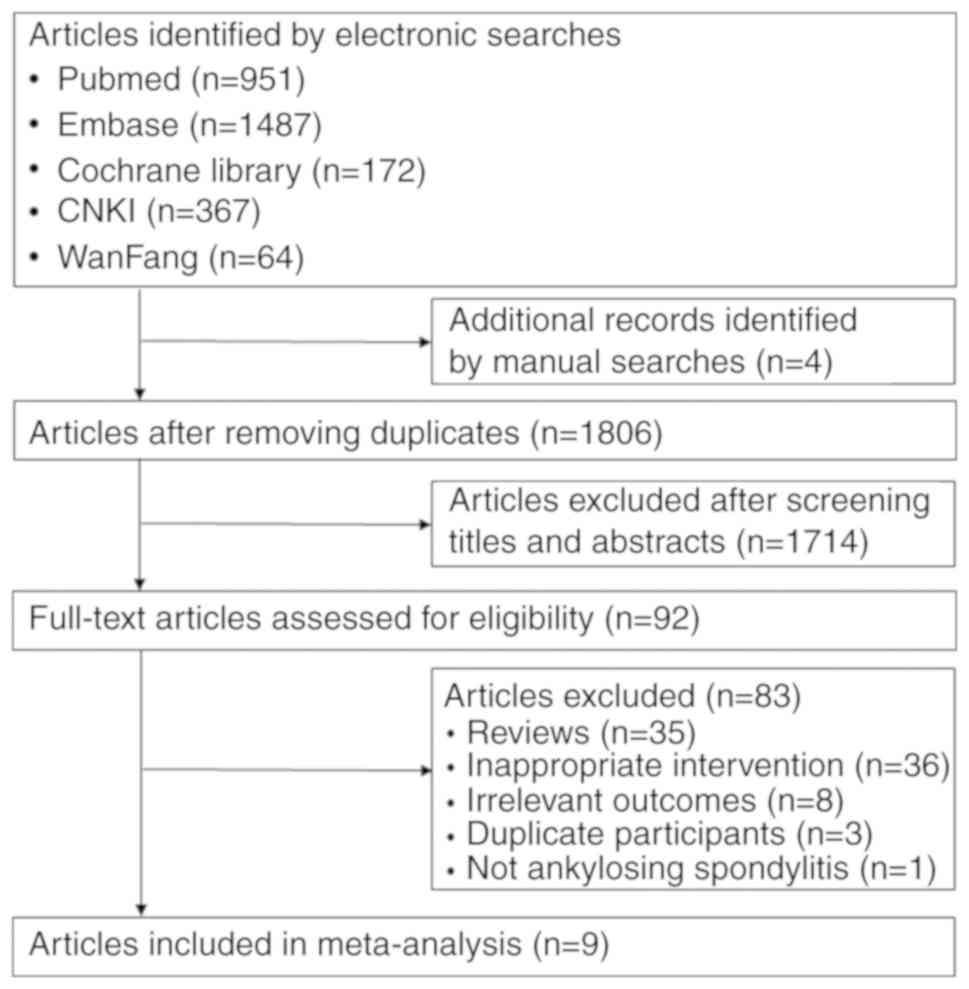
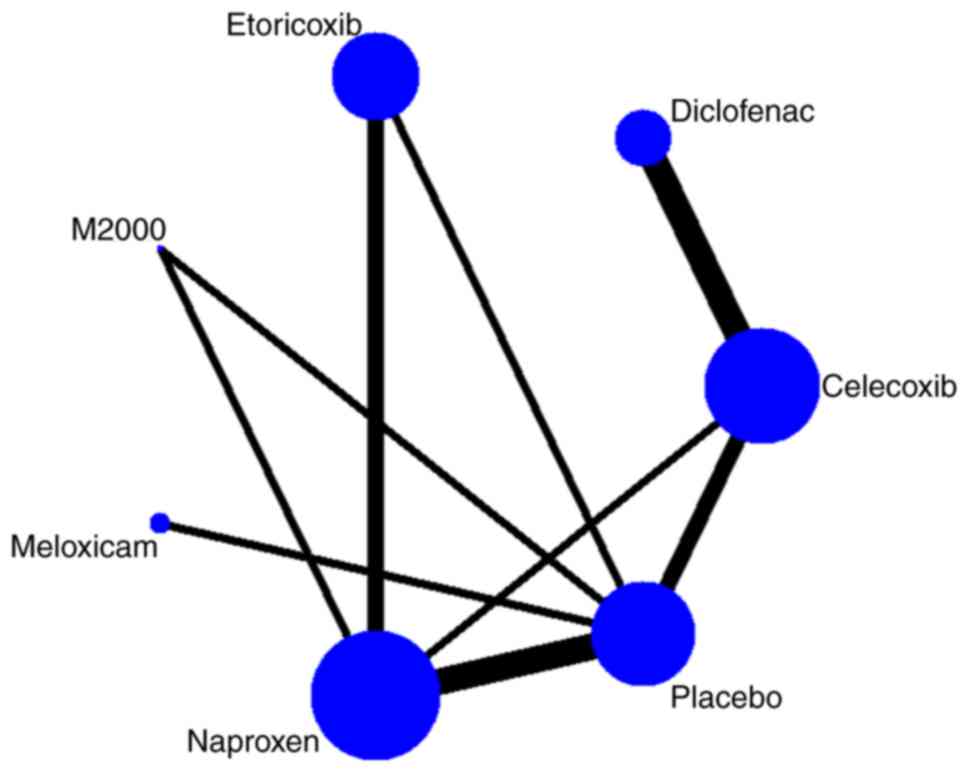
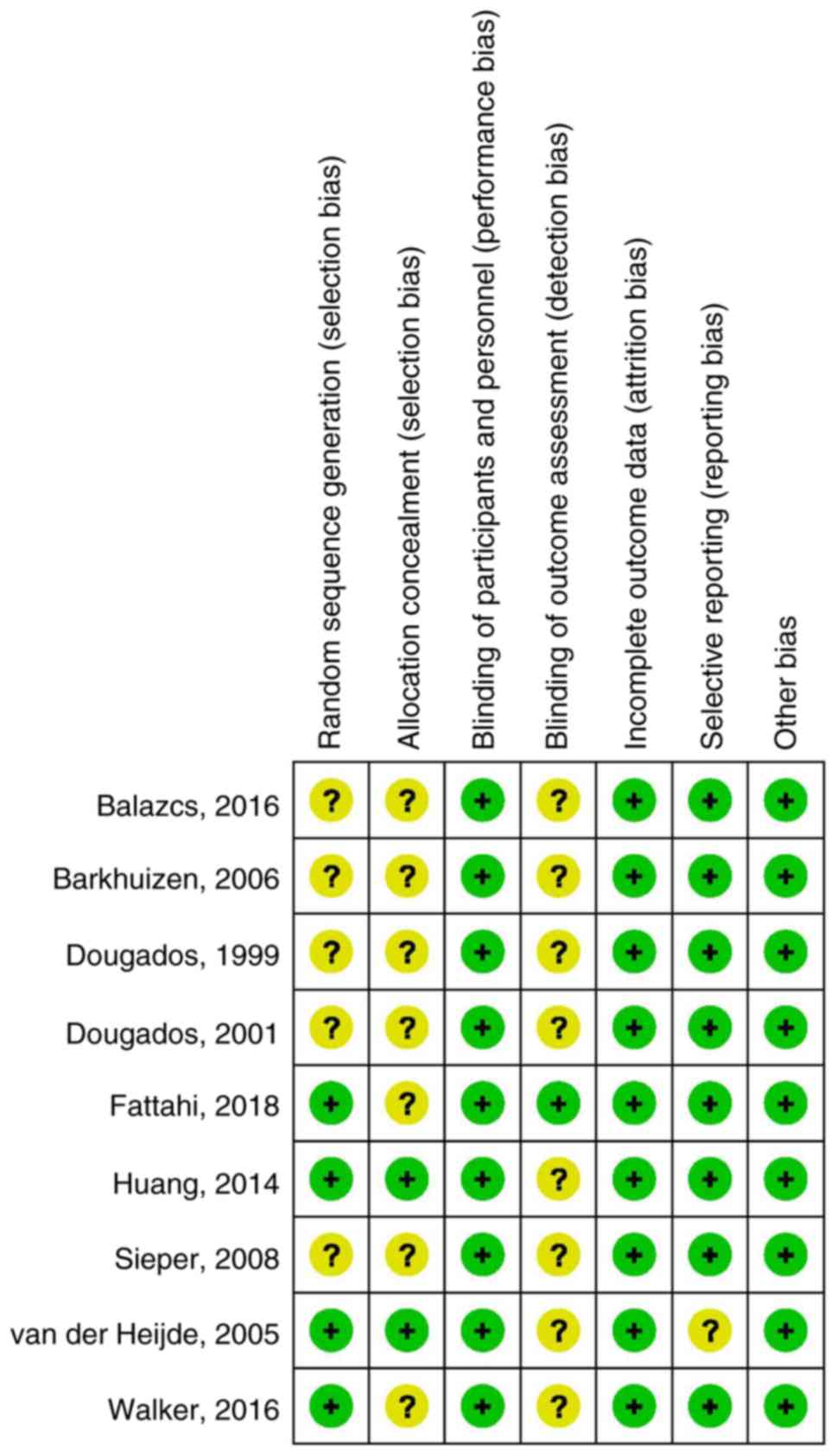
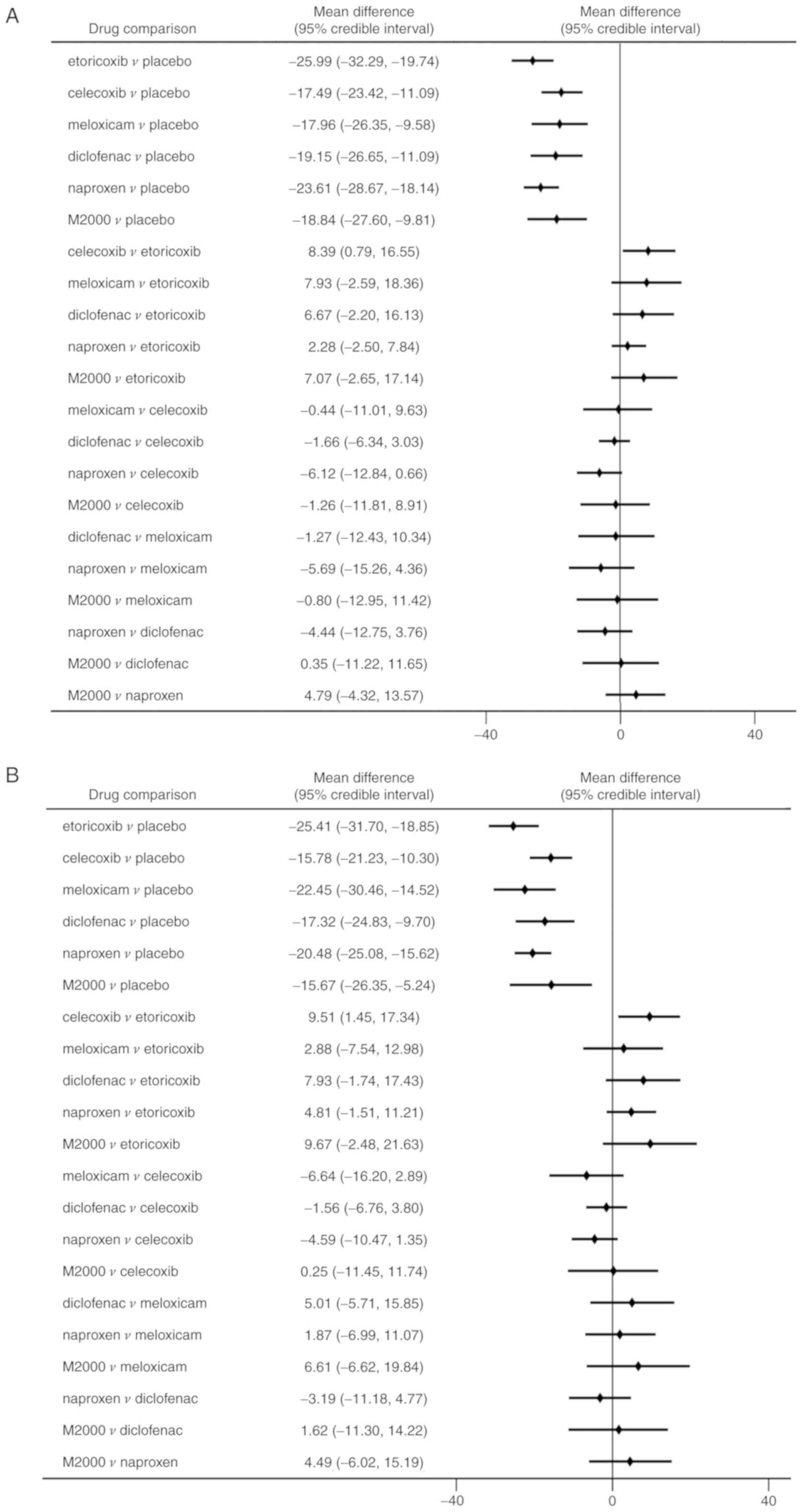
|
1
|
Sieper J and Poddubnyy D: Axial
spondyloarthritis. Lancet. 390:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guan M, Wang J, Zhao L, Xiao J, Li Z and
Shi Z: Management of hip involvement in ankylosing spondylitis.
Clin Rheumatol. 32:1115–1120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang J, Zhang Y, Zhao L, Li ZH and Shi ZJ:
The efficacy and safety of infliximab used in patients with
ankylosing spondylitis after unilateral total hip arthroplasty. Hip
Int. 23:406–410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guan M, Wang J, Zhu Z, Xiao J, Zhao L, Li
Z and Shi Z: Comparison in clinical features and life impact
