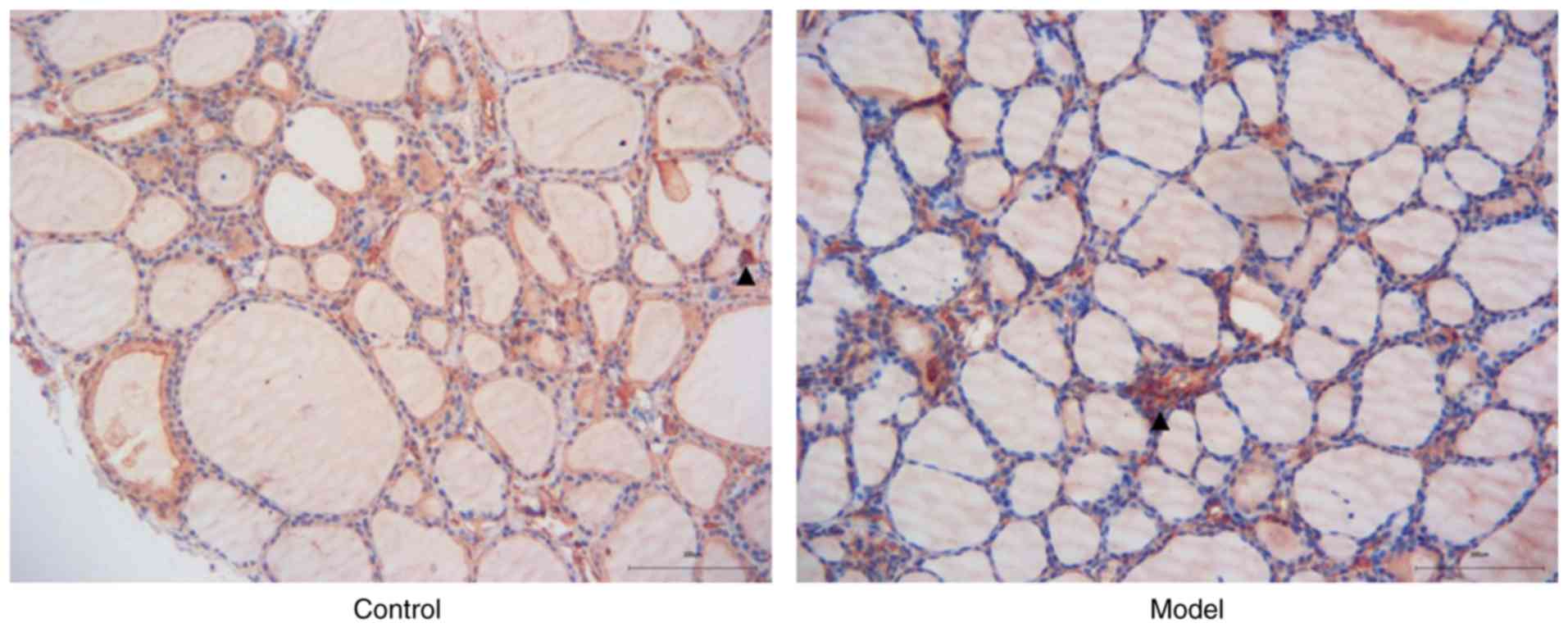
The rats were injected with porcine thyroglobulin to
induce HT model, and IHC was performed to detect Nampt protein in
the thyroid tissues of HT rats. As shown in Fig. 2, Nampt was widely expressed in
thyroid follicles and blood cells. The immunological staining of
inflammatory infiltrating cells was obviously stronger in the model
group than that noted in the control group.
