Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disease characterized by lower back pain, enthesitis
and asymmetrical peripheral arthritis (1). It predominantly affects the axial
skeleton (2). Hip involvement
usually causes severe functional impairment (3,4). The
disease commonly occurs in the second or third decade of life.
Without adequate treatment, it may lead to disability and a
significant decrease in quality of life (5,6).
Non-steroidal anti-inflammatory drugs (NSAIDs) are
commonly used to reduce pain and inflammation, and are recommended
as first-line agents for the treatment of AS (7-9).
Furthermore, continuous treatment with NSAIDs is preferred for
symptomatic patients (8,10,11).
However, the use of NSAIDs may increase the risk of
gastrointestinal (GI), cardiovascular and renal adverse events
(AEs) (12-14).
Traditional non-selective NSAIDs are associated with an increased
risk of GI events due to their inhibition of cyclooxygenase (COX)-1
isoenzyme (15). Furthermore, COX-2
selective inhibitors may reduce GI toxicity but increase the risk
of cardiovascular events (16,17).
Several randomized controlled trials (RCTs) have
indicated that in AS, COX-2 inhibitors, including etoricoxib and
celecoxib, have comparable or superior efficacy and improved GI
tolerability compared to non-selective NSAIDs (18-22).
However, comprehensive comparisons among various NSAIDs are scarce.
It is necessary to compare the benefits and disadvantages among the
most commonly used NSAIDs. In the present study, an indirect
comparison was performed using a Bayesian network meta-analysis to
assess the efficacy and safety of NSAIDs in the treatment of
AS.
Materials and methods
Literature search
A systematic literature search on PubMed, Embase,
the Cochrane Library, China National Knowledge Infrastructure and
WanFang databases (with entries up to August 12, 2019 considered)
was performed without any restrictions regarding the region,
publication date or language. Titles/abstracts were searched using
logic combinations of the following terms: (‘ankylosing
spondylitis’ OR ‘spondyloarthritis’) AND (‘non-steroidal
anti-inflammatory drugs’ OR ‘etoricoxib’ OR ‘celecoxib’ OR
‘meloxicam’ OR ‘diclofenac’ OR ‘naproxen’) AND ‘randomized
controlled trial’. The web-based search was supplemented with
manual searches of references of relevant reviews on AS. When
multiple studies describing the same population were published, the
most complete study was used.
All double-blinded RCTs that enrolled patients
fulfilling the modified 1984 AS New York criteria (23) were included and comparisons between
different NSAIDs or of an NSAID with placebo were performed. The
outcomes reported within 12 weeks were used. Studies were excluded
if none of the quantitative outcomes of interest (see below) was
reported. Studies on patients with concomitant treatment with
prednisone >10 mg/d or biologics were excluded, while those
using sulfasalazine for patients who had been using them with
stable doses prior to the study were considered.
Data extraction and quality
assessment
Two researchers (MDF and JL) independently screened
the studies retrieved for eligibility and extracted data from the
trials included. The following data were extracted for each
eligible RCT: First author, publication year, interventions, study
duration, endpoints and patients' characteristics. Disagreements
were resolved through discussion. Mean differences (MDs) and
standard deviations were used to describe continuous outcomes and
the number of events was used for dichotomous outcomes. When the
values were not provided in the published article, the data were
extracted from graphs. For trials that assessed more than one dose
of an NSAID, the effects of different doses were pooled together.
The present analysis was based on the intent-to-treat principle,
which included all patients receiving at least one dose of the
studied drug (20,22,24-27).
Evaluations of methodological quality of the RCTs
included were performed independently by two reviewers (MDF and JL)
according to the standard criteria of The Cochrane Collaboration
(28), which consists of seven
items: Random sequence generation, allocation concealment, blinding
of participants, blinding of outcome assessment, incomplete outcome
data, selective reporting and other bias. Regarding each of the
above items, each study received a rating as low, high or unclear
and two reviewers assessed each trial. The studies were then
divided into three categories: i) Low risk of bias: Low risk of
bias for all key domains; ii) moderate risk of bias: Unclear risk
of bias for one or more key domains; iii) high risk of bias: High
risk of bias for one or more key domains.
Endpoints of interest
The primary efficacy endpoints included the mean
change in total pain score, patients' global assessment of disease
activity (PGA) (29) and the Bath
Ankylosing Spondylitis Functional Index (BASFI) (30). Pain and PGA scores were assessed
using a 0-100 mm visual analog scale (VAS). BASFI assessment was
performed using a series of 10 specific questions, each answered on
a 0-100 mm VAS where 0 indicated ‘easy’ and 100 indicated
‘impossible’ (30). The secondary
efficacy endpoints were the proportions of patients reaching the
Assessment in Ankylosing Spondylitis 20 improvement criteria
(ASAS20) (29), which were defined
as an improvement of ≥20% and absolute improvement of ≥10 units
(0-100 mm VAS) from baseline in at least 3 of the following 4
domains: PGA, total back pain, BASFI and inflammation/morning
stiffness, without any worsening of ≥20% and 10 units in the
remaining domain.
The safety endpoints included total AEs, GI events,
withdrawals due to AEs and serious AEs during the study. The GI
events were defined as any abdominal complaints, including nausea,
vomiting, dyspepsia, heartburn, diarrhea, constipation and
abdominal pain.
Statistical analysis
Indirect comparisons were performed using a
random-effects Bayesian network meta-analysis with WinBUGS version
1.4.3 (MRC Biostatistics Unit). Bayesian network meta-analysis
incorporates direct and indirect comparisons of treatments, so as
to derive estimates of effect of one treatment against another and
perform a ranking of treatments (31-34).
For continuous data, the MDs were reported from the
median of the posterior distribution with the accompanying 95%
credible intervals (CrIs). For dichotomous data, the odds ratios
(ORs) with the 95% CrIs were presented. Furthermore, the
probability of being the best (Pbest) for each treatment
was estimated (35). The goodness of
model fit was assessed using the residual deviance to examine the
validity of the network models, which should be close to the data
points. To assess the robustness of the results, a sensitivity
analysis was performed by including only trials with full daily
doses of NSAIDs, i.e. etoricoxib 90 mg/d, celecoxib 400 mg/d,
meloxicam 15 mg/d, diclofenac 150 mg/d, naproxen 1,000 mg/d and
beta-D-mannuronic acid (M2000) 1,000 mg/d (36).
A traditional meta-analysis was also performed using
RevMan version 5.3.3 (Cochrane Collaboration) for the outcomes of
withdrawals due to AEs and serious AEs. As these outcomes were rare
events (<10%), the Peto ORs with corresponding 95% confidence
intervals (CIs) were calculated.