between juvenile-onset and adult-onset ankylosing spondylitis. Turk
J Med Sci. 44:601–605. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boonen A, Chorus A, Miedema H, van der
Heijde D, van der Tempel H and van der Linden S: Employment, work
disability, and work days lost in patients with ankylosing
spondylitis: A cross sectional study of Dutch patients. Ann Rheum
Dis. 60:353–358. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ward MM, Deodhar A, Akl EA, Lui A, Ermann
J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman
RD, et al: American College of Rheumatology/Spondylitis Association
of America/Spondyloarthritis Research and Treatment Network 2015
recommendations for the treatment of ankylosing spondylitis and
nonradiographic axial spondyloarthritis. Arthritis Care Res
(Hoboken). 68:151–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braun J, van den Berg R, Baraliakos X,
Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H,
Dijkmans B, Dougados M, Emery P, et al: 2010 update of the
ASAS/EULAR recommendations for the management of ankylosing
spondylitis. Ann Rheum Dis. 70:896–904. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zochling J, van der Heijde D,
Burgos-Vargas R, Collantes E, Davis JC, Jr..Dijkmans B, Dougados M,
Géher P, Inman RD, Khan MA, et al: ASAS/EULAR recommendations for
the management of ankylosing spondylitis. Ann Rheum Dis.
65:442–452. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Poddubnyy D, Rudwaleit M, Haibel H,
Listing J, Märker-Hermann E, Zeidler H, Braun J and Sieper J:
Effect of non-steroidal anti-inflammatory drugs on radiographic
spinal progression in patients with axial spondyloarthritis:
Results from the German Spondyloarthritis Inception Cohort. Ann
Rheum Dis. 71:1616–1622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wanders A, Heijde D, Landewé R, Béhier JM,
Calin A, Olivieri I, Zeidler H and Dougados M: Nonsteroidal
antiinflammatory drugs reduce radiographic progression in patients
with ankylosing spondylitis: A randomized clinical trial. Arthritis
Rheum. 52:1756–1765. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shukla A, Rai MK, Prasad N and Agarwal V:
Short-term non-steroid anti-inflammatory drug use in
spondyloarthritis patients induces subclinical acute kidney injury:
Biomarkers study. Nephron. 135:277–286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song IH, Poddubnyy DA, Rudwaleit M and
Sieper J: Benefits and risks of ankylosing spondylitis treatment
with nonsteroidal antiinflammatory drugs. Arthritis Rheum.