Effect of Nampt on TLR4 expression in
HT rats
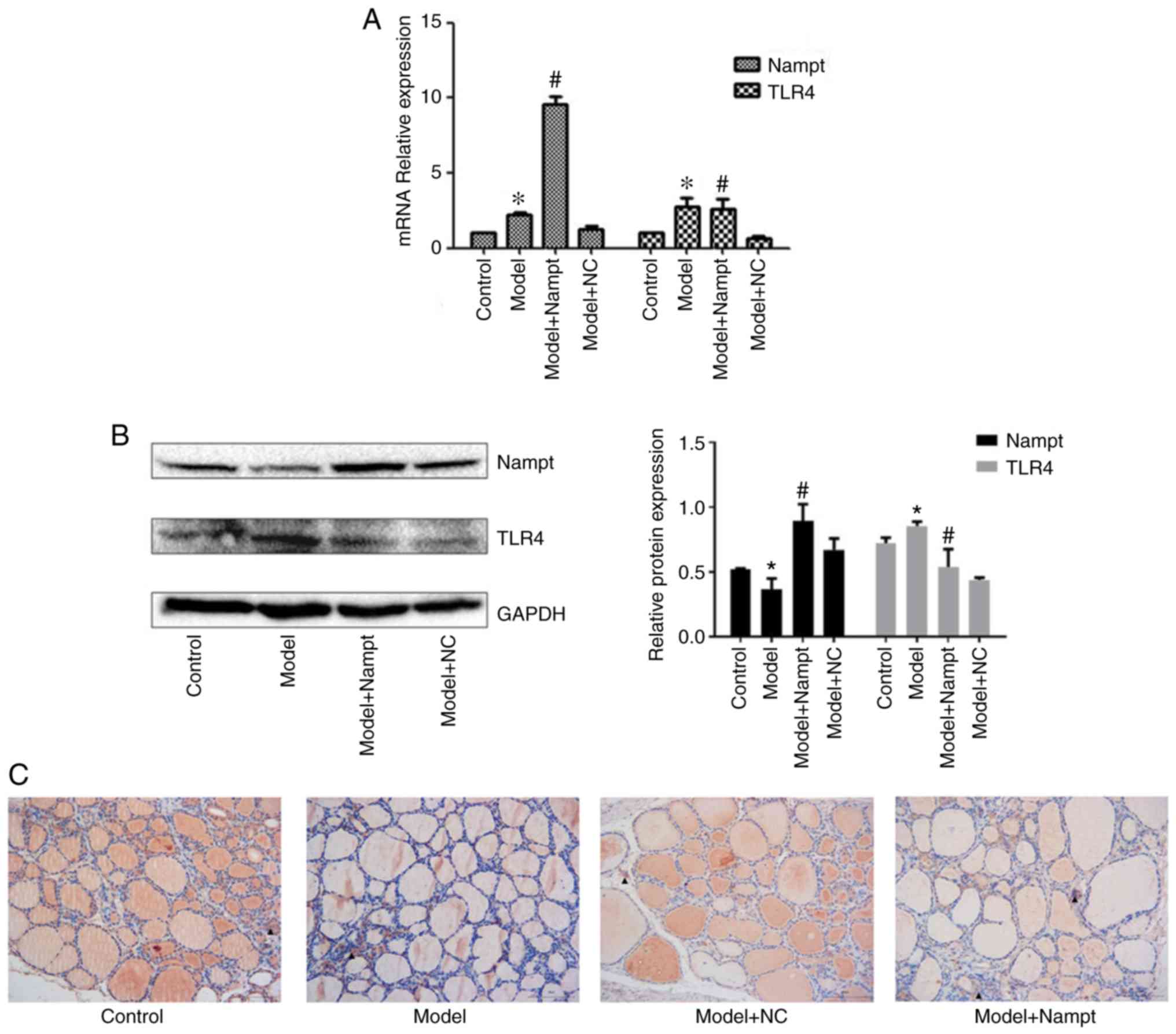
We examined Nampt mRNA and protein levels in the
thyroid tissues using RT-qPCR and western blot analyses. The
Nampt mRNA level was significantly increased in rats with HT
compared with the control rats; however, the Nampt protein level
was decreased in the model group. Compared with the HT rats
injected with empty adenoviral vector, we found Nampt expression
was significantly increased both at the mRNA level and the protein
level in the HT rats injected with adenoviral expression vector
carrying the Nampt gene (Fig. 3A and
B).
To evaluate the effect of Nampt on TLR4 expression,
the sections of rat thyroid tissues were stained with an antibody
against TLR4. It was shown that the thyroid tissues had more
extensive follicular atrophy and collapse, as well as more serious
inflammatory infiltration in the model+Nampt group than in the
model+NC group. TLR4 was mainly expressed in monocytes/macrophages
and endothelial cells. We found that TLR4 was more strongly
positive in the model+Nampt group than in the model+NC group
(Fig. 3C). In addition, we examined
TLR4 mRNA and protein levels in the thyroid tissues. The results
revealed that TLR4 mRNA and protein levels were significantly
upregulated in the rats with HT compared with the control rats.