Results
Literature search
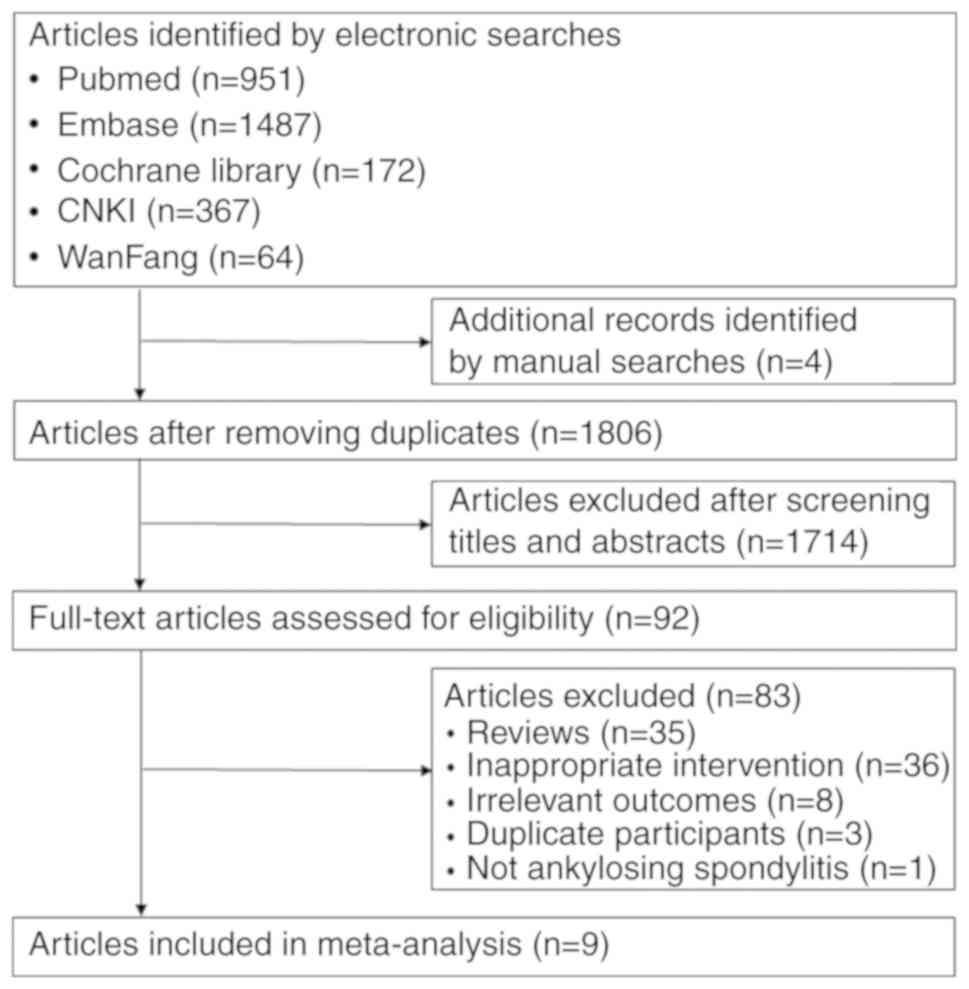
The selection process of trials for inclusion in the
present study is summarized in Fig.
1. The literature research identified 1,806 records in total,
1,714 of which were excluded after screening their titles and
abstracts. The full text of the 92 remaining, potentially eligible
articles was reviewed. Finally, 9 RCTs (18-22,24-27)
comprising 3,647 patients focusing on 6 NSAIDs, including
etoricoxib, celecoxib, meloxicam, diclofenac, naproxen and M2000,
were selected for analysis. All studies compared an NSAID with
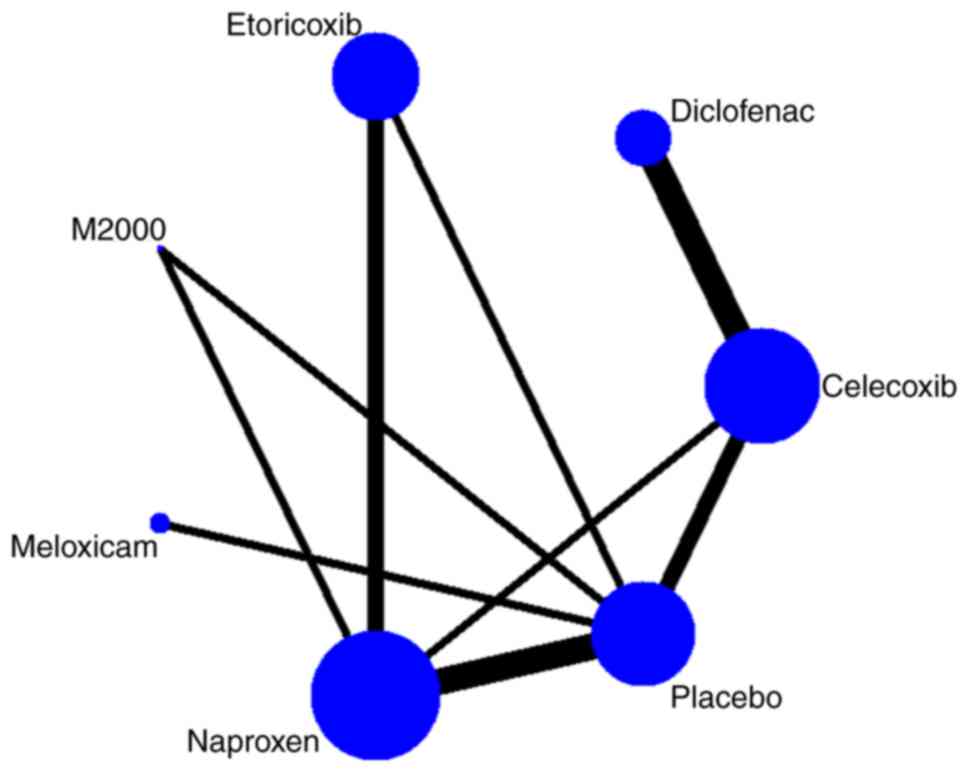
placebo or a different NSAID. A network diagram of treatment
comparisons among these trials is provided in Fig. 2.