58:929–938. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fosbøl EL, Køber L, Torp-Pedersen C and
Gislason GH: Cardiovascular safety of non-steroidal
anti-inflammatory drugs among healthy individuals. Expert Opin Drug
Saf. 9:893–903. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Combe B, Swergold G, McLay J, McCarthy T,
Zerbini C, Emery P, Connors L, Kaur A, Curtis S, Laine L and Cannon
CP: Cardiovascular safety and gastrointestinal tolerability of
etoricoxib vs diclofenac in a randomized controlled clinical trial
(The MEDAL study). Rheumatology (Oxford). 48:425–432.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rostom A, Muir K, Dubé C, Jolicoeur E,
Boucher M, Joyce J, Tugwell P and Wells GW: Gastrointestinal safety
of cyclooxygenase-2 inhibitors: A Cochrane Collaboration systematic
review. Clin Gastroenterol Hepatol. 5:818–828, 828.e1-e5; quiz 768.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trelle S, Reichenbach S, Wandel S,
Hildebrand P, Tschannen B, Villiger PM, Egger M and Jüni P:
Cardiovascular safety of non-steroidal anti-inflammatory drugs:
Network meta-analysis. BMJ. 342(c7086)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang F, Gu J, Liu Y, Zhu P, Zheng Y, Fu
J, Pan S and Le S: Efficacy and safety of celecoxib in chinese
patients with ankylosing spondylitis: A 6-week randomized,
double-blinded study with 6-week open-label extension treatment.
Curr Ther Res Clin Exp. 76:126–133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sieper J, Klopsch T, Richter M, Kapelle A,
Rudwaleit M, Schwank S, Regourd E and May M: Comparison of two
different dosages of celecoxib with diclofenac for the treatment of
active ankylosing spondylitis: Results of a 12-week randomised,
double-blind, controlled study. Ann Rheum Dis. 67:323–329.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Heijde D, Baraf HS, Ramos-Remus C,
Calin A, Weaver AL, Schiff M, James M, Markind JE, Reicin AS,
Melian A and Dougados M: Evaluation of the efficacy of etoricoxib
in ankylosing spondylitis: Results of a fifty-two-week, randomized,
controlled study. Arthritis Rheum. 52:1205–1215. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Balazcs E, Sieper J, Bickham K, Mehta A,
Frontera N, Stryszak P, Popmihajlov Z and Peloso PM: A randomized,
clinical trial to assess the relative efficacy and tolerability of
two doses of etoricoxib versus naproxen in patients with ankylosing
spondylitis. BMC Musculoskelet Disord. 17(426)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Walker C, Essex MN, Li C and Park PW:
Celecoxib versus diclofenac for the treatment of ankylosing
spondylitis: 12-week randomized study in Norwegian patients. J Int
Med Res. 44:483–495. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Barkhuizen A, Steinfeld S, Robbins J, West
C, Coombs J and Zwillich S: Celecoxib is efficacious and well
tolerated in treating signs and symptoms of ankylosing spondylitis.
J Rheumatol. 33:1805–1812. 2006.PubMed/NCBI
|
|
25
|
Dougados M, Béhier JM, Jolchine I, Calin
A, van der Heijde D, Olivieri I, Zeidler H and Herman H: Efficacy
of celecoxib, a cyclooxygenase 2-specific inhibitor, in the
treatment of ankylosing spondylitis: A six-week controlled study
with comparison against placebo and against a conventional
nonsteroidal antiinflammatory drug. Arthritis Rheum. 44:180–185.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dougados M, Gueguen A, Nakache JP,
Velicitat P, Veys EM, Zeidler H and Calin A: Ankylosing
spondylitis: What is the optimum duration of a clinical study? A
one year versus a 6 weeks non-steroidal anti-inflammatory drug
trial. Rheumatology (Oxford). 38:235–244. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fattahi MJ, Jamshidi AR, Mahmoudi M,
Vojdanian M, Yekaninejad MS, Jafarnezhad-Ansariha F, Ahmadi H, Rehm
BHA, Matsuo H, Cuzzocrea S, et al: Evaluation of the efficacy and
safety of beta-d-mannuronic acid in patients with ankylosing
spondylitis: A 12-week randomized, placebo-controlled, phase I/II
clinical trial. Int Immunopharmacol. 54:112–117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nieves-Cintrón M, Syed AU, Nystoriak MA
and Navedo MF: Regulation of voltage-gated potassium channels in
vascular smooth muscle during hypertension and metabolic disorders.
Microcirculation. 25:2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Calin A, Garrett S, Whitelock H, Kennedy
LG, O'Hea J, Mallorie P and Jenkinson T: A new approach to defining
functional ability in ankylosing spondylitis: The development of
the Bath Ankylosing Spondylitis Functional Index. J Rheumatol.
21:2281–2285. 1994.PubMed/NCBI
|
|
30
|
Anderson JJ, Baron G, van der Heijde D,
Felson DT and Dougados M: Ankylosing spondylitis assessment group
preliminary definition of short-term improvement in ankylosing
spondylitis. Arthritis Rheum. 44:1876–1886. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lu G and Ades AE: Combination of direct
and indirect evidence in mixed treatment comparisons. Stat Med.
23:3105–3124. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Sutton AJ and Abrams KR: Bayesian methods
in meta-analysis and evidence synthesis. Stat Methods Med Res.
10:277–303. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: Combining direct
and indirect evidence. BMJ. 331:897–900. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao BC, Huang TY, Deng QW, Liu WF, Liu J,
Deng WT, Liu KX and Li C: Prophylaxis against atrial fibrillation
after general thoracic surgery: Trial sequential analysis and
network meta-analysis. Chest. 151:149–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Salanti G, Ades AE and Ioannidis JP:
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial. J
Clin Epidemiol. 64:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dougados M, Simon P, Braun J,
Burgos-Vargas R, Maksymowych WP, Sieper J and van der Heijde D:
ASAS recommendations for collecting, analysing and reporting NSAID
intake in clinical trials/epidemiological studies in axial
spondyloarthritis. Ann Rheum Dis. 70:249–251. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang R, Dasgupta A and Ward MM:
Comparative efficacy of non-steroidal anti-inflammatory drugs in
ankylosing spondylitis: A Bayesian network meta-analysis of
clinical trials. Ann Rheum Dis. 75:1152–1160. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jansen JP and Taylor SD:
Cost-effectiveness evaluation of etoricoxib versus celecoxib and
nonselective NSAIDs in the treatment of ankylosing spondylitis in
Norway. Int J Rheumatol. 2011(160326)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fattahi MJ, Abdollahi M, Agha Mohammadi A,
Rastkari N, Khorasani R, Ahmadi H, Tofighi Zavareh F, Sedaghat R,
Tabrizian N and Mirshafiey A: Preclinical assessment of
β-D-mannuronic acid (M2000) as a nonsteroidal anti-inflammatory
drug. Immunopharmacol Immunotoxicol. 37:535–540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kristensen LE, Jakobsen AK, Askling J,
Nilsson F and Jacobsson LT: Safety of etoricoxib, celecoxib, and
nonselective nonsteroidal antiinflammatory drugs in ankylosing
spondylitis and other spondyloarthritis patients: A Swedish
National Population-Based Cohort study. Arthritis Care Res
(Hoboken). 67:1137–1149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kroon F, Landewé R, Dougados M and van der
Heijde D: Continuous NSAID use reverts the effects of inflammation
on radiographic progression in patients with ankylosing
spondylitis. Ann Rheum Dis. 71:1623–1629. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sieper J, Listing J, Poddubnyy D, Song IH,
Hermann KG, Callhoff J, Syrbe U, Braun J and Rudwaleit M: Effect of
continuous versus on-demand treatment of ankylosing spondylitis
with diclofenac over 2 years on radiographic progression of the
spine: Results from a randomised multicentre trial (ENRADAS). Ann
Rheum Dis. 75:1438–1443. 2016.PubMed/NCBI View Article : Google Scholar
|