Introduction
Chronic obstructive pulmonary disease (COPD) remains
one of the major causes of morbidity and mortality worldwide and is
a large global public health burden. Cigarette smoking is the major
risk factor for COPD with long-term exposure to cigarette smoke the
most common method for establishing COPD in animal models. However,
there is increasing attention on the role of infection in the
development of COPD, with the opportunistic pathogen
Pneumocystis jirovecii (P. jirovecii) likely to be a key
contributor (1).
Recently, the role of P. jirovecii
colonization in respiratory diseases has become the focus of
research due to a high frequency of P. jirovecii
colonization among HIV-negative patients, especially those with
COPD (2-5).
Although the underlying physiopathology remains unclear, results
from animal model experiments support the hypothesis that P.
jirovecii colonization serves a significant role in COPD
pathogenesis (6-8).
COPD is a chronic inflammatory disorder characterized by airflow
limitation, which correlates with a complex inflammatory response
in the lungs. P. jirovecii colonization leading to infection
in the lung tissue can cause pulmonary tissue damage and the
deterioration of lung function via inflammation and the related
inflammatory mediators. Therefore, P. jirovecii colonization
affects COPD progression (8).
COPD is accompanied by hypoxia and increased
cellular stress in addition to increased inflammation. High
expression of matrix metalloproteases (MMPs), including MMP-2,
MMP-8, MMP-9, and MMP-12, and chaperone heat shock protein-27
(HSP-27) have been confirmed in both lung tissues of COPD rat
models and in COPD patients (9-15).
These markers are also closely correlated with COPD development
(11,16,17).
The present study aimed to establish a
steroid-induced Pneumocystis pneumonia (PCP) rat model to
study the role of P. jirovecii infection in the pathogenesis
and development of COPD. Investigation into the expression levels
of various COPD-related MMPs and also HSP-27 in PCP rats compared
to healthy controls was performed. The present findings provided
new insights into the contribution of Pneumocystis to COPD
pathogenesis and may lead to novel prophylactic treatment
options.
Materials and methods
Establishment of a rat PCP model.
The protocol for animal experimentation was approved
by the Experimental Animal Care and Ethics Committee of China
Medical University. A total of 20 female Wistar rats (mean weight,
150±20 g; age, 5-7 weeks) were purchased from the Department of
Experimental Animal Center, China Medical University. Rats were
randomly divided into two groups: A control group and a PCP group
with ten rats per group. All rats were housed in a temperature
(20-25˚C) and humidity (53-58%)-controlled facility with a 12-h
light/dark cycle with food and water provided ad libitum.
The antibiotic oxytetracycline (1 g/l; Yichuang Pharmaceutical Co.,
Ltd.) was added to the drinking water to prevent bacterial
infections. The immune response of the PCP group rats was
suppressed by intraperitoneal dexamethasone sodium phosphate
injections (3 mg per rat; Rongsheng Pharmaceutical Co., Ltd) twice
per week, for 8 successive weeks. The control group was injected
with physiological saline. The rats were weighed once a week. Fur
color and thickness, activity, breathing, death rate, and other
symptoms and signs of respiratory distress were monitored
throughout the study.
Processing of lung tissue
specimens.
After 8 weeks, rats were weighed, anesthetized with
an intraperitoneal injection of sodium pentobarbital (50 mg/kg)
then sacrificed by rapid exsanguination. Blood was collected via
the abdominal aorta and serum was isolated for detection of MMPs
and HSP-27. The lower lobe of the right lung was fixed for further
histopathological assessment. The middle lobe of the right lung was
removed for preparation of lung imprint smears. After ligation of
the right lung, bronchoalveolar lavage (BAL) was performed in the
left lung. All remaining lung tissue was frozen and stored in
liquid nitrogen for further use.
Pathogen identification.
The presence of P. jirovecii organisms was
confirmed by Giemsa and Gomori's methenamine silver nitrate
staining assays (GMS) of the lung imprint smears from the
cross-sections of the middle lobe of the right lungs, according to
a previously described protocol (18).
Histopathological examination.
Two-thirds of the lower lobe of the right lung were
fixed with 4% paraformaldehyde (Dingguo Biotechnology Co. Ltd.) at
room temperature for >24 h. The lung tissue sections were then
washed, dehydrated, embedded in paraffin and sectioned at 5 µm
thick preparations. The sections were placed onto glass slides,
dewaxed, dehydrated and stained with hematoxylin (5 min) and eosin
(for 3-5 min) (HE) at room temperature or subsequently used for
immunohistochemical staining. Histological changes in the lung
tissues were respectively observed under a light microscope
(Magnifications, x200 and x400; Olympus Coporation).
Flow cytometry.
A direct immunofluorescence staining method was
used. Homogenates made from splenic tissues were collected and
passed through a screen mesh (pores size, 70 µm; cat. no. 258368;
Wuxi NEST Biotechnology Co., Ltd.). Antibodies were added after
lysis of red blood cells (at 4˚C for 4-5 min) and adjustment of
cell concentrations (1x106 cells/ml). Pre-cooled PBS
(cat no. FG701-01; Beijing Transgen Biotech Co., Ltd.) was used to
terminate the lysis reaction at 4˚C. T lymphocytes were stained
with PerCP-anti-CD8 (0.2 mg/ml; cat. no. 558824; BD Biosciences)
and BV421-anti-CD4 (0.2 mg/ml, cat no: 740040, BD Biosciences). M1
macrophages were stained with PE-Anti-CD86 (0.2 mg/ml; cat no:
551396, BD Biosciences) and granulocytes with fluorescein
isothiocyanate-anti-granulocytes (0.5 mg/ml, cat no: 554907, BD
Biosciences). BD Pharmingen™ Stain Buffer (FBS; cat no. 554656; BD
Biosciences) was applied as the washing reagent. Corresponding
isotype-matched monoclonal antibodies were used as negative
controls. All antibodies used in flow cytometry were purchased from
BD Biosciences. Inflammatory cell counts were analyzed using BD
FACSDiva™ Software (version 6.2; BD Biosciences)
combined with a BD LSRFortessa™ flow cytometer (BD
Biosciences).
ELISA.
Expression levels of MMPs and HSP-27 in the
bronchoalveolar lavage fluid (BALF) and serum were quantified using
the following ELISA kits (Cusabio Biotech Co., Ltd.): MMP-2 ELISA
kit (cat. no. CSB-E07411r); Rat MMP8 (neutrophil collagenase) ELISA
kit (cat. no. CSB-E07406r); Rat MMP-9 (Gelatinase B) ELISA Kit
(cat. no. CSB-E08008r); Rat MMP12 (Macrophage metalloelastase)
ELISA kit (cat. no. CSB-EL014659RA); and Rat heat shock protein 27
(HSP-27) ELISA Kit (cat. no. CSB-E09240r) according to the
manufacturer's protocols. In brief, standards and samples (100 µl)
were added to appropriate wells and incubated for 2 h at 37˚C. The
liquid was removed from all wells, and 100 µl of biotin-antibody
(1X) were added to each well, and incubated for 1 h at 37˚C. The
residual liquid in all wells was removed and the microtiter plate
was washed three times with wash buffer (200 µl) using a
multi-channel pipette for 2 min per wash. Then, 100 µl of
horseradish peroxidase-avidin (1X) was added to each well and
incubated for 1 h at 37˚C. The aspiration/wash process was repeated
five times. Then, 90 µl of the 2,2'5,5'-Tetramethylbenzidine
substrate were added to each well and incubated for 15-30 min at
37˚C. The chromogenic reaction was terminated with the addition of
50 µl stop solution. Optical density at 450 nm wavelength was
determined immediately using a Thermo Scientific
Varioskan® Flash (Thermo Fisher Scientific, Inc.).
Immunohistochemical detection.
Immunohistochemical detection of MMPs and HSP-27 in
lung tissue was performed using the Streptavidin-Peroxidase (SP)
method and the UltraSensitive™ SP IHC kit (cat. no.
Kit-9720; Fuzhou Maixin Biotech Co., Ltd.) according to the
manufacturer's protocols. Two-thirds of the lower lobe of the right
lung were fixed with 4% paraformaldehyde (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) at room temperature for >24
h. The lung tissue was then washed, dehydrated, embedded in
paraffin and sectioned into 5-µm thick preparations. Antigen
retrieval was performed in sodium citrate buffer (pH 6.0) at 100˚C
for 1 min followed by cooling at room temperature for 1 min, a
process which was repeated a further three times. After blocking
for 30 min at 37˚C with goat serum (included in the UltraSensitive
SP IHC kit), slices were incubated with primary antibodies against
MMP-2 (1:250; cat. no. ab37150; Abcam), MMP-8 (1:250; cat. no.
ab81286; Abcam), MMP-9 (1:250; cat. no. ab76003; Abcam), MMP-12
(1:250; cat. no. ab52897; Abcam), and HSP-27 (1:250; cat. no.
ab5579; Abcam) overnight at 4˚C. After washing with PBS three
times, a horseradish peroxidase-conjugated secondary antibody
(included in the UltraSensitive SP IHC kit) was added to samples
and incubated for 30 min at 37˚C. PBS was used as a negative
control. The DAB detection kit (Fuzhou Maixin Biotech, Co., Ltd.)
was applied to visualize the distribution and location of MMPs and
HSP-27 expression. Brown staining indicated positive protein
detection. Five regions of positive expression on each slide were
selected randomly and photographed under light microscopy (Olympus
Corporation). Integrated optical density (IOD) was analyzed by
Image pro plus 6.0 (Media Cybernetics, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR).
Total RNA was extracted from lung tissue using
RNAiso Plus (Takara Bio, Inc.). RNA concentration was quantified
using a NanoDrop™ 2000 (Thermo Fisher Scientific, Inc.)
and samples were diluted to 1 µg of RNA. Reverse transcription was
performed with a PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Bio, Inc.) according to manufacturer's protocols.
qPCR was subsequently performed using the ABI 7500 real-time PCR
instrument (Thermo Fisher Scientific, Inc.) with TB
Green™ Premix Ex Taq™ II (Tli RNaseH Plus; Takara Bio,
Inc) in 20 µl reaction mixtures. The thermocycling conditions for
qPCR amplification were as follows: Initial denaturation at 95˚C
for 30 sec, followed by 40 cycles of 95˚C for 5 sec and 60˚C for 34
sec. All primers used in the present study and their sequences are
listed in Table I. Data were
analyzed using the 2-ΔΔCq method
(19). GAPDH was used as
housekeeping gene for normalizing the levels of MMPs and HSP-27
mRNA.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5'-3') | Product size
(bp) |
|---|
| MMP-2 forward |
GTGGCAATGGAGATGGACAG | 127 |
| MMP-2 reverse |
CGGTCATAATCCTCGGTGGT | |
| MMP-8 forward |
CAGTGCCTCCAGAACACCTG | 120 |
| MMP-8 reverse |
CGGCAATCATAGTGGCATTC | |
| MMP-9 forward |
GTGACACCGCTCACCTTCAC | 122 |
| MMP-9 reverse |
GCGTGTGCCAGTAGACCATC | |
| MMP-12 forward |
CGATGTGGAGTGCCTGATGT | 113 |
| MMP-12 reverse |
GCACGCTTCATGTCTGGAGT | |
| HSP-27 forward |
CAACTCAGCAGCGGTGTCTC | 115 |
| HSP-27 reverse |
CCACGCCTTCCTTGGTCTTA | |
| GAPDH forward |
GACATGCCGCCTGGAGAAAC | 92 |
| GAPDH reverse |
AGCCCAGGATGCCCTTTAGT | |
Western blot analysis.
Protein was extracted from the lung tissues using
the ProteinExt® Mammalian Total Protein Extraction Kit
(cat no. DE101-01; Beijing TransGen Biotech Co., Ltd.) and
quantified using the Bicinchonininc protein assay kit (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.). Protein samples were
separated using gel electrophoresis (Bio-Rad Laboratories, Inc.) by
SDS-PAGE on a 10% gel. The proteins were transferred to a
polyvinylidene difluoride membrane. Blocking was performed with 5%
bovine serum albumin (BSA; Beijing Solarbio Science &
Technology Co., Ltd.). in Tris-buffered saline with 0.05% Tween-20
(TBST) for 2 h at room temperature. The membranes were incubated
with the following primary antibodies diluted in 5% BSA TBST:
Anti-MMP-2 (1:2,000; cat. no. ab37150; Abcam), anti-MMP-8 (1:2,000;
cat. no. ab81286; Abcam), anti-MMP-9 (1:2,000; cat. no. ab76003;
Abcam), anti-MMP-12 (1:2,000; cat. no. ab52897; Abcam) and
anti-HSP-27 (1:1,000; cat. no. ab5579; Abcam) then incubated
overnight at 4˚C. Following 10 min washes three times, the
membranes were incubated with goat anti-Rabbit IgG (cat. no.
ZB-2301) and goat anti-Mouse IgG (cat. no. ZB-2305) secondary
antibodies conjugated to horseradish peroxidase (both 1:5,000;
Beijing Zhongshan Jinqiao Biotechnology Co. Ltd.; OriGene
Technologies, Inc.) for 2 h at room temperature. Following the
second washing step (six times for 5 min each), membranes were
visualized with SuperSignal West Pico PLUS Chemiluminescent
Substrate, an enhanced chemiluminescent (ECL) reagent (Thermo
Fisher Scientific, Inc.) and analyzed using the Tanon-5200
automatic chemiluminescence imaging analysis system. GAPDH was used
to normalize the relative density of each protein band in each
group. The intensity of the protein bands was analyzed with Image J
software (version 1.8.0; National Institutes of Health).
Gelatin zymography.
The prepared protein samples were separated by 10%
SDS-PAGE containing 1% w/v gelatin by gel electrophoresis (Bio-Rad
Laboratories, Inc.) at 4˚C. The gelatin zymography assay kit
(Wanlei Biotech Co., Ltd) was used to detect MMP-2 and MMP-9
activity. The gel was subsequently washed four times in eluate for
15 min per wash, washed with rinse buffer twice for 20 min each,
and then incubated in incubation buffer for 48 h at 37˚C. After
staining with Coomassie Brilliant Blue R-250 for 3 h, incubation
with decolorized buffer was performed at room temperature for 2 h
resulting in the appearance of white bands on a blue
background.
Statistical analysis.
All statistical analyses were performed using the
statistical software SPSS 13.0 (SPSS Inc.) and results were
presented using Prism 7 (GraphPad Software, Inc.). All values were
expressed as mean ± standard deviation. The means of two groups
were compared using the Student's t test after testing for
normality and homogeneity of variance. P<0.05 was considered to
indicate statistical significance.
Results
Clinical features and gross
findings.
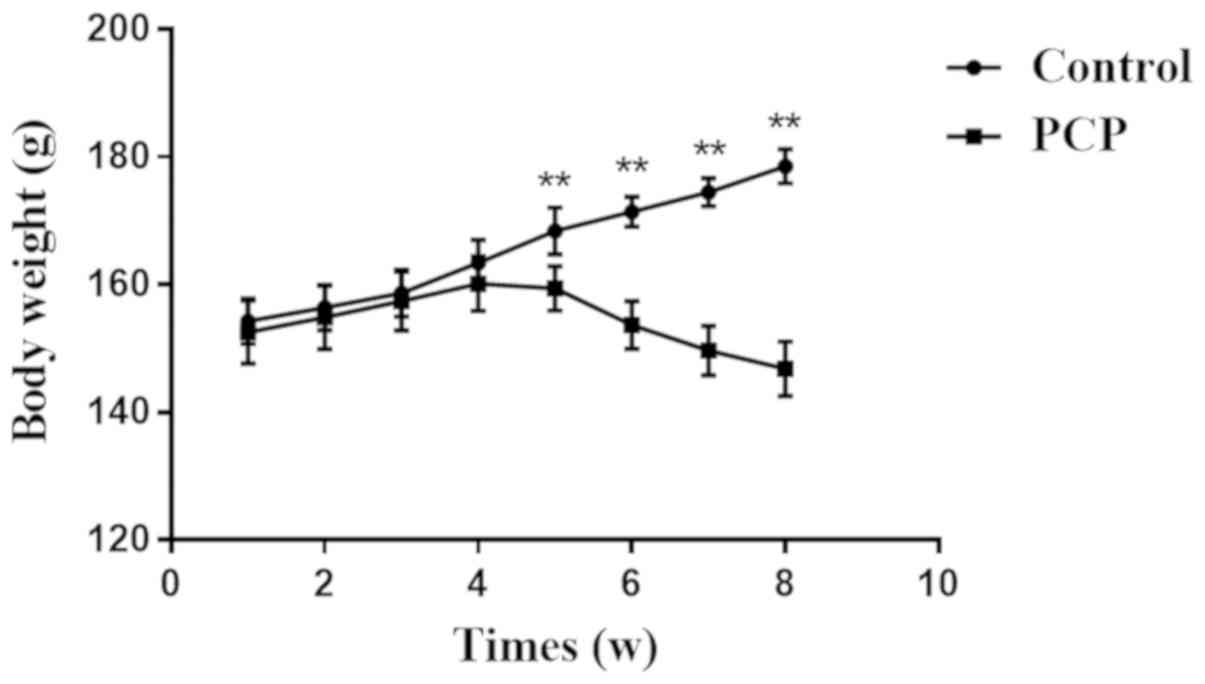
Compared with the control, rats in the PCP group had
decreased body weight after 4 weeks (Fig. 1), and their fur density was reduced
and fur color was darker. PCP rats displayed decreased activity and
always herded in the corner of the cage. PCP rats also occasionally
displayed wheezing.
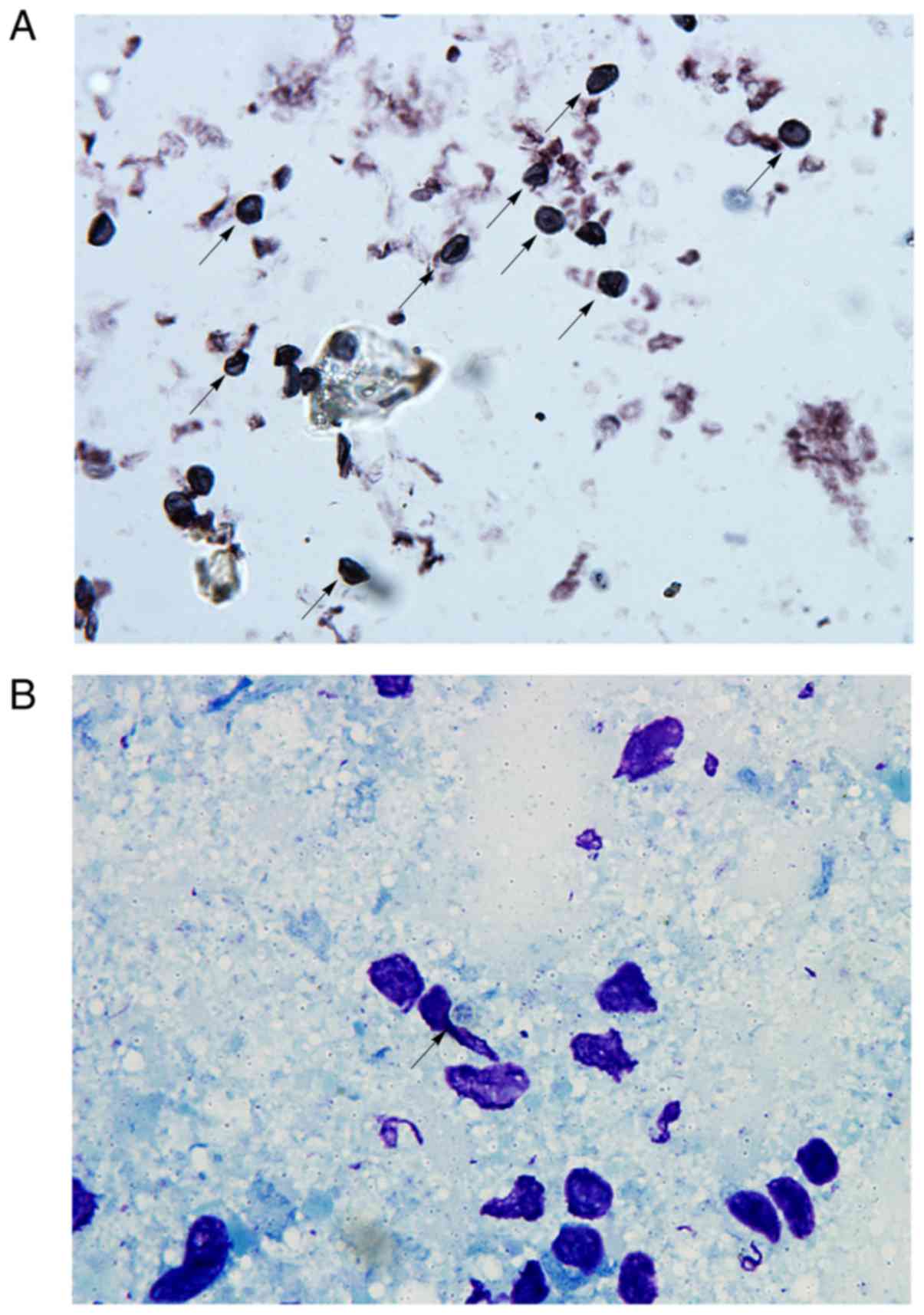
Pathogenic identification.
Pneumocystis
cysts, identified as brown or brown-reddish spheres
or ovoids with a small black stick-shape figure in the middle, were
visualized using GMS staining (Fig.
2A). On Giemsa stained lung imprint smears, cyst walls were not
stained and thus formed transparent zones. Every cyst contained 2-8
blue-stained intracystic bodies that were arranged in the shape of
a ring (Fig. 2B). Typical
Pneumocystis cysts assumed a crescent or irregular spherical
shape. No signs of Pneumocystis cysts were observed in the
control group (data not shown).
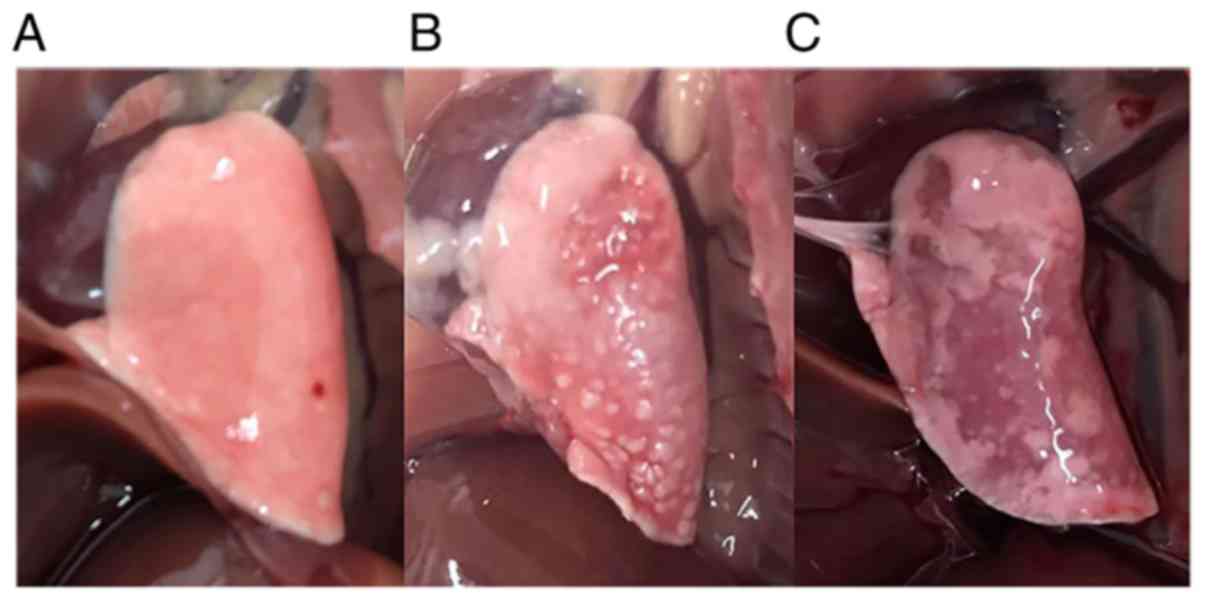
Lung appearance.
Shrinkage of lung volume and hardening of the lung
tissue was observed in the PCP group (Fig. 3). Gray or white dots were
interspersed on the surface of the lung tissue in PCP rats. No
serious gross pulmonary lesions, such as hemorrhage or necrosis,
were observed in the PCP group. None of the rats died during
steroid treatment.
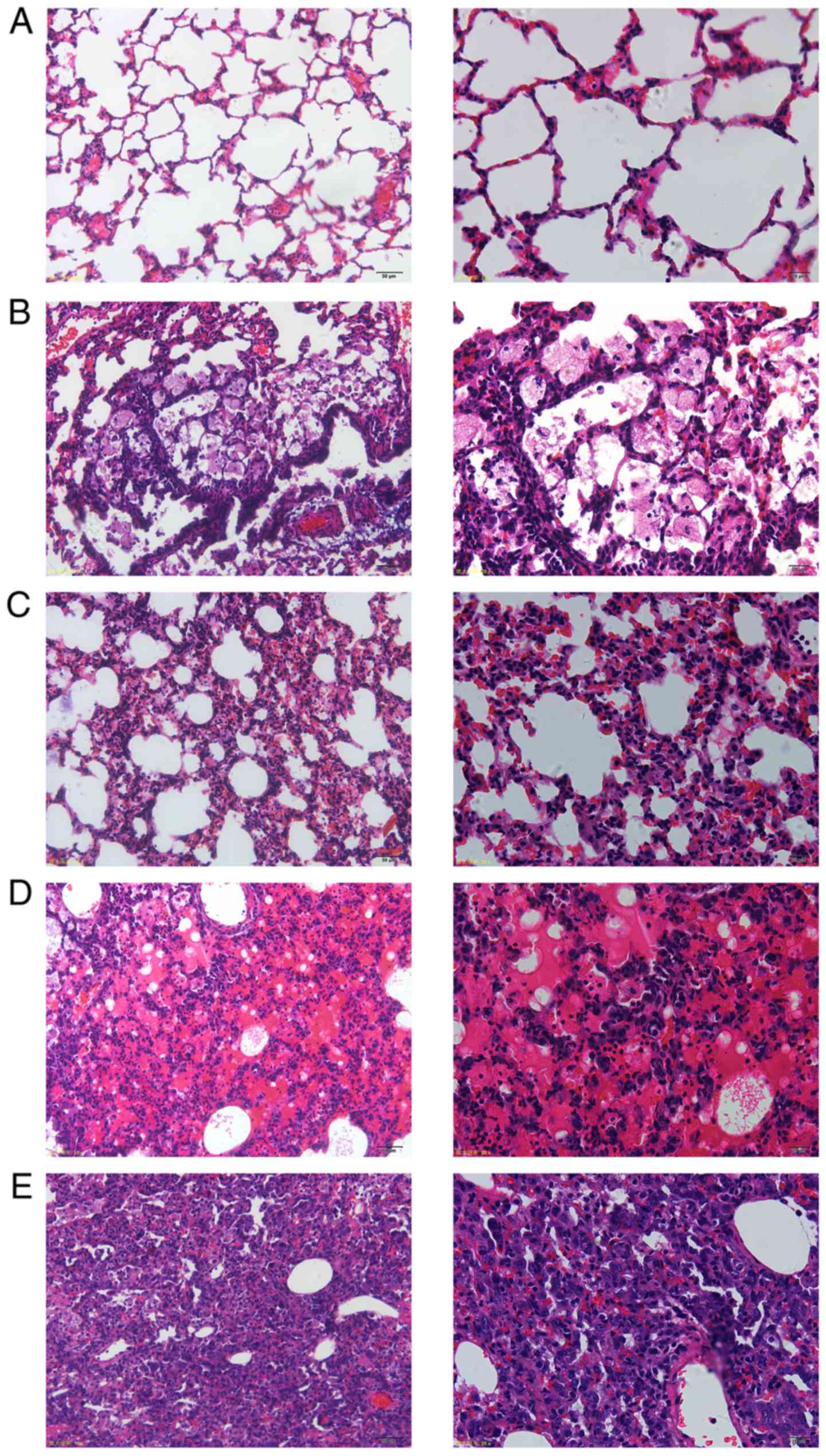
PCP induces histopathological changes
in rat lungs.
Histopathologic examination was performed under
light microscopy at magnifications of x200 and x400.
Morphologically, lung sections of rats in the control group were
free of cellular infiltrate and mucus, and did not show any
pathological abnormality. The structure of the lung interstitial
tissue was intact and the alveolar space was clear (Fig. 4A). The degree of histopathological
changes varied in lungs of the PCP rats. The inflammatory cell
infiltrate in the pulmonary interstitial tissue primarily included
lymphocytes and macrophages (Fig.
4B). Diffuse pulmonary interstitial tissue hyperplasia and
interstitial edema was observed (Fig.
4C). Lesions in the form of red foamy alveolar exudates
(Fig. 4D) and consolidated areas in
lung tissue (Fig. 4E) were
identified. Morphological signs of emphysema, including alveolar
fusion in the sectional tissue and pulmonary bullae due to alveolar
wall destruction were also present.
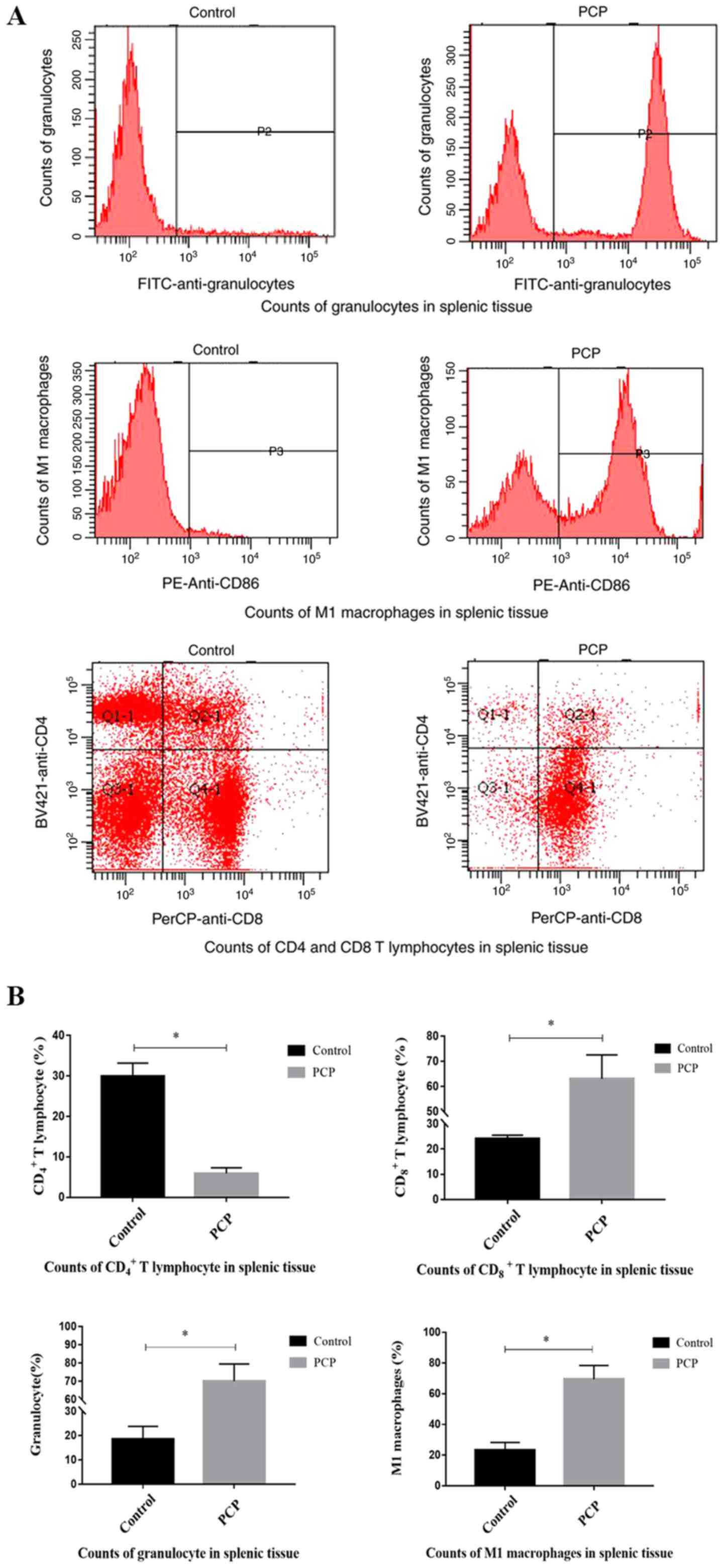
PCP increases inflammatory cell
counts.
Flow cytometry analysis determined that compared
with the control group, the numbers of CD8+ T
lymphocytes (P<0.01; Fig. 5), M1
macrophages (P<0.01; Fig. 5), and
granulocytes (P<0.01; Fig. 5)
were significantly increased in the splenic tissue of PCP rats,
whilst the number of CD4+ T lymphocytes was
significantly reduced (P<0.01; Fig.
5).
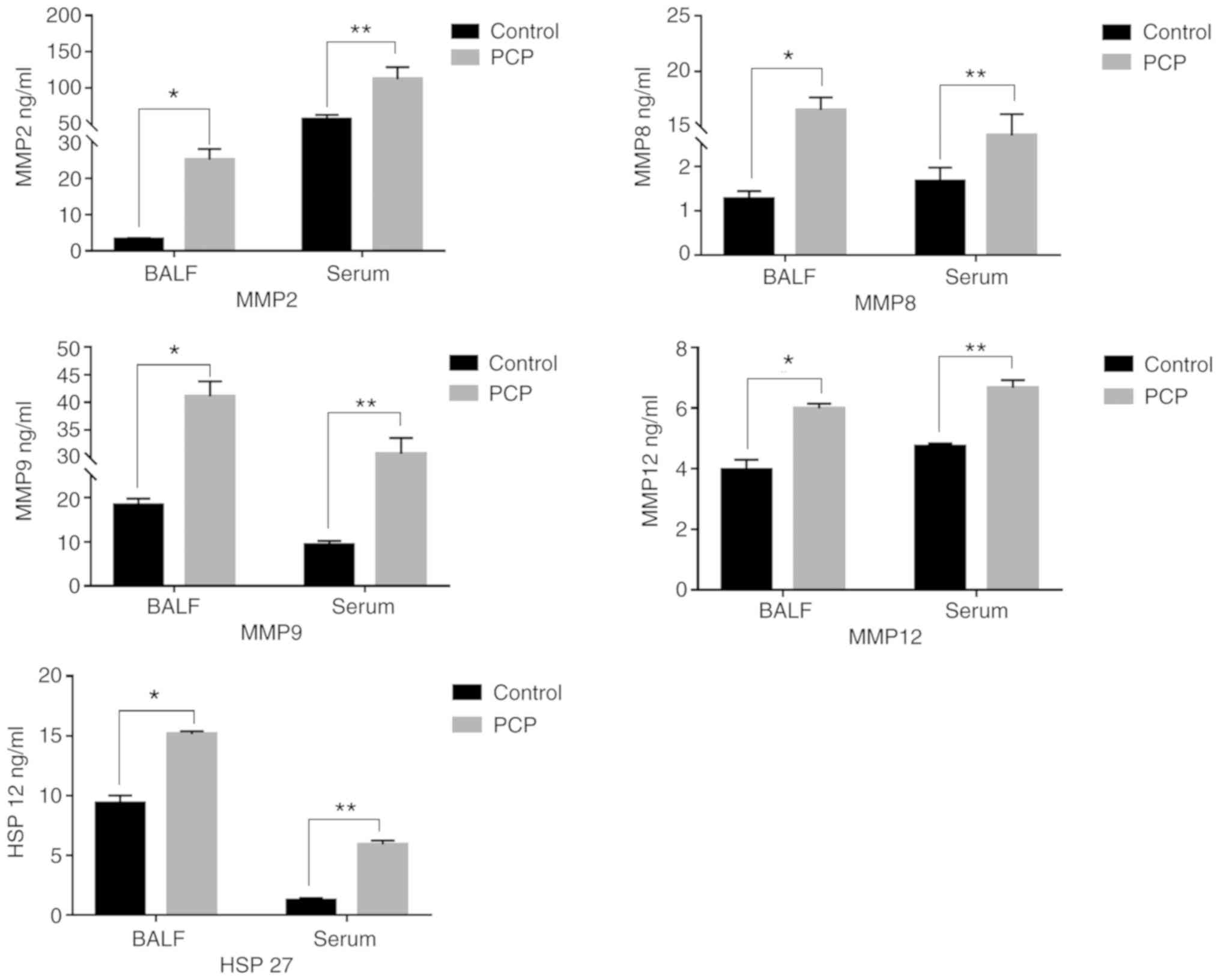
PCP increases MMPs and HSP-27 secreted
levels in serum and BALF.
The concentrations of MMPs and HSP-27 in rat serum
and BALF were determined by ELISA (Fig.
6). MMPs and HSP-27 expression levels in the serum and BALF
were significantly higher in the PCP group compared with the
control group.
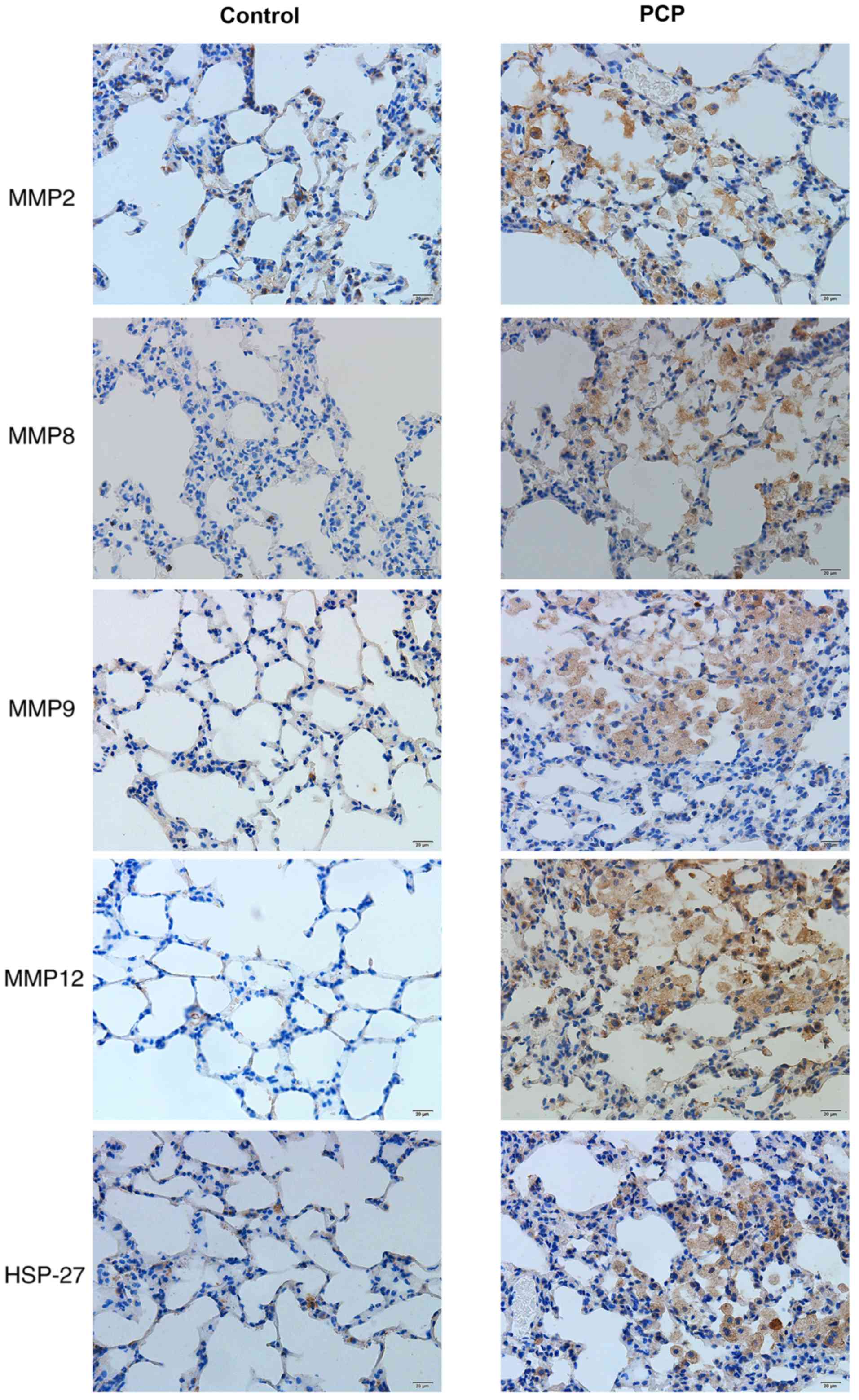
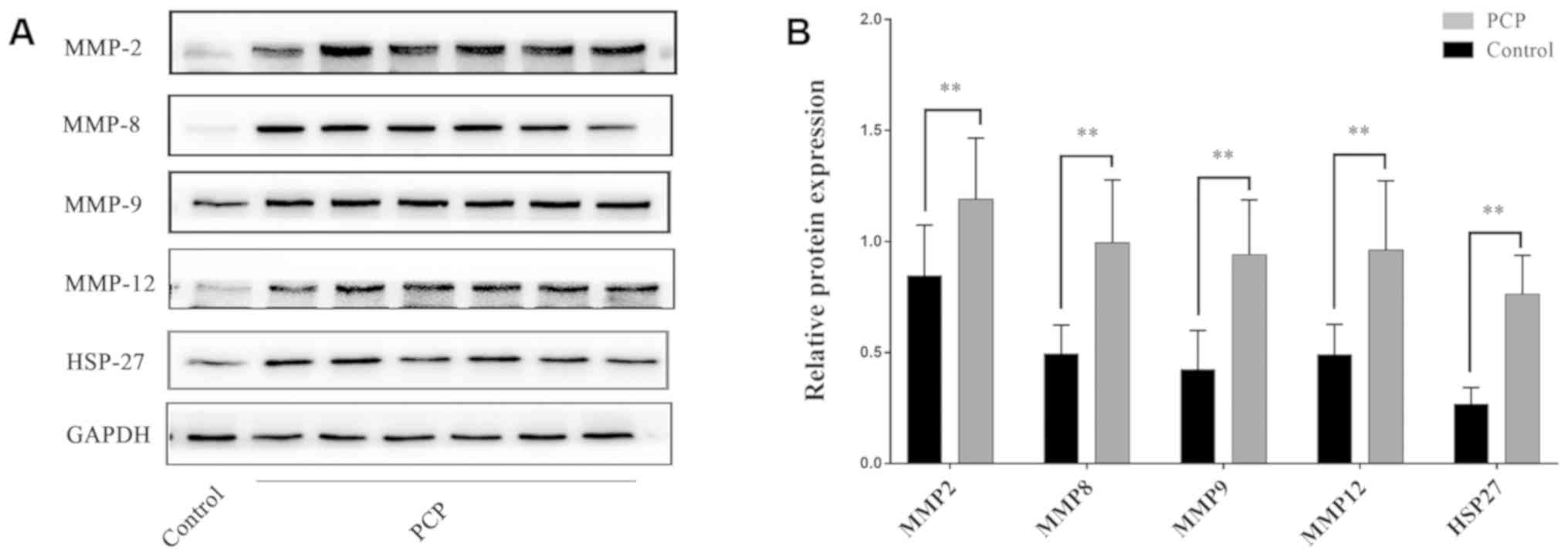
PCP increases MMPs and HSP-27 protein
expression in pulmonary tissue.
Positive protein expression was detected by brown
staining upon immunohistochemical analysis. HSP-27 expression was
largely located in the bronchial epithelial cells and a few other
cell types in the PCP group (Fig.
7). Positive MMP expression was clearly detected in the
cytoplasm and nuclei of cells in the PCP group, particularly in
macrophages and alveolar epithelial cells (Fig. 7). However, there was weak or no
staining for MMPs and HSP-27 in the control group. In the PCP model
group, positive expression was demonstrated by darker staining
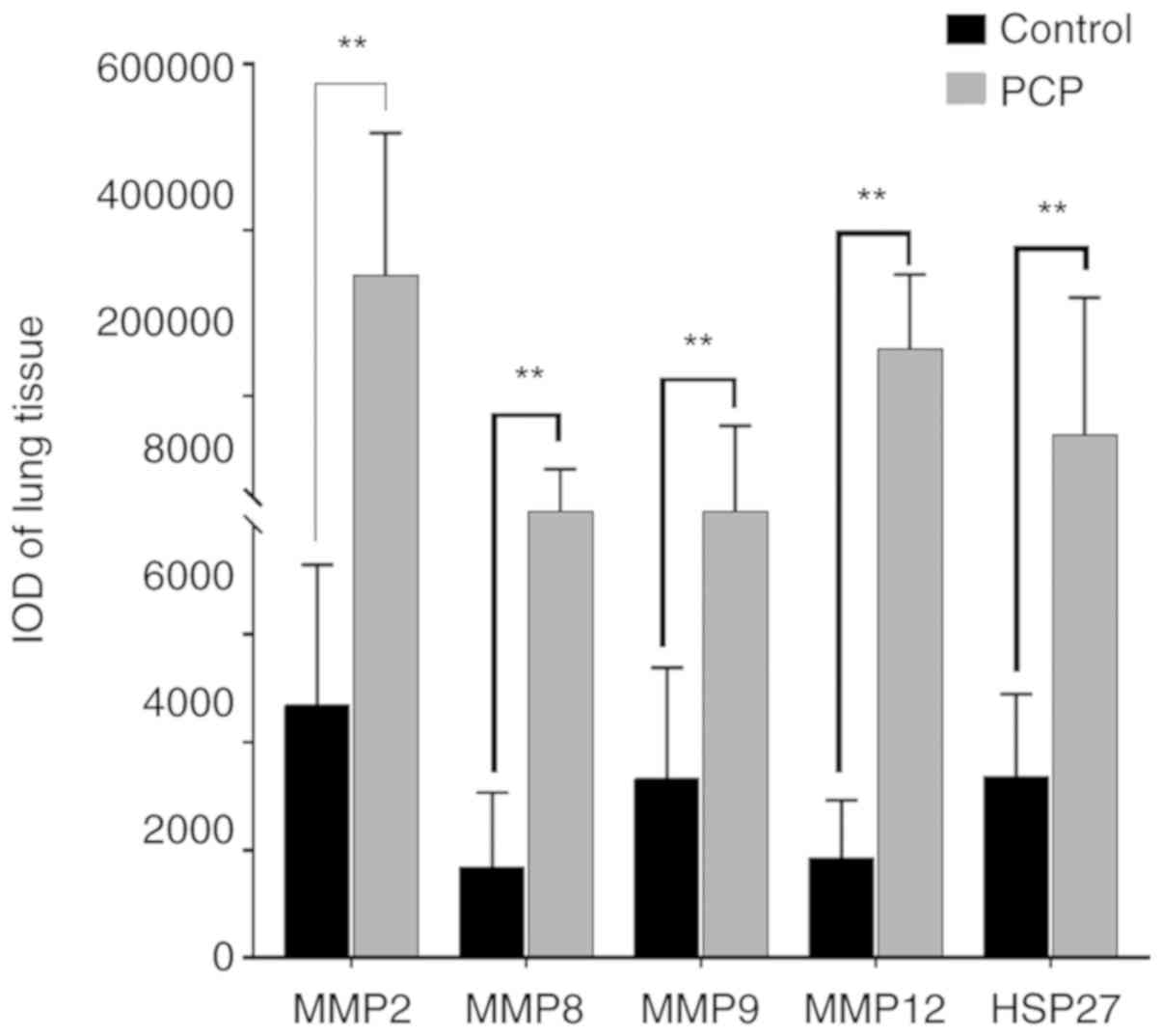
(Fig. 7). The IOD values of MMPs and
HSP-27 were significantly higher in the PCP group compared to the
control group (P<0.01; Fig.
8).
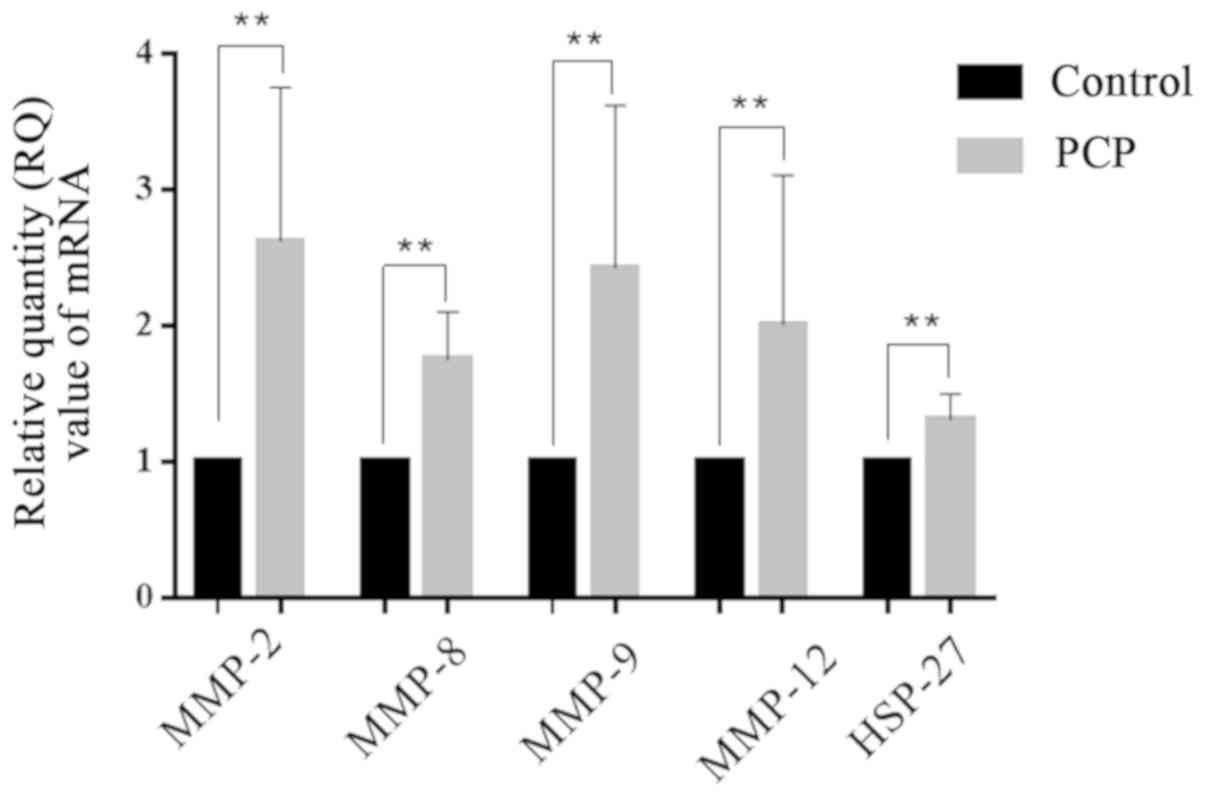
PCP increases MMP and HSP-27 mRNA and
protein expression levels in lung tissue.
RT-qPCR and western blot results demonstrated that
MMPs and HSP-27 mRNA and protein expression levels in the lung
tissues were significantly upregulated in the PCP group compared to
the control group (P<0.01; Figs.
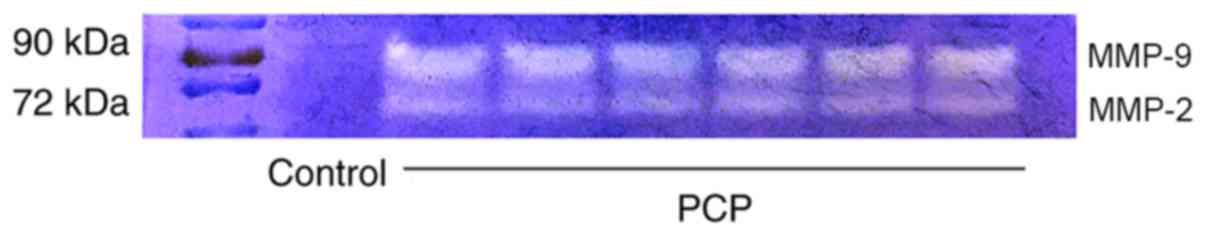
9 and 10). Gelatin zymography
revealed that there was higher activity of MMP-9 and MMP-2 in the
PCP model group compared to the control group (Fig. 11).
Discussion
The underlying pathological processes leading to the
development of COPD remain unclear at present however several
studies have demonstrated that colonization of pathogenic
microorganisms in the tracheobronchial tree could serve as
inflammatory stimuli in the airways of stable COPD patients and
possibly lead to disease progression (20). Recurrent acute infections are
strongly correlated with the occurrence of acute exacerbation of
COPD (21). The lungs of patients
with COPD become susceptible to airway mucosal infections by
pathogenic microorganisms. Morris et al (1,8)
demonstrated that an increase in P. jirovecii colonization
in COPD is independently correlated with the degree of airway
obstruction. PCP is a serious complication that can lead to death
for patients infected with HIV. Compared with HIV negative
patients, lung function of COPD patients infected with HIV rapidly
declines. Acute PCP has been associated with obstructive pulmonary
changes (7). The present study
investigated the pathogenicity of Pneumocystis infection and
its role in the progression of COPD using an immunosuppressed rat
PCP model to determine the expression of COPD-related cytokine
expression and inflammatory cell populations.
Advances in research of P. jirovecii
pathogenicity have been limited by the difficulties in cultivating
P. jirovecii in vitro for an extended time period.
Therefore, in vitro Pneumocystis studies are scarce. For
in vivo studies of Pneumocystis, rats are the most
commonly used animal model. Immunosuppressed rats induced by
steroids can be spontaneously infected with P. jirovecii,
resulting in pathologic features closely resembling PCP in humans
(22-24).
The present study demonstrated that various degrees
of pulmonary pathology appeared in steroid-induced PCP model rats.
Gross clinical changes included body weight loss, fur density and
color changes, decreased activity level, and respiratory symptoms.
Histopathological changes included inflammatory cell infiltration,
diffuse lung interstitial tissue hyperplasia, and appearance of
consolidation areas. Similar histopathological changes have been
described previously in cigarette smoke-induced COPD model rats
(25). Morris et al (1,7,8) reported that the frequency of fibrous
degeneration in lung tissue and obstructive pathological changes
were higher in patients with PCP compared with patients without
PCP. Consequently, animal and human studies investigating the
changes of pulmonary pathology support the hypothesis that P.
jirovecii infection has an important role in COPD
progression.
The present study determined that there was a close
correlation between the immune response and severity of pulmonary
impairment. The severity of pathological lesions increased with
declining levels of CD4+ T lymphocytes and increasing
levels of CD8+ T lymphocytes, M1 macrophages, and
granulocytes. Lung tissue may be damaged directly by the
inflammatory responses of organisms. It has been previously
demonstrated that CD8+ T lymphocytes serve a significant
role in the pathogenic process of PCP (26). Similar inflammatory cell count
results were reported in rat studies of cigarette-smoke-induced
COPD (27-29).
COPD is a chronic inflammatory disorder characterized by
irreversible airflow limitation, which correlates with a complex
inflammatory response in the lungs. CD8+ T lymphocytes,
M1 macrophages, and granulocytes cause pathological injury to the
pulmonary airway, blood vessels, and lung tissue which decreases
pulmonary function, and leads to chronic inflammation and COPD
pathogenesis. Importantly, COPD patients with higher GOLD (The
Global Initiative for Chronic Obstructive Lung Disease) stages
demonstrate a more severe inflammatory response (30-32).
At present, there are various theories concerning
the mechanism of COPD (33) with an
imbalance in protease-antiprotease one of the most widely accepted.
MMPs, neutrophil elastase, serine protease, and other proteases are
regarded to be the key drivers. MMPs are mainly secreted and
produced by alveolar macrophages, neutrophils, and lung structural
cells. Basement membrane and extracellular matrix (ECM) are
degraded by MMPs, which contributes to progressive destructive
inflammation in the airway and pulmonary tissue leading to COPD
development. MMPs are also the principal inflammatory mediators of
emphysema and have a regulatory role in the inflammatory process.
Elevated levels of MMPs have been detected in COPD patients,
particularly in the patients with exacerbated COPD (12,13,25,34).
MMP-2 and MMP-9 are released into the intercellular space in the
form of inactive zymogens. Following activation, they have pivotal
roles in proteolysis. The present study demonstrated by western
blot analysis that MMP-2, MMP-8, MMP-9 and MMP-12 expression levels
were upregulated in PCP model rats compared with control rats. In
addition, gelatin zymography assay revealed that MMP-2 and MMP-9
activity was increased in PCP model rats. Therefore, it was
hypothesized that P. jirovecii infection may lead to the
release of endogenous proteases which is in accordance with the
literature (6,35-37).
In addition, upregulated levels of MMPs suggested that the
occurrence and progression of emphysema may be caused by P.
jirovecii colonization due to release of endogenous proteases
and stimulation of protease release in the lung tissue of PCP model
rats which is in agreement with a previous study (38) Similar upregulation of MMP levels have
also been previously reported in COPD rats (33). Findings suggested that ECM
degradation caused by a protease-antiprotease imbalance due to
P. jirovecii infection may have an important role in COPD
progression.
HSP-27, an important small heat shock protein, is a
COPD biomarker. HSP-27 levels increase during oxidative stress,
hypoxia, and inflammation. Among these stressors, oxidative
responses of macrophages represent an important component of
microbicidal effector cell function against a variety of potential
pathogens (39). The phagocytosis of
pathogens mediated by macrophages has been correlated with the
release of oxygen radicals (40).
Previous studies have confirmed that Pneumocystis is
associated with the induction of oxidative stresses in alveolar
macrophages (41,42). The present study determined that
HSP-27 expression was higher in the PCP model group compared with
the control group, which suggested that P. jirovecii
infection was involved in inducing an oxidative burst in alveolar
macrophages. Upregulated levels of HSP-27 have also been identified
in COPD smokers compared with non-COPD smokers (16,17,43,44).
Elevated HSP-27 expression indicates that P. jirovecii
infections may serve a role in COPD progression.
The complex mechanisms of COPD remain unclear
however cigarette smoking and pathogenic infection are currently
regarded as the two major risk factors. Christensen et al
(38) demonstrated that cigarette
smoke exposure can increase the pulmonary Pneumocystis
burden whilst Pneumocystis and cigarette smoke exposure can
cause airspace enlargement. A limitation of the present study was
that the causal relationship between P. jirovecii
colonization or infection and COPD was not definitively
established. The interaction between cigarette smoke exposure and
Pneumocystis may synergistically speed up the progression of
COPD. Further confirmation of the role of Pneumocystis
infections in the progression of COPD and the causal relationship
between these are crucial for understanding the pathogenesis of
COPD and for the development of novel prophylactic treatment
options.
In conclusion, the present study established a rat
model of PCP via steroid injection, and then tested the
pathogenicity of P. jirovecii infection, investigated the
inflammatory response following PCP, and evaluated the expression
of factors closely related to COPD, including MMPs and HSP-27. The
present findings suggested that Pneumocystis caused
pulmonary inflammation similar to COPD, which may contribute to
lung tissue destruction, as well as the development of pulmonary
emphysema, protease-antiprotease imbalance, and induction of
oxidative stress in alveolar macrophages. It was demonstrated that
P. jirovecii infection may have an indirect role in the
progression of COPD therefore providing evidence for
Pneumocystis pathogenicity and its role in COPD development
and progression.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Natural
Science Foundation of China (grant no. 81370189).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The current study was designed and conceived by TX
and CLA, and performed and analyzed by TX. Data acquisition,
statistical analysis, and manuscript preparation were performed by
TX. CLA reviewed and edited the manuscript. All authors contributed
to the drafting and revision of the paper, as well as data
analysis. All authors read and approved the final manuscript and
are responsible for all aspects of this study.
Ethics approval and consent to
participate
The protocol for animal experimentation was approved
by the Experimental Animal Care and Ethics Committee of China
Medical University (Liaoning, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morris A and Norris KA: Colonization by
Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev.
25:297–317. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang DD, Zheng MQ, Zhang N and An CL:
Investigation of Pneumocystis jirovecii colonization in patients
with chronic pulmonary diseases in the People's Republic of China.
Int J Chron Obstruct Pulmon Dis. 10:2079–2085. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gutiérrez S, Respaldiza N, Campano E,
Martinez-Risquez MT, Calderón EJ and De La Horra C: Pneumocystis
jirovecii colonization in chronic pulmonary disease. Parasite.
18:121–126. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Khodavaisy S, Mortaz E, Mohammadi F,
Aliyali M, Fakhim H and Badali H: Pneumocystis jirovecii
colonization in Chronic Obstructive Pulmonary Disease (COPD). Curr
Med Mycol. 1:42–48. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martínez Lamas L, Pérez Rodríguez MT,
Álvarez Ramos I, Bouza Soage ME, Figueroa Lamas MP and Álvarez
Fernández M: Role of Pneumocystis jirovecii in patients with
different pulmonary underlying condition using a nested-PCR. Rev
Esp Quimioter. 31:336–343. 2018.PubMed/NCBI
|
|
6
|
Kling HM, Shipley TW, Patil SP, Kristoff
J, Bryan M, Montelaro RC, Morris A and Norris KA: Relationship of
Pneumocystis jiroveci humoral immunity to prevention of
colonization and chronic obstructive pulmonary disease in a primate
model of HIV infection. Infect Immun. 78:4320–4330. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morris A, Sciurba FC, Lebedeva IP,
Githaiga A, Elliott WM, Hogg JC, Huang L and Norris KA: Association
of chronic obstructive pulmonary disease severity and Pneumocystis
colonization. Am J Respir Crit Care Med. 170:408–413.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morris A, Sciurba FC and Norris KA:
Pneumocystis: A novel pathogen in chronic obstructive pulmonary
disease? COPD. 5:43–51. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ankersmit HJ, Lambers C, Zimmermann M,
Hacker S and Moser B: Serendipity and technical considerations for
the measurement of serum heat shock protein HSP27 in patients with
COPD and lung cancer. Cell Stress Chaperones. 20:727–728.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen W, Cui X, Xing J and Wu T: Response
to: Hsp27 and Hsp70 in chronic obstructive pulmonary disease. Cell
Stress Chaperones. 20:725–726. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ünver R, Deveci F, Kirkil G, Telo S, Kaman
D and Kuluöztürk M: Serum heat shock protein levels and the
relationship of heat shock proteins with various parameters in
chronic obstructive pulmonary disease patients. Turk Thorac J.
17:153–159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cane JL, Mallia-Millanes B, Forrester DL,
Knox AJ, Bolton CE and Johnson SR: Matrix metalloproteinases -8 and
-9 in the airways, blood and urine during exacerbations of COPD.
COPD. 13:26–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koo HK, Hong Y, Lim MN, Yim JJ and Kim WJ:
Relationship between plasma matrix metalloproteinase levels,
pulmonary function, bronchodilator response, and emphysema
severity. Int J Chron Obstruct Pulmon Dis. 11:1129–1137.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sng JJ, Prazakova S, Thomas PS and Herbert
C: MMP-8, MMP-9 and neutrophil elastase in peripheral blood and
exhaled breath condensate in COPD. COPD. 14:238–244.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Esquivel AL, Pérez-Ramos J, Cisneros J,
Herrera I, Rivera-Rosales R, Montaño M and Ramos C: The effect of
obesity and tobacco smoke exposure on inflammatory mediators and
matrix metalloproteinases in rat model. Toxicol Mech Methods.
24:633–643. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jan Ankersmit H, Nickl S, Hoeltl E,
Toepker M, Lambers C, Mitterbauer A, Kortuem B, Zimmermann M, Moser
B, Bekos C, et al: Increased serum levels of HSP27 as a marker for
incipient chronic obstructive pulmonary disease in young smokers.
Respiration. 83:391–399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu R, Ouyang Q, Dai A, Tan S, Xiao Z and
Tang C: Heat shock protein 27 and cyclophilin A associate with the
pathogenesis of COPD. Respirology. 16:983–993. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
An CL, Li S, Jiang L and Masanobu T:
Detection of the Pneumocystis carinii by PCR and organism staining
method. J China Med Univ. 28:27–28. 1999.(In Chinese). PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sethi S, Maloney J, Grove L, Wrona C and
Berenson CS: Airway inflammation and bronchial bacterial
colonization in chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 173:991–998. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sethi S: Infection as a comorbidity of
COPD. Eur Respir J. 35:1209–1215. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fan H, Guo JY, Ma SL, Zhang N and An CL:
Synthetic p55 tandem DNA vaccine against Pneumocystis carinii in
rats. Microbiol Immunol. 60:397–406. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Feng Y, Guo S, Jiang T, Han X, Liu P, Wu T
and Luo Y: Active immunization against Pneumocystis carinii with
p55-v3 DNA vaccine in rats. Can J Microbiol. 57:375–381.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Liu AB, Pu Y, Zheng YQ, Cai H and Ye B:
Therapeutic efficacies of chitosan against Pneumocystis pneumonia
of immunosuppressed rat. Parasite Immunol. 36:292–302.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun J, Bao J, Shi Y, Zhang B, Yuan L, Li
J, Zhang L, Sun M, Zhang L and Sun W: Effect of simvastatin on MMPs
and TIMPs in cigarette smoke-induced rat COPD model. Int J Chron
Obstruct Pulmon Dis. 12:717–724. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
An CL, Su XP and Harmsen AG: The role of
CD8+ T cells in the pathogenesis of Pneumocystis carinii pneumonia
in mice depleted of CD4+ T cells. Zhongguo Ji Sheng Chong Xue Yu Ji
Sheng Chong Bing Za Zhi. 18:207–212. 2000.PubMed/NCBI
|
|
27
|
Eppert BL, Wortham BW, Flury JL and
Borchers MT: Functional characterization of T cell populations in a
mouse model of chronic obstructive pulmonary disease. J Immunol.
190:1331–1340. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jiang SL, Li Y, Tian YG, Li JS, Li SY,
Wang Y, Wang YY and Deng L: Influence and long-term effects of
three methods for regulating and invigorating fei-shen on T
lymphocyte subsets and CD4+ CD25+ in COPD rats. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 33:1538–1544. 2013.(In Chinese). PubMed/NCBI
|
|
29
|
Zhu X, Gadgil AS, Givelber R, George MP,
Stoner MW, Sciurba FC and Duncan SR: Peripheral T cell functions
correlate with the severity of chronic obstructive pulmonary
disease. J Immunol. 182:3270–3277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cazzola M, Page CP, Calzetta L and Matera
MG: Emerging anti-inflammatory strategies for COPD. Eur Respir J.
40:724–741. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chung KF and Adcock IM: Multifaceted
mechanisms in COPD: Inflammation, immunity, and tissue repair and
destruction. Eur Respir J. 31:1334–1356. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thatcher TH, Hsiao HM, Pinner E, Laudon M,
Pollock SJ, Sime PJ and Phipps RP: Neu-164 and Neu-107, two novel
antioxidant and anti-myeloperoxidase compounds, inhibit acute
cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell
Mol Physiol. 305(L165-L174)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mocchegiani E, Giacconi R and Costarelli
L: Metalloproteases/anti-metalloproteases imbalance in chronic
obstructive pulmonary disease: Genetic factors and treatment
implications. Curr Opin Pulm Med. 17 (Suppl
1)(S11-S19)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vlahos R, Bozinovski S, Chan SP, Ivanov S,
Lindén A, Hamilton JA and Anderson GP: Neutralizing
granulocyte/macrophage colony-stimulating factor inhibits cigarette
smoke-induced lung inflammation. Am J Respir Crit Care Med.
182:34–40. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Qu J, Rong Z, He L, Pan J and Chen X:
Relationship between the burden of Pneumocystis carinii, the
inflammatory reaction and lung injury in Pneumocystis carinii
pneumonia. Chin Med J (Engl). 113:1071–1074. 2000.PubMed/NCBI
|
|
36
|
Sukura A, Konttinen YT, Sepper R,
Kaartinen L, Sorsa T and Lindberg LA: Collagenases and the serine
proteinases elastase and cathepsin G in steroid-induced
Pneumocystis carinii pneumonia. J Clin Microbiol. 33:829–834.
1995.PubMed/NCBI
|
|
37
|
Norris KA, Morris A, Patil S and Fernandes
E: Pneumocystis colonization, airway inflammation, and pulmonary
function decline in acquired immunodeficiency syndrome. Immunol
Res. 36:175–187. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Christensen PJ, Preston AM, Ling T, Du M,
Fields WB, Curtis JL and Beck JM: Pneumocystis murina infection and
cigarette smoke exposure interact to cause increased organism
burden, development of airspace enlargement, and pulmonary
inflammation in mice. Infect Immun. 76:3481–3490. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sibille Y and Reynolds HY: Macrophages and
polymorphonuclear neutrophils in lung defense and injury. Am Rev
Respir Dis. 141:471–501. 1990.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Johnston RB Jr, Godzik CA and Cohn ZA:
Increased superoxide anion production by immunologically activated
and chemically elicited macrophages. J Exp Med. 148:115–127.
1978.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pesanti EL: Interaction of cytokines and
alveolar cells with Pneumocystis carinii in vitro. J Infect Dis.
163:611–616. 1991.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hidalgo HA, Helmke RJ, German VF and
Mangos JA: Pneumocystis carinii induces an oxidative burst in
alveolar macrophages. Infect Immun. 60:1–7. 1992.PubMed/NCBI
|
|
43
|
Hacker S, Lambers C, Hoetzenecker K,
Pollreisz A, Aigner C, Lichtenauer M, Mangold A, Niederpold T,
Zimmermann M, Taghavi S, et al: Elevated HSP27, HSP70 and HSP90
alpha in chronic obstructive pulmonary disease: Markers for immune
activation and tissue destruction. Clin Lab. 55:31–40.
2009.PubMed/NCBI
|
|
44
|
Kelsen SG, Duan X, Ji R, Perez O, Liu C
and Merali S: Cigarette smoke induces an unfolded protein response
in the human lung: A proteomic approach. Am J Respir Cell Mol Biol.
38:541–550. 2008.PubMed/NCBI View Article : Google Scholar
|