Characteristics of the included
trials
The major characteristics of the RCTs included are
summarized in Table I. While all of
the patients included were diagnosed with AS, there were certain
differences, e.g. in terms of the pain score, PGA and BASFI at
baseline. The treatment duration varied from 6 to 12 weeks. All of
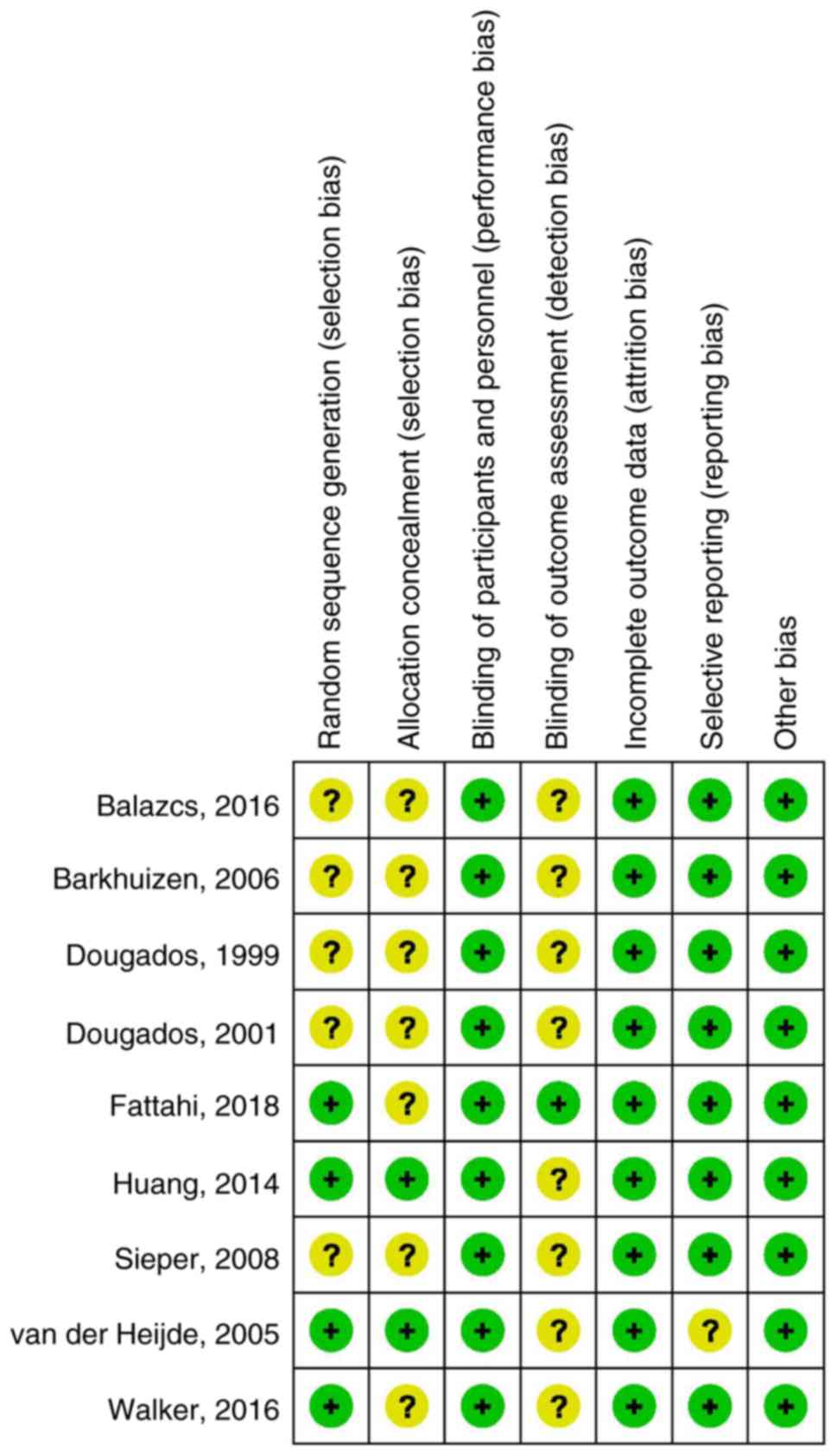
the included studies were double-blinded RCTs. The results of the
risk of bias assessment of the included trials are presented in
Fig. 3. There was a moderate risk of
bias in all trials, mostly due to lack of blinding of outcome
assessment or allocation concealment.
 | Table IMajor characteristics of the trials
included. |
Table I
Major characteristics of the trials
included.
| | | | Patients | | | | |
|---|
| First author
(year) | Interventions | Study duration
(weeks) | N (% males) | Age (years) | Pain score at
baseline (mm) | PGA at baseline
(mm) | BASFI at baseline
(mm) | (Refs.) |
|---|
| Balazcs (2016) | Etoricoxib 60/90
mg/d Naproxen 1,000 mg/d | 6 | 1015 (70.9) | 45.2 | 76.8 | NR | NR | (21) |
| Barkhuizen
(2006) | Celecoxib 200/400
mg/d Naproxen 1,000 mg/d Placebo | 12 | 611 (73.8) | 44.6 | 71.9 | 66.6 | 52.1 | (24) |
| Dougados
(1999) | Meloxicam 15/22.5
mg/d Placebo | 6 | 365 (78.7) | 42.0 | 71.0 | NR | NR | (26) |
| Dougados
(2001) | Celecoxib 200 mg/d
Placebo | 6 | 156 (70.5) | 39.0 | 70.0 | 44.5 | 44.5 | (25) |
| Fattahi (2018) | M2000 1,000 mg/d
Naproxen 1,000 mg/d Placebo | 12 | 85 (70.6) | 30.5 | 61.5 | 62.8 | 44.3 | (27) |
| Huang (2014) | Celecoxib 200 mg/d
Diclofenac 75 mg/d | 6 | 240 (85.8) | 29.3 | 63.4 | NR | NR | (18) |
| Sieper (2008) | Celecoxib 200/400
mg/d Diclofenac 150 mg/d | 12 | 458 (69.2) | 44.8 | 66.0 | 44.0 | 44.0 | (19) |
| van der Heijde
(2005) | Etoricoxib 90/120
mg/d Naproxen 1,000 mg/d Placebo | 6 | 387 (77.8) | 43.6 | 77.6 | 55.1 | 55.1 | (20) |
| Walker (2016) | Celecoxib 200/400
mg/d Diclofenac 150 mg/d | 12 | 330 (72.4) | 43.8 | 65.5 | 65.4 | 47.3 | (22) |
Efficacy of NSAIDs
All of the 9 trials reported the mean change of the
pain score (18-22,24-27).
A total of 7 trials reported the PGA (19,20,22,24-27),
7 reported the BASFI (18-20,22,24,25,27)
and 6 reported the ASAS20 (18-20,22,24,27).
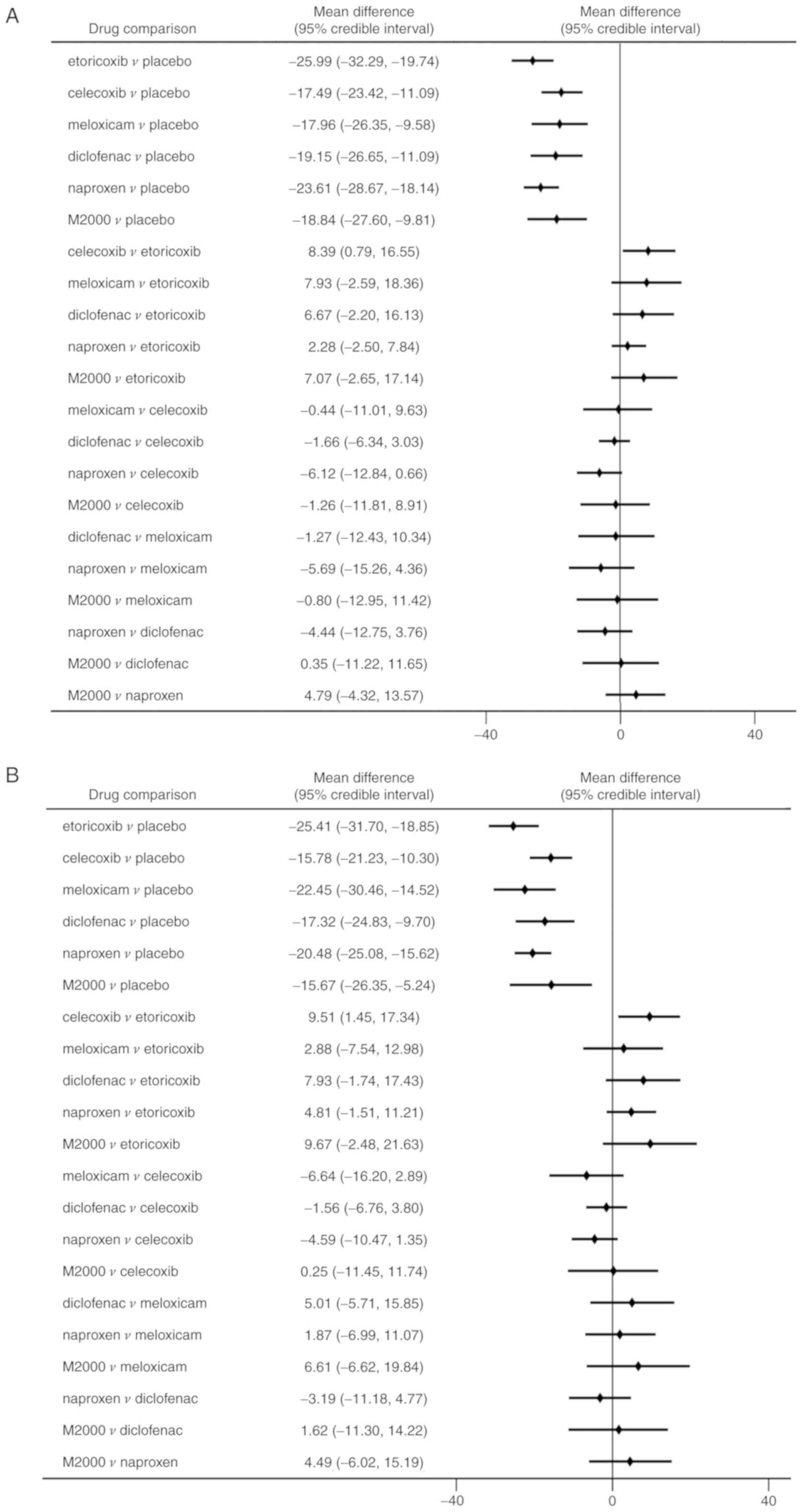
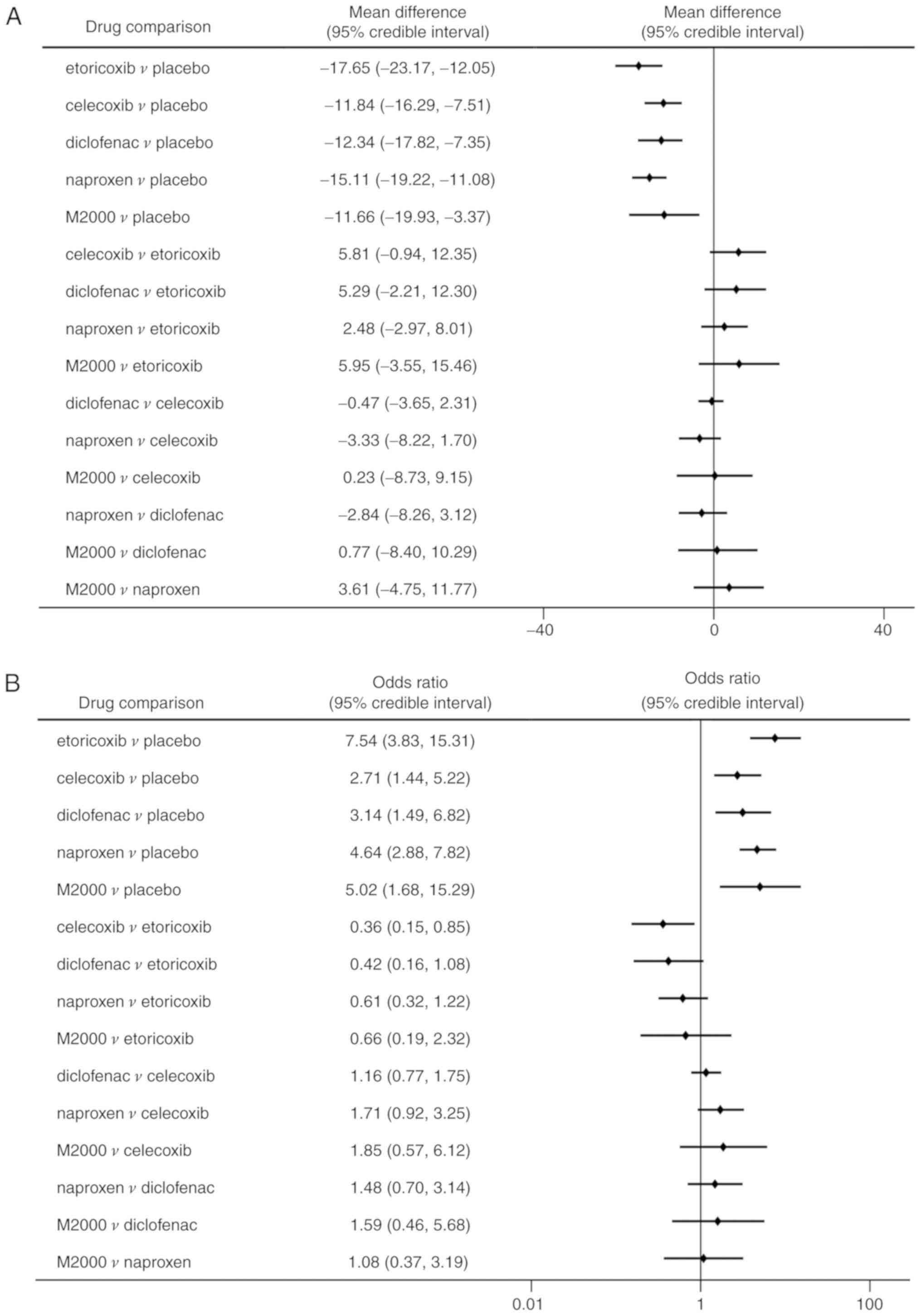
Compared with placebo, all NSAIDs were significantly
more efficacious in reducing pain severity (MDs between -17.49 and
-25.99 with a lower value indicating higher efficacy; Fig. 4A). Etoricoxib was significantly more
effective than celecoxib in terms of pain alleviation (MD=-8.39,
95% CrI: -16.55 to -0.79). Analysis of ranking probabilities
indicated that etoricoxib had the highest probability of being the
best treatment in decreasing pain severity (Pbest,
73.8%) (Table II).
 | Table IIDetermination of the most effective
non-steroidal anti-inflammatory drug in the treatment of ankylosing
spondylitis. |
Table II
Determination of the most effective
non-steroidal anti-inflammatory drug in the treatment of ankylosing
spondylitis.
| Agent | Change in pain
score (%) | Change in PGA score
(%) | Change in BASFI
score (%) | ASAS20 (%) |
|---|
| Etoricoxib | 73.8 | 67.2 | 76.1 | 71.8 |
| Celecoxib | 0.19 | 0.00 | 0.58 | 0.22 |
| Meloxicam | 4.62 | 25.1 | NA | NA |
| Diclofenac | 4.28 | 2.06 | 3.54 | 1.53 |
| Naproxen | 11.8 | 2.10 | 12.1 | 2.62 |
| M2000 | 5.33 | 3.50 | 7.70 | 23.8 |
Similarly, significant improvements in PGA and BASFI
were determined in patients receiving NSAIDs compared to placebo
(Figs. 4B and 5A). Etoricoxib was superior to celecoxib in
reducing the PGA score with statistical significance (MD=-9.51, 95%
CrI: -17.34 to -1.45). However, there were no significant
differences among the NSAIDs in decreasing the BASFI. All NSAIDs
had a significantly higher rate of ASAS20 compared with placebo
(ORs between 2.71 and 7.54; Fig.
5B). But celecoxib was significantly less efficacious in
reaching ASAS20 than etoricoxib (OR=0.36, 95% CrI: 0.15-0.85). The
probability analysis suggested that etoricoxib remained the most
effective option for the outcomes of PGA, BASFI and ASAS20
(Pbest of 67.2, 76.1 and 71.8%, respectively; Table II).
The test for goodness of model fit suggested that
the models of efficacy outcomes were appropriate. In the
sensitivity analysis of full-dose NSAIDs trials, all NSAIDs were
highly effective in improving pain, PGA, BASFI and achieving the
ASAS20, except that diclofenac had an inconclusive higher rate of
ASAS20 than placebo (data not shown). Etoricoxib remained the best
therapy regarding the outcomes of pain, PGA, BASFI and ASAS20
(Pbest of 77.8, 65.6, 66.5 and 67.1%, respectively).
However, no significant differences were obtained between
etoricoxib and celecoxib in improving pain, PGA scores and
ASAS20.
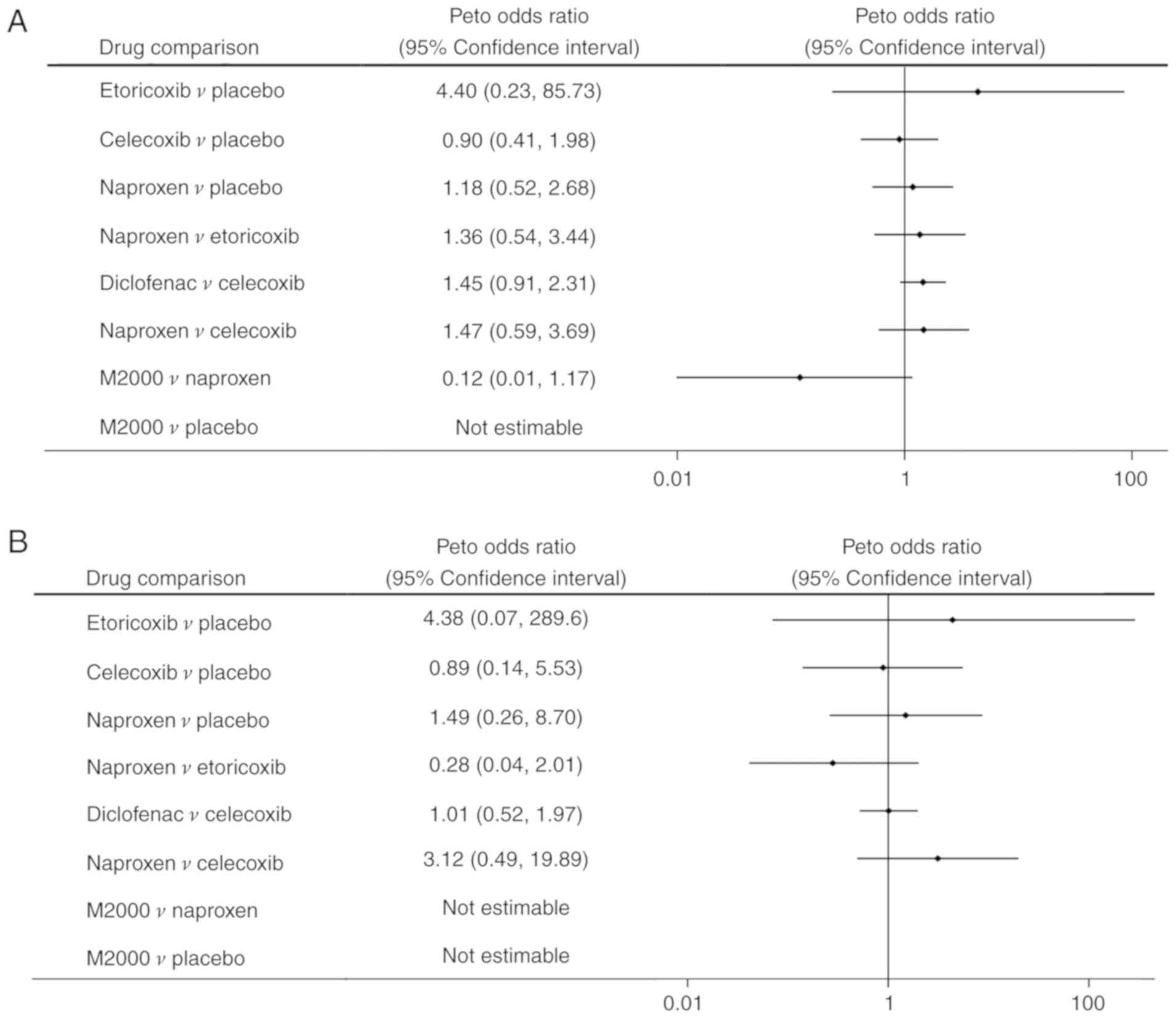
Safety of NSAIDs
A total of 8 RCTs reported on the occurrence of
total AEs, GI events, withdrawals due to AEs and serious AEs
(18-22,24,25,27).
Meloxicam was not included in the analysis due to a lack of
data.
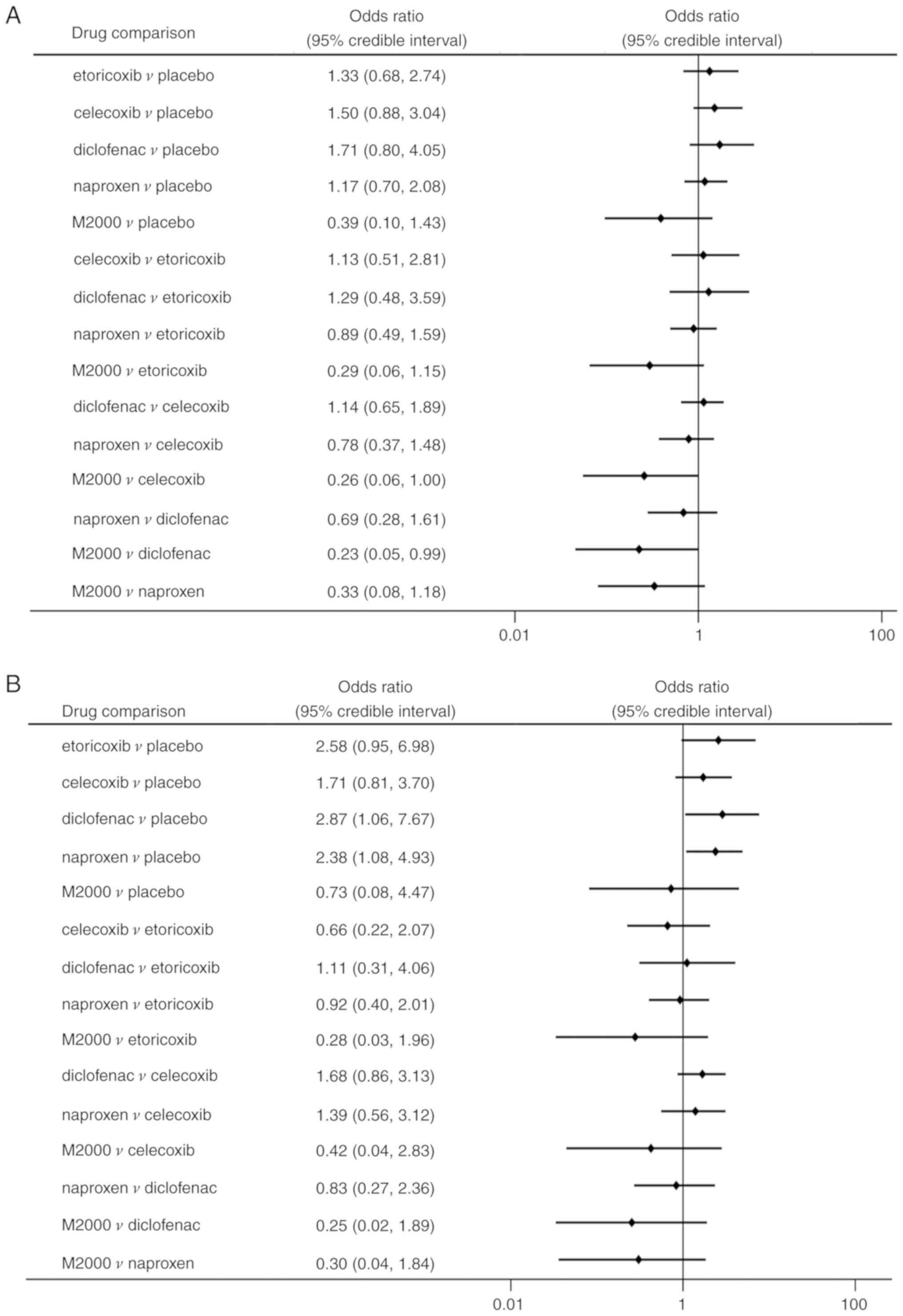
Network meta-analysis demonstrated no statistically
significant differences among NSAIDs and placebo regarding the risk
of total AEs (Fig. 6A).
Additionally, no significant differences were identified in AEs of
the M2000 and placebo (OR=0.39, 95% CrI: 0.10-1.43). The
probability analysis indicated that M2000 had the greatest
probability of being the safest treatment (Pbest,
90.5%). Furthermore, M2000 was associated with a lower incidence of
AEs than celecoxib and diclofenac (OR=0.26, 95% CrI: 0.06-1.00 and
OR=0.23, 95% CrI: 0.05-0.99, respectively).
Regarding the risk of GI events, patients treated
with diclofenac and naproxen had a significantly higher risk than
those taking placebo (OR=2.87, 95% CrI: 1.06-7.67 and OR=2.38, 95%
CrI: 1.08-4.93, respectively; Fig.
6B). No significant differences in terms of GI events were
determined among the different NSAIDs. However, M2000 had a
considerable probability of being ranked as the safest drug for
decreasing GI events (62.2%). Regarding withdrawals due to AEs and
serious AEs, there were no statistically significant differences
among NSAIDs and placebo (Fig. 7A
and B).
The test for goodness of model fit suggested that
the models of safety endpoints were appropriate. In the sensitivity
analysis of full-dose trials, no significant differences in total
AEs were identified among NSAIDs and placebo groups. Additionally,
no significant differences were identified in the GI events for
diclofenac and naproxen when compared with the placebo. M2000
remained the safest therapy for the outcomes of AEs and GI events
(Pbest of 87.3 and 61.4%, respectively). The results of
withdrawals due to AEs and serious AEs remained stable during
sensitivity analysis.
Discussion
The present meta-analysis provides comparative
information on the efficacy and safety of commonly used NSAIDs in
the treatment of AS. The analysis confirmed that NSAIDs were
consistently more effective than placebo in improving pain, disease
activity and physical function in patients with AS. Furthermore,
etoricoxib was significantly superior to celecoxib in reducing
pain, PGA scores and achievement of ASAS20. However, in the
sensitivity analysis of full-dose NSAID trials, no significant
differences in efficacy outcomes were obtained between etoricoxib
and celecoxib. However, etoricoxib still ranked as the most
efficacious treatment for AS.
Likewise, the trials comparing etoricoxib to
naproxen directly identified that etoricoxib had superior or
comparable efficacy compared with naproxen (20,21). In
their recent indirect comparison of NSAIDs for AS, Wang et
al (37) also demonstrated that
etoricoxib was more effective than certain other NSAIDs in reducing
pain, which is in accordance with the present results. Furthermore,
an economic evaluation indicated that etoricoxib was a more
cost-effective treatment for AS compared to celecoxib, diclofenac
and naproxen for a duration of >5 years (38).
With regard to safety, there were no significant
differences between NSAIDs and placebo in terms of total AEs,
withdrawals due to AEs or serious AEs. Additionally, no significant
differences in AEs were identified between M2000 and the placebo.
However, M2000 was ranked as the safest drug for AEs and GI events.
M2000, a novel NSAID with immunosuppressive properties, has been
indicated to be well tolerated with a high safety profile for the
digestive system and kidney (39).
In a recent RCT, Fattahi et al demonstrated that M2000 had
similar efficacy, but lower risk of GI and other AEs than naproxen
for the treatment of AS (27).
In addition, patients taking diclofenac or naproxen
complained of more GI events than those with placebo. Thus,
clinicians should take the risk of GI events into account when
prescribing NSAIDs. In the present analysis, there were no
significant differences in safety among etoricoxib, celecoxib and
non-selective NSAIDs. The reason may be that AS patients are on
average young and, therefore, have a lower risk of GI events. These
results are consistent with those of a recent cohort study, which
identified no significantly increased risks of GI and
cardiovascular events for etoricoxib, celecoxib and non-selective
NSAIDs (40).
The 2016 update of the Assessment of
SpondyloArthritis International Society/European League Against
Rheumatism recommendations for managing AS suggests that NSAIDs are
the mainstay of treatment for patients with AS (8). Several studies have indicated that
continuous use of NSAIDs may slow radiographic progression in
symptomatic patients, as assessed by the modified Stoke Ankylosing
Spondylitis spinal score (10,11,41).
Conversely, a recent study demonstrated that continuous treatment
with diclofenac over 2 years was not able to reduce radiographic
progression compared with on-demand treatment (42). There is an ongoing debate regarding
whether NSAIDs are effective in inhibiting new bone formation
(1). To date, the available data are
too scant to draw any conclusions for clinical practice. Further
long-term prospective studies should be performed.
Of note, the present study has several limitations.
Firstly, the literature review only identified a limited number of
the RCTs available, and meloxicam and M2000 were studied in only
one trial, which may affect the robustness of the comparison.
Secondly, different doses of an NSAID were pooled together, which
may have introduced certain heterogeneity into the analysis.
However, in the sensitivity analysis of full-dose trials, the
results were not markedly affected. In addition, there were certain
variations in the pain score, PGA, BASFI at baseline and treatment
duration among trials, which may have affected the efficacy of
NSAIDs. Furthermore, NSAIDs were not assessed in patients with
non-radiographic axial spondyloarthritis due to the paucity of
data. Finally, only the short-term efficacy and safety of NSAIDs
were investigated. Further studies with adequate follow-up are
required to assess the long-term efficacy and safety of NSAIDs in
patients with AS.
In summary, NSAIDs are all highly effective and
well-tolerated compared to placebo in the treatment of AS.
Clinicians should take GI toxicity into account when prescribing
NSAIDs.
Acknowledgements
The results of this study were previously presented
at the 18th Asia Pacific League of Associations for Rheumatology
Congress in Shanghai (China) in 2016 (abstract no.
APL16-0754).
Funding
This work was supported by the National Science
Foundation of China (grant nos. 81871294, 81972204 and 81702327),
the Natural Science Foundation of Guangdong Province (grant no.
2019A1515011097), the President Foundation of Nanfang Hospital,
Southern Medical University (grant no. 2016C010), the China
Postdoctoral Science Foundation (grant nos. 2018M640834 and
2019T120756), the Science and Technology Planning Project of
Guangzhou (grant no. 201904010089), Innovation Program of Shenzhen
(grant no. JCYJ20180508165208399) and the 111 Project from the
Ministry of Education of China (grant no. D18010).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG conceived the study. JG, XL, MF, JL and XW
designed the study. MF and JL performed the literature search and
data extraction. MF and BZ performed the network meta-analysis. JG,
XL, MF, JL and XW interpreted the data. MF and JL wrote the first
draft of the manuscript. XL and JG critically revised the
manuscript and provided final approval of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sieper J and Poddubnyy D: Axial
spondyloarthritis. Lancet. 390:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guan M, Wang J, Zhao L, Xiao J, Li Z and
Shi Z: Management of hip involvement in ankylosing spondylitis.
Clin Rheumatol. 32:1115–1120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang J, Zhang Y, Zhao L, Li ZH and Shi ZJ:
The efficacy and safety of infliximab used in patients with
ankylosing spondylitis after unilateral total hip arthroplasty. Hip
Int. 23:406–410. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guan M, Wang J, Zhu Z, Xiao J, Zhao L, Li
Z and Shi Z: Comparison in clinical features and life impact
between juvenile-onset and adult-onset ankylosing spondylitis. Turk
J Med Sci. 44:601–605. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Boonen A, Chorus A, Miedema H, van der
Heijde D, van der Tempel H and van der Linden S: Employment, work
disability, and work days lost in patients with ankylosing
spondylitis: A cross sectional study of Dutch patients. Ann Rheum
Dis. 60:353–358. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ward MM, Deodhar A, Akl EA, Lui A, Ermann
J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman
RD, et al: American College of Rheumatology/Spondylitis Association
of America/Spondyloarthritis Research and Treatment Network 2015
recommendations for the treatment of ankylosing spondylitis and
nonradiographic axial spondyloarthritis. Arthritis Care Res
(Hoboken). 68:151–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braun J, van den Berg R, Baraliakos X,
Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H,
Dijkmans B, Dougados M, Emery P, et al: 2010 update of the
ASAS/EULAR recommendations for the management of ankylosing
spondylitis. Ann Rheum Dis. 70:896–904. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zochling J, van der Heijde D,
Burgos-Vargas R, Collantes E, Davis JC, Jr..Dijkmans B, Dougados M,
Géher P, Inman RD, Khan MA, et al: ASAS/EULAR recommendations for
the management of ankylosing spondylitis. Ann Rheum Dis.
65:442–452. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Poddubnyy D, Rudwaleit M, Haibel H,
Listing J, Märker-Hermann E, Zeidler H, Braun J and Sieper J:
Effect of non-steroidal anti-inflammatory drugs on radiographic
spinal progression in patients with axial spondyloarthritis:
Results from the German Spondyloarthritis Inception Cohort. Ann
Rheum Dis. 71:1616–1622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wanders A, Heijde D, Landewé R, Béhier JM,
Calin A, Olivieri I, Zeidler H and Dougados M: Nonsteroidal
antiinflammatory drugs reduce radiographic progression in patients
with ankylosing spondylitis: A randomized clinical trial. Arthritis
Rheum. 52:1756–1765. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shukla A, Rai MK, Prasad N and Agarwal V:
Short-term non-steroid anti-inflammatory drug use in
spondyloarthritis patients induces subclinical acute kidney injury:
Biomarkers study. Nephron. 135:277–286. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song IH, Poddubnyy DA, Rudwaleit M and
Sieper J: Benefits and risks of ankylosing spondylitis treatment
with nonsteroidal antiinflammatory drugs. Arthritis Rheum.
58:929–938. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fosbøl EL, Køber L, Torp-Pedersen C and
Gislason GH: Cardiovascular safety of non-steroidal
anti-inflammatory drugs among healthy individuals. Expert Opin Drug
Saf. 9:893–903. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Combe B, Swergold G, McLay J, McCarthy T,
Zerbini C, Emery P, Connors L, Kaur A, Curtis S, Laine L and Cannon
CP: Cardiovascular safety and gastrointestinal tolerability of
etoricoxib vs diclofenac in a randomized controlled clinical trial
(The MEDAL study). Rheumatology (Oxford). 48:425–432.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rostom A, Muir K, Dubé C, Jolicoeur E,
Boucher M, Joyce J, Tugwell P and Wells GW: Gastrointestinal safety
of cyclooxygenase-2 inhibitors: A Cochrane Collaboration systematic
review. Clin Gastroenterol Hepatol. 5:818–828, 828.e1-e5; quiz 768.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Trelle S, Reichenbach S, Wandel S,
Hildebrand P, Tschannen B, Villiger PM, Egger M and Jüni P:
Cardiovascular safety of non-steroidal anti-inflammatory drugs:
Network meta-analysis. BMJ. 342(c7086)2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang F, Gu J, Liu Y, Zhu P, Zheng Y, Fu
J, Pan S and Le S: Efficacy and safety of celecoxib in chinese
patients with ankylosing spondylitis: A 6-week randomized,
double-blinded study with 6-week open-label extension treatment.
Curr Ther Res Clin Exp. 76:126–133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sieper J, Klopsch T, Richter M, Kapelle A,
Rudwaleit M, Schwank S, Regourd E and May M: Comparison of two
different dosages of celecoxib with diclofenac for the treatment of
active ankylosing spondylitis: Results of a 12-week randomised,
double-blind, controlled study. Ann Rheum Dis. 67:323–329.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Heijde D, Baraf HS, Ramos-Remus C,
Calin A, Weaver AL, Schiff M, James M, Markind JE, Reicin AS,
Melian A and Dougados M: Evaluation of the efficacy of etoricoxib
in ankylosing spondylitis: Results of a fifty-two-week, randomized,
controlled study. Arthritis Rheum. 52:1205–1215. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Balazcs E, Sieper J, Bickham K, Mehta A,
Frontera N, Stryszak P, Popmihajlov Z and Peloso PM: A randomized,
clinical trial to assess the relative efficacy and tolerability of
two doses of etoricoxib versus naproxen in patients with ankylosing
spondylitis. BMC Musculoskelet Disord. 17(426)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Walker C, Essex MN, Li C and Park PW:
Celecoxib versus diclofenac for the treatment of ankylosing
spondylitis: 12-week randomized study in Norwegian patients. J Int
Med Res. 44:483–495. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Barkhuizen A, Steinfeld S, Robbins J, West
C, Coombs J and Zwillich S: Celecoxib is efficacious and well
tolerated in treating signs and symptoms of ankylosing spondylitis.
J Rheumatol. 33:1805–1812. 2006.PubMed/NCBI
|
|
25
|
Dougados M, Béhier JM, Jolchine I, Calin
A, van der Heijde D, Olivieri I, Zeidler H and Herman H: Efficacy
of celecoxib, a cyclooxygenase 2-specific inhibitor, in the
treatment of ankylosing spondylitis: A six-week controlled study
with comparison against placebo and against a conventional
nonsteroidal antiinflammatory drug. Arthritis Rheum. 44:180–185.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dougados M, Gueguen A, Nakache JP,
Velicitat P, Veys EM, Zeidler H and Calin A: Ankylosing
spondylitis: What is the optimum duration of a clinical study? A
one year versus a 6 weeks non-steroidal anti-inflammatory drug
trial. Rheumatology (Oxford). 38:235–244. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fattahi MJ, Jamshidi AR, Mahmoudi M,
Vojdanian M, Yekaninejad MS, Jafarnezhad-Ansariha F, Ahmadi H, Rehm
BHA, Matsuo H, Cuzzocrea S, et al: Evaluation of the efficacy and
safety of beta-d-mannuronic acid in patients with ankylosing
spondylitis: A 12-week randomized, placebo-controlled, phase I/II
clinical trial. Int Immunopharmacol. 54:112–117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nieves-Cintrón M, Syed AU, Nystoriak MA
and Navedo MF: Regulation of voltage-gated potassium channels in
vascular smooth muscle during hypertension and metabolic disorders.
Microcirculation. 25:2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Calin A, Garrett S, Whitelock H, Kennedy
LG, O'Hea J, Mallorie P and Jenkinson T: A new approach to defining
functional ability in ankylosing spondylitis: The development of
the Bath Ankylosing Spondylitis Functional Index. J Rheumatol.
21:2281–2285. 1994.PubMed/NCBI
|
|
30
|
Anderson JJ, Baron G, van der Heijde D,
Felson DT and Dougados M: Ankylosing spondylitis assessment group
preliminary definition of short-term improvement in ankylosing
spondylitis. Arthritis Rheum. 44:1876–1886. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lu G and Ades AE: Combination of direct
and indirect evidence in mixed treatment comparisons. Stat Med.
23:3105–3124. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Sutton AJ and Abrams KR: Bayesian methods
in meta-analysis and evidence synthesis. Stat Methods Med Res.
10:277–303. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Caldwell DM, Ades AE and Higgins JP:
Simultaneous comparison of multiple treatments: Combining direct
and indirect evidence. BMJ. 331:897–900. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao BC, Huang TY, Deng QW, Liu WF, Liu J,
Deng WT, Liu KX and Li C: Prophylaxis against atrial fibrillation
after general thoracic surgery: Trial sequential analysis and
network meta-analysis. Chest. 151:149–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Salanti G, Ades AE and Ioannidis JP:
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial. J
Clin Epidemiol. 64:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dougados M, Simon P, Braun J,
Burgos-Vargas R, Maksymowych WP, Sieper J and van der Heijde D:
ASAS recommendations for collecting, analysing and reporting NSAID
intake in clinical trials/epidemiological studies in axial
spondyloarthritis. Ann Rheum Dis. 70:249–251. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang R, Dasgupta A and Ward MM:
Comparative efficacy of non-steroidal anti-inflammatory drugs in
ankylosing spondylitis: A Bayesian network meta-analysis of
clinical trials. Ann Rheum Dis. 75:1152–1160. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jansen JP and Taylor SD:
Cost-effectiveness evaluation of etoricoxib versus celecoxib and
nonselective NSAIDs in the treatment of ankylosing spondylitis in
Norway. Int J Rheumatol. 2011(160326)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fattahi MJ, Abdollahi M, Agha Mohammadi A,
Rastkari N, Khorasani R, Ahmadi H, Tofighi Zavareh F, Sedaghat R,
Tabrizian N and Mirshafiey A: Preclinical assessment of
β-D-mannuronic acid (M2000) as a nonsteroidal anti-inflammatory
drug. Immunopharmacol Immunotoxicol. 37:535–540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kristensen LE, Jakobsen AK, Askling J,
Nilsson F and Jacobsson LT: Safety of etoricoxib, celecoxib, and
nonselective nonsteroidal antiinflammatory drugs in ankylosing
spondylitis and other spondyloarthritis patients: A Swedish
National Population-Based Cohort study. Arthritis Care Res
(Hoboken). 67:1137–1149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kroon F, Landewé R, Dougados M and van der
Heijde D: Continuous NSAID use reverts the effects of inflammation
on radiographic progression in patients with ankylosing
spondylitis. Ann Rheum Dis. 71:1623–1629. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sieper J, Listing J, Poddubnyy D, Song IH,
Hermann KG, Callhoff J, Syrbe U, Braun J and Rudwaleit M: Effect of
continuous versus on-demand treatment of ankylosing spondylitis
with diclofenac over 2 years on radiographic progression of the
spine: Results from a randomised multicentre trial (ENRADAS). Ann
Rheum Dis. 75:1438–1443. 2016.PubMed/NCBI View Article : Google Scholar
|