Introduction
Radiation-induced liver damage (RILD) refers to a
series of pathophysiological changes that occur as a result of
liver cell damage caused by exposure of the liver to a certain dose
of radiation (1). RILD is a common
complication of radiotherapy for abdominal tumors. In most cases,
early acute RILD may be repaired. However, if clinical symptomatic
treatment is not administered, it may continue to develop into
subacute RILD, eventually leading to liver failure with a high rate
of mortality (2). Therefore, early
detection and diagnosis of acute RILD are of great significance for
restorative treatment of the liver.
Several studies have explored effective ways to
diagnose and treat early-stage RILD, including the establishment of
animal models of RILD and investigation of/using certain imaging
methods. However, previous imaging studies using modalities
including CT, MRI or single-photon emission CT, focused mainly on
the discovery of morphological changes associated with RILD and
were limited by the duration of the disease, and accordingly, their
clinical application is currently limited (3,4).
Previous experimental studies on RILD in animals have demonstrated
that contrast-enhanced ultrasound (CEUS) is able to directly
reflect changes in the circulating dynamics of microvessels in
early-stage RILD (5). The aim of the
present study was therefore to assess the performance of CEUS in
the quantitative evaluation of microcirculation changes in the
liver parenchyma of patients with acute RILD, so as to explore
effective diagnostic parameters.
Materials and methods
Participants
Patients who had undergone radiotherapy for the
treatment of abdominal malignant tumors at the Second Hospital of
Wuxi Affiliated to Nanjing Medical University (Wuxi, China) between
August 2015 and August 2018 were recruited for the present study. A
total of 32 patients were selected, including 21 males and 11
females aged 52-86 years (mean age, 68.8±10.6 years). Five cases of
pancreatic head carcinoma, 7 of pancreatic body and tail tumor, 8
of gastric cancer following radiotherapy and 12 of abdominal
metastatic lymph node treated with radiotherapy were enrolled in
the present study. The tumor diameters were 3-6 cm and pancreatic
cancer stages were IB-IIB (6). The
exclusion criteria were as follows: i) Imaging examination prior to
radiotherapy indicated diffuse liver lesions or benign or malignant
occupying lesions in the liver; and ii) serological examination
prior to radiotherapy suggested abnormal liver function.
The grouping criteria were as follows: According to
the time of radiotherapy received for the treatment of malignant
abdominal tumors, the patients were divided into 4 groups: i) Prior
to radiotherapy; ii) 2 weeks after radiotherapy; iii) 3 weeks after
radiotherapy; and iv) 4 weeks after radiotherapy.
Instruments and methods
The following instruments were used in the present
study: A Primus linear accelerator (6MV-X; Siemens AG) and a LOGIQ
E9 ultrasound instrument (GE Healthcare) with a low mechanical
index enhancement mode, time-intensity curve (TIC) analysis
software and convex array probe (frequency, 1-5 MHz). The contrast
medium was SonoVue (Bracco Imaging SpA).
A CT simulator was used for positioning and an
XIO4.80 treatment planning system (Elekta Instrument AB) was used
to delineate the target areas (gross tumor volume, clinical target
volume and planning target volume) and design the exposure field.
The entire liver was delineated according to the endangered organs,
followed by exposure of 4-5 fields to conformal radiotherapy, in
which the dose was divided conventionally, with an exposure dose
rate of 200 cGy/min.
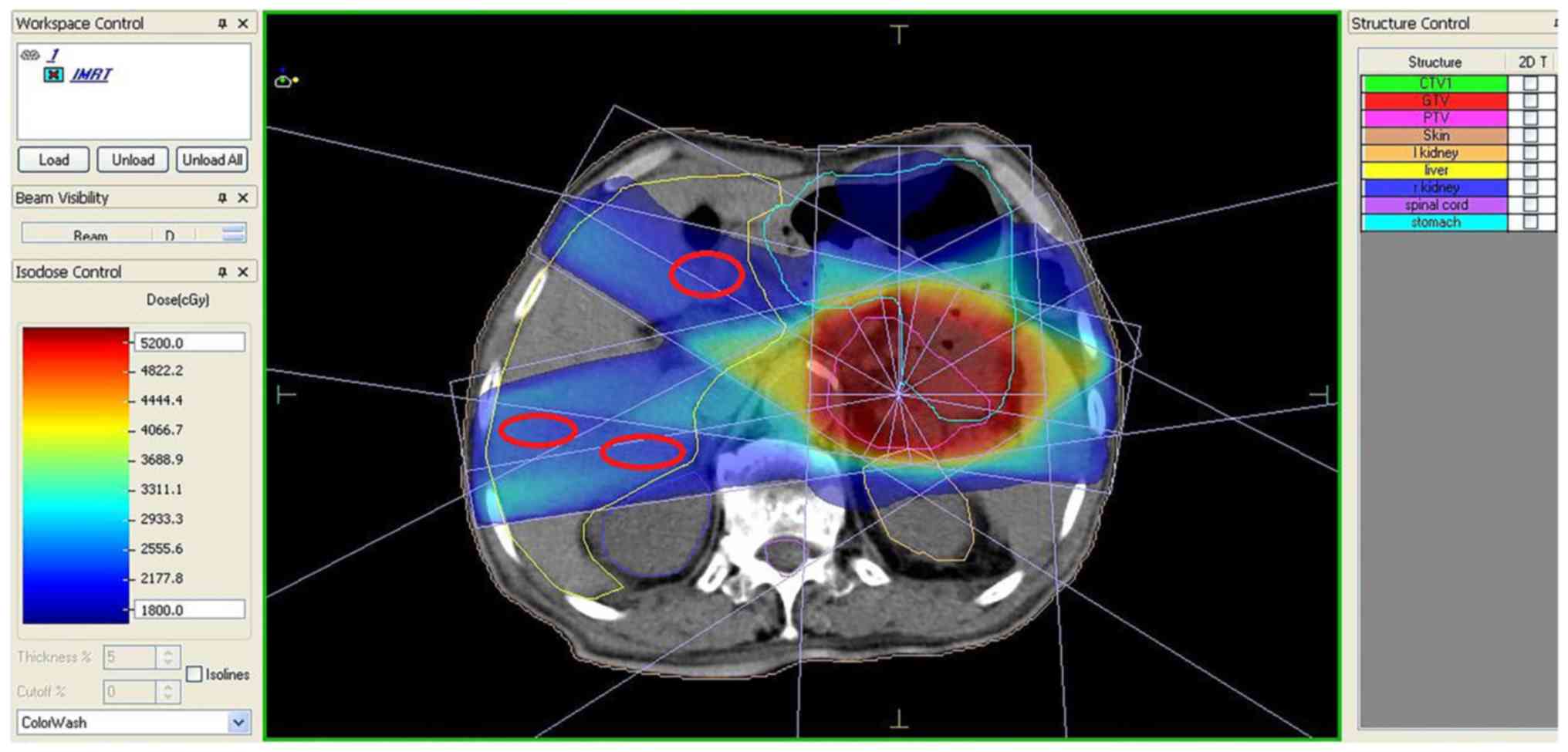
The exposure doses of the liver were evaluated by a
dose-volume histogram (Fig. 1) and
the areas with exposure doses of 2,400-2,600 cGy/25-30 f were
selected for ultrasonography, according to the isodose curve
(Fig. 2).
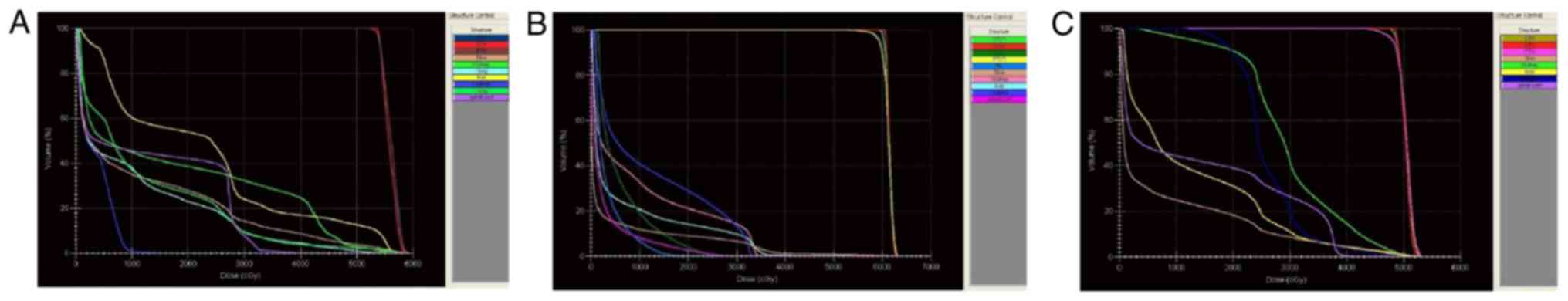 | Figure 1.DVHs of three different patients.
X-axis, total irradiation dose; Y-axis, organ exposure volume. The
key chart on the right denotes different organs with varying colors
and the yellow line refers to the DVH of the liver after exposure.
(A) Radiotherapy for pancreatic head carcinoma. According to the
DVH diagram, the volume of liver irradiation at the radiation dose
of 2,500 cGy was ~48%. (B) Radiotherapy for left abdominal lymph
node metastasis. According to the DVH diagram, the volume of liver
irradiation at the radiation dose of 2,500 cGy was ~12%. (C)
Radiotherapy for pancreatic tail tumor. According to the DVH
diagram, the volume of liver irradiation at the radiation dose of
2,500 cGy was ~18%. DVH, dose-volume histogram; GTV, gross tumor
volume; CTV, clinical target volume; PTV, planning target
volume. |
Two-dimensional and color Doppler ultrasound was
used to examine the liver prior to CEUS to observe the liver
parenchyma and intrahepatic vessels. According to the isodose
curve, suitable areas in the hepatic parenchyma were selected,
avoiding the large blood vessels, and CEUS was performed after
fixing the section. A bolus of SonoVue suspension (2.4 ml) was
injected via the elbow vein using a 16G intravenous indwelling
needle, following which 5 ml normal saline was injected. The
contrast enhancement mode was turned on and the timer was triggered
at the time of contrast medium injection. The dynamic data were
acquired over 180 sec and stored in digital imaging and
communications in medicine (DICOM) format for offline analysis.
Data from all patients were analyzed using the same
sonographer, who has >15 years of experience in
contrast-enhanced ultrasound. A circular region of interest (ROI;
diameter, 5 mm) was selected in the preselected parenchyma region
of the isodose curve. For each patient, the incision section for
CEUS was kept consistent as much as possible. The TIC of the liver
parenchyma was obtained using the software complementary to the
instrument. The time until the appearance of the first contrast
agent microbubble in the liver was defined as the arrival time, and
the time-point 160 sec after that as the end time, during which the
hepatic blood perfusion was analyzed. In addition, the time to peak
(TTP), gradient (Grad), and area under the curve (AUC) of the
contrast in the liver parenchyma of the ROI were analyzed to
determine the hemodynamic changes in the liver.
Statistical analysis
Data were statistically analyzed using SPSS 20.0
software (IBM Corp.). Measurement data following a normal
distribution are expressed as the mean ± standard deviation. The
changes in the three indicators at each time-point were compared
using single-factor repeated-measures analysis of variance and the
changes prior to and after the exposure were compared using paired
t-tests.
Results
General information
The present study included 32 patients that had
undergone radiotherapy for abdominal tumors, conformed to the
inclusion criteria and had stage IB-IIB pancreatic cancer. CEUS was
performed on the liver of each patient prior to, as well as 2, 3
and 4 weeks after the exposure. The complete information obtained
with CEUS was stored in DICOM. The TTP, Grad and AUC were analyzed
and expressed by the TIC, which was obtained from the quantitative
analysis of CEUS, so as to explore potential diagnostic parameters
for RILD.
Ultrasonography results
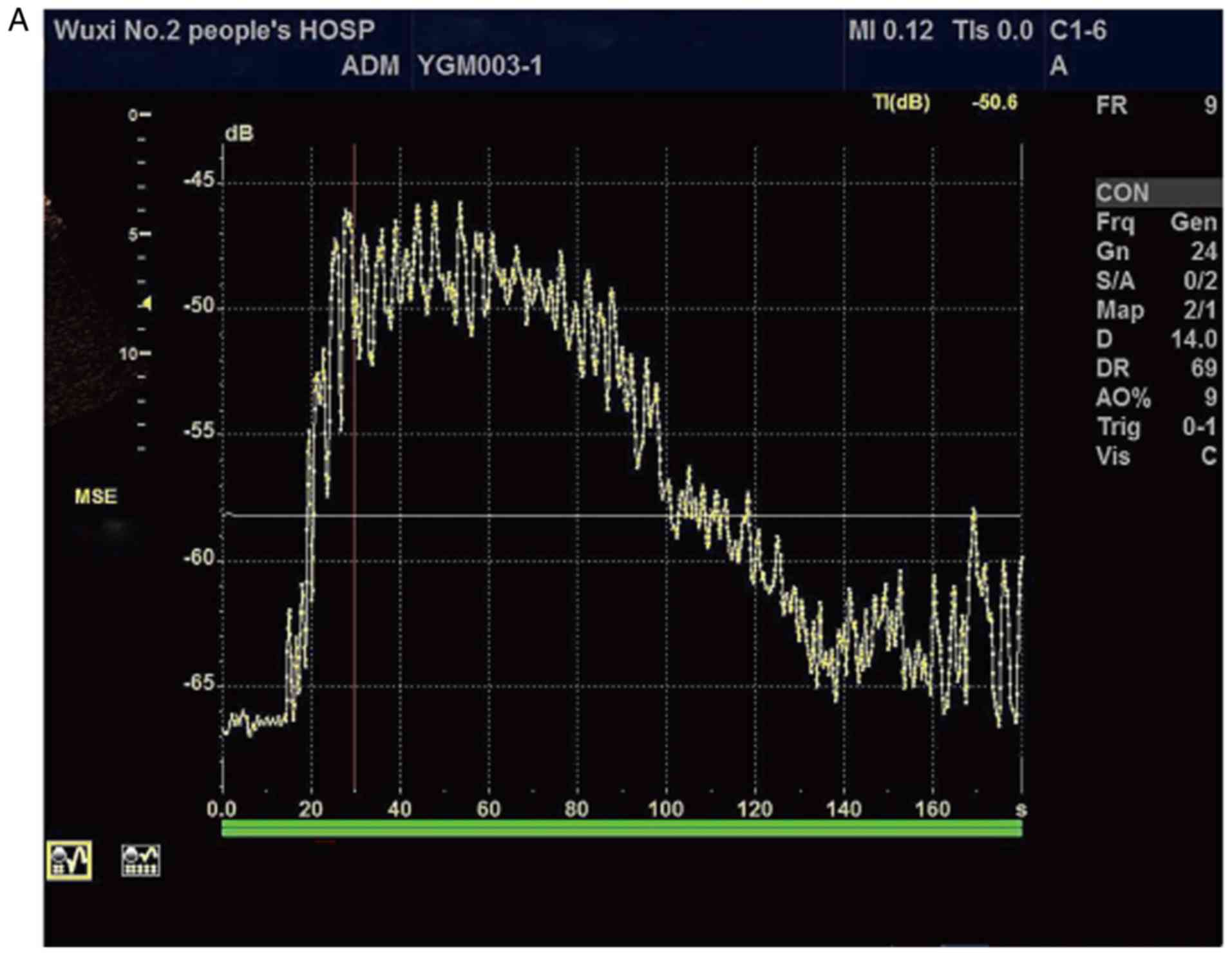
CEUS was successfully performed on all patients. The
TIC obtained by quantitative analysis is presented in Fig. 3, with the yellow curve representing
the perfusion curve of the contrast in the liver parenchyma. Under
normal liver tissue conditions, liver parenchyma enhancement caused
by hepatic artery alone was performed for 10-20 sec after the
contrast agent was injected into the peripheral vein for 10-15 sec.
Subsequently, the portal vein phase was initiated, which occurred
for 2 min after the contrast agent was injected. When contrast
microbubbles began to clear from the liver parenchyma, it entered
the delayed phase, which occurred from 4-6 min after the injection
of SonoVue. Hence, in the present study, the TIC presented as a
single-peak curve with a steep upward rise and a slow decline after
a short plateau. With the aggravation of liver damage, the rise and
fall of TIC gradually slowed down and the peak value gradually
decreased. Consequently, the TTP was gradually delayed and the Grad
decreased slowly.
Results of TIC analysis
The TIC of the current study presented a single-peak
curve with a steep upward rise and a slow decline after a short
plateau. With the aggravation of liver damage, the rise and decline
of TIC gradually slowed and the peak value gradually decreased.
Consequently, the TTP was gradually delayed and the Grad gradually
decreased. In terms of the three parameters of the TIC, significant
differences were identified in the Grad, TTP and AUC of the 2-, 3-
or 4-week groups, as compared with those prior to exposure
(P<0.05); the differences among the 2-, 3- and 4-week groups
were also statistically significant (P<0.05). Compared with that
prior to exposure, the AUC decreased at 2 and 3 weeks, but
increased at 4 weeks after the exposure (Table I).
 | Table IComparison of time-intensity curve
parameters in quantitative analysis of contrast-enhanced
ultrasonography for RILD. |
Table I
Comparison of time-intensity curve
parameters in quantitative analysis of contrast-enhanced
ultrasonography for RILD.
| Time-point | TTP (sec) | Grad | AUC |
|---|
| Pre-exposure | 14.371±2.491 | 2.145±0.160 | 3,220.75±128.381 |
| 2 weeks after
exposure |
18.723±3.096a |
1.555±0.090a |
2,992.91±104.907a |
| 3 weeks after
exposure |
23.086±3.395a,b |
1.038±0.088a,b |
2,787.66±101.623a,b |
| 4 weeks after
exposure |
30.174±2.017a-c |
0.737±0.050a-c |
3,248.78±87.264a-c |
AST and ALT results
Serological examination of 32 patients revealed that
alanine aminotransamine (ALT) and aspartate transaminase (AST)
increased gradually when the irradiation time was prolonged. There
was a statistically significant difference in ALT and AST values of
the 3- and 4-week groups, as compared with those prior to the
exposure (P<0.05). There was no statistically significant
difference between the 2-week group and the pre-exposure group
(Table II).
 | Table IIAST and ALT levels in patients. |
Table II
AST and ALT levels in patients.
| Groups | ALT (U/l) | AST (U/l) |
|---|
| Pre-exposure | 24.44±11.68 | 25.63±10.39 |
| 2 weeks after
radiotherapy | 25.13±12.17 | 28.52±12.94 |
| 3 weeks after
radiotherapy |
30.30±10.20a |
29.73±10.76a |
| 4 weeks after
radiotherapy |
30.79±11.73a |
31.26±13.55a |
Discussion
In previous years, three-dimensional conformal
intensity-modulated radiotherapy has been widely used for the
treatment of abdominal malignant tumors; however, post-radioactive
liver damage has become the most important factor affecting their
radiation dose tolerance (7).
Therefore, the early detection and diagnosis of acute RILD are of
great significance for the restorative treatment of the liver. At
present, the common examination methods for RILD are CT and MRI,
but there is no specific imaging manifestation of early-stage RILD
on CT and MRI (3,4). CEUS displays the microcirculation of
tissues and organs and the imaging effect is consistent with that
of enhanced CT and MRI. To the best of our knowledge, no previous
studies have assessed RILD in the hepatic microcirculation of the
perfused liver, as previous study has only focused on liver tumors,
cirrhosis and liver reperfusion injury (8).
In the present study, Grad, TTP and AUC were
selected as the quantitative analysis parameters for CEUS. TTP is
an index for the velocity of blood flowing in and out of the
tissues, Grad reflects the average perfusion rate of the tissue and
the AUC is associated to blood volume and flow velocity in the
region. The results suggested that with the aggravation of liver
injury, the liver parenchyma Grad exhibited a downward trend, the
TTP gradually increased and the AUC decreased at 2 and 3 weeks, but
increased at 4 weeks after the exposure. Based on the present
results, it may be concluded that CEUS is able to reflect the
changes of hepatic perfusion in patients with early-stage RILD.
Timely and accurate assessment of the existence of RILD is of great
significance for the selection of clinical treatment plans, disease
monitoring and clinical prognosis.
Previous studies have indicated that typically, RILD
pathologically manifests as a hepatic veno-occlusive disease
(9). It is characterized by diffuse
swelling and degeneration of liver cells, accompanied by necrosis
and bleeding of liver cells, a significant increase of inflammatory
cells in the portal area, as well as occlusion and congestion of
the hepatic sinus and central venous lobule. Early lesions of RILD
are mainly hepatic sinus congestion and inflammatory cell exudation
following injury of sinusoidal endothelial cells. In the present
study, CEUS was able to clearly reflect the changes in hepatic
perfusion. The reasons may be as follows: i) In the early stage of
acute RILD, the liver parenchyma undergoes fatty degeneration and
the hepatocytes gradually swell, resulting in the compression and
degeneration of the intrahepatic lumen structure, and a persistent
decrease in blood flow perfusion in the liver parenchyma; ii) as
liver injury progresses, infiltration of inflammatory cells in the
liver parenchyma occurs, hepatocytes undergo degeneration and
necrosis, and the inflammatory and necrotic tissues block the
microcirculation pathway of the liver. At the same time, hepatic
sinus congestion and inflammatory cell exudation caused by hepatic
sinusoidal endothelial cell injury may cause narrowing and
obstruction of the hepatic sinusoids, decreasing the blood flow
into the hepatic sinusoids and resulting in a decrease in the blood
volume in the hepatic sinusoids (5,8,10); and iii) further aggravation of liver
injury leads to fibrotic changes in the liver parenchyma. The
increase in the intrahepatic fibrous structure compresses the
peripheral sublobular vein of the liver, central vein and hepatic
sinus, resulting in increased arteriovenous pressure, decreased
blood flow in the microvessels and decreased total blood flow in
the liver (11). The aforementioned
causes lead to a decrease in the Grad of liver parenchyma and an
increase in the TTP, which is gradually aggravated with the
severity of RILD.
The present study also indicated that the AUC
gradually decreased with the severity of liver damage at 2 and 3
weeks, but increased at 4 weeks after the exposure, with
significant differences between the three exposure and the one
pre-exposure group. The reasons may be as follows: i) Hepatic sinus
capillarization, which is similar to continuous capillaries and
manifests as the damage of sinusoidal endothelial cells, reduction
or disappearance of endothelial fenestrae and formation of a
continuous basement membrane under the endothelium, may occur in
the early stage of RILD. This change causes a disturbance in the
exchange of substances and oxygen in the liver cells, eventually
leading to hepatocyte atrophy and hepatic sinus collapse, a
decrease in blood flow in the hepatic sinus and a gradual reduction
of the AUC (8,12); ii) as the liver damage intensifies,
the hepatic sinus occlusion is aggravated, resulting in slow blood
backflow, prolonged blood perfusion time in the liver at the late
stage of the disease and thus an increase of the AUC; iii)
intrahepatic cytokines (e.g. transforming growth factor β1,
connective tissue growth factor, platelet-derived growth factor)
act as a driving factor to promote hepatic stellate cells to
synthesize collagen fibers and accelerate their transformation into
fibroblasts (13). The proliferation
of fibroblasts and their connection with the surrounding fiber
bundles lead to the formation of a fibrous septum. The blood
vessels in the fibrous septum form a communication branch between
the hepatic artery and portal and hepatic veins, which shunts the
blood and causes an upturn in the overall blood flow in the liver.
However, RILD progresses gradually; the cytokines are not
significantly expressed in the early stage and their expression
gradually increases after 2 weeks (11). In the present study, the AUC
gradually decreased at 2 and 3 weeks after the exposure and
significantly increased at 4 weeks.
In conclusion, quantitative analysis of CEUS may be
used to accurately and objectively assess the changes and quantify
the intrahepatic microcirculation perfusion in acute RILD. The TTP,
Grad and AUC may be used as important reference indicators for the
early diagnosis of acute RILD. Therefore, CEUS may be used to
monitor liver hemodynamic changes in tumor patients following
radiotherapy. For patients with abnormal TIC parameters, the
corresponding liver protection treatment should be administered
early and the radiation dose should be adjusted to delay or even
prevent the occurrence and development of RILD. CEUS is expected to
become a novel method for evaluating acute RILD.
Acknowledgements
Not applicable.
Funding
The current study was awarded two grants from the
Wuxi Health Committee of Jiangsu Province (grant nos. YGM1126 and
YGZXM1507) and one grant from the Science and Technology
Development Fund of Nanjing Medical University (grant no.
NMUB2018236).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JF designed the study. TYX and SBC performed the
research. YYG and PS analyzed the data. XC and YZC collected
experimental data, created the database and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of the Second Hospital of Wuxi Affiliated to Nanjing
Medical University (Wuxi, China) and all patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan CC, Kavanagh BD, Dawson LA, Li XA, Das
SK, Miften M and Ten Haken RK: Radiation-associated liver injury.
Int J Radiat Oncol Biol Phys 76. (Suppl 3):S94–S100.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peixoto A, Pereira P, Bessa de Melo R and
Macedo G: Radiation-induced liver disease secondary to adjuvant
therapy for extra-hepatic cholangiocarcinoma. Dig Liver Dis.
49(227)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jung J, Yoon SM, Cho B, Choi YE, Kwak J,
Kim SY, Lee SW, Ahn SD, Choi EK and Kim JH: Hepatic reaction dose
for parenchymal changes on Gd-EOB-DTPA-enhanced magnetic resonance
images after stereotactic body radiation therapy for hepatocellular
carcinoma. J Med Imaging Radiat Oncol. 60:96–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guha C and Kavanagh BD: Hepatic radiation
toxicity: Avoidance and amelioration. Semin Radiat Oncol.
21:256–263. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng J, Chen SB, Wu SJ, Sun P, Xin TY and
Chen YZ: Quantitative analysis of contrast-enhanced ultrasonography
in acute radiation-induced liver injury: An animal model. Exp Ther
Med. 10:1807–1811. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chapman TR, Kumarapeli AR, Nyflflot MJ,
Bowen SR, Yeung RS, Vesselle HJ, Yeh MM and Apisarnthanarax S:
Functional imaging of radiation liver injury in a liver metastasis
patient: Imaging and pathologic correlation. J Gastrointest Oncol.
6:E44–E47. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Da Silveira EB, Jeffers L and Schiff ER:
Diagnostic laparoscopy in radiation-induced liver disease.
Gastrointest Endosc. 55:432–434. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang Y, Chen SW, Fan CC, Ting LL, Kuo CC
and Chiou JF: Clinical parameters for predicting radiation-induced
liver disease after intrahepatic reirradiation for hepatocellular
carcinoma. Radiat Oncol. 11(89)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xia H, Deng L and Jin Y: An experimental
study on acute radiation-induced liver damage and its protection in
rats. J Cancer Control Treat. 24:9–13. 2011.
|
|
12
|
Benson R, Madan R, Kilambi R and Chander
S: Radiation induced liver disease: A clinical update. J Egypt Natl
Canc Inst. 28:7–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee IJ, Seong J, Shim SJ and Han KH:
Radiotherapeutic parameters predictive of liver complications
induced by liver tumor radiotherapy. Int J Radiat Oncol Biol Phys.
73:154–158. 2009.PubMed/NCBI View Article : Google Scholar
|