Nampt overexpression resulted in increased TLR4 mRNA and protein
levels in rats with HT (Fig. 3A and
B) compared with the model+NC
group.
Effect of Nampt on TGAb and TPOAb in
HT rats
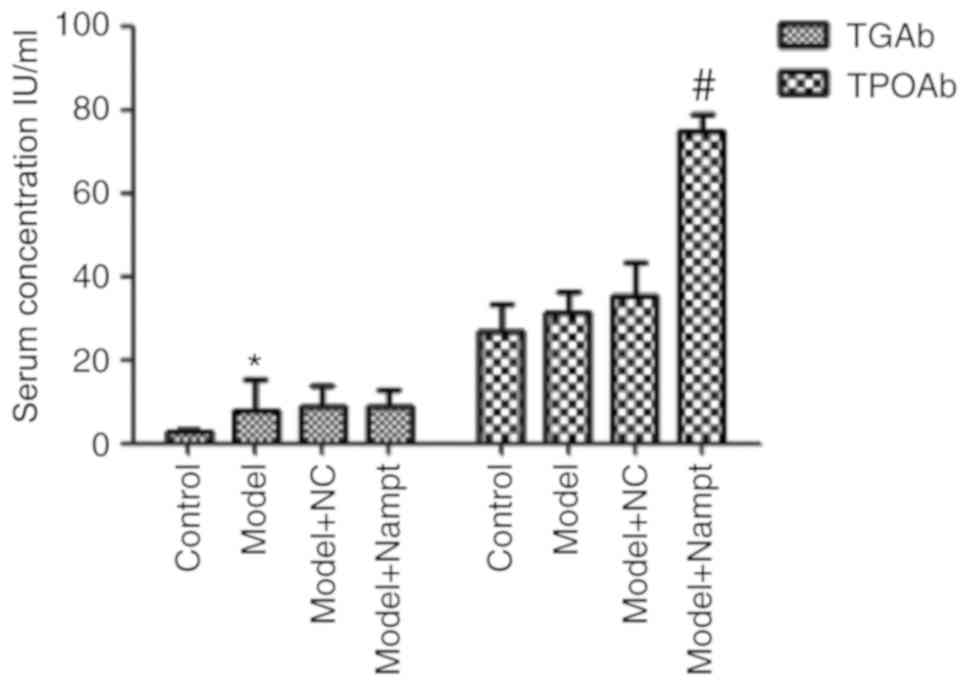
We detected serum concentrations of TGAb and TPOAb
using chemiluminescence method. As shown in Fig. 4, the TGAb level was significantly
higher in the model group than in the control group, while no
difference in TPOAb level was detected between the two groups.
Moreover, we found there was a significant increase in TPOAb level
in the model+Nampt group compared with that in the model+NC group;
however, we did not observe a change in TGAb level between the two
groups.
Discussion
Nampt is involved in many important biological
processes, including survival, apoptosis, metabolism and
angiogenesis (16). As a cytokine,
an elevated concentration of Nampt has been observed in several
inflammatory and autoimmune disorders, such as sepsis,
atherosclerosis, systemic lupus erythematosus and Crohn's disease
(17-20).
Previous studies have demonstrated an elevated plasma Nampt
concentration in Hashimoto's thyroiditis (HT) patients (14). Nampt is present in plasma to behave
both as a cytokine and an adipokine. The blood brings Nampt to all
tissues of the body. In the present study, adenoviral expression
vector carrying the Nampt gene was injected into rats
through the tail vein to elevate the Nampt level in plasma.
Elevated Nampt was transported to the thyroid gland by the blood to
exert its local effects. The administration method used in this
study could mimic the effect of Nampt on HT in human bodies. In
this study, we found that Nampt was strongly expressed in the
capillary region of HT rat thyroid tissues. The Nampt mRNA
level was increased but the Nampt protein level was decreased in
the thyroid tissues of rats with HT. Nampt has two different forms:
intranuclear and secreted Nampt. In the thyroid tissues of the
model group, the staining of Nampt in the capillary region of
thyroid tissues was stronger in the model group than in the control
group, suggesting that Nampt is more likely to enter the blood in a
secreted form. The elevated secreted Nampt may lead to the
decreased Nampt protein level in the thyroid tissues. Our
hypothesis was consistent with a previous study reporting that
plasma Nampt concentration was elevated in HT patients (14).
Autoimmune thyroid diseases are
characterized by antibodies against thyroglobuline
and thyroid peroxidase. Positive serum TPOAb and TGAb
are found in the majority of HT patients. In the present study, an
HT animal model was established by injecting porcine thyroglobulin.
We found inflammatory infiltration in the thyroid tissues of the HT
rats; however, only TGAb was significantly increased in the model
group, suggesting that there were some differences between the
animal model and HT subjects. This may be due to the fact that the
thyroid injury was mild in the model group, and the disease
duration of the model group was short. Furthermore, Nampt
overexpression was demonstrated to lead to increased severity of
inflammatory infiltration in thyroid tissues as well as increased
TPOAb levels in the serum of HT rats; however, the serum TGAb level
was not affected by Nampt overexpression. TPOAb is a diagnostic
marker of HT, and TPOAb could cause thyroid cell damage through
antibody-complement system-mediated T cell activation (21-23).
Nampt overexpression led to the increased serum TPOAb level in HT
rats, indicating that Nampt promotes HT progression. However, TGAb
did not increase after Nampt overexpression, suggesting that
thyroid follicle cells may not be attacked by Nampt
overexpression.
Toll-like receptor 4 (TLR4) is a member of the TLR
family which plays a key role in the innate immune system. TLR4
triggers inflammatory response, leading to NF-κB (NF-κB) activation
and cytokine secretion (24-26).
In periodontal ligament cells, there was a positive correlation
between TLR2/4 and Nampt expression (27). A study by Camp et al reported
that TLR4 is a receptor for Nampt; Nampt induces lung NF-κB
transcriptional activities and inflammatory injury via direct
ligation of TLR4(28). In the
present study, compared with the control rats, the mRNA and protein
expression levels of TLR4 were significantly increased in rats with
HT. Nampt overexpression promoted TLR4 expression in HT rats. It
has been reported that TLR4 protein expression is regulated by many
other factors in the inflammatory process, such as HMGB1,
hyaluronan fragments, HSP60 and fibrinogen (29-32).
This study demonstrated that TLR4 expression was also regulated by
Nampt.
In the present study, the rats in the Model+NC group
were injected with empty adenoviral vector. It has been reported
that bioengineered adenovirus can stimulate the immune system
(33), causing an in vivo
immune response in rats. Therefore, a significant difference
between the Model group and Model+NC group may exist.
Interestingly, we found the difference between the Model+NC and
Model groups was even more significant than that between the
Model+Nampt and Model groups. This result indicated that
intravenous injection of adenovirus inhibited the autoimmune
response to the thyroid gland. Adenovirus particles act as antigens
to induce humoral immune response. Humoral immune response competes
with the autoimmune response to the thyroid gland, leading to a
decrease in autoimmunity.
A previous study reported that the Nampt inhibitor
FK866 modulated T cell-mediated immune responses and thereby was
beneficial in immune-mediated disorders (34). We expected an inhibitory effect of
Nampt silencing on thyroid inflammation and HT progression. Our
further studies may focus on the effect of FK866 on HT in animal
models.
A limitation of this study was that there was a
nonspecific staining of colloid of thyroid follicles, and the
staining influenced the evaluation of Nampt and TLR4 expression.
Therefore, we did not evaluate the relative expression of Nampt and
TLR4 in the IHC study.
In conclusion, this study revealed that Nampt was
strongly expressed in the capillary region of HT rat thyroid
tissues. The Nampt mRNA level was increased but the Nampt
protein level was decreased in the thyroid tissues of rats with HT.
Nampt overexpression had a promotive effect on HT progression, and
this effect was related to TLR4. These results suggest that
inhibition of Nampt activity may be valuable in the treatment of
HT.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Jiangxi Province (grant no. 20171BAB205038) and the
Jiangxi Provincial Department of Education (grant no. 150256).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZT and WQX designed the study. JZT, FJW, YZJ and
XXZ performed the experiments and collected the data. JZT, WQX and
FJW conducted the statistical analysis and participated in data
interpretation. JZT wrote the manuscript, and WQX revised the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All the animal procedures were approved by the
Ethics Committee of The First Affiliated Hospital of Nanchang
University (Nanchang, Jiangxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caturegli P, De Remigis A and Rose NR:
Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun
Rev. 13:391–397. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Umar H, Muallima N, Adam JM and Sanusi H:
Hashimoto's thyroiditis following Graves' disease. Acta Med
Indones. 42:31–35. 2010.PubMed/NCBI
|
|
3
|
Ohye H and Sugawara M: Dual oxidase,
hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood).
235:424–433. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sawicka-Gutaj N, Zybek-Kocik A, Klimowicz
A, Kloska M, Mańkowska-Wierzbicka D, Sowiński J and Ruchała M:
Circulating visfatin in hypothyroidism is associated with free
thyroid hormones and antithyroperoxidase antibodies. Int J
Endocrinol. 2016(7402469)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dahl TB, Holm S, Aukrust P and Halvorsen
B: Visfatin/NAMPT: A multifaceted molecule with diverse roles in
physiology and pathophysiology. Annu Rev Nutr. 32:229–243.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Revollo JR, Grimm AA and Imai S: The
regulation of nicotinamide adenine dinucleotide biosynthesis by
Nampt/PBEF/visfatin in mammals. Curr Opin Gastroenterol.
23:164–170. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee
KW, Kang Y, Li X, Kemper B and Kemper JK: Elevated microRNA-34a in
obesity reduces NAD+ levels and SIRT1 activity by
directly targeting NAMPT. Aging Cell. 12:1062–1072. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Garten A, Schuster S, Penke M, Gorski T,
de Giorgis T and Kiess W: Physiological and pathophysiological
roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 11:535–546.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sawicka-Gutaj N, Zybek-Kocik A, Kloska M,
Czarnywojtek A, Sowiński J, Budny B, Woliński K, Ziemnicka K,
Mańkowska-Wierzbicka D and Ruchała M: Determinants of
visfatin/NAMPT serum concentration and its leukocyte expression in
hyperthyroidism. Horm Metab Res. 50:653–660. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sawicka-Gutaj N, Andrusiewicz M,
Czarnywojtek A, Waligórska-Stachura J, Biczysko M, Skrobisz J,
Sowiński J and Ruchała M: Changes of nicotinamide
phosphoribosyltransferase expressions in thyroid glands of patients
with different thyroid pathologies. Biomed Res Int.
2018(1316390)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sawicka-Gutaj N, Budny B, Zybek-Kocik A,
Sowiński J, Ziemnicka K, Waligórska-Stachura J and Ruchała M:
Nicotinamide phosphoribosyltransferase leukocyte overexpression in
Graves' opthalmopathy. Endocrine. 53:497–504. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ozkaya M, Sahin M, Cakal E, Yuzbasioglu F,
Sezer K, Kilinc M and Imrek SS: Visfatin plasma concentrations in
patients with hyperthyroidism and hypothyroidism before and after
control of thyroid function. J Endocrinol Invest. 32:435–439.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han J, Zhang TO, Xiao WH, Chang CQ and Ai
H: Up-regulation of visfatin expression in subjects with
hyperthyroidism and hypothyroidism is partially relevant to a
nonlinear regulation mechanism between visfatin and
tri-iodothyronine with various concentrations. Chin Med J (Engl).
125:874–881. 2012.PubMed/NCBI
|
|
14
|
Li JB and Li YB: The relationship between
visfatin and autoimmune thyroid disease. Labeled Immunoassays Clin
Med. 17:355–358. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bi TQ and Che XM: Nampt/PBEF/visfatin and
cancer. Cancer Biol Ther. 10:119–125. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chung CP, Long AG, Solus JF, Rho YH, Oeser
A, Raggi P and Stein CM: Adipocytokines in systemic lupus
erythematosus: Relationship to inflammation, insulin resistance and
coronary atherosclerosis. Lupus. 18:799–806. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Y, Ke J, Peng C, Wu F and Song Y:
microRNA-300/NAMPT regulates inflammatory responses through
activation of AMPK/mTOR signaling pathway in neonatal sepsis.
Biomed Pharmacother. 108:271–279. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kong YY, Li GQ, Zhang WJ, Hua X, Zhou CC,
Xu TY, Li ZY, Wang P and Miao CY: Nicotinamide
phosphoribosyltransferase aggravates inflammation and promotes
atherosclerosis in ApoE knockout mice. Acta Pharmacol Sin.
40:1184–1192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Neubauer K, Bednarz-Misa I,
Walecka-Zacharska E, Wierzbicki J, Agrawal A, Gamian A and
Krzystek-Korpacka M: Oversecretion and overexpression of
nicotinamide phosphoribosyltransferase/pre-B colony-enhancing
factor/visfatin in inflammatory bowel disease reflects the disease
activity, severity of inflammatory response and hypoxia. Int J Mol
Sci. 20(pii: E166)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dayan CM and Daniels GH: Chronic
autoimmune thyroiditis. N Engl J Med. 335:99–107. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grani G, Carbotta G, Nesca A, D'Alessandri
M, Vitale M, Del Sordo M and Fumarola A: A comprehensive score to
diagnose Hashimoto's thyroiditis: A proposal. Endocrine.
49:361–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ehlers M, Thiel A, Bernecker C, Porwol D,
Papewalis C, Willenberg HS, Schinner S, Hautzel H, Scherbaum WA and
Schott M: Evidence of a combined cytotoxic thyroglobulin and
thyroperoxidase epitope-specific cellular immunity in Hashimoto's
thyroiditis. J Clin Endocrinol Metab. 97:1347–1354. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397.
1997.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Arbour NC, Lorenz E, Schutte BC, Zabner J,
Kline JN, Jones M, Frees K, Watt JL and Schwartz DA: TLR4 mutations
are associated with endotoxin hyporesponsiveness in humans. Nat
Genet. 25:187–191. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Tatematsu M, Yoshida R, Morioka Y, Ishii
N, Funami K, Watanabe A, Saeki K, Seya T and Matsumoto M: Raftlin
controls lipopolysaccharide-induced TLR4 internalization and
TICAM-1 signaling in a cell type-specific manner. J Immunol.
196:3865–3876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nogueira AV, Nokhbehsaim M, Eick S,
Bourauel C, Jäger A, Jepsen S, Cirelli JA and Deschner J:
Regulation of visfatin by microbial and biomechanical signals in
PDL cells. Clin Oral Investig. 18:171–178. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Camp SM, Ceco E, Evenoski CL, Danilov SM,
Zhou T, Chiang ET, Moreno-Vinasco L, Mapes B, Zhao J, Gursoy G, et
al: Unique Toll-like receptor 4 activation by NAMPT/PBEF induces
NFκB signaling and inflammatory lung injury. Sci Rep.
5(13135)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun S, He M, VanPatten S and Al-Abed Y:
Mechanistic insights into high mobility group box-1 (HMGb1)-induced
Toll-like receptor 4 (TLR4) dimer formation. J Biomol Struct Dyn.
37:3721–3730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Taylor KR, Trowbridge JM, Rudisill JA,
Termeer CC, Simon JC and Gallo RL: Hyaluronan fragments stimulate
endothelial recognition of injury through TLR4. J Biol Chem.
279:17079–17084. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Swaroop S, Sengupta N, Suryawanshi AR,
Adlakha YK and Basu A: HSP60 plays a regulatory role in
IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J
Neuroinflammation. 13(27)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Smiley ST, King JA and Hancock WW:
Fibrinogen stimulates macrophage chemokine secretion through
Toll-like receptor 4. J Immunol. 167:2887–2894. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Alba R, Bosch A and Chillon M: Gutless
adenovirus: Last-generation adenovirus for gene therapy. Gene Ther.
12 (Suppl 1):S18–S27. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bruzzone S, Fruscione F, Morando S,
Ferrando T, Poggi A, Garuti A, D'Urso A, Selmo M, Benvenuto F, Cea
M, et al: Catastrophic NAD+ depletion in activated T
lymphocytes through Nampt inhibition reduces demyelination and
disability in EAE. PLoS One. 4(e7897)2009.PubMed/NCBI View Article : Google Scholar
|