Introduction
Diabetic nephropathy (DN) is one of most frequent
complications of diabetes, and is the major cause of end-stage
disease in diabetic patients (1). DN
is characterized by mesangial expansion, glomerular extracellular
matrix (ECM) accumulation and renal interstitial fibrosis, and
these pathological changes lead to chronic renal dysfunction
(2). Human mesangial cells (HMCs)
which produce mesangial ECM constituents are located in the
interpapillary space of the glomerular tufts (3).
Ye et al (4)
reported that Norcantharidin could inhibit HMC proliferation and
induce apoptosis in a dose and time-dependent manner. Zhou et
al (5) observed that mevalonate
could stimulate HMC proliferation, increase the expression of Bcl-2
and downregulate the expression of Bax in the HMCs. The present
study demonstrated that Rhein-8-O-β-D-glucopyranoside (Rg) could
significantly inhibit high glucose (HG)-induced HMC apoptosis;
however, the underlying mechanisms were largely unknown.
A previous study demonstrated that transforming
growth factor-β (TGF-β) plays a key role in the progression of DN
(6). Smad2, a member of receptor
Smads, is phosphorylated when TGF-β1 binds to TGF-β receptor.
Smad7, which is an inhibitory Smad, could bind to type I receptors
and prevent phosphorylation of receptor Smads (7). The TGF-β1/Smad signaling pathway was
activated in DN, and could also be induced by HG treatment in HMCs
(8). The present study further
investigated the roles of the TGF-β1/Smad signaling pathway in
Rg-treated HMCs.
In the present study, the roles and mechanisms of Rg
on HG-induced apoptosis of HMCs were examined. The present study
suggested that Rg alleviated HG-induced apoptosis of HMCs, and Rg
increased HG-reduced Bcl-2 expression and decreased HG-induced
caspase-3 expression. Rg inhibited the HG-activated TGF-β1/Smad
signaling pathway by regulating long intervening non-coding RNA
(lincRNA) ANRIL/let-7a expressions.
Materials and methods
Purification of Rg
Rhubarb was purchased from Tong Ren Tang
Technologies Co., Ltd (http://www.tongrentangkj.com). Rg was extracted from
rhubarb according to the method from a previous study (9).
Cell culture and transfection
HMCs were purchased from ScienCell Research
Laboratories, Inc. and cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml) and
streptomycin (100 mg/ml). High glucose culture media was made by
supplementing normal DMEM medium with additional D-glucose at a
final concentration of 25 mM (HG). All of these cells were
maintained at 37˚C with 5% CO2.
Cells were seeded in the six-well plates at a
density of 5x105 cells per well and treated with Rg (20
or 80 µM) or transfected with lincRNA ANRIL small interfering
(siRNA; 50 nM), let-7a mimics (50 nM) or negative control siRNA (50
nM) using Lipofectamine® 3000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and cultured using HG
DMEM medium according to the manufacturer's protocol at 37˚C for 48
h. The lincRNA ANRIL siRNA sequence was 5'-GGUCAUCUCAUUGCUCUAU-3',
and let-7a mimics sequence was 5'-UGAGGUAGUAGGUUGUAUAGUU-3', and
negative control siRNA sequence was 5'-UUCUCCGAACGUGUCACGUTT-3'.
The lincRNA ANRIL siRNA, let-7a mimics and negative control were
synthesized by Shanghai GenePharma Co., Ltd.
CCK-8 assay
Cell proliferation was detected using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.) as
previously described (10).
Flow cytometry assay
Apoptosis was detected using flow cytometry. In
apoptosis assay, harvested cells were double-stained with Annexin V
(room temperature for 15 min) and propidium iodide (PI; room
temperature for 5 min) according to the protocol of a FITC-Annexin
V cell apoptosis assay kit (BD Biosciences). Then the cells were
analyzed using a flow cytometer (FACScan; BD Biosciences) equipped
with CellQuest pro software (v5.2, BD Biosciences).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) assay
RNA of cells was extracted using a Total RNA Rapid
Extraction kit (BioTeke Corporation) according to the
manufacturer's protocol. After detecting the concentration, 1 µg
RNA sample was reverse transcribed into cDNA with M-MLV reverse
transcriptase (BioTeke Corporation) in the presence of oligo(dT)
and 50 random primers (Invitrogen; Thermo Fisher Scientific, Inc.).
The RT procedure is 42˚C for 60 min, and 70˚C for 10 min. The
instruments used for this experiment were pre-treated using surface
RNase Erase (Tiandz, Inc.) and the reagents were RNase-free. The
cDNA (1 µl each reaction) was used for real-time PCR to detect the
gene expressions using 2X Power Taq PCR MasterMix (BioTeke
Corporation) and SYBR Green (Beijing Solarbio Science &
Technology Co., Ltd.), with GAPDH as the internal control. The PCR
procedure was as follows: 95˚C for 10 min, 38 cycles of 95˚C for 12
sec, 60˚C for 18 sec and 72˚C for 30 sec, and finally 4˚C for 5
min. Calculations were performed using the
2-ΔΔCq method (11). The following primer pairs were used
for amplification: ANRIL forward, 5'-GGACTACAGATGCACCACCAT-3',
ANRIL reverse, 5'-TGAGCACTGTGTCCATAGCA-3'; GAPDH forward,
5'-AAATCCCATCACCATCTTCCAG-3', and GAPDH reverse,
5'-GAGTCCTTCCACGATACCAAAGTTG-3'.
Hairpin-it™ let-7a RT-qPCR Primer Set
(Shanghai GenePharma Co., Ltd.) was used for the measurement of the
relative quantity of let-7a. The reaction conditions were as
follows: 95˚C for 4 min, 30 cycles of 95˚C for 30 sec, 57˚C for 30
sec and 72˚C for 30 sec. The mRNA expression of let-7a was
normalized to the endogenous expression of U6. The primer sequences
were as follows: Let-7a forward: 5'-CACCCACCACTGGGAGATAAC-3', and
let-7a reverse, 5'-TATGGTTGTTCACGACTCCTTCAC-3'; U6 forward:
5'-GCTTCGGCAGCACATATACTAAAAT-3', and U6 reverse,
5'-CGCTTC.ACGAATTTGCGTGTCAT-3'.
Western blot analysis
Protein was extracted using a whole-cell lysis kit
(CWBio) from cells and the concentration of protein was measured
using a BCA protein quantitative kit (Beyotime Institute of
Biotechnology). After being denatured by boiling, the protein
sample (40 µg for each lane) was separated by 10% SDS-PAGE and
transferred to a PVDF membrane (EMD Millipore). After blocking with
5% skim milk (Inner Mongolia Yili Industrial Group Co., Ltd.) at
room temperature for 1 h, the membrane was incubated with the
primary antibodies at 4˚C overnight. After rinsing with TBS with
Tween-20, the membrane was incubated with goat anti-rabbit Ig G
labeled with horseradish peroxidase (HRP; cat. no. sc-2004;
1:5,000; Santa Cruz Biotechnologies, Inc.) or goat anti-mouse Ig
G-HRP (cat. no. sc-2005; 1:5,000; Santa Cruz Biotechnologies, Inc.)
at 37˚C for 45 min, and exposed with ECL reagent (Thermo Fisher
Scientific, Inc.). Optical density values of bands were analyzed
using a gel image processing system ImageLab software (version:
3.0, Bio Rad Laboratories, Inc.). The primary antibodies were as
follows: Bcl-2 antibody (cat. no. ab185002; 1:1,000; Abcam);
cleaved caspase-3 antibody (cat. no. ab2302; 1:1,000; Cell
Signaling Technology, Inc.); TGF-β1 antibody (cat. no. ab92486;
1:1,000; Abcam); phosphorylated (p)-Smad2 antibody (cat. no.
ab188334; 1:1,000; Abcam); Smad2 antibody (cat. no. ab33875;
1:1,000; Abcam); Smad7 antibody (cat. no. ab216428; 1:1,000;
Abcam); and β-actin antibody (cat. no. ab179467; 1:1,000;
Abcam).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 6 (GraphPad Software, Inc.). The data in the present study
are presented as the mean ± SD of three or five individual
experiments, and analyzed by one-way ANOVA followed by Tukey's
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
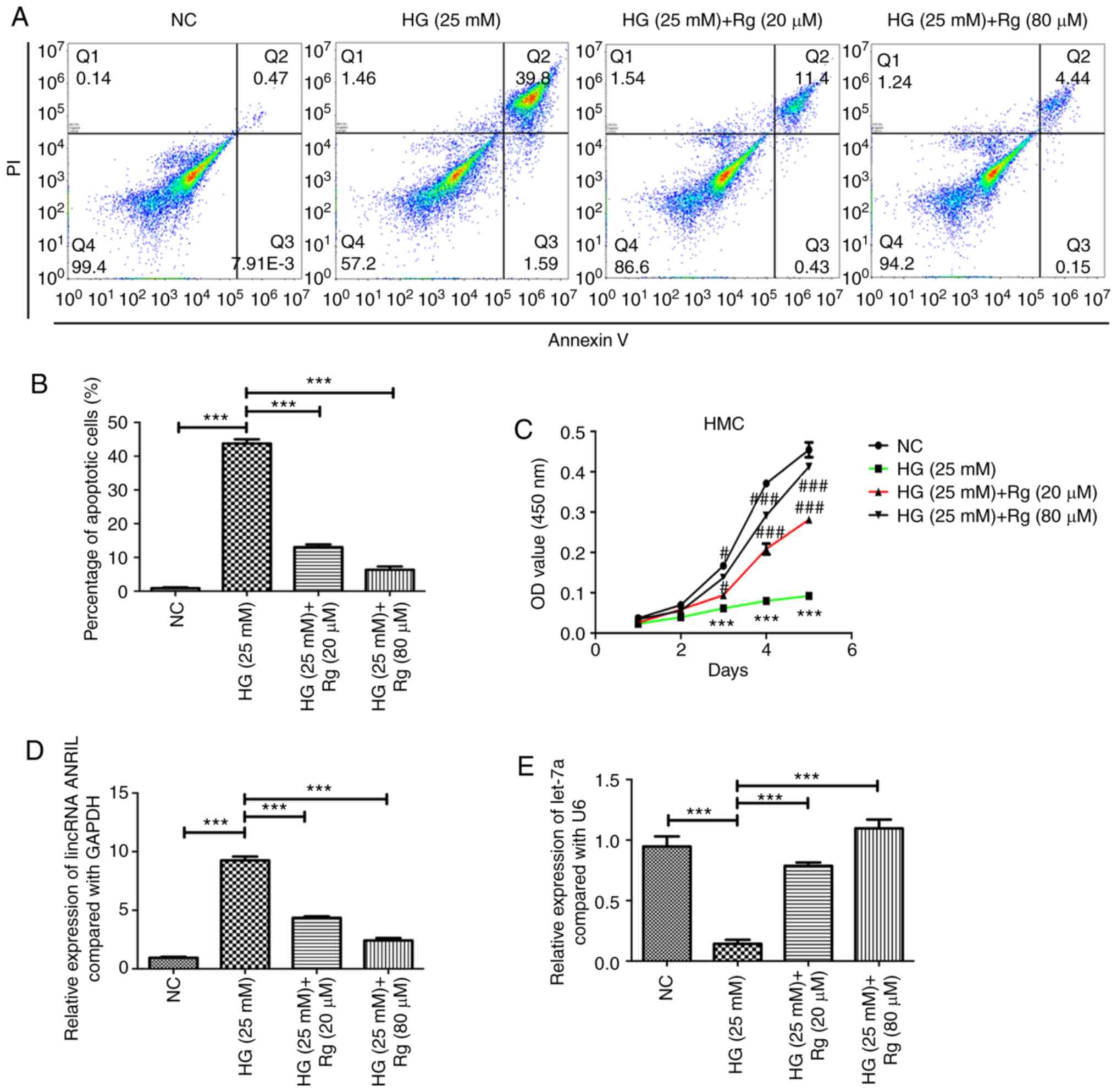
Rg inhibits HG-induced apoptosis and
promotes HG-suppressed growth of HMCs
HMCs were cultured in HG (25 mM) DMEM for 24 h, and
then treated with Rg for 48 h. The results showed that HG
significantly induced the apoptosis of HMCs, and 20 and 80 µM Rg
could both inhibit the apoptosis induced by HG (Fig. 1A and B). Using a Cell Counting Kit-8 assay, it
was also observed that HG significantly suppressed the cell
proliferation of HMCs, and 20 and 80 µM Rg could both promote the
cell growth, which was inhibited by HG (Fig. 1C).
Rg reduces HG-induced lincRNA ANRIL
expression, increases HG-reduced let-7a expression and inhibits the
HG-activated TGF-β/Smad signaling pathway
A previous study reported that HG and diabetes could
upregulate lincRNA ANRIL in human retinal endothelial cells and in
the retina, and lincRNA ANRIL could also regulate vascular
endothelial growth factor expression (12). The present study examined whether HG
regulates the expression of lincRNA ANRIL in HMCs, and whether Rg
inhibits the apoptosis of HMCs induced by HG through lincRNA ANRIL.
The present study demonstrated that HG significantly upregulated
the expression of lincRNA ANRIL, and 20 and 80 µM Rg decreased the
HG-induced lincRNA ANRIL expression (Fig. 1D). HG decreased the expression of
let-7a, and 20 and 80 µM Rg could both increase the HG-reduced
let-7a expression (Fig. 1E).
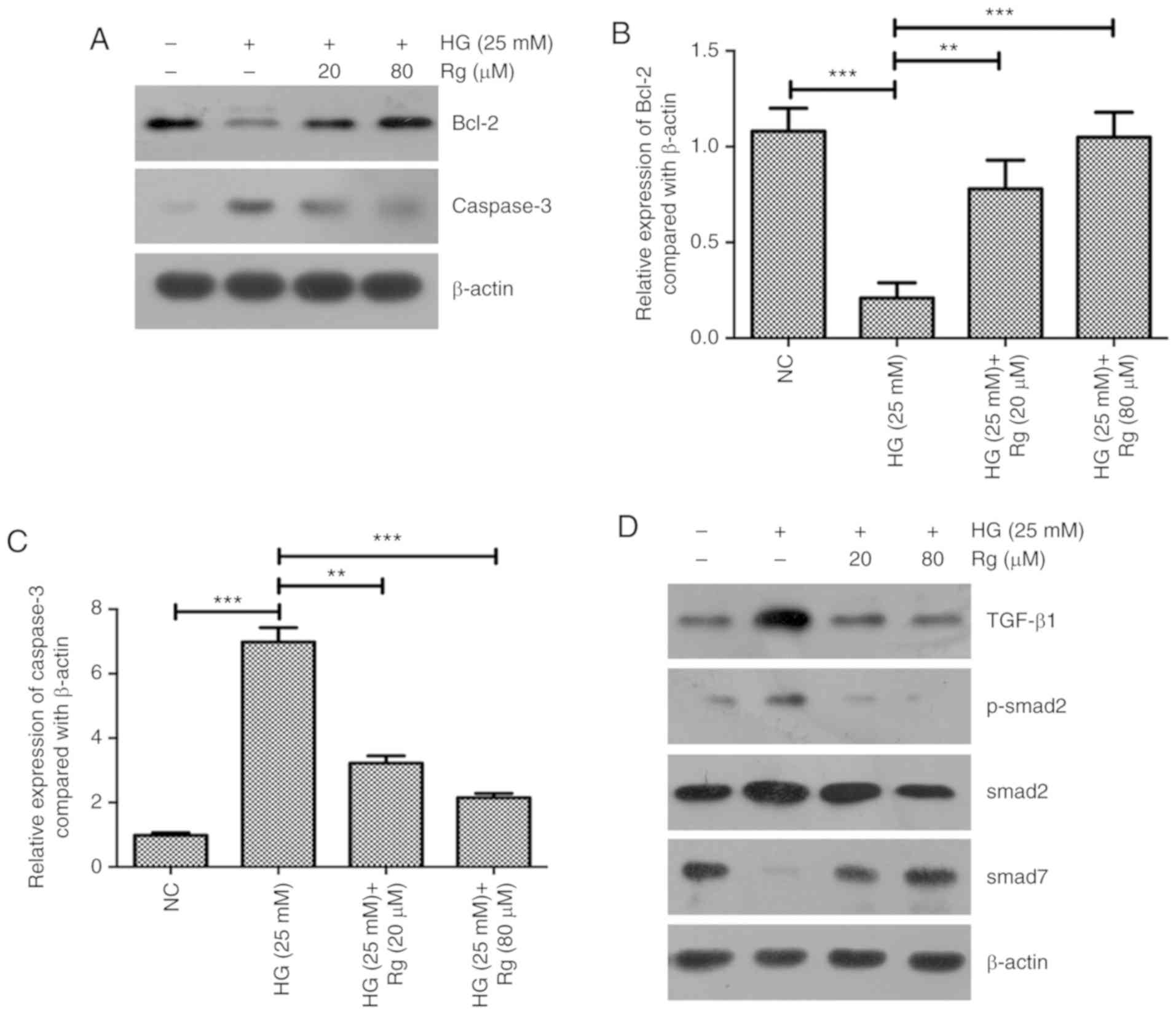
The expressions of apoptosis-associated proteins
Bcl-2 and active caspase-3 were further detected. The present
results showed that HG significantly inhibited Bcl-2 expression and
increased active caspase-3 expression, and Rg treatment recovered
the expressions of Bcl-2 and caspase-3 affected by HG (Fig. 2A-C). The present results suggested
that Rg inhibited the apoptosis of HMCs induced by HG by
upregulating Bcl-2 and downregulating caspase-3.
Previous studies demonstrated that dencichine could
ameliorate kidney injury in induced type II DN via the TGF-β/Smad
signaling pathway and 1,25(OH)2D3/VDR could attenuate HG-induced
epithelial-mesenchymal transition in human peritoneal mesothelial
cells via the TGF-β/Smad3 pathway (13,14). The
present study aimed to determine whether the TGF-β/Smad signaling
pathway is involved in the regulation of Rg on the HG-affected cell
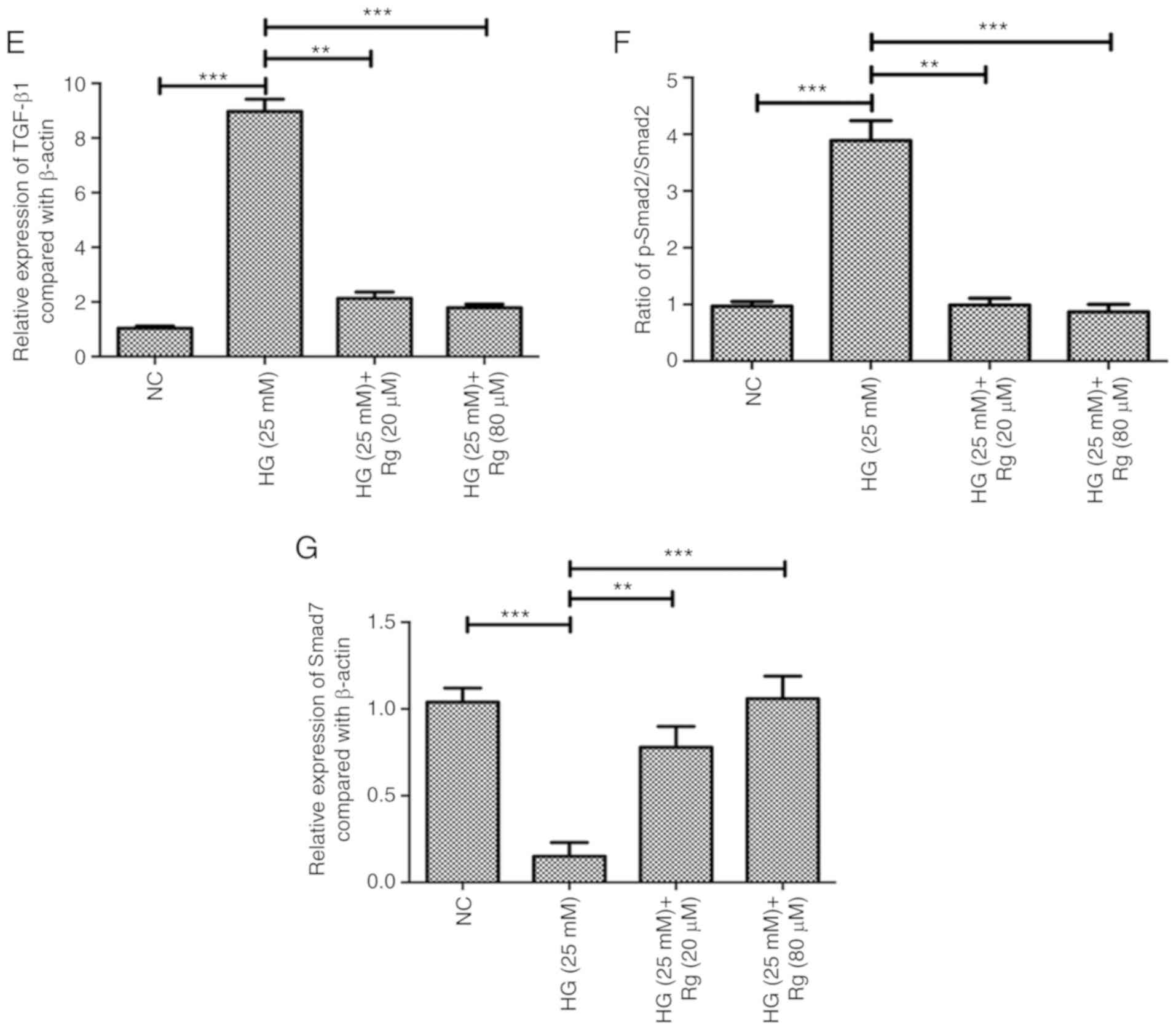
apoptosis and proliferation. Using a western blotting assay, HG
significantly upregulated TGF-β1 expression, downregulated Smad7
expression and activated the phosphorylation of Smad2. Both 20 and
80 µM Rg treatment recovered the expression levels of TGF-β1 and
Smad7, and the phosphorylation status of Smad2 regulated by HG
(Fig. 2D-G).
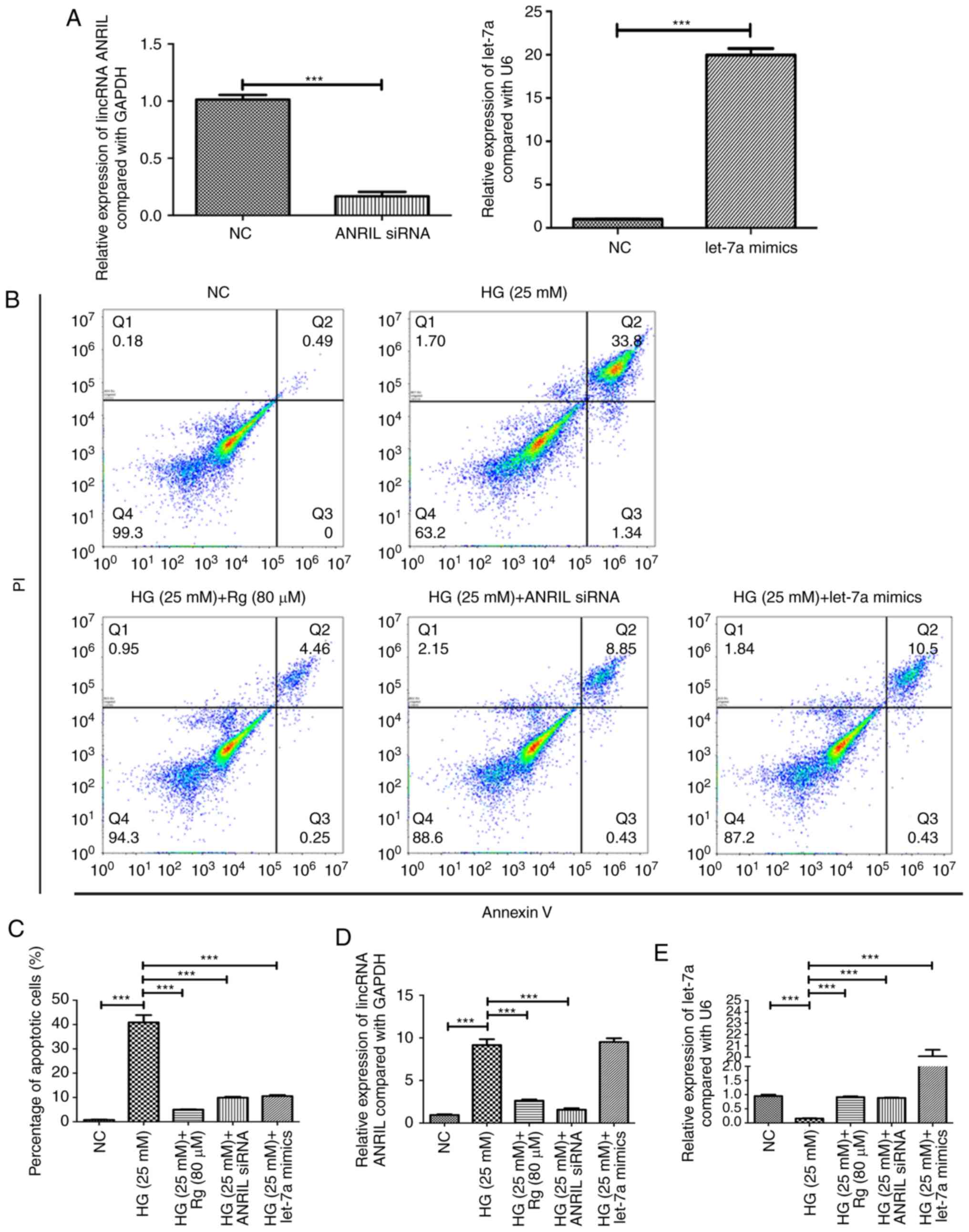
Knockdown of lincRNA ANRIL and
overexpression of let-7a have similar effects to Rg on HG-induced
apoptosis and HG-suppressed growth of HMCs
To further confirm whether lincRNA ANRIL and let-7a
are involved in regulation of cell apoptosis and the TGF-β1/Smad
signaling pathway by Rg treatment in the condition of HG, the
present study detected whether knockdown of lincRNA ANRIL and
overexpression of let-7a had the same results as Rg treatment.
RT-qPCR was used to confirm the efficacy of lincRNA ANRIL knockdown
and let-7a overexpression (Fig. 3A).
The results showed that knockdown of lincRNA ANRIL and
overexpression of let-7a significantly inhibited HG-induced
apoptosis, similar to Rg treatment (Fig.
3B and C). Overexpression of
let-7a had no effect on the expression of lincRNA ANRIL in an HG
condition (Fig. 3D), but knockdown
of lincRNA ANRIL significantly upregulated the level of let-7a
compared with the HG group (Fig.
3E). The present results suggested that lincRNA ANRIL could
negatively regulate let-7a in an HG condition and that lincRNA
ANRIL was an upstream regulator of let-7a.
Knockdown of lincRNA ANRIL and
overexpression of let-7a have similar effects to Rg on
apoptosis-associated proteins and the TGF-β1/Smad signaling pathway
in an HG condition
Knockdown of lincRNA ANRIL and overexpression of
let-7a significantly increased the HG-suppressed Bcl-2 expression
and decreased the HG-induced caspase-3 expression, similar to the
Rg treatment (Fig. 4A-C). Knockdown
of lincRNA ANRIL and overexpression of let-7a significantly reduced
the HG-induced TGF-β1 level, inhibited the HG-activated
phosphorylation of Smad2, and increased the HG-reduced Smad7 level
(Fig. 4D-G). The present results
suggested that Rg attenuated HG-induced apoptosis by regulating the
lincRNA ANRIL/let-7a/TGF-β1/Smad signaling pathway.
Discussion
The present study hypothesized that Rg inhibited
HG-induced apoptosis of HMCs by inactivating the TGF-β1/Smad
signaling pathway. The present results showed that HG significantly
induced HMC apoptosis and Rg markedly attenuated the HG-induced
apoptosis. HG was also demonstrated to decrease the Bcl-2
expression and increase the caspase-3 expression, and Rg treatment
recovered the expressions of Bcl-2 and caspase-3 affected by HG.
The underlying mechanisms were further examined and it was observed
that HG significantly upregulated the lincRNA ANRIL level,
downregulated let-7a expression and activated the TGF-β1/Smad
signaling pathway; Rg treatment recovered the expressions of
lincRNA ANRIL and let-7a, and inhibited the TGF-β1/Smad signaling
pathway in an HG condition.
The TGF-β1/Smad signaling pathway plays a key role
in the progression of DN. Xie et al (15) observed that relaxin inhibited
HG-induced matrix accumulation in HMCs by interfering with TGF-β1
production and mesangial cells phenotypic transition; telmisartan
activated peroxisome proliferator-activated receptor-σ and had
anti-fibrotic effects in HMCs. The present results suggested that
Rg treatment could inhibit the HG-activated TGF-β1/Smad signaling
pathway in HMCs.
The present study further investigated the
mechanisms underlying the regulation between Rg treatment and the
TGF-β1/Smad signaling pathway. The lincRNA ANRIL, which is
associated with atherosclerosis, periodontitis and several types of
cancer, was reported to regulate adiponectin 1, vesicle associated
membrane protein 3 and chromosome 11 open reading frame 10(16). In nasopharyngeal carcinoma, lincRNA
ANRIL was upregulated, and could promote cancer progression by
increasing proliferation, reprograming cell glucose metabolism and
inducing side-population stem-like cancer cells (17). However, the role and mechanism of
lincRNA ANRIL in HG-induced HMC apoptosis and Rg treatment are
still unknown to the best of the authors' knowledge. let-7a was
downregulated in both rats with DN and mesangial cells in an HG
condition, and could negatively regulate the expression of TGFβ
receptor 1(18). Katayama et
al (19) reported that glucose
could significantly regulate the expression of let-7a by affecting
promoter activity. Wang et al (20) found that lincRNA ANRIL could
negatively regulate the expression of let-7a. The present study
demonstrated that HG could upregulate lincRNA ANRIL expression and
downregulate the level of let-7a, and Rg treatment decreased
HG-induced lincRNA ANRIL expression and increased HG-suppressed
let-7a expression. Knockdown of lincRNA ANRIL upregulated let-7a
expression. The present results suggested that Rg treatment
recovered lincRNA ANRIL and let-7a expression in an HG condition.
Although the current study indicated that Rg attenuated HG-induced
HMC apoptosis by regulating the ANRIL/let-7a axis and the
downstream TGF-β1/Smad signaling pathway, the mechanism of how
ANRIL/let-7a axis regulated TGF-β1/Smad signaling pathway is still
yet to be determined.
In conclusion, Rg inhibited HG-induced apoptosis by
regulating the lincRNA ANRIL/let-7a/TGF-β1/Smad signaling pathway.
In the future, Rg may be developed as a new therapeutic method in
diabetic nephropathy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 31360083).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LSZ and JPL designed the study. LSZ, JL and JPL
performed the experiments. LSZ and JL analyzed the data. LSZ and
JPL wrote the manuscript.
Ethics and approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhee CM, Leung AM, Kovesdy CP, Lynch KE,
Brent GA and Kalantar-Zadeh K: Updates on the management of
diabetes in dialysis patients. Semin Dial. 27:135–145.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cove-Smith A and Hendry BM: The regulation
of mesangial cell proliferation. Nephron Exp Nephrol.
108(e74-e79)2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ye K, Wei Q, Gong Z, Huang Y, Liu H, Li Y
and Peng X: Effect of norcantharidin on the proliferation,
apoptosis, and cell cycle of human mesangial cells. Ren Fail.
39:458–464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou X, Wang C, Tian J, Wang Y, Li Y, Hu Z
and Li R: Mitogen-activated protein kinase mediates
mevalonate-stimulated human mesangial cell proliferation. Mol Med
Rep. 12:2643–2649. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li JL, Wu B, Hu H, Fang X, Liu Z and Wu S:
GdCl3 attenuates the glomerular sclerosis of streptozotocin (STZ)
induced diabetic rats via inhibiting TGF-β/Smads signal pathway. J
Pharmacol Sci. 19:34173–34178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vander Ark A, Cao J and Li X: TGF-β
receptors: In and beyond TGF-β signaling. Cell Signal. 52:112–120.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Namgung S, Yoon JJ, Yoon CS, Han BH, Choi
ES, Oh H, Kim YC, Lee YJ, Kang DG and Lee HS: Prunella vulgaris
attenuates diabetic renal injury by suppressing glomerular fibrosis
and inflammation. Am J Chin Med. 45:475–495. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takayama K, Tsutsumi H, Ishizu T and
Okamura N: The influence of rhein 8-O-β-D-glucopyranoside on the
purgative action of sennoside A from rhubarb in mice. Biol Pharm
Bull. 35:2204–2208. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mei LL, Wang WJ, Qiu YT, Xie XF, Bai J and
Shi ZZ: miR-145-5p suppresses tumor cell migration, invasion and
epithelial to mesenchymal transitionby regulating the Sp1/NF-κ
signaling pathway in esophageal squamous cell carcinoma. Int J Mol
Sci. 18(E1833)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thomas AA, Feng B and Chakrabarti S:
ANRIL: A regulator of VEGF in diabetic retinopathy. Invest
Ophthalmol Vis Sci. 58:470–480. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang L, Wu L, Zhang X, Hu Y, Fan Y and Ma
J: 1,25(OH)2D3/VDR attenuates high glucoseinduced
epithelialmesenchymal transition in human peritoneal mesothelial
cells via the TGFβ/Smad3 pathway. Mol Med Rep. 15:2273–2279.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jie L, Pengcheng Q, Qiaoyan H, Linlin B,
Meng Z, Fang W, Min J, Li Y, Ya Z, Qian Y and Siwang W: Dencichine
ameliorates kidney injury in induced type II diabetic nephropathy
via the TGF-β/Smad signalling pathway. Eur J Pharmacol.
812:196–205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xie X, Xia W, Fei X, Xu Q, Yang X, Qiu D
and Wang M: Relaxin inhibits high glucose-induced matrix
accumulation in human mesangial cells by interfering with TGF-β1
production and mesangial cells phenotypic transition. Biol Pharm
Bull. 38:1464–1469. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bochenek G, Hasler R, El Mokhtari NE,
König IR, Loos BG, Jepsen S, Rosenstiel P, Schreiber S and Schaefer
AS: The large non-coding RNA ANRIL, which is associated with
atherosclerosis, periodontitis and several forms of cancer,
regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 22:4516–4527.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zou ZW, Ma C, Medoro L, Chen L, Wang B,
Gupta R, Liu T, Yang XZ, Chen TT, Wang RZ, et al: LncRNA ANRIL is
up-regulated in nasopharyngeal carcinoma and promotes the cancer
progression via increasing proliferation, reprograming cell glucose
metabolism and inducing side-population stem-like cancer cells.
Oncotarget. 7:61741–61754. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yan N, Wen L, Peng R, Li H, Liu H, Peng H,
Sun Y, Wu T, Chen L, Duan Q, et al: Naringenin ameliorated kidney
injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J
Diabetes Res. 2016(8738760)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Katayama M, Sjogren RJ, Egan B and Krook
A: miRNA let-7 expression is regulated by glucose and TNF-α by a
remote upstream promoter. Biochem J. 472:147–156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Cheng N and Luo J: Downregulation
of lncRNA ANRIL represses tumorigenicity and enhances
cisplatin-induced cytotoxicity via regulating microRNA let-7a in
nasopharyngeal carcinoma. J Biochem Mol Toxicol. 31:2017.PubMed/NCBI View Article : Google Scholar